Brain Activation for Social Cognition and Emotion Processing Tasks in Borderline Personality Disorder: A Meta-Analysis of Neuroimaging Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Task Selection
| Study | n1 | n2 | Task | Contrast |
|---|---|---|---|---|
| Narrow task selection (n = 19) | ||||
| Beeney [60] | 17 | 21 | Judging traits for self and other | Avg. activation for all judgments * |
| Bertsch [65] | 48 | 28 | Social Threat Aggression Paradigm | Aggressive > neutral cues in interaction |
| Cullen [47] | 12 | 12 | Viewing faces, implicit task | Covert fear > neutral faces |
| Guit.-M. [48] | 10 | 10 | Discriminating emotions/orientations | Fearful faces > neutral figures |
| Doell [66] | 21 | 24 | Monetary/social reward feedback task | Social feedback > non-social feedback |
| Domsalla [61] | 20 | 20 | Virtual ball-tossing game (cyberball) | Exclusion > obligatory inclusion |
| Fertuck [56] | 16 | 17 | Rating faces for trustworthiness or fear | Trustw.-to-untrustw. > fearful-to-neutral |
| Fertuck [62] | 23 | 22 | Virtual ball-tossing game (cyberball) | High > low rejection distress |
| Frick [57] | 21 | 20 | Reading Mind in the Eyes (RMET) task | Neg. > neutral emo. (affective mentalizing) |
| Goettlich [68] | 19 | 22 | Read scenarios and imagine taking part | Guilt scenarios (social content) > neutral |
| Herpertz [69] | 33 | 30 | Listen to script, imagine the scene | Avg. activation for interpersonal rejection * |
| Lamers [49] | 20 | 20 | Viewing movie sequences showing faces | Negative > neutral faces |
| Mier [58] | 13 | 13 | Judge intentions from emotional faces | Avg. activation for all judgments * |
| Nicol [50] | 20 | 16 | View faces and judge gender | Negative > neutral faces |
| Olie [63] | 20 | 23 | Virtual ball-tossing game (cyberball) | Exclusion > inclusion |
| Peters [70] | 13 | 16 | Directed Rumination Task | Content previous provocation > neutral |
| v. Schie [67] | 26 | 32 | Receiving feedback about an interview | Negative > positive feedback |
| Wrege [64] | 39 | 29 | Virtual ball-tossing game (cyberball) | Exclusion > inclusion |
| Wrege [51] | 39 | 25 | View faces and judge gender | Negative > neutral faces |
| Additional tasks for extended task selection (combined n = 29) | ||||
| Hazlett [72] | 33 | 32 | Judging valence of repeated IAPS pict. | Repeated unpleasant pictures * |
| Herpertz [73] | 6 | 6 | Passive viewing of IAPS pictures | Negative > neutral IAPS pictures |
| Koenigsb. [74] | 18 | 16 | Rating own emotion for IAPS pictures | Negative > neutral IAPS pictures |
| Koenigsb. [75] | 19 | 17 | Passive viewing of IAPS pictures | Negative IAPS > resting baseline * |
| Koenigsb. [82] | 19 | 25 | Viewing IAPS and Empathy 1 Pictures | Avg. activ. negative pictures * |
| Niedtfeld [76] | 20 | 23 | Passive viewing of IAPS pictures | Avg. activation for negative pictures * |
| Scherpiet [77] | 18 | 18 | Passive viewing of IAPS pictures | Negative > neutral IAPS pictures |
| Schnell [78] | 14 | 14 | Passive viewing of IAPS pictures | Avg. activation for negative pictures * |
| Schulze [79] | 15 | 15 | Passive viewing of IAPS pictures | Negative > neutral IAPS pictures |
| v. Zutph. [80] | 55 | 42 | Passive viewing of IAPS pictures | Negative > neutral IAPS pictures |
2.3. Meta-Analysis Methods
3. Results
4. Discussion
4.1. Meta-Analysis of the Extended Task Selection
4.2. Meta-Analysis of the Narrow Task Selection
4.3. The Relation between Results of the Extended and Narrow Task Selection
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gunderson, J.G.; Links, P.S. Borderline Personality Disorder: A Clinical Guide, 2nd ed.; APA: Washington, DC, USA, 2008. [Google Scholar]
- Kernberg, O.F.; Michels, R. Borderline Personality Disorder. Am. J. Psychiatry 2009, 166, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Lieb, K.; Zanarini, M.C.; Schmahl, C.; Linehan, M.M.; Bohus, M. Borderline personality disorder. Lancet 2004, 364, 453–461. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Ebner-Priemer, U.W.; Houben, M.; Santangelo, P.; Kleindienst, N.; Tuerlinckx, F.; Oravecz, Z.; Verleysen, G.; Van Deun, K.; Bohus, M.; Kuppens, P. Unraveling affective dysregulation in borderline personality disorder: A theoretical model and empirical evidence. J. Abnorm. Psychol. 2015, 124, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Brendel, G.R.; Stern, E.; Silbersweig, D.A. Defining the neurocircuitry of borderline personality disorder: Functional neuroimaging approaches. Dev. Psychopathol. 2005, 17, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Sicorello, M.; Schmahl, C. Emotion dysregulation in borderline personality disorder: A fronto–limbic imbalance? Curr. Opin. Psychol. 2021, 37, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Schulze, L.; Schulze, A.; Renneberg, B.; Schmahl, C.; Niedtfeld, I. Neural Correlates of Affective Disturbances: A Comparative Meta-analysis of Negative Affect Processing in Borderline Personality Disorder, Major Depressive Disorder, and Posttraumatic Stress Disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2019, 4, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Degasperi, G.; Cristea, I.A.; Di Rosa, E.; Costa, C.; Gentili, C. Parsing variability in borderline personality disorder: A meta-analysis of neuroimaging studies. Transl. Psychiatry 2021, 11, 314. [Google Scholar] [CrossRef] [PubMed]
- Pan, N.; Wang, S.; Qin, K.; Li, L.; Chen, Y.; Zhang, X.; Lai, H.; Suo, X.; Long, Y.; Yu, Y.; et al. Common and Distinct Neural Patterns of Attention-Deficit/Hyperactivity Disorder and Borderline Personality Disorder: A Multimodal Functional and Structural Meta-analysis. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2023, 8, 640–650. [Google Scholar] [CrossRef] [PubMed]
- McTeague, L.M.; Rosenberg, B.M.; Lopez, J.W.; Carreon, D.M.; Huemer, J.; Jiang, Y.; Chick, C.F.; Eickhoff, S.B.; Etkin, A. Identification of Common Neural Circuit Disruptions in Emotional Processing Across Psychiatric Disorders. Am. J. Psychiatry 2020, 177, 411–421. [Google Scholar] [CrossRef] [PubMed]
- McTeague, L.M.; Huemer, J.; Carreon, D.M.; Jiang, Y.; Eickhoff, S.B.; Etkin, A. Identification of Common Neural Circuit Disruptions in Cognitive Control Across Psychiatric Disorders. Am. J. Psychiatry 2017, 174, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Buchheim, A. Borderline Persönlichkeitsstörung und Bindungserfahrung. In Handbuch der Borderline Persönlichkeitsstörungen; Dulz, B., Herpertz, S.C., Sachsse, U., Kernberg, O.F., Eds.; Schattauer: Stuttgart, Germany, 2011; pp. 158–167. [Google Scholar]
- Buchheim, A.; Diamond, D. Attachment and Borderline Personality Disorder. Psychiatr. Clin. North. Am. 2018, 41, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Viviani, R.; Kächele, H.; Buchheim, A. Models of Change in the Psychotherapy of Borderline Personality Disorders. Neuropsychoanalysis 2011, 13, 147–160. [Google Scholar] [CrossRef]
- Buchheim, A.; Erk, S.; George, C.; Kächele, H.; Kircher, T.; Martius, P.; Pokorny, D.; Ruchsow, M.; Spitzer, M.; Walter, H. Neural correlates of attachment trauma in borderline personality disorder:: A functional magnetic resonance imaging study. Psychiat Res.-Neuroim 2008, 163, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Buchheim, A.; Erk, S.; George, C.; Kächele, H.; Martius, P.; Pokorny, D.; Spitzer, M.; Walter, H. Neural Response during the Activation of the Attachment System in Patients with Borderline Personality Disorder: An fMRI Study. Front. Hum. Neurosci. 2016, 10, 389. [Google Scholar] [CrossRef] [PubMed]
- Buchheim, A.G.; GeorGe, C. Attachment disorganization in borderline personality disorder and anxiety disorder. In Disorganization of Attachment and Caregiving; Solomon, J., George, V., Eds.; Guilford Press: New York, NY, USA, 2011; pp. 343–383. [Google Scholar]
- De Meulemeester, C.; Lowyck, B.; Luyten, P. The role of impairments in self–other distinction in borderline personality disorder: A narrative review of recent evidence. Neurosci. Biobehav. Rev. 2021, 127, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Németh, N.; Mátrai, P.; Hegyi, P.; Czéh, B.; Czopf, L.; Hussain, A.; Pammer, J.; Szabó, I.; Solymár, M.; Kiss, L.; et al. Theory of mind disturbances in borderline personality disorder: A meta-analysis. Psychiatry Res. 2018, 270, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Luyten, P.; Campbell, C.; Allison, E.; Fonagy, P. The Mentalizing Approach to Psychopathology: State of the Art and Future Directions. Annu. Rev. Clin. Psychol. 2020, 16, 297–325. [Google Scholar] [CrossRef] [PubMed]
- Fischer-Kern, M.; Doering, S.; Taubner, S.; Horz, S.; Zimmermann, J.; Rentrop, M.; Schuster, P.; Buchheim, P.; Buchheim, A. Transference-focused psychotherapy for borderline personality disorder: Change in reflective function. Br. J. Psychiatry 2015, 207, 173–174. [Google Scholar] [CrossRef] [PubMed]
- Keefe, J.R.; Levy, K.N.; Sowislo, J.F.; Diamond, D.; Doering, S.; Horz-Sagstetter, S.; Buchheim, A.; Fischer-Kern, M.; Clarkin, J.F. Reflective functioning and its potential to moderate the efficacy of manualized psychodynamic therapies versus other treatments for borderline personality disorder. J. Consult. Clin. Psychol. 2023, 91, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Fonagy, P.; Luyten, P. A developmental, mentalization-based approach to the understanding and treatment of borderline personality disorder. Dev. Psychopathol. 2009, 21, 1355–1381. [Google Scholar] [CrossRef] [PubMed]
- Labek, K.; Viviani, R.; Buchheim, A. Konzeption der borderline-persönlichkeitsstörung aus neurobiologischer Sicht. PTT-Persönlichkeitsstörungen Theor. Und Ther. 2019, 23, 310–320. [Google Scholar]
- Ripoll, L.H.; Snyder, R.; Steele, H.; Siever, L.J. The Neurobiology of Empathy in Borderline Personality Disorder. Curr. Psychiatry Rep. 2013, 15, 344. [Google Scholar] [CrossRef] [PubMed]
- Molenberghs, P.; Johnson, H.; Henry, J.D.; Mattingley, J.B. Understanding the minds of others: A neuroimaging meta-analysis. Neurosci. Biobehav. Rev. 2016, 65, 276–291. [Google Scholar] [CrossRef] [PubMed]
- Van Overwalle, F. Social cognition and the brain: A meta-analysis. Hum. Brain Mapp. 2009, 30, 829–858. [Google Scholar] [CrossRef] [PubMed]
- Maliske, L.Z.; Schurz, M.; Kanske, P. Interactions within the social brain: Co-activation and connectivity among networks enabling empathy and Theory of Mind. Neurosci. Biobehav. Rev. 2023, 147, 105080. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Duncan, N.W.; de Greck, M.; Northoff, G. Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neurosci. Biobehav. Rev. 2011, 35, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Lamm, C.; Decety, J.; Singer, T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage 2011, 54, 2492–2502. [Google Scholar] [CrossRef] [PubMed]
- Schurz, M.; Radua, J.; Tholen, M.G.; Maliske, L.; Margulies, D.S.; Mars, R.B.; Sallet, J.; Kanske, P. Toward a hierarchical model of social cognition: A neuroimaging meta-analysis and integrative review of empathy and theory of mind. Psychol. Bull. 2021, 147, 293–327. [Google Scholar] [CrossRef] [PubMed]
- Timmers, I.; Park, A.L.; Fischer, M.D.; Kronman, C.A.; Heathcote, L.C.; Hernandez, J.M.; Simons, L.E. Is Empathy for Pain Unique in Its Neural Correlates? A Meta-Analysis of Neuroimaging Studies of Empathy. Front. Behav. Neurosci. 2018, 12, 289. [Google Scholar] [CrossRef] [PubMed]
- Gallese, V. The Roots of Empathy: The Shared Manifold Hypothesis and the Neural Basis of Intersubjectivity. Psychopathology 2003, 36, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Titchener, E.B. Experimental Psychology of the Thought-Processes; MacMillan: New York, NY, USA, 1909. [Google Scholar]
- Adolphs, R. The social brain: Neural basis of social knowledge. Annu. Rev. Psychol. 2009, 60, 693–716. [Google Scholar] [CrossRef] [PubMed]
- Kanske, P. The social mind: Disentangling affective and cognitive routes to understanding others. Interdiscip. Sci. Rev. 2018, 43, 115–124. [Google Scholar] [CrossRef]
- Perner, J.; Aichhorn, M.; Tholen, M.G.; Schurz, M. Mental Files and Teleology. In The Neural Basis of Mentalizing; Springer International Publishing: Cham, Switzerland, 2021; pp. 257–281. [Google Scholar] [CrossRef]
- Premack, D.; Woodruff, G. Does the chimpanzee have a theory of mind? Behav. Brain Sci. 1978, 1, 515–526. [Google Scholar] [CrossRef]
- Hardwick, R.M.; Caspers, S.; Eickhoff, S.B.; Swinnen, S.P. Neural correlates of action: Comparing meta-analyses of imagery, observation, and execution. Neurosci. Biobehav. Rev. 2018, 94, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Dricu, M.; Frühholz, S. A neurocognitive model of perceptual decision-making on emotional signals. Hum. Brain Mapp. 2020, 41, 1532–1556. [Google Scholar] [CrossRef]
- Vijayakumar, N.; Cheng, T.W.; Pfeifer, J.H. Neural correlates of social exclusion across ages: A coordinate-based meta-analysis of functional MRI studies. Neuroimage 2017, 153, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Schilbach, L.; Timmermans, B.; Reddy, V.; Costall, A.; Bente, G.; Schlicht, T.; Vogeley, K. Toward a second-person neuroscience. Behav. Brain Sci. 2013, 36, 393–414. [Google Scholar] [CrossRef] [PubMed]
- Albajes-Eizagirre, A.; Solanes, A.; Fullana, M.A.; Ioannidis, J.P.A.; Fusar-Poli, P.; Torrent, C.; Solé, B.; Bonnín, C.M.; Vieta, E.; Mataix-Cols, D.; et al. Meta-analysis of Voxel-Based Neuroimaging Studies using Seed-based d Mapping with Permutation of Subject Images (SDM-PSI). J. Vis. Exp. 2019, 153, e59841. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Radua, J.; Mataix-Cols, D.; Phillips, M.L.; El-Hage, W.; Kronhaus, D.M.; Cardoner, N.; Surguladze, S. A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur. Psychiatry 2011, 27, 605–611. [Google Scholar] [CrossRef]
- Cullen, K.R.; LaRiviere, L.L.; Vizueta, N.; Thomas, K.M.; Hunt, R.H.; Miller, M.J.; Lim, K.O.; Schulz, S.C. Brain activation in response to overt and covert fear and happy faces in women with borderline personality disorder. Brain Imaging Behav. 2016, 10, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Guitart-Masip, M.; Pascual, J.C.; Carmona, S.; Hoekzema, E.; Bergé, D.; Pérez, V.; Soler, J.; Soliva, J.C.; Rovira, M.; Bulbena, A.; et al. Neural correlates of impaired emotional discrimination in borderline personality disorder: An fMRI study. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2009, 33, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Lamers, A.; Toepper, M.; Fernando, S.C.; Schlosser, N.; Bauer, E.; Woermann, F.; Driessen, M.; Beblo, T. Nonacceptance of negative emotions in women with borderline personality disorder: Association with neuroactivity of the dorsal striatum. J. Psychiatry Neurosci. 2019, 44, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Nicol, K.; Pope, M.; Romaniuk, L.; Hall, J. Childhood trauma, midbrain activation and psychotic symptoms in borderline personality disorder. Transl. Psychiatry 2015, 5, e559. [Google Scholar] [CrossRef] [PubMed]
- Wrege, J.S.; Ruocco, A.C.; Carcone, D.; Lang, U.E.; Lee, A.C.H.; Walter, M. Facial Emotion Perception in Borderline Personality Disorder: Differential Neural Activation to Ambiguous and Threatening Expressions and Links to Impairments in Self and Interpersonal Functioning. J. Affect. Disord. 2021, 284, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Baron-Cohen, S. The extreme male brain theory of autism. Trends Cogn. Sci. 2002, 6, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Schulte-Ruther, M.; Markowitsch, H.J.; Fink, G.R.; Piefke, M. Mirror neuron and theory of mind mechanisms involved in face-to-face interactions: A functional magnetic resonance imaging approach to empathy. J. Cogn. Neurosci. 2007, 19, 1354–1372. [Google Scholar] [CrossRef] [PubMed]
- Carr, L.; Iacoboni, M.; Dubeau, M.C.; Mazziotta, J.C.; Lenzi, G.L. Neural mechanisms of empathy in humans: A relay from neural systems for imitation to limbic areas. Proc. Natl. Acad. Sci. USA 2003, 100, 5497–5502. [Google Scholar] [CrossRef] [PubMed]
- Wicker, B.; Keysers, C.; Plailly, J.; Royet, J.P.; Gallese, V.; Rizzolatti, G. Both of us disgusted in Insula: The common neural basis of seeing and feeling disgust. Neuron 2003, 40, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Fertuck, E.A.; Grinband, J.; Mann, J.J.; Hirsch, J.; Ochsner, K.; Pilkonis, P.; Erbe, J.; Stanley, B. Trustworthiness appraisal deficits in borderline personality disorder are associated with prefrontal cortex, not amygdala, impairment. Neuroimage Clin. 2019, 21, 101616. [Google Scholar] [CrossRef] [PubMed]
- Frick, C.; Lang, S.; Kotchoubey, B.; Sieswerda, S.; Dinu-Biringer, R.; Berger, M.; Veser, S.; Essig, M.; Barnow, S. Hypersensitivity in borderline personality disorder during mindreading. PLoS ONE 2012, 7, e41650. [Google Scholar] [CrossRef] [PubMed]
- Mier, D.; Lis, S.; Esslinger, C.; Sauer, C.; Hagenhoff, M.; Ulferts, J.; Gallhofer, B.; Kirsch, P. Neuronal correlates of social cognition in borderline personality disorder. Soc. Cogn. Affect. Neurosci. 2013, 8, 531–537. [Google Scholar] [CrossRef] [PubMed]
- BaronCohen, S.; Jolliffe, T.; Mortimore, C.; Robertson, M. Another advanced test of theory of mind: Evidence from very high functioning adults with autism or Asperger syndrome. J. Child. Psychol. Psyc 1997, 38, 813–822. [Google Scholar] [CrossRef]
- Beeney, J.E.; Hallquist, M.N.; Ellison, W.D.; Levy, K.N. Self-other disturbance in borderline personality disorder: Neural, self-report, and performance-based evidence. Pers. Disord. 2016, 7, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Domsalla, M.; Koppe, G.; Niedtfeld, I.; Vollstädt-Klein, S.; Schmahl, C.; Bohus, M.; Lis, S. Cerebral processing of social rejection in patients with borderline personality disorder. Soc. Cogn. Affect. Neurosci. 2014, 9, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Fertuck, E.A.; Stanley, B.; Kleshchova, O.; Mann, J.J.; Hirsch, J.; Ochsner, K.; Pilkonis, P.; Erbe, J.; Grinband, J. Rejection Distress Suppresses Medial Prefrontal Cortex in Borderline Personality Disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2023, 8, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Olié, E.; Doell, K.C.; Corradi-Dell’Acqua, C.; Courtet, P.; Perroud, N.; Schwartz, S. Physical pain recruits the nucleus accumbens during social distress in borderline personality disorder. Soc. Cogn. Affect. Neurosci. 2018, 13, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Wrege, J.S.; Ruocco, A.C.; Euler, S.; Preller, K.H.; Busmann, M.; Meya, L.; Schmidt, A.; Lang, U.E.; Borgwardt, S.; Walter, M. Negative affect moderates the effect of social rejection on frontal and anterior cingulate cortex activation in borderline personality disorder. Cogn. Affect. Behav. Neurosci. 2019, 19, 1273–1285. [Google Scholar] [CrossRef] [PubMed]
- Bertsch, K.; Buades-Rotger, M.; Krauch, M.; Ueltzhöffer, K.; Kleindienst, N.; Herpertz, S.C.; Krämer, U.M. Abnormal processing of interpersonal cues during an aggressive encounter in women with borderline personality disorder: Neural and behavioral findings. J. Psychopathol. Clin. Sci. 2022, 131, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Doell, K.C.; Olié, E.; Courtet, P.; Corradi-Dell’Acqua, C.; Perroud, N.; Schwartz, S. Atypical processing of social anticipation and feedback in borderline personality disorder. Neuroimage Clin. 2020, 25, 102126. [Google Scholar] [CrossRef] [PubMed]
- van Schie, C.C.; Chiu, C.-D.; Rombouts, S.A.R.B.; Heiser, W.J.; Elzinga, B.M. Stuck in a negative me: fMRI study on the role of disturbed self-views in social feedback processing in borderline personality disorder. Psychol. Med. 2020, 50, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Göttlich, M.; Westermair, A.L.; Beyer, F.; Bußmann, M.L.; Schweiger, U.; Krämer, U.M. Neural basis of shame and guilt experience in women with borderline personality disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2020, 270, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Herpertz, S.C.; Nagy, K.; Ueltzhöffer, K.; Schmitt, R.; Mancke, F.; Schmahl, C.; Bertsch, K. Brain Mechanisms Underlying Reactive Aggression in Borderline Personality Disorder—Sex Matters. Biol. Psychiatry 2017, 82, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.R.; Chester, D.S.; Walsh, E.C.; DeWall, C.N.; Baer, R.A. The rewarding nature of provocation-focused rumination in women with borderline personality disorder: A preliminary fMRI investigation. Borderline Pers. Disord. Emot. Dysregul 2018, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Eickhoff, S.B.; Li, T.; Wang, L.; Becker, B.; Camilleri, J.A.; Hétu, S.; Luo, Y. Common brain networks underlying human social interactions: Evidence from large-scale neuroimaging meta-analysis. Neurosci. Biobehav. Rev. 2021, 126, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Hazlett, E.A.; Zhang, J.; New, A.S.; Zelmanova, Y.; Goldstein, K.E.; Haznedar, M.M.; Meyerson, D.; Goodman, M.; Siever, L.J.; Chu, K.-W. Potentiated amygdala response to repeated emotional pictures in borderline personality disorder. Biol. Psychiatry 2012, 72, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Herpertz, S.C.; Dietrich, T.M.; Wenning, B.; Krings, T.; Erberich, S.G.; Willmes, K.; Thron, A.; Sass, H. Evidence of abnormal amygdala functioning in borderline personality disorder: A functional MRI study. Biol. Psychiatry 2001, 50, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Koenigsberg, H.W.; Fan, J.; Ochsner, K.N.; Liu, X.; Guise, K.G.; Pizzarello, S.; Dorantes, C.; Guerreri, S.; Tecuta, L.; Goodman, M.; et al. Neural correlates of the use of psychological distancing to regulate responses to negative social cues: A study of patients with borderline personality disorder. Biol. Psychiatry 2009, 66, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Koenigsberg, H.W.; Siever, L.J.; Lee, H.; Pizzarello, S.; New, A.S.; Goodman, M.; Cheng, H.; Flory, J.; Prohovnik, I. Neural correlates of emotion processing in borderline personality disorder. Psychiatry Res. 2009, 172, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Niedtfeld, I.; Schulze, L.; Kirsch, P.; Herpertz, S.C.; Bohus, M.; Schmahl, C. Affect Regulation and Pain in Borderline Personality Disorder: A Possible Link to the Understanding of Self-Injury. Biol. Psychiatry 2010, 68, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Scherpiet, S.; Brühl, A.B.; Opialla, S.; Roth, L.; Jäncke, L.; Herwig, U. Altered emotion processing circuits during the anticipation of emotional stimuli in women with borderline personality disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2013, 264, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Schnell, K.; Herpertz, S.C. Effects of dialectic-behavioral-therapy on the neural correlates of affective hyperarousal in borderline personality disorder. J. Psychiatr. Res. 2007, 41, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Schulze, L.; Domes, G.; Krüger, A.; Berger, C.; Fleischer, M.; Prehn, K.; Schmahl, C.; Grossmann, A.; Hauenstein, K.; Herpertz, S.C. Neuronal Correlates of Cognitive Reappraisal in Borderline Patients with Affective Instability. Biol. Psychiatry 2011, 69, 564–573. [Google Scholar] [CrossRef] [PubMed]
- van Zutphen, L.; Siep, N.; Jacob, G.A.; Domes, G.; Sprenger, A.; Willenborg, B.; Goebel, R.; Arntz, A. Always on guard: Emotion regulation in women with borderline personality disorder compared to nonpatient controls and patients with cluster-C personality disorder. J. Psychiatry Neurosci. 2018, 43, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Lang, P.J.; Bradley Margaret, M.; Cuthbert Bruce, N. International Affective Picture System (IAPS): Instruction Manual and Affective Ratings, Technical Report A-8; The Center for Research in Psychophysiology, University of Florida: Gainesville, FL, USA, 2008. [Google Scholar]
- Koenigsberg, H.W.; Denny, B.T.; Fan, J.; Liu, X.; Guerreri, S.; Mayson, S.J.; Rimsky, L.; New, A.S.; Goodman, M.; Siever, L.J. The neural correlates of anomalous habituation to negative emotional pictures in borderline and avoidant personality disorder patients. Am. J. Psychiatry 2014, 171, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Geday, J.; Gjedde, A.; Boldsen, A.S.; Kupers, R. Emotional valence modulates activity in the posterior fusiform gyrus and inferior medial prefrontal cortex in social perception. Neuroimage 2003, 18, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Albajes-Eizagirre, A.; Solanes, A.; Vieta, E.; Radua, J. Voxel-based meta-analysis via permutation of subject images (PSI): Theory and implementation for SDM. NeuroImage 2019, 186, 174–184. [Google Scholar] [CrossRef]
- Zhang, E.; Hauson, A.O.; Pollard, A.A.; Meis, B.; Lackey, N.S.; Carson, B.; Khayat, S.; Fortea, L.; Radua, J. Lateralized grey matter volume changes in adolescents versus adults with major depression: SDM-PSI meta-analysis. Psychiatry Res. Neuroimaging 2023, 335, 111691. [Google Scholar] [CrossRef] [PubMed]
- Margulies, D.S.; Ghosh, S.S.; Goulas, A.; Falkiewicz, M.; Huntenburg, J.M.; Langs, G.; Bezgin, G.; Eickhoff, S.B.; Castellanos, F.X.; Petrides, M.; et al. Situating the default-mode network along a principal gradient of macroscale cortical organization. Proc. Natl. Acad. Sci. USA 2016, 113, 12574–12579. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Ruocco, A.C.; Amirthavasagam, S.; Choi-Kain, L.W.; McMain, S.F. Neural Correlates of Negative Emotionality in Borderline Personality Disorder: An Activation-Likelihood-Estimation Meta-Analysis. Biol. Psychiatry 2013, 73, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Messina, I.; Sambin, M.; Beschoner, P.; Viviani, R. Changing views of emotion regulation and neurobiological models of the mechanism of action of psychotherapy. Cogn. Affect. Behav. Neurosci. 2016, 16, 571–587. [Google Scholar] [CrossRef] [PubMed]
- Crowell, S.E.; Beauchaine, T.P.; Linehan, M.M. A biosocial developmental model of borderline personality: Elaborating and extending Linehan’s theory. Psychol. Bull. 2009, 135, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Schoenbaum, G.; Setlow, B.; Saddoris, M.P.; Gallagher, M. Encoding predicted outcome and acquired value in orbitofrontal cortex during cue sampling depends upon input from basolateral amygdala. Neuron 2003, 39, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Seymour, B.; Dolan, R. Emotion, decision making, and the amygdala. Neuron 2008, 58, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Labek, K.; Sittenberger, E.; Kienhofer, V.; Rabl, L.; Messina, I.; Schurz, M.; Stingl, J.C.; Viviani, R. The gradient model of brain organization in decisions involving “empathy for pain”. Cereb. Cortex 2023, 33, 5839–5850. [Google Scholar] [CrossRef] [PubMed]
- Viviani, R. Emotion regulation, attention to emotion, and the ventral attentional network. Front. Hum. Neurosci. 2013, 7, 746. [Google Scholar] [CrossRef] [PubMed]
- Yeo, B.T.T.; Krienen, F.M.; Sepulcre, J.; Sabuncu, M.R.; Lashkari, D.; Hollinshead, M.; Roffman, J.L.; Smoller, J.W.; Zöllei, L.; Polimeni, J.R.; et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011, 106, 1125–1165. [Google Scholar] [CrossRef] [PubMed]
- Yarkoni, T.; Poldrack, R.A.; Nichols, T.E.; Van Essen, D.C.; Wager, T.D. Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods 2011, 8, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Andrews-Hanna, J.R.; Smallwood, J.; Spreng, R.N. The default network and self-generated thought: Component processes, dynamic control, and clinical relevance. Ann. N. Y Acad. Sci. 2014, 1316, 29–52. [Google Scholar] [CrossRef]
- Buckner, R.L.; Carroll, D.C. Self-projection and the brain. Trends Cogn. Sci. 2007, 11, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Bzdok, D.; Langner, R.; Schilbach, L.; Jakobs, O.; Roski, C.; Caspers, S.; Laird, A.R.; Fox, P.T.; Zilles, K.; Eickhoff, S.B. Characterization of the temporo-parietal junction by combining data-driven parcellation, complementary connectivity analyses, and functional decoding. Neuroimage 2013, 81, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.L.; Davachi, L.; Ochsner, K.N.; Lieberman, M.D. Evidence That Default Network Connectivity During Rest Consolidates Social Information. Cereb. Cortex 2019, 29, 1910–1920. [Google Scholar] [CrossRef] [PubMed]
- Spreng, R.N.; Grady, C.L. Patterns of Brain Activity Supporting Autobiographical Memory, Prospection, and Theory of Mind, and Their Relationship to the Default Mode Network. J. Cogn. Neurosci. 2010, 22, 1112–1123. [Google Scholar] [CrossRef] [PubMed]
- Spreng, R.N.; Mar, R.A.; Kim, A.S.N. The Common Neural Basis of Autobiographical Memory, Prospection, Navigation, Theory of Mind, and the Default Mode: A Quantitative Meta-analysis. J. Cogn. Neurosci. 2009, 21, 489–510. [Google Scholar] [CrossRef] [PubMed]
- Spunt, R.P.; Meyer, M.L.; Lieberman, M.D. The Default Mode of Human Brain Function Primes the Intentional Stance. J. Cogn. Neurosci. 2015, 27, 1116–1124. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bernheim, D.; Buchheim, A.; Domin, M.; Mentel, R.; Lotze, M. Neural Correlates of Attachment Representation in Patients With Borderline Personality Disorder Using a Personalized Functional Magnet Resonance Imaging Task. Front. Hum. Neurosci. 2022, 16, 810417. [Google Scholar] [CrossRef] [PubMed]
- Flechsig, A.; Bernheim, D.; Buchheim, A.; Domin, M.; Mentel, R.; Lotze, M. One Year of Outpatient Dialectical Behavioral Therapy and Its Impact on Neuronal Correlates of Attachment Representation in Patients with Borderline Personality Disorder Using a Personalized fMRI Task. Brain Sci. 2023, 13, 1001. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.P. Inferences about mental states. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.P.; Banaji, M.R.; Macrae, C.N. The Link between Social Cognition and Self-referential Thought in the Medial Prefrontal Cortex. J. Cogn. Neurosci. 2005, 17, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Tamir, D.I.; Mitchell, J.P. Neural correlates of anchoring-and-adjustment during mentalizing. Proc. Natl. Acad. Sci. USA 2010, 107, 10827–10832. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.P.; Macrae, C.N.; Banaji, M.R. Dissociable Medial Prefrontal Contributions to Judgments of Similar and Dissimilar Others. Neuron 2006, 50, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Eddy, C.M. The junction between self and other? Temporo-parietal dysfunction in neuropsychiatry. Neuropsychologia 2016, 89, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Lamm, C.; Bukowski, H.; Silani, G. From shared to distinct self-other representations in empathy: Evidence from neurotypical function and socio-cognitive disorders. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150083. [Google Scholar] [CrossRef] [PubMed]
- Quesque, F.; Brass, M. The Role of the Temporoparietal Junction in Self-Other Distinction. Brain Topogr. 2019, 32, 943–955. [Google Scholar] [CrossRef] [PubMed]
- Bowlby, J. Attachment and Loss. In Seperation: Anxiety and Anger; Basic Books: New York, NY, USA, 1973. [Google Scholar]
- Milrod, B.; Markowitz, J.C.; Gerber, A.J.; Cyranowski, J.; Altemus, M.; Shapiro, T.; Hofer, M.; Glatt, C. Childhood separation anxiety and the pathogenesis and treatment of adult anxiety. Am. J. Psychiatry 2014, 171, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Mosquera, D.; Gonzalez, A.; Leeds, A.M. Early experience, structural dissociation, and emotional dysregulation in borderline personality disorder: The role of insecure and disorganized attachment. Borderline Pers. Disord. Emot. Dysregul 2014, 1, 15. [Google Scholar] [CrossRef] [PubMed]
- Brune, M.; Walden, S.; Edel, M.A.; Dimaggio, G. Mentalization of complex emotions in borderline personality disorder: The impact of parenting and exposure to trauma on the performance in a novel cartoon-based task. Compr. Psychiatry 2016, 64, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Buchheim, A.; Hörz-Sagstetter, S.; Doering, S.; Rentrop, M.; Schuster, P.; Buchheim, P.; Pokorny, D.; Fischer-Kern, M. Change of Unresolved Attachment in Borderline Personality Disorder: RCT Study of Transference-Focused Psychotherapy. Psychother. Psychosom. 2017, 86, 314–316. [Google Scholar] [CrossRef] [PubMed]
- Bandelow, B.; Krause, J.; Wedekind, D.; Broocks, A.; Hajak, G.; Ruther, E. Early traumatic life events, parental attitudes, family history, and birth risk factors in patients with borderline personality disorder and healthy controls. Psychiatry Res. 2005, 134, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Gunderson, J.G.; Daversa, M.T.; Grilo, C.M.; McGlashan, T.H.; Zanarini, M.C.; Shea, M.T.; Skodol, A.E.; Yen, S.; Sanislow, C.A.; Bender, D.S.; et al. Predictors of 2-year outcome for patients with borderline personality disorder. Am. J. Psychiatry 2006, 163, 822–826. [Google Scholar] [CrossRef] [PubMed]
- Zanarini, M.C.; Frankenburg, F.R.; Hennen, J.; Reich, D.B.; Silk, K.R. Prediction of the 10-year course of borderline personality disorder. Am. J. Psychiatry 2006, 163, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Pagura, J.; Stein, M.B.; Bolton, J.M.; Cox, B.J.; Grant, B.; Sareen, J. Comorbidity of borderline personality disorder and posttraumatic stress disorder in the U.S. population. J. Psychiatr. Res. 2010, 44, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.G.; Felker, B.L.; Liu, C.F.; Yano, E.M.; Kirchner, J.E.; Chan, D.; Rubenstein, L.V.; Chaney, E.F. Prevalence of depression-PTSD comorbidity: Implications for clinical practice guidelines and primary care-based interventions. J. Gen. Intern. Med. 2007, 22, 711–718. [Google Scholar] [CrossRef] [PubMed]

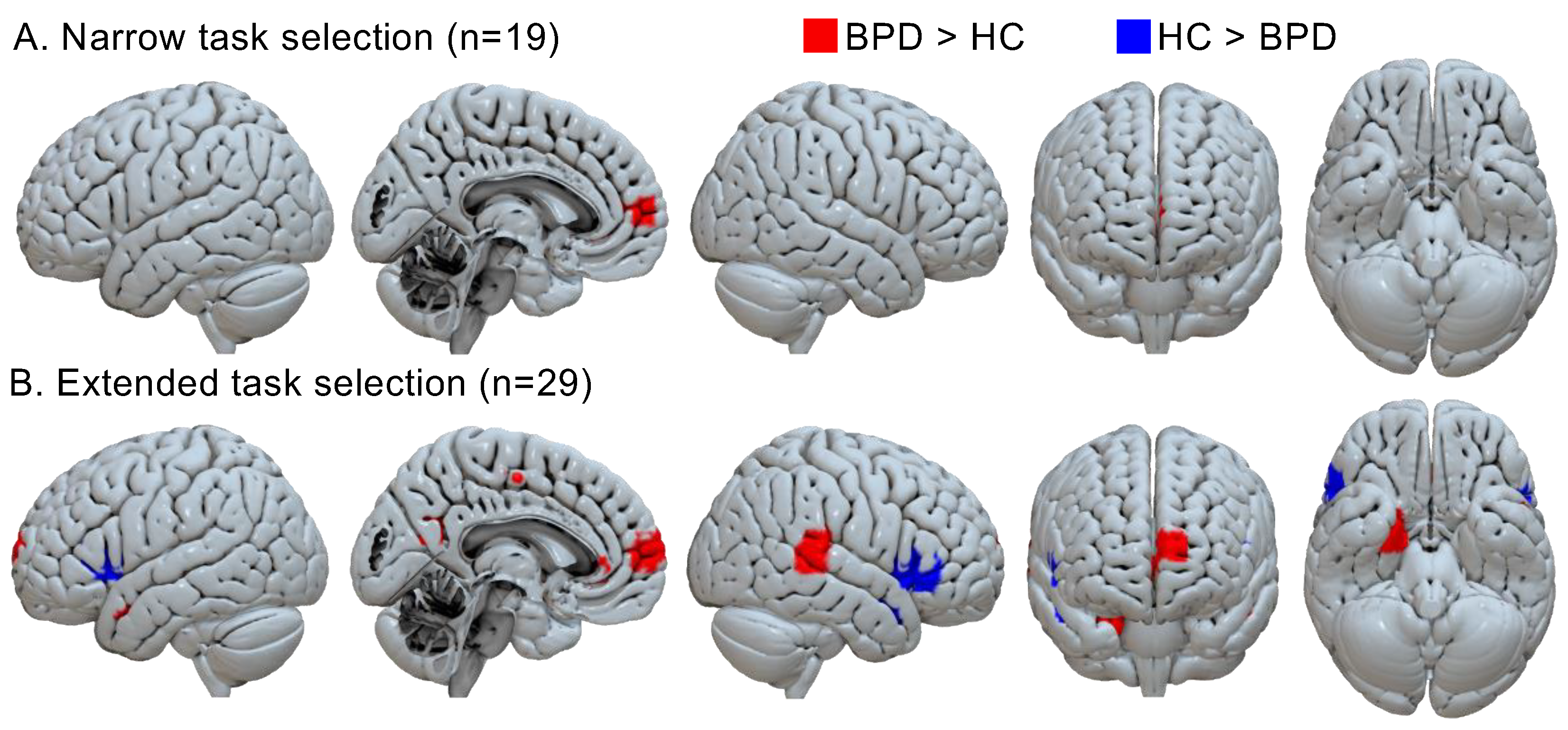
| Cluster Peak | Sub-Peaks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Label | x | y | z | z-Val | jk/Perm. | vx | x | y | z | Label |
| Narrow task selection (n = 19) | ||||||||||
| Borderline personality disorder > healthy controls | ||||||||||
| R ant. cing. g. | 8 | 38 | 0 | 3.10 | 17/19 | 30 | 12 | 46 | 2 | R ant. cing. g. |
| L sup. front. g. | −8 | 58 | 10 | 3.23 | 17/19 | 13 | ||||
| L ant. cing. g. | −12 | 46 | 8 | 3.31 | 17/19 | 12 | ||||
| Healthy controls > borderline personality disorder | ||||||||||
Extended task selection (including IAPS tasks, n = 29) | ||||||||||
| Borderline personality disorder > healthy controls | ||||||||||
| R parahipp. g. | 22 | 0 | −26 | 3.43 | 100 | 246 | 26 | −4 | −24 | R parahipp. g. |
| 22 | −3 | −16 | R amygdala | |||||||
| R ant. cing. g. | 12 | 44 | 4 | 3.94 | 29 | 118 | 10 | 38 | −2 | R ant. cing. g. |
| R sup. temp. g. | 64 | −32 | 12 | 3.25 | 98 | 93 | 64 | −38 | 4 | R mid. temp. g. |
| L sup. front. g. | −8 | 60 | 12 | 3.75 | 28 | 72 | ||||
| R med. cing. g. | 8 | −16 | 50 | 3.41 | 78 | 44 | ||||
| L prec. g. | −42 | 2 | 28 | 3.03 | 8 | 16 | ||||
| L cuneus | −8 | −62 | 22 | 2.78 | 21 | 14 | ||||
| L temp. pole | −46 | 4 | −16 | 2.74 | 31 | 14 | ||||
| Healthy controls > borderline personality disorder | ||||||||||
| R inf. front. g. | 46 | 18 | 2 | 3.55 | 69 | 212 | 42 | 24 | −2 | R insula |
| 50 | 30 | 2 | R inf. front. g. | |||||||
| L inf. front. g. | −48 | 20 | 4 | 3.25 | 84 | 60 | −40 | 18 | 0 | L insula |
| R temp. pole | 48 | 6 | −22 | 2.92 | 31 | 16 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schurz, M.; Berenz, J.-P.; Maerz, J.; Perla, R.; Buchheim, A.; Labek, K. Brain Activation for Social Cognition and Emotion Processing Tasks in Borderline Personality Disorder: A Meta-Analysis of Neuroimaging Studies. Brain Sci. 2024, 14, 395. https://doi.org/10.3390/brainsci14040395
Schurz M, Berenz J-P, Maerz J, Perla R, Buchheim A, Labek K. Brain Activation for Social Cognition and Emotion Processing Tasks in Borderline Personality Disorder: A Meta-Analysis of Neuroimaging Studies. Brain Sciences. 2024; 14(4):395. https://doi.org/10.3390/brainsci14040395
Chicago/Turabian StyleSchurz, Matthias, Jan-Patrick Berenz, Jeff Maerz, Raphael Perla, Anna Buchheim, and Karin Labek. 2024. "Brain Activation for Social Cognition and Emotion Processing Tasks in Borderline Personality Disorder: A Meta-Analysis of Neuroimaging Studies" Brain Sciences 14, no. 4: 395. https://doi.org/10.3390/brainsci14040395
APA StyleSchurz, M., Berenz, J.-P., Maerz, J., Perla, R., Buchheim, A., & Labek, K. (2024). Brain Activation for Social Cognition and Emotion Processing Tasks in Borderline Personality Disorder: A Meta-Analysis of Neuroimaging Studies. Brain Sciences, 14(4), 395. https://doi.org/10.3390/brainsci14040395






