The Effectiveness of Mindfulness in the Treatment of Methamphetamine Addiction Symptoms: Does Neuroplasticity Play a Role?
Abstract
1. Introduction
1.1. Methamphetamine Use Disorder
1.2. Mindfulness
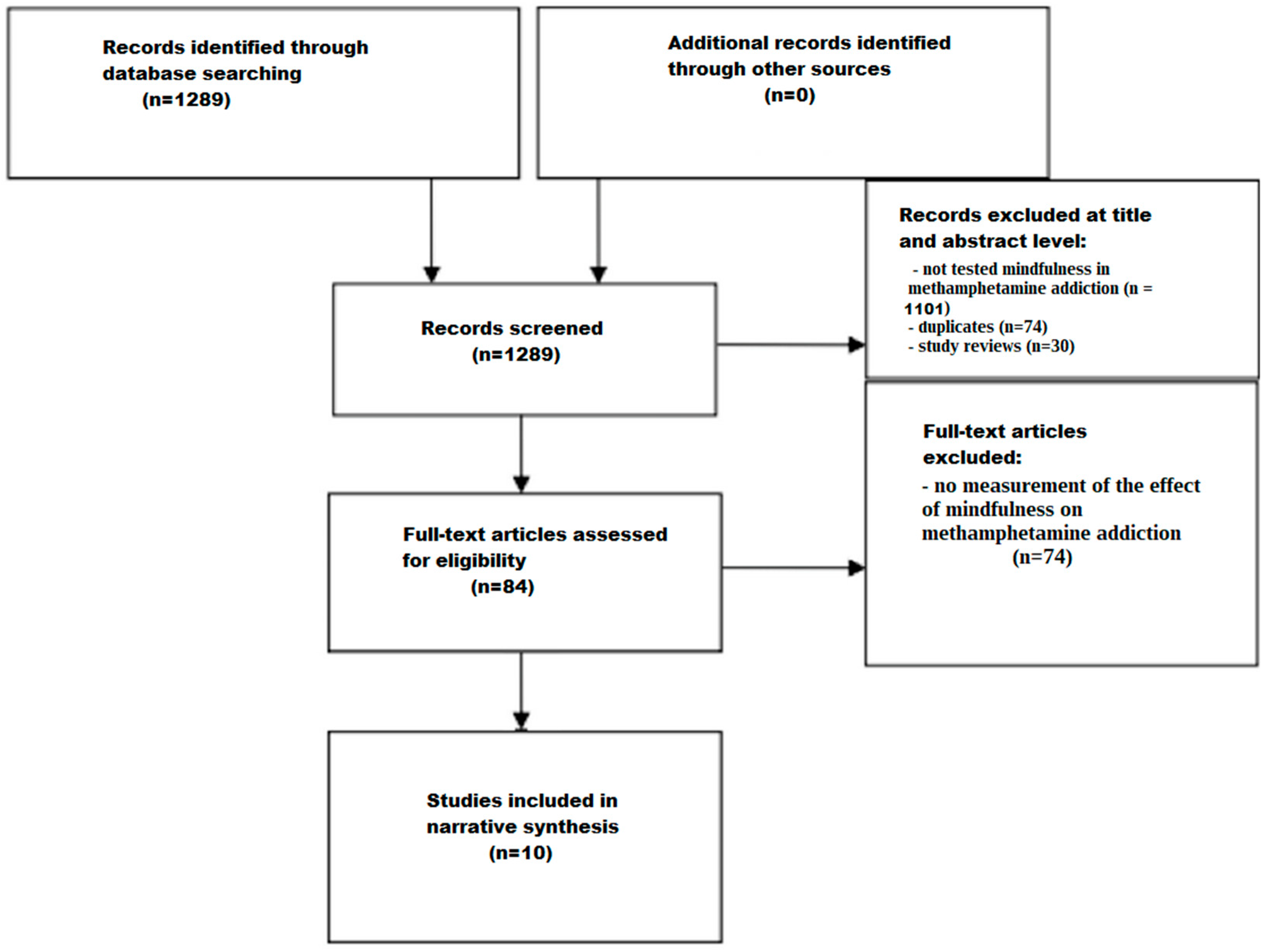
2. Methods
2.1. Data Sources and Search Strategy
2.2. Study Selection Criteria
2.3. Screening Process
2.3.1. Title and Abstract Screening
2.3.2. Full-Text Assessment
3. Results
3.1. Summary of Included Studies
3.2. Effect of Mindfulness on Different Outcomes in Methamphetamine Dependency
4. Discussion
5. Potential Mechanisms of Mindfulness in the Treatment of Methamphetamine Dependency
5.1. Impact on Craving
5.2. Impact on Executive Functions
5.3. Impact on Stress Reactivity
5.4. Impact on Reward System
5.5. Impact on Emotion Regulation
6. Can Mindfulness Induce Neuroplasticity in Addictions?
6.1. Effect on Neurogenesis in Several Brain Structures, Primarily in the Hippocampus
6.2. Effects on Neuroinflammation
6.3. Neurotrophic and Vascular Effect of Mindfulness on Neuroplasticity in Methamphetamine Dependency
7. Research Limitations and Future Perspectives
7.1. Heterogeneity in Study Designs
7.2. Assessment of Variables Influencing Treatment Success for Methamphetamine Dependency
7.3. Small Sample Size
7.4. Lack of Long-Term Follow-Up
7.5. Potential Bias in Self-Reported Measures
7.6. Lack of Studies Examining the Impact of Mindfulness on Neuroplasticity in the Context of Methamphetamine Dependency
7.7. Investigating the Impact of Communication with a Mindfulness Therapist on Addiction Treatment Outcomes
7.8. Examining the Impact of Gender on the Outcome of Methamphetamine Dependency Treatment through Mindfulness
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schep, L.J.; Slaughter, R.J.; Beasley, D.M. The clinical toxicology of metamfetamine. Clin. Toxicol. 2010, 48, 675–694. [Google Scholar] [CrossRef] [PubMed]
- Elkashef, A.; Vocci, F.; Hanson, G.; White, J.; Wickes, W.; Tiihonen, J. Pharmacotherapy of methamphetamine addiction: An update. Subst. Abus. 2008, 29, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Yun, J.; Park, B. Methamphetamine-Induced Neuronal Damage: Neurotoxicity and Neuroinflammation. Biomol. Ther. 2020, 28, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, R.R.; Seminerio, M.J.; Turner, R.C.; Robson, M.J.; Nguyen, L.; Miller, D.B.; O’Callaghan, J.P. Methamphetamine-induced toxicity: An updated review on issues related to hyperthermia. Pharmacol. Ther. 2014, 144, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Melnikova, N.; Welles, W.L.; Wilburn, R.E.; Rice, N.; Wu, J.; Stanbury, M. Hazards of illicit methamphetamine production and efforts at reduction: Data from the hazardous sub-stances emergency events surveillance system. Public Health Rep. 2011, 12 (Suppl. S1), 116–123. [Google Scholar] [CrossRef] [PubMed]
- Blum, K.; Cadet, J.L.; Gold, M.S. Psychostimulant use disorder emphasizing methamphetamine and the opioid -dopamine connection: Digging out of a hypodopaminergic ditch. J. Neurol. Sci. 2021, 420, 117252. [Google Scholar] [CrossRef]
- Johnson, K.; Stollings, J.L.; Ely, E.W. Breaking Bad Delirium: Methamphetamine and Boric Acid Toxicity with Hallucinations and Pseudosepsis. South. Med. J. 2017, 110, 138–141. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stoneberg, D.M.; Shukla, R.K.; Magness, M.B. Global Methamphetamine Trends: An Evolving Problem. Int. Crim. Justice Rev. 2018, 28, 136–161. [Google Scholar] [CrossRef]
- Jones, C.M.; Houry, D.; Han, B.; Baldwin, G.; Vivolo-Kantor, A.; Compton, W.M. Methamphetamine use in the United States: Epidemiological update and implications for preven-tion, treatment, and harm reduction. Ann. N. Y Acad. Sci. 2022, 1508, 3–22. [Google Scholar] [CrossRef]
- Daiwile, A.P.; Jayanthi, S.; Cadet, J.L. Sex differences in methamphetamine use disorder perused from pre-clinical and clinical studies: Potential therapeutic impacts. Neurosci. Biobehav. Rev. 2022, 137, 104674. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.M.; Compton, W.M.; Mustaquim, D. Patterns and Characteristics of Methamphetamine Use Among Adults—United States, 2015–2018. MMWR Morb. Mortal Wkly. Rep. 2020, 69, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, M.; Limanaqi, F.; Ryskalin, L.; Biagioni, F.; Busceti, C.L.; Fornai, F. The Effects of Amphetamine and Methamphetamine on the Release of Norepinephrine, Dopamine and Acetylcholine from the Brainstem Reticular Formation. Front. Neuroanat. 2019, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Jaehne, E.J.; Ameti, D.; Paiva, T.; van den Buuse, M. Investigating the Role of Serotonin in Methamphetamine Psychosis: Unaltered Behavioral Effects of Chronic Methampheta-mine in 5-HT1A Knockout Mice. Front. Psychiatry 2017, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Yorgason, J.T.; Hedges, D.M.; Obray, J.D.; Jang, E.Y.; Bills, K.B.; Woodbury, M.; Williams, B.; Parsons, M.J.; Andres, M.A.; Steffensen, S.C. Methamphetamine increases dopa-mine release in the nucleus accumbens through calcium-dependent processes. Psychopharmacology 2020, 237, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Nickell, J.R.; Siripurapu, K.B.; Vartak, A.; Crooks, P.A.; Dwoskin, L.P. The vesicular monoamine transporter-2: An important pharmacological target for the discovery of novel therapeutics to treat methamphetamine abuse. Adv. Pharmacol. 2014, 69, 71–106. [Google Scholar] [PubMed]
- Moszczynska, A. Neurobiology and Clinical Manifestations of Methamphetamine Neurotoxicity. Psychiatr. Times 2016, 33, 16–18. [Google Scholar] [PubMed]
- Winslow, B.T.; Voorhees, K.I.; Pehl, K.A. Methamphetamine abuse. Am. Fam. Physician 2007, 76, 1169–1174. [Google Scholar] [PubMed]
- Juárez-Portilla, C.; Molina-Jiménez, T.; Morin, J.P.; Roldán-Roldán, G.; Zepeda, R.C. Influence of Drugs on Cognitive Functions. In Health and Academic Achievement; InTech: London, UK, 2018. [Google Scholar]
- Prakash, M.D.; Tangalakis, K.; Antonipillai, J.; Stojanovska, L.; Nurgali, K.; Apostolopoulos, V. Methamphetamine: Effects on the brain, gut and immune system. Pharmacol. Res. 2017, 120, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Rawstorne, P.; Digiusto, E.; Worth, H.; Zablotska, I. Associations between crystal methamphetamine use and potentially unsafe sexual activity among gay men in Australia. Arch. Sex. Behav. 2007, 36, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Vocci, F.J.; Appel, N.M. Approaches to the development of medications for the treatment of methamphetamine dependence. Addiction 2007, 102 (Suppl. S1), 96–106. [Google Scholar] [CrossRef]
- Edinoff, A.N.; Kaufman, S.E.; Green, K.M.; Provenzano, D.A.; Lawson, J.; Cornett, E.M.; Murnane, K.S.; Kaye, A.M.; Kaye, A.D. Methamphetamine Use: A Narrative Review of Adverse Effects and Related Toxicities. Health Psychol. Res. 2022, 10, 38161. [Google Scholar] [CrossRef] [PubMed]
- Chiu, V.M.; Schenk, J.O. Mechanism of action of methamphetamine within the catecholamine and serotonin areas of the central nervous system. Curr. Drug Abus. Rev. 2012, 5, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Ling, W.; Rawson, R.; Shoptaw, S.; Ling, W. Management of methamphetamine abuse and dependence. Curr. Psychiatry Rep. 2006, 8, 345–354. [Google Scholar] [CrossRef]
- McKetin, R.; Lubman, D.I.; Najman, J.M.; Dawe, S.; Butterworth, P.; Baker, A.L. Does methamphetamine use increase violent behaviour? Evidence from a prospective longitudinal study. Addiction 2014, 109, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Jayanthi, S.; Daiwile, A.P.; Cadet, J.L. Neurotoxicity of methamphetamine: Main effects and mechanisms. Exp. Neurol. 2021, 344, 113795. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.L.; Wise, R.A.; Kiyatkin, E.A. Brain hyperthermia is induced by methamphetamine and exacerbated by social interaction. J. Neurosci. 2003, 23, 3924–3929. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Nguyen, N.V.; Mammo, D.A.; Albini, T.A.; Hayek, B.R.; Timperley, B.D.; Krueger, R.R.; Yeh, S. Vision health perspectives on Breaking Bad: Ophthalmic sequelae of methamphetamine use disorder. Front. Toxicol. 2023, 5, 1135792. [Google Scholar] [CrossRef] [PubMed]
- Fleckenstein, A.E.; Volz, T.J.; Riddle, E.L.; Gibb, J.W.; Hanson, G.R. New insights into the mechanism of action of amphetamines. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 681–698. [Google Scholar] [CrossRef] [PubMed]
- Paulus, M.P.; Stewart, J.L. Neurobiology, Clinical Presentation, and Treatment of Methamphetamine Use Disorder: A Review. JAMA Psychiatry 2020, 77, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Glasner-Edwards, S.; Mooney, L.J. Methamphetamine psychosis: Epidemiology and management. CNS Drugs 2014, 28, 1115–1126. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Sun, Y.; Zhang, Y.; Muhai, J.; Lu, L.; Shi, J. A Review of Risk Factors for Methamphetamine-Related Psychiatric Symptoms. Front. Psychiatry 2018, 9, 603. [Google Scholar] [CrossRef]
- Li, K.X.; Loshak, H. Treatment for Methamphetamine Addiction: A Review of Guidelines; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2019. [Google Scholar]
- Scott, J.C.; Woods, S.P.; Matt, G.E.; Meyer, R.A.; Heaton, R.K.; Atkinson, J.H.; Grant, I. Neurocognitive effects of methamphetamine: A critical review and meta-analysis. Neuro-Psychol Rev. 2007, 17, 275–297. [Google Scholar] [CrossRef]
- Todd, G.; Pearson-Dennett, V.; Wilcox, R.A.; Chau, M.T.; Thoirs, K.; Thewlis, D.; Vogel, A.P.; White, J.M. Adults with a history of illicit amphetamine use exhibit abnormal sub-stantia nigra morphology and parkinsonism. Park. Relat. Disord. 2016, 25, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, R.C.; Cunningham, J.K.; Sykes, J.; Kish, S.J. Increased risk of Parkinson’s disease in individuals hospitalized with conditions related to the use of methamphetamine or other amphetamine-type drugs. Drug Alcohol Depend. 2012, 120, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.C.; Lyoo, I.K.; Sung, Y.H.; Yoo, J.; Chung, A.; Yoon, S.J.; Kim, D.J.; Hwang, J.; Kim, S.J.; Renshaw, P.F. Increased white matter hyperintensities in male methamphetamine abusers. Drug Alcohol Depend. 2006, 81, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.G.; Alhassoon, O.M.; Stern, M.J.; Wollman, S.C.; Kimmel, C.L.; Perez-Figueroa, A.; Radua, J. Gray matter abnormalities in cocaine versus methamphetamine-dependent patients: A neuroimaging meta-analysis. Am. J. Drug Alcohol Abus. 2015, 41, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Ghahremani, D.G.; Mandelkern, M.A.; Dean, A.C.; Luo, W.; Ren, A.; Li, J.; London, E.D. The relationship between duration of abstinence and gray-matter brain struc-ture in chronic methamphetamine users. Am. J. Drug Alcohol Abus. 2021, 47, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Kevil, C.G.; Goeders, N.E.; Woolard, M.D.; Bhuiyan, M.S.; Dominic, P.; Kolluru, G.K.; Arnold, C.L.; Traylor, J.G.; Orr, A.W. Methamphetamine Use and Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1739–1746. [Google Scholar] [CrossRef] [PubMed]
- Courtney, K.E.; Ray, L.A. Methamphetamine: An update on epidemiology, pharmacology, clinical phenomenology, and treatment literature. Drug Alcohol Depend. 2014, 143, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Shearer, R.D.; Howell, B.A.; Bart, G.; Winkelman, T.N.A. Substance use patterns and health profiles among US adults who use opioids, methamphetamine, or both, 2015–2018. Drug Alcohol Depend. 2020, 214, 108162. [Google Scholar] [CrossRef] [PubMed]
- Morasco, B.J.; OʼNeil, M.E.; Duckart, J.P.; Ganzini, L. Comparison of health service use among veterans with methamphetamine versus alcohol use disorders. J. Addict. Med. 2014, 8, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Galanter, M.; Dermatis, H.; Post, S.; Santucci, C. Abstinence from drugs of abuse in community-based members of Narcotics Anonymous. J. Stud. Alcohol Drugs 2013, 74, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.K.; Rawson, R.A. A systematic review of cognitive and behavioural therapies for methamphetamine dependence. Drug Alcohol Rev. 2008, 27, 309–317. [Google Scholar] [CrossRef]
- AshaRani, P.V.; Hombali, A.; Seow, E.; Ong, W.J.; Tan, J.H.; Subramaniam, M. Non-pharmacological interventions for methamphetamine use disorder: A systematic review. Drug Alcohol Depend. 2020, 212, 108060. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, F.; Ghaderi, E.; Mardani, R.; Hamidi, S.; Hassanzadeh, K. Topiramate for the management of methamphetamine dependence: A pilot randomized, double-blind, placebo-controlled trial. Fundam. Clin. Pharmacol. 2016, 30, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Karila, L.; Weinstein, A.; Aubin, H.J.; Benyamina, A.; Reynaud, M.; Batki, S.L. Pharmacological approaches to methamphetamine dependence: A focused review. Br. J. Clin. Phar-Macol 2010, 69, 578–592. [Google Scholar] [CrossRef] [PubMed]
- Hölzel, B.K.; Lazar, S.W.; Gard, T.; Schuman-Olivier, Z.; Vago, D.R.; Ott, U. How Does Mindfulness Meditation Work? Proposing Mechanisms of Action from a Conceptual and Neural Perspective. Perspect. Psychol. Sci. 2011, 6, 537–559. [Google Scholar] [CrossRef] [PubMed]
- Timbers, V.L.; Hollenberger, J.C. Christian Mindfulness and Mental Health: Coping through Sacred Traditions and Embodied Awareness. Religions 2022, 13, 62. [Google Scholar] [CrossRef]
- Kopel, J.; Habermas, G.R. Neural Buddhism and Christian mindfulness in medicine. Bayl. Univ. Med Cent. Proc. 2019, 32, 308–310. [Google Scholar] [CrossRef] [PubMed]
- Trousselard, M.; Steiler, D.; Claverie, D.; Canini, F. L’histoire de la Mindfulness à l’épreuve des données actuelles de la littérature: Questions en suspens [The history of Mindful-ness put to the test of current scientific data: Unresolved questions]. Encephale 2014, 40, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Fox, K.C.R.; Andrews-Hanna, J.R.; Mills, C.; Dixon, M.L.; Markovic, J.; Thompson, E.; Christoff, K. Affective neuroscience of self-generated thought. Ann. N. Y Acad. Sci. 2018, 1426, 25–51. [Google Scholar] [CrossRef] [PubMed]
- Zorn, J.; Abdoun, O.; Sonié, S.; Lutz, A. Cognitive Defusion Is a Core Cognitive Mechanism for the Sensory-Affective Uncoupling of Pain During Mindfulness Meditation. Psychosom Med. 2021, 83, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Serfaty, S.; Gale, G.; Beadman, M.; Froeliger, B.; Kamboj, S.K. Mindfulness, Acceptance and Defusion Strategies in Smokers: A Systematic Review of Laboratory Studies. Mindfulness 2018, 9, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Birtwell, K.; Williams, K.; van Marwijk, H.; Armitage, C.J.; Sheffield, D. An Exploration of Formal and Informal Mindfulness Practice and Associations with Wellbeing. Mindfulness 2019, 10, 89–99. [Google Scholar] [CrossRef]
- Schuman-Olivier, Z.; Trombka, M.; Lovas, D.A.; Brewer, J.A.; Vago, D.R.; Gawande, R.; Dunne, J.P.; Lazar, S.W.; Loucks, E.B.; Fulwiler, C. Mindfulness and Behavior Change. Harv. Rev. Psychiatry 2020, 28, 371–394. [Google Scholar] [CrossRef] [PubMed]
- Leça, S.; Tavares, I. Research in Mindfulness Interventions for Patients with Fibromyalgia: A Critical Review. Front. Integr. Neurosci. 2022, 16, 920271. [Google Scholar] [CrossRef]
- Grossman, P.; Niemann, L.; Schmidt, S.; Walach, H. Mindfulness-based stress reduction and health benefits. A meta-analysis. J. Psychosom. Res. 2004, 57, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Poissant, H.; Mendrek, A.; Talbot, N.; Khoury, B.; Nolan, J. Behavioral and Cognitive Impacts of Mindfulness-Based Interventions on Adults with Attention-Deficit Hyperactivity Disorder: A Systematic Review. Behav. Neurol. 2019, 2019, 5682050. [Google Scholar] [CrossRef] [PubMed]
- Hartley, M.; Dorstyn, D.; Due, C. Mindfulness for Children and Adults with Autism Spectrum Disorder and Their Caregivers: A Meta-analysis. J. Autism Dev. Disord. 2019, 49, 4306–4319. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Ni, C.X.; Liu, Y.Z.; Zhang, Y.; Su, W.J.; Lian, Y.J.; Peng, W.; Jiang, C.L. Mindfulness meditation for insomnia: A meta-analysis of randomized controlled trials. J. Psycho-Som Res. 2016, 89, 1–6. [Google Scholar] [CrossRef]
- Lin, H.W.; Tam, K.W.; Kuan, Y.C. Mindfulness or meditation therapy for Parkinson’s disease: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Neurol. 2023, 30, 2250–2260. [Google Scholar] [CrossRef] [PubMed]
- Hilton, L.; Hempel, S.; Ewing, B.A.; Apaydin, E.; Xenakis, L.; Newberry, S.; Colaiaco, B.; Maher, A.R.; Shanman, R.M.; Sorbero, M.E.; et al. Mindfulness Meditation for Chronic Pain: Systematic Review and Meta-analysis. Ann. Behav. Med. 2017, 51, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, T.; Barnhofer, T.; Acabchuk, R.; Cohen, A.; Lee, M.; Schlosser, M.; Marchant, N.L. The Effect of Mindfulness-based Programs on Cognitive Function in Adults: A Systematic Review and Meta-analysis. Neuropsychol. Rev. 2022, 32, 677–702. [Google Scholar] [CrossRef] [PubMed]
- Cásedas, L.; Vadillo, M.A.; Lupiáñez, J. Does Mindfulness Meditation Training Enhance Executive Control? A Systematic Review and Meta-analysis of Randomized Controlled Trials in Adults. Mindfulness 2019, 11, 411–424. [Google Scholar] [CrossRef]
- Killingsworth, M.A.; Gilbert, D.T. A wandering mind is an unhappy mind. Science 2010, 330, 932. [Google Scholar] [CrossRef] [PubMed]
- Farb, N.A.; Anderson, A.K.; Mayberg, H.; Bean, J.; McKeon, D.; Segal, Z.V. Minding one’s emotions: Mindfulness training alters the neural expression of sadness. Emotion 2010, 10, 25–33. [Google Scholar] [CrossRef]
- Reive, C. The Biological Measurements of Mindfulness-based Stress Reduction: A Systematic Review. Explore 2019, 15, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Taylor, V.A.; Grant, J.; Daneault, V.; Scavone, G.; Breton, E.; Roffe-Vidal, S.; Courtemanche, J.; Lavarenne, A.S.; Beauregard, M. Impact of mindfulness on the neural responses to emotional pictures in experienced and beginner meditators. Neuroimage 2011, 57, 1524–1533. [Google Scholar] [CrossRef]
- Hu, Z.; Wen, Y.; Wang, Y.; Lin, Y.; Shi, J.; Yu, Z.; Lin, Y.; Wang, Y. Effectiveness of mindfulness-based interventions on empathy: A meta-analysis. Front. Psychol. 2022, 13, 992575. [Google Scholar] [CrossRef] [PubMed]
- Jerath, R.; Barnes, V.A.; Crawford, M.W. Mind-body response and neurophysiological changes during stress and meditation: Central role of homeostasis. J. Biol. Regul. Homeost. Agents 2014, 28, 545–554. [Google Scholar] [PubMed]
- Farias, M.; Maraldi, E.; Wallenkampf, K.C.; Lucchetti, G. Adverse events in meditation practices and meditation-based therapies: A systematic review. Acta Psychiatr. Scand. 2020, 142, 374–393. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, P.S.; Eftekhar Saadi, Z.; Johari Fard, R. Effects of Mindfulness-Based Relapse Prevention Therapy on Drug Craving and Emotion Regulation of Therapeutic Community Centers Clients. J. Health Rep. Technol. 2023, 9, e136888. [Google Scholar] [CrossRef]
- Maneesang, W.; Hengpraprom, S.; Kalayasiri, R. Effectiveness of Mindfulness—Based Therapy and Counseling programs (MBTC) on relapses to methamphetamine dependence at a substance dependency treatment center. Psychiatry Res. 2022, 317, 114886. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Long, Y.; Shi, J.; Shi, D.; Ren, Q.; Zhao, M.; Du, J. The Effectiveness of Mindfulness-Based Relapse Prevention on Chinese Methamphetamine Dependent Patients: A Pilot Study. Front. Psychiatry 2022, 13, 819075. [Google Scholar] [CrossRef] [PubMed]
- Dsouza, L.; Aghili, M.; Nasiri, Z.; Asghari, A. Investigating the Effectiveness of Mindfulness on Reducing Cravings for Drugs and Stress Levels and Cortisol Levels of Men Addicted to Glass in Mashhad. Iran. J. Health Psychol. 2021, 4, 17–26. [Google Scholar]
- Hamidi, F.; Kheiran, S. Mindfulness-Based Relapse Prevention to Reduce High Risk Behaviors of People Addicted to Methamphetamine. Int. J. High Risk Behav. Addict. 2019, 8, e92609. [Google Scholar] [CrossRef]
- Alizadehgoradel, J.; Imani, S.; Nejati, V.; Fathabadi, J. Mindfulness-based substance abuse treatment (MBSAT) improves executive functions in adolescents with substance use disorders. Neurol. Psychiatry Brain Res. 2019, 34, 21–34. [Google Scholar] [CrossRef]
- Shareh, H.; Gholami, Z.; Jafari, M. Effectiveness of mindfulness-based group therapy in relapse prevention for methamphetamine dependent males. J. Fundam. Ment. Health 2018, 20, 167–175. [Google Scholar]
- Alizadehgoradel, J.; Imani, S.; Nejati, V.; Vanderhasselt, M.A.; Molaei, B.; Salehinejad, M.A.; Ahmadi, S.; Taherifard, M. Improved Executive Functions and Reduced Craving in Youths with Methamphetamine Addiction: Evidence from Combined Transcranial Direct Current Stimulation with Mindfulness Treatment. Clin. Psychopharmacol. Neurosci. 2021, 19, 653–668. [Google Scholar] [CrossRef] [PubMed]
- Alizadehgoradel, J. Modification of attentional bias and reduced craving from combined mindfulness+tDCS therapy in methamphetamine addiction: A randomized, sham-controlled, single-blinded clinical trial. J. Res. Psychopathol. 2023, 4, 6–15. [Google Scholar]
- Alizadehgoradel, J. The Effects of Combined Transcranial Direct Current Stimulation (tDCS) with Mindfulness on Negative Emotions and Craving in Adolescents with Methamphetamine Dependence. Int. J. High. Risk Behav. Addict. 2021, 10, e100909. [Google Scholar] [CrossRef]
- Lorenzetti, V.; Gaillard, A.; Beyer, E.; Kowalczyk, M.; Kamboj, S.K.; Manning, V.; Gleeson, J. Do mindfulness-based interventions change brain function in people with substance dependence? A systematic review of the fMRI evidence. BMC Psychiatry 2023, 23, 407. [Google Scholar] [CrossRef] [PubMed]
- Pearson, M.R.; Roos, C.R.; Brown, D.B.; Witkiewitz, K. Neuroscience and mindfulness-based interventions: Translating neural mechanisms to addiction treatment. In Neuroimaging and Psychosocial Addiction Treatment: An Integrative Guide for Researchers and Clinicians; Feldstein Ewing, S.W., Witkiewitz, K., Filbey, F.M., Eds.; Palgrave Macmillan/Springer Nature: Berlin/Heidelberg, Germany, 2015; pp. 85–96. [Google Scholar]
- Kober, H.; Mende-Siedlecki, P.; Kross, E.F.; Weber, J.; Mischel, W.; Hart, C.L.; Ochsner, K.N. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proc. Natl. Acad. Sci. USA 2010, 107, 14811–14816. [Google Scholar] [CrossRef] [PubMed]
- Taren, A.A.; Gianaros, P.J.; Greco, C.M.; Lindsay, E.K.; Fairgrieve, A.; Brown, K.W.; Rosen, R.K.; Ferris, J.L.; Julson, E.; Marsland, A.L.; et al. Mindfulness Meditation Training and Executive Control Network Resting State Functional Connectivity: A Randomized Controlled Trial. Psychosom. Med. 2017, 79, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Luders, E.; Toga, A.W.; Lepore, N.; Gaser, C. The underlying anatomical correlates of long-term meditation: Larger hippocampal and frontal volumes of gray matter. Neuroimage 2009, 45, 672–678. [Google Scholar] [CrossRef]
- Garland, E.L.; Howard, M.O. Mindfulness-based treatment of addiction: Current state of the field and envisioning the next wave of research. Addict. Sci. Clin. Pract. 2018, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Garland, E.L.; Baker, A.K.; Howard, M.O. Mindfulness-oriented recovery enhancement reduces opioid attentional bias among prescription opioid-treated chronic pain patients. J. Soc. Social. Work. Res. 2017, 8, 493–509. [Google Scholar] [CrossRef]
- Garland, E.L.; Froeliger, B.; Howard, M.O. Effects of Mindfulness-Oriented Recovery Enhancement on reward responsiveness and opioid cue-reactivity. Psychopharmacology 2014, 231, 3229–3238. [Google Scholar] [CrossRef] [PubMed]
- Froeliger, B.; Mathew, A.R.; McConnell, P.A.; Eichberg, C.; Saladin, M.E.; Carpenter, M.J.; Garland, E.L. Restructuring Reward Mechanisms in Nicotine Addiction: A Pilot fMRI Study of Mindfulness-Oriented Recovery Enhancement for Cigarette Smokers. Evid. Based Complement. Altern. Med. 2017, 2017, 7018014. [Google Scholar] [CrossRef] [PubMed]
- Spears, C.A.; Hedeker, D.; Li, L.; Wu, C.; Anderson, N.K.; Houchins, S.C.; Vinci, C.; Hoover, D.S.; Vidrine, J.I.; Cinciripini, P.M.; et al. Mechanisms under-lying mindfulness-based addiction treatment versus cognitive behavioral therapy and usual care for smoking cessation. J. Consult Clin. Psychol. 2017, 85, 1029–1040. [Google Scholar] [CrossRef] [PubMed]
- Garland, E.L.; Froeliger, B.; Howard, M.O. Mindfulness training targets neurocognitive mechanisms of addiction at the attention-appraisal-emotion interface. Front. Psychiatry 2014, 4, 173. [Google Scholar] [CrossRef] [PubMed]
- Tiffany, S.T. A cognitive model of drug urges and drug-use behavior: Role of automatic and nonautomatic processes. Psychol. Rev. 1990, 97, 147–168. [Google Scholar] [CrossRef]
- Zeidan, F.; Martucci, K.T.; Kraft, R.A.; Gordon, N.S.; McHaffie, J.G.; Coghill, R.C. Brain mechanisms supporting the modulation of pain by mindfulness meditation. J. Neurosci. 2011, 31, 5540–5548. [Google Scholar] [CrossRef] [PubMed]
- Hyman, S.E.; Malenka, R.C.; Nestler, E.J. Neural mechanisms of addiction: The role of reward-related learning and memory. Annu. Rev. Neurosci. 2006, 29, 565–598. [Google Scholar] [CrossRef] [PubMed]
- Dutcher, J.M.; Boyle, C.C.; Eisenberger, N.I.; Cole, S.W.; Bower, J.E. Neural responses to threat and reward and changes in inflammation following a mindfulness intervention. Psychoneuroendocrinology 2021, 125, 105114. [Google Scholar] [CrossRef] [PubMed]
- Zsadanyi, S.E.; Kurth, F.; Luders, E. The Effects of Mindfulness and Meditation on the Cingulate Cortex in the Healthy Human Brain: A Review. Mindfulness 2021, 12, 2371–2387. [Google Scholar] [CrossRef]
- Alfonso, J.P.; Caracuel, A.; Delgado-Pastor, L.C.; Verdejo-García, A. Combined Goal Management Training and Mindfulness meditation improve executive functions and decision-making performance in abstinent polysubstance abusers. Drug Alcohol Depend. 2011, 117, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Valls-Serrano, C.; Caracuel, A.; Verdejo-Garcia, A. Goal Management Training and Mindfulness Meditation improve executive functions and transfer to ecological tasks of daily life in polysubstance users enrolled in therapeutic community treatment. Drug Alcohol Depend. 2016, 165, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.Y.; Tang, R.; Posner, M.I. Brief meditation training induces smoking reduction. Proc. Natl. Acad. Sci. USA 2013, 110, 13971–13975. [Google Scholar] [CrossRef] [PubMed]
- Soler, J.; Elices, M.; Pascual, J.C.; Martín-Blanco, A.; Feliu-Soler, A.; Carmona, C.; Portella, M.J. Effects of mindfulness training on different components of impulsivity in border-line personality disorder: Results from a pilot randomized study. Borderline Personal. Disord. Emot. Dysregul 2016, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Sahdra, B.K.; MacLean, K.A.; Ferrer, E.; Shaver, P.R.; Rosenberg, E.L.; Jacobs, T.L.; Zanesco, A.P.; King, B.G.; Aichele, S.R.; Bridwell, D.A.; et al. Enhanced response inhibition during intensive meditation training predicts improvements in self-reported adaptive socioemotional functioning. Emotion 2011, 11, 299–312. [Google Scholar] [CrossRef]
- Friese, M.; Messner, C.; Schaffner, Y. Mindfulness meditation counteracts self-control depletion. Conscious. Cogn. 2012, 21, 1016–1022. [Google Scholar] [CrossRef]
- Schoenberg, P.L.; Hepark, S.; Kan, C.C.; Barendregt, H.P.; Buitelaar, J.K.; Speckens, A.E. Effects of mindfulness-based cognitive therapy on neurophysiological correlates of per-formance monitoring in adult attention-deficit/hyperactivity disorder. Clin. Neurophysiol. 2014, 125, 1407–1416. [Google Scholar] [CrossRef]
- Andreu, C.I.; Cosmelli, D.; Slagter, H.A.; Franken, I.H.A. Effects of a brief mindfulness-meditation intervention on neural measures of response inhibition in cigarette smokers. PLoS ONE 2018, 13, e0191661. [Google Scholar] [CrossRef]
- Garland, E.L.; Bryan, M.A.; Priddy, S.E.; Riquino, M.R.; Froeliger, B.; Howard, M.O. Effects of Mindfulness-Oriented Recovery Enhancement Versus Social Support on Negative Affective Interference During Inhibitory Control Among Opioid-Treated Chronic Pain Patients: A Pilot Mechanistic Study. Ann. Behav. Med. 2019, 53, 865–876. [Google Scholar] [CrossRef]
- Allen, M.; Dietz, M.; Blair, K.S.; van Beek, M.; Rees, G.; Vestergaard-Poulsen, P.; Lutz, A.; Roepstorff, A. Cognitive-affective neural plasticity following active-controlled mindfulness intervention. J. Neurosci. 2012, 32, 15601–15610. [Google Scholar] [CrossRef]
- Tang, Y.Y.; Lu, Q.; Fan, M.; Yang, Y.; Posner, M.I. Mechanisms of white matter changes induced by meditation. Proc. Natl. Acad. Sci. USA 2012, 109, 10570–10574. [Google Scholar] [CrossRef]
- Tang, Y.Y.; Lu, Q.; Geng, X.; Stein, E.A.; Yang, Y.; Posner, M.I. Short-term meditation induces white matter changes in the anterior cingulate. Proc. Natl. Acad. Sci. USA 2010, 107, 15649–15652. [Google Scholar] [CrossRef]
- Rodriguez, E.A.; Yamamoto, B.K. Toxic Effects of Methamphetamine on Perivascular Health: Co-morbid Effects of Stress and Alcohol Use Disorders. Curr. Neuropharmacol. 2021, 19, 2092–2107. [Google Scholar] [CrossRef]
- May, A.C.; Aupperle, R.L.; Stewart, J.L. Dark Times: The Role of Negative Reinforcement in Methamphetamine Addiction. Front. Psychiatry 2020, 11, 114. [Google Scholar] [CrossRef]
- Thayer, J.F.; Ahs, F.; Fredrikson, M.; Sollers, J.J., 3rd; Wager, T.D. A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev. 2012, 36, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Holzman, J.B.; Bridgett, D.J. Heart rate variability indices as bio-markers of top-down self-regulatory mechanisms: A meta-analytic review. Neurosci. Biobehav. Rev. 2017, 74 Pt A, 233–255. [Google Scholar] [CrossRef]
- Carroll, H.B.; Lustyk, M.K. Mindfulness-Based Relapse Prevention for Substance Use Disorders: Effects on Cardiac Vagal Control and Craving Under Stress. Mindfulness 2018, 9, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Brewer, J.A.; Sinha, R.; Chen, J.A.; Michalsen, R.N.; Babuscio, T.A.; Nich, C.; Grier, A.; Bergquist, K.L.; Reis, D.L.; Potenza, M.N.; et al. Mindfulness training and stress reactivity in substance abuse: Results from a randomized, controlled stage I pilot study. Subst. Abus. 2009, 30, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Paz, R.; Zvielli, A.; Goldstein, P.; Bernstein, A. Brief mindfulness training de-couples the anxiogenic effects of distress intolerance on reactivity to and recovery from stress among deprived smokers. Behav. Res. Ther. 2017, 95, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Garland, E.L.; Gaylord, S.A.; Boettiger, C.A.; Howard, M.O. Mindfulness training modifies cognitive, affective, and physiological mechanisms implicated in alcohol dependence: Results of a randomized controlled pilot trial. J. Psychoact. Drugs 2010, 42, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Kober, H.; Brewer, J.A.; Height, K.L.; Sinha, R. Neural stress reactivity relates to smoking outcomes and differentiates between mindfulness and cognitive-behavioral treatments. Neuroimage 2017, 151, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Garland, E.L. Trait Mindfulness Predicts Attentional and Autonomic Regulation of Alcohol Cue-Reactivity. J. Psychophysiol. 2011, 25, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Hölzel, B.K.; Ott, U.; Hempel, H.; Hackl, A.; Wolf, K.; Stark, R.; Vaitl, D. Differential engagement of anterior cingulate and adjacent medial frontal cortex in adept meditators and non-meditators. Neurosci. Lett. 2007, 421, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Farb, N.A.; Segal, Z.V.; Mayberg, H.; Bean, J.; McKeon, D.; Fatima, Z.; Anderson, A.K. Attending to the present: Mindfulness meditation reveals distinct neural modes of self-reference. Soc. Cogn. Affect Neurosci. 2007, 2, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Garland, E.L. Restructuring reward processing with Mindfulness-Oriented Recovery Enhancement: Novel therapeutic mechanisms to remediate hedonic dysregulation in addic-tion, stress, and pain. Ann. N. Y Acad. Sci. 2016, 1373, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Priddy, S.E.; Howard, M.O.; Hanley, A.W.; Riquino, M.R.; Friberg-Felsted, K.; Garland, E.L. Mindfulness meditation in the treatment of substance use disorders and preventing future relapse: Neurocognitive mechanisms and clinical implications. Subst. Abus. Rehabil. 2018, 9, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Garland, E.L.; Farb, N.A.; Goldin, P.; Fredrickson, B.L. Mindfulness Broadens Awareness and Builds Eudaimonic Meaning: A Process Model of Mindful Positive Emotion Regulation. Psychol. Inq. 2015, 26, 293–314. [Google Scholar] [CrossRef] [PubMed]
- Gayner, B.; Esplen, M.J.; DeRoche, P.; Wong, J.; Bishop, S.; Kavanagh, L.; Butler, K. A randomized controlled trial of mindfulness-based stress reduction to manage affective symptoms and improve quality of life in gay men living with HIV. J. Behav. Med. 2011, 35, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Geschwind, N.; Peeters, F.; Drukker, M.; van Os, J.; Wichers, M. Mindfulness training increases momentary positive emotions and reward experience in adults vulnerable to depression: A randomized controlled trial. J. Consult. Clin. Psychol. 2011, 79, 618. [Google Scholar] [CrossRef] [PubMed]
- Garland, E.L.; Bryan, M.A.; Hanley, A.W.; Howard, M.O. Neurocognitive mechanisms of mindfulness-based interventions for addiction–sciencedirect. Cogn. Addict. 2020, 11, 283–293. [Google Scholar]
- Thompson, R.A. Emotion regulation: A theme in search of definition. Monogr. Soc. Res. Child. Dev. 1994, 59, 25–52. [Google Scholar] [CrossRef] [PubMed]
- McRae, K.; Misra, S.; Prasad, A.K.; Pereira, S.C.; Gross, J.J. Bottom-up and top-down emotion generation: Implications for emotion regulation. Soc. Cogn. Affect. Neurosci. 2012, 7, 253–262. [Google Scholar] [CrossRef] [PubMed]
- García-Cabezas, M.Á.; Barbas, H. Anterior Cingulate Pathways May Affect Emotions Through Orbitofrontal Cortex. Cereb. Cortex 2017, 27, 4891–4910. [Google Scholar] [CrossRef] [PubMed]
- Etkin, A.; Egner, T.; Kalisch, R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci. 2011, 15, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.Y.; Posner, M.I. Training brain networks and states. Trends Cogn. Sci. 2014, 18, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Di Giuseppe, M.; Orrù, G.; Gemignani, A.; Ciacchini, R.; Miniati, M.; Conversano, C. Mindfulness and Defense Mechanisms as Explicit and Implicit Emotion Regulation Strategies against Psychological Distress during Massive Catastrophic Events. Int. J. Environ. Res. Public Health 2022, 19, 12690. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Tang, Y.Y.; Tang, R.; Posner, M.I. Improving creativity performance by short-term meditation. Behav. Brain Funct. 2014, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.E.; Pommy, J.M.; Adinoff, B. Neural Circuitry of Impaired Emotion Regulation in Substance Use Disorders. Am. J. Psychiatry 2016, 173, 344–361. [Google Scholar] [CrossRef] [PubMed]
- Payer, D.E.; Lieberman, M.D.; London, E.D. Neural correlates of affect processing and aggression in methamphetamine dependence. Arch. Gen. Psychiatry 2011, 68, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Dean, A.C.; Kohno, M.; Hellemann, G.; London, E.D. Childhood maltreatment and amygdala connectivity in methamphetamine dependence: A pilot study. Brain Behav. 2014, 4, 867–876. [Google Scholar] [CrossRef]
- Tang, Y.Y.; Posner, M.I.; Rothbart, M.K.; Volkow, N.D. Circuitry of self-control and its role in reducing addiction. Trends Cogn. Sci. 2015, 19, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.Y.; Hölzel, B.K.; Posner, M.I. The neuroscience of mindfulness meditation. Nat. Rev. Neurosci. 2015, 16, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.Y.; Ma, Y.; Wang, J.; Fan, Y.; Feng, S.; Lu, Q.; Yu, Q.; Sui, D.; Rothbart, M.K.; Fan, M.; et al. Short-term meditation training improves attention and self-regulation. Proc. Natl. Acad. Sci. USA 2007, 104, 17152–17156. [Google Scholar] [CrossRef]
- Tang, Y.Y.; Ma, Y.; Fan, Y.; Feng, H.; Wang, J.; Feng, S.; Lu, Q.; Hu, B.; Lin, Y.; Li, J.; et al. Central and autonomic nervous system interaction is altered by short-term meditation. Proc. Natl. Acad. Sci. USA 2009, 106, 8865–8870. [Google Scholar] [CrossRef] [PubMed]
- Goldin, P.R.; Gross, J.J. Effects of mindfulness-based stress reduction (MBSR) on emotion regulation in social anxiety disorder. Emotion 2010, 10, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Quaglia, J.T.; Zeidan, F.; Grossenbacher, P.G.; Freeman, S.P.; Braun, S.E.; Martelli, A.; Goodman, R.J.; Brown, K.W. Brief mindfulness training enhances cognitive control in socioemotional contexts: Behavioral and neural evidence. PLoS ONE 2019, 14, e0219862. [Google Scholar] [CrossRef] [PubMed]
- Puderbaugh, M.; Emmady, P.D. Neuroplasticity 2023, May 1. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Weerasinghe-Mudiyanselage, P.D.E.; Ang, M.J.; Kang, S.; Kim, J.S.; Moon, C. Structural Plasticity of the Hippocampus in Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 3349. [Google Scholar] [CrossRef] [PubMed]
- Madsen, H.B.; Brown, R.M.; Lawrence, A.J. Neuroplasticity in addiction: Cellular and transcriptional perspectives. Front. Mol. Neurosci. 2012, 5, 99. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.T.; Bowers, M.S.; Martin, M.; Hopf, F.W.; Guillory, A.M.; Carelli, R.M.; Chou, J.K.; Bonci, A. Cocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA. Neuron 2008, 59, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Kutlu, M.G.; Gould, T.J. Effects of drugs of abuse on hippocampal plasticity and hippocampus-dependent learning and memory: Contributions to development and maintenance of addiction. Learn. Mem. 2016, 23, 515–533. [Google Scholar] [CrossRef] [PubMed]
- North, A.; Swant, J.; Salvatore, M.F.; Gamble-George, J.; Prins, P.; Butler, B.; Mittal, M.K.; Heltsley, R.; Clark, J.T.; Khoshbouei, H. Chronic methamphetamine exposure produces a delayed, long-lasting memory deficit. Synapse 2013, 67, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Swant, J.; Chirwa, S.; Stanwood, G.; Khoshbouei, H. Methamphetamine reduces LTP and increases baseline synaptic transmission in the CA1 region of mouse hippocampus. PLoS ONE 2010, 5, e11382. [Google Scholar] [CrossRef] [PubMed]
- Ólafsdóttir, H.F.; Bush, D.; Barry, C. The Role of Hippocampal Replay in Memory and Planning. Curr. Biol. 2018, 28, R37–R50. [Google Scholar] [CrossRef] [PubMed]
- Castilla-Ortega, E.; Serrano, A.; Blanco, E.; Araos, P.; Suárez, J.; Pavón, F.J.; Rodríguez de Fonseca, F.; Santín, L.J. A place for the hippocampus in the cocaine addiction circuit: Potential roles for adult hippocampal neurogenesis. Neurosci. Biobehav. Rev. 2016, 66, 15–32. [Google Scholar] [CrossRef]
- Canales, J.J. Deficient plasticity in the hippocampus and the spiral of addiction: Focus on adult neurogenesis. Curr. Top. Behav. Neurosci. 2013, 15, 293–312. [Google Scholar] [PubMed]
- Belujon, P.; Grace, A.A. Hippocampus, amygdala, and stress: Interacting systems that affect susceptibility to addiction. Ann. N. Y. Acad. Sci. 2011, 1216, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Tipps, M.E.; Raybuck, J.D.; Lattal, K.M. Substance abuse, memory, and post-traumatic stress disorder. Neurobiol. Learn. Mem. 2014, 112, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Knierim, J.J.; Lee, I.; Hargreaves, E.L. Hippocampal place cells: Parallel input streams, subregional processing, and implications for episodic memory. Hippocampus 2006, 16, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Takashima, Y.; Mandyam, C.D. The role of hippocampal adult neurogenesis in methamphetamine addiction. Brain Plast. 2018, 3, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Jessberger, S.; Clark, R.E.; Broadbent, N.J.; Clemenson, G.D., Jr.; Consiglio, A.; Lie, D.C.; Squire, L.R.; Gage, F.H. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn. Mem. 2009, 16, 147–154. [Google Scholar] [CrossRef]
- Shors, T.J.; Miesegaes, G.; Beylin, A.; Zhao, M.; Rydel, T.; Gould, E. Neurogenesis in the adult is involved in the formation of trace memories. Nature 2001, 410, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y. Reorganization and plastic changes of the human brain associated with skill learning and expertise. Front. Hum. Neurosci. 2014, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.T.; Lee, J.J.; Song, H.J.; Kim, J.H.; Kwon, D.H.; Kim, M.N.; Yoo, D.S.; Lee, H.J.; Kim, H.J.; Chang, Y. Alterations in cortical activity of male methamphetamine abusers performing an empathy task: fMRI study. Hum. Psychopharmacol. 2010, 25, 63–70. [Google Scholar] [CrossRef]
- Mandyam, C.D.; Wee, S.; Crawford, E.F.; Eisch, A.J.; Richardson, H.N.; Koob, G.F. Varied access to intravenous methamphetamine self-administration differentially alters adult hippocampal neurogenesis. Biol. Psychiatry 2008, 64, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.L.; Dean, A.C.; Cordova, X.; Monterosso, J.R.; London, E.D. Methamphetamine dependence and neuropsychological functioning: Evaluating change during early abstinence. J. Stud. Alcohol Drugs 2010, 71, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.L.; Mitchell, A.D.; Lahna, D.L.; Luber, H.S.; Huckans, M.S.; Mitchell, S.H.; Hoffman, W.F. Global and local morphometric differences in recently abstinent meth-amphetamine-dependent individuals. Neuroimage 2010, 50, 1392–1401. [Google Scholar] [CrossRef] [PubMed]
- Recinto, P.; Samant, A.R.; Chavez, G.; Kim, A.; Yuan, C.J.; Soleiman, M.; Grant, Y.; Edwards, S.; Wee, S.; Koob, G.F.; et al. Levels of neural progenitors in the hippocampus predict memory impairment and relapse to drug seeking as a function of excessive methamphetamine self-administration. Neuropsychopharmacology 2012, 37, 1275–1287. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.L.; De Santis, S.; See, R.E. Extended methamphetamine self-administration enhances reinstatement of drug seeking and impairs novel object recognition in rats. Psychopharmacology 2008, 199, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Zatorre, R.J.; Fields, R.D.; Johansen-Berg, H. Plasticity in gray and white: Neuroimaging changes in brain structure during learning. Nat. Neurosci. 2012, 15, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Pernet, C.R.; Belov, N.; Delorme, A.; Zammit, A. Mindfulness related changes in grey matter: A systematic review and meta-analysis. Brain Imaging Behav. 2021, 15, 2720–2730. [Google Scholar] [CrossRef] [PubMed]
- Kempermann, G. What Is Adult Hippocampal Neurogenesis Good? Front. Neurosci. 2022, 16, 852680. [Google Scholar] [CrossRef] [PubMed]
- Kempermann, G. Activity Dependency and Aging in the Regulation of Adult Neurogenesis. Cold Spring Harb. Perspect. Biol. 2015, 7, a018929. [Google Scholar] [CrossRef] [PubMed]
- Tronel, S.; Fabre, A.; Charrier, V.; Oliet, S.H.; Gage, F.H.; Abrous, D.N. Spatial learning sculpts the dendritic arbor of adult-born hippocampal neurons. Proc. Natl. Acad. Sci. USA 2010, 107, 7963–7968. [Google Scholar] [CrossRef] [PubMed]
- Sahay, A.; Scobie, K.N.; Hill, A.S.; O’Carroll, C.M.; Kheirbek, M.A.; Burghardt, N.S.; Fenton, A.A.; Dranovsky, A.; Hen, R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 2011, 472, 466–470. [Google Scholar] [CrossRef]
- Deng, W.; Saxe, M.D.; Gallina, I.S.; Gage, F.H. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J. Neurosci. 2009, 29, 13532–13542. [Google Scholar] [CrossRef] [PubMed]
- Castino, R.; Bellio, N.; Nicotra, G.; Follo, C.; Trincheri, N.F.; Isidoro, C. Cathepsin D-Bax death pathway in oxidative stressed neuroblastoma cells. Free Radic. Biol. Med. 2007, 42, 1305–1316. [Google Scholar] [CrossRef]
- Kohno, M.; Link, J.; Dennis, L.E.; McCready, H.; Huckans, M.; Hoffman, W.F.; Loftis, J.M. Neuroinflammation in addiction: A review of neuroimaging studies and potential immunotherapies. Pharmacol. Biochem. Behav. 2019, 179, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Wang, H.J.; Qiao, D.F. Effect of methamphetamine on the microglial cells and activity of nitric oxide synthases in rat striatum. Nan Fang Yi Ke Da Xue Xue Bao 2008, 28, 1789–1791. (In Chinese) [Google Scholar] [PubMed]
- Loftis, J.M.; Janowsky, A. Neuroimmune basis of methamphetamine toxicity. Int. Rev. Neurobiol. 2014, 118, 165–197. [Google Scholar] [PubMed]
- Sekine, Y.; Ouchi, Y.; Sugihara, G.; Takei, N.; Yoshikawa, E.; Nakamura, K.; Iwata, Y.; Tsuchiya, K.J.; Suda, S.; Suzuki, K.; et al. Methamphetamine causes microglial activation in the brains of human abusers. J. Neurosci. 2008, 28, 5756–5761. [Google Scholar] [CrossRef] [PubMed]
- Du, S.H.; Qiao, D.F.; Chen, C.X.; Chen, S.; Liu, C.; Lin, Z.; Wang, H.; Xie, W.B. Toll-like receptor 4 mediates methamphetamine-induced neuroinflammation through Caspase-11 signaling pathway in astrocytes. Front. Mol. Neurosci. 2017, 10, 409. [Google Scholar] [CrossRef] [PubMed]
- Wan, F.; Zang, S.; Yu, G.; Xiao, H.; Wang, J.; Tang, J. Ginkgolide B suppresses methamphetamine-induced microglial activation through TLR4-NF-kappaB signaling pathway in BV2 cells. Neurochem. Res. 2017, 42, 2881–2891. [Google Scholar] [CrossRef]
- Sanada, K.; Alda Díez, M.; Salas Valero, M.; Pérez-Yus, M.C.; Demarzo, M.M.; Montero-Marín, J.; García-Toro, M.; García-Campayo, J. Effects of mindfulness-based interventions on biomarkers in healthy and cancer populations: A systematic review. BMC Complement. Altern. Med. 2017, 17, 125. [Google Scholar] [CrossRef] [PubMed]
- Black, D.S.; Slavich, G.M. Mindfulness meditation and the immune system: A systematic review of randomized controlled trials. Ann. N. Y Acad. Sci. 2016, 1373, 13–24. [Google Scholar] [CrossRef]
- Ryan, S.M.; Nolan, Y.M. Neuroinflammation negatively affects adult hippocampal neurogenesis and cognition: Can exercise compensate? Neurosci. Biobehav. Rev. 2016, 61, 121–131. [Google Scholar] [CrossRef]
- Gomutbutra, P.; Yingchankul, N.; Chattipakorn, N.; Chattipakorn, S.; Srisurapanont, M. The Effect of Mindfulness-Based Intervention on Brain-Derived Neurotrophic Factor (BDNF): A Systematic Review and Meta-Analysis of Controlled Trials. Front. Psychol. 2020, 11, 2209. [Google Scholar] [CrossRef]
- Gallegos, A.M.; Hoerger, M.; Talbot, N.L.; Krasner, M.S.; Knight, J.M.; Moynihan, J.A.; Duberstein, P.R. Toward identifying the effects of the specific components of Mindful-ness-Based Stress Reduction on biologic and emotional outcomes among older adults. J. Altern. Complement. Med. 2013, 19, 787–792. [Google Scholar] [CrossRef]
- Numakawa, T.; Odaka, H.; Adachi, N. Actions of Brain-Derived Neurotrophin Factor in the Neurogenesis and Neuronal Function, and Its Involvement in the Pathophysiology of Brain Diseases. Int. J. Mol. Sci. 2018, 19, 3650. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Estévez, V.; Defterali, Ç.; Vicario-Abejón, C. IGF-I: A Key Growth Factor that Regulates Neurogenesis and Synaptogenesis from Embryonic to Adult Stages of the Brain. Front. Neurosci. 2016, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Kermani, P.; Hempstead, B. Brain-derived neurotrophic factor: A newly described mediator of angiogenesis. Trends Cardiovasc. Med. 2007, 17, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Hofer, M.; Pagliusi, S.R.; Hohn, A.; Leibrock, J.; Barde, Y.A. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. EMBO J. 1990, 9, 2459–2464. [Google Scholar] [CrossRef]
- Rasmussen, P.; Brassard, P.; Adser, H.; Pedersen, M.V.; Leick, L.; Hart, E.; Secher, N.H.; Pedersen, B.K.; Pilegaard, H. Evidence for a release of brain-derived neurotrophic fac-tor from the brain during exercise. Exp. Physiol. 2009, 94, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Banks, W.A.; Fasold, M.B.; Bluth, J.; Kastin, A.J. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology 1998, 37, 1553–1561. [Google Scholar] [CrossRef]
- Gejl, A.K.; Enevold, C.; Bugge, A.; Andersen, M.S.; Nielsen, C.H.; Andersen, L.B. Associations between serum and plasma brain-derived neurotrophic factor and influence of storage time and centrifugation strategy. Sci. Rep. 2019, 9, 9655. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.W.; Chu, E.; Hui, T.; Helmeste, D.; Law, C. Influence of exercise on serum brain-derived neurotrophic factor concentrations in healthy human subjects. Neurosci. Lett. 2008, 431, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef] [PubMed]
- Inoue, D.S.; Monteiro, P.A.; Gerosa-Neto, J.; Santana, P.R.; Peres, F.P.; Edwards, K.M.; Lira, F.S. Acute increases in brain-derived neurotrophic factor following high or moder-ate-intensity exercise is accompanied with better cognition performance in obese adults. Sci. Rep. 2020, 10, 13493. [Google Scholar] [CrossRef] [PubMed]
- Charlton, T.; Prowse, N.; McFee, A.; Heiratifar, N.; Fortin, T.; Paquette, C.; Hayley, S. Brain-derived neurotrophic factor (BDNF) has direct anti-inflammatory effects on micro-glia. Front. Cell Neurosci. 2023, 17, 1188672. [Google Scholar] [CrossRef] [PubMed]
- Porter, G.A.; O’Connor, J.C. Brain-derived neurotrophic factor and inflammation in depression: Pathogenic partners in crime? World J. Psychiatry 2022, 12, 77–97. [Google Scholar] [CrossRef] [PubMed]
- García-Suárez, P.C.; Rentería, I.; Plaisance, E.P.; Moncada-Jiménez, J.; Jiménez-Maldonado, A. The effects of interval training on peripheral brain derived neurotrophic factor (BDNF) in young adults: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 8937. [Google Scholar] [CrossRef] [PubMed]
- Collo, G.; Cavalleri, L.; Spano, P. Structural plasticity in mesencephalic dopaminergic neurons produced by drugs of abuse: Critical role of BDNF and dopamine. Front. Pharmacol. 2014, 5, 259. [Google Scholar] [CrossRef] [PubMed]
- Numakawa, T.; Kajihara, R. Involvement of brain-derived neurotrophic factor signaling in the pathogenesis of stress-related brain diseases. Front. Mol. Neurosci. 2023, 16, 1247422. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Ma, M.; Yang, C.; Zhang, J.C.; Yao, W.; Hashimoto, K. BDNF-TrkB signaling in the nucleus accumbens shell of mice has key role in methamphetamine withdrawal symptoms. Transl. Psychiatry 2015, 5, e666. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Tang, W.; Liu, W.; Zhang, Y.; Wang, L.; Wang, W. Bilateral methamphetamine-induced ischemic retinopathy. Am. J. Ophthalmol. Case Rep. 2019, 15, 100473. [Google Scholar] [CrossRef] [PubMed]
- Schnaubelt, S.; Hammer, A.; Koller, L.; Niederdoeckl, J.; Kazem, N.; Spiel, A.; Niessner, A.; Sulzgruber, P. Expert Opinion: Meditation and Cardiovascular Health: What is the Link? Eur. Cardiol. 2019, 14, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Gathright, E.C.; Wu, W.C.; Salmoirago-Blotcher, E. Mindfulness-Based Interventions for Patients with Cardiovascular Disease: A Focused Review for Practicing Clinicians. Curr. Cardiol. Rep. 2023, 25, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Luo, A. The efficacy of mindfulness-based intervention for heart diseases: A meta-analysis of randomized controlled trials. Medicine 2022, 101, e29649. [Google Scholar] [CrossRef]
- Reske, M.; Paulus, M.P. Predicting treatment outcome in stimulant dependence. Ann. N. Y Acad. Sci. 2008, 1141, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, D.; Giannouli, V. Lesch Type III Alcoholism in Bulgarian Women: Implications and Recommendations for Psychotherapy. Int. J. Caring Sci. 2017, 10, 1569–1576. [Google Scholar]
- Dluzen, D.E.; Liu, B. Gender differences in methamphetamine use and responses: A review. Gend. Med. 2008, 5, 24–35. [Google Scholar] [CrossRef] [PubMed]
| Study | Type of Mindfulness Intervention | Participants | Number of Mindfulness Sessions | Results |
|---|---|---|---|---|
| [74] | Mindfulness-based relapse prevention | N = 40, 20 in the mindfulness group, 20 in the control group | Eight 90-min therapy sessions, one per week | - drug craving was alleviated, - improved emotion regulation. |
| [75] | Mindfulness-based therapy and counseling | N = 70, 35 in the mindfulness group, 35 in the control group | Weekly 90–120-min mindfulness practice sessions over an eight-week period | - decrease in craving levels, - significantly lower depression scores, - lower stress scores, - the rate of people experiencing a methamphetamine relapse was notably lower. |
| [76] | Mindfulness-based relapse prevention | N = 41, 21 in the mindfulness group, 20 in the control group | Sixteen sessions of 2-h MBRP interventions, twice a week over an eight-week period | - no effect of MBRP on craving, - no effect of MBRP on depression scores, - no effect of MBRP on anxiety scores. |
| [77] | Mindfulness-based cognitive therapy | N = 30, 15 in the mindfulness group, 15 in the control group | Eight 60-min sessions | - reduction of craving, - reduction of stress scores, - reduction of cortisol levels. |
| [78] | Mindfulness-based relapse prevention | N = 30, 15 in the mindfulness group, 15 in the control group | Eight 1.5-h sessions over a two-month period | - reduction of aggression, - reduction of craving. |
| [79] | Mindfulness-based substance abuse treatment | N = 40, 20 in the mindfulness group, 20 in the control group | Twelve sessions, with two sessions lasting 50–60-min per week | - reduction of risky behaviour and improved decision-making, - improved cognitive flexibility, - improved working memory, - improved response inhibition. |
| [80] | Mindfulness-based relapse prevention | N = 30, 15 in the mindfulness group, 15 in the control group | Nine 1-h mindfulness sessions (twice a week) | - decreased likelihood of drug use and cravings, - decreased depression score. |
| [81] | Mindfulness-based substance abuse treatment | N = 80, 20 in the tDCS group, 20 in the mindfulness group 20 in the combined intervention group 20 in the sham group | Twelve mindfulness sessions were held over the course of six weeks, with two sessions per week and a 72-h gap between them. Sessions were 45–50 min | - craving scores improved with the combination of MBSAT and tDCS and MBSAT alone, but the improvement was greater with the combined intervention. increase and decrease of Accuracy Go and Accuracy No-Go, respectively, after the combined tDCS + MBSAT intervention, but not after MBSAT alone, - MBSAT and the combination of tDCS and MBSAT improved accuracy and response time in the WM task, but the improvement was greater after the combined intervention, - significant decrease and increase of WCST persistent errors and completed categories after MBSAT and the combination of tDCS and MBSAT, but the improvement was greater after the combined intervention, - significant decrease of BART Adjusted value and Max pumping, respectively, after MBSAT and combined tDCS and MBSAT, but the improvement was greater after the combined intervention. |
| [82] | Mindfulness-based substance abuse treatment | N = 48, 15 in the mindfulness group, 17 in the combined intervention group, 16 in the sham group | Twelve mindfulness sessions were held over the course of six weeks, with two sessions per week and a 72-h gap between them. Sessions were 45–50 minn | - craving scores improved with the combination of MBSAT and tDCS and MBSAT alone, but the improvement was greater with the combined intervention, - improvement in AB after MBSAT and the combination of tDCS and MBSAT, but after the combined intervention the improvement was greater |
| [83] | Mindfulness-based substance abuse treatment | N = 80, 20 in the tDCS group, 20 in the mindfulness group 20 in the combined intervention group 20 in the sham group | The 12-session mindfulness regimen (two sessions per week) was followed by the MBSAT group. The duration of each mindfulness treatment session was 40–50 min | - craving scores improved with the combination of MBSAT and tDCS and MBSAT alone, but the improvement was greater with the combined intervention. - both MBSAT alone and the combination of tDCS and MBSAT improved anxiety and stress scores, while the improvement was greater after the combined intervention, - both MBSAT alone and the combination of tDCS and MBSAT improved depression scores, while the improvement was greater after combined intervention. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chmiel, J.; Malinowska, A.; Rybakowski, F.; Leszek, J. The Effectiveness of Mindfulness in the Treatment of Methamphetamine Addiction Symptoms: Does Neuroplasticity Play a Role? Brain Sci. 2024, 14, 320. https://doi.org/10.3390/brainsci14040320
Chmiel J, Malinowska A, Rybakowski F, Leszek J. The Effectiveness of Mindfulness in the Treatment of Methamphetamine Addiction Symptoms: Does Neuroplasticity Play a Role? Brain Sciences. 2024; 14(4):320. https://doi.org/10.3390/brainsci14040320
Chicago/Turabian StyleChmiel, James, Agnieszka Malinowska, Filip Rybakowski, and Jerzy Leszek. 2024. "The Effectiveness of Mindfulness in the Treatment of Methamphetamine Addiction Symptoms: Does Neuroplasticity Play a Role?" Brain Sciences 14, no. 4: 320. https://doi.org/10.3390/brainsci14040320
APA StyleChmiel, J., Malinowska, A., Rybakowski, F., & Leszek, J. (2024). The Effectiveness of Mindfulness in the Treatment of Methamphetamine Addiction Symptoms: Does Neuroplasticity Play a Role? Brain Sciences, 14(4), 320. https://doi.org/10.3390/brainsci14040320






