Machine Learning-Assisted Classification of Paraffin-Embedded Brain Tumors with Raman Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Data
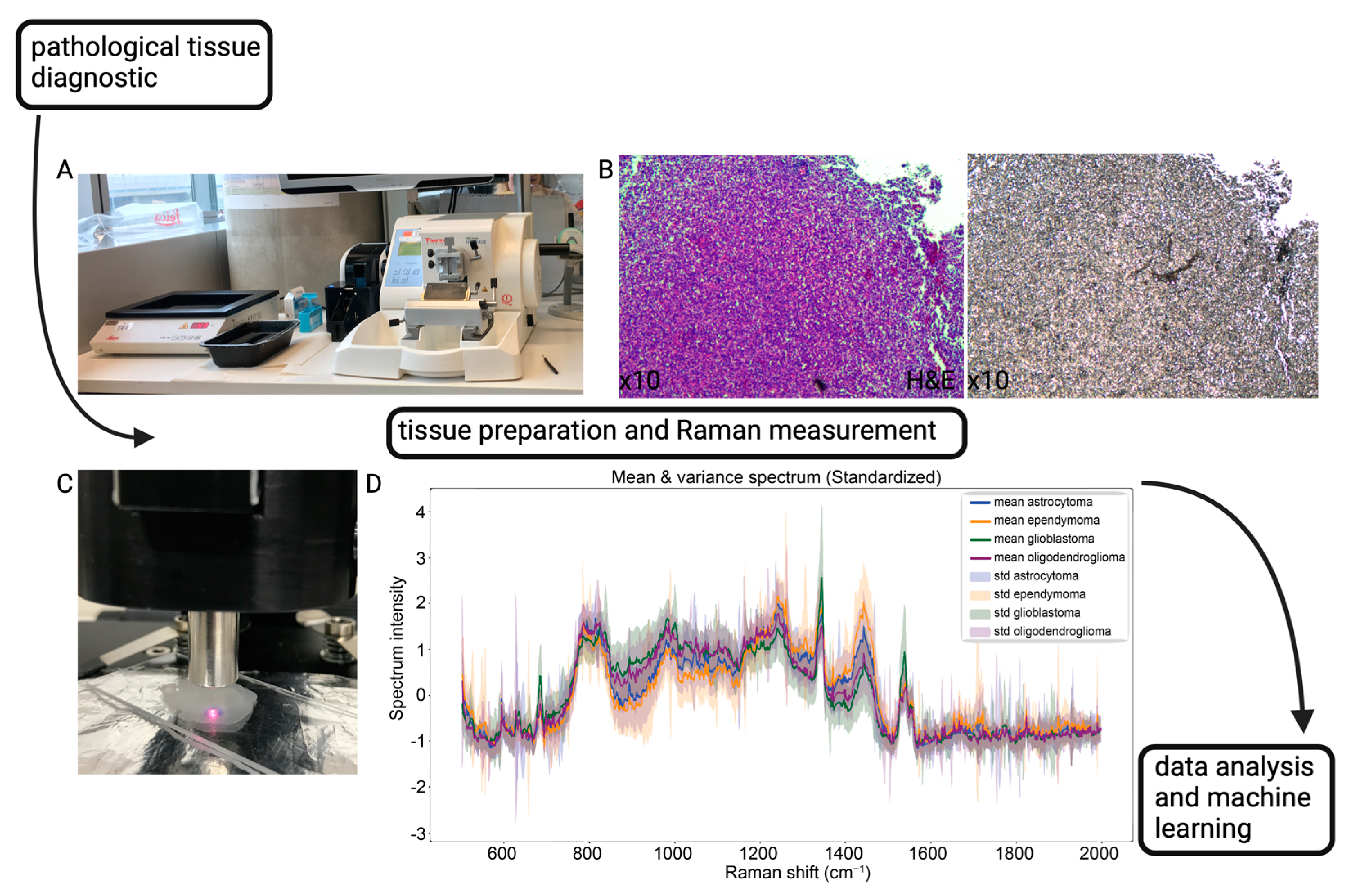
2.2. Sample Preparation
2.3. Data Acquisition and Raman Spectroscopy
2.4. Machine Learning
3. Results
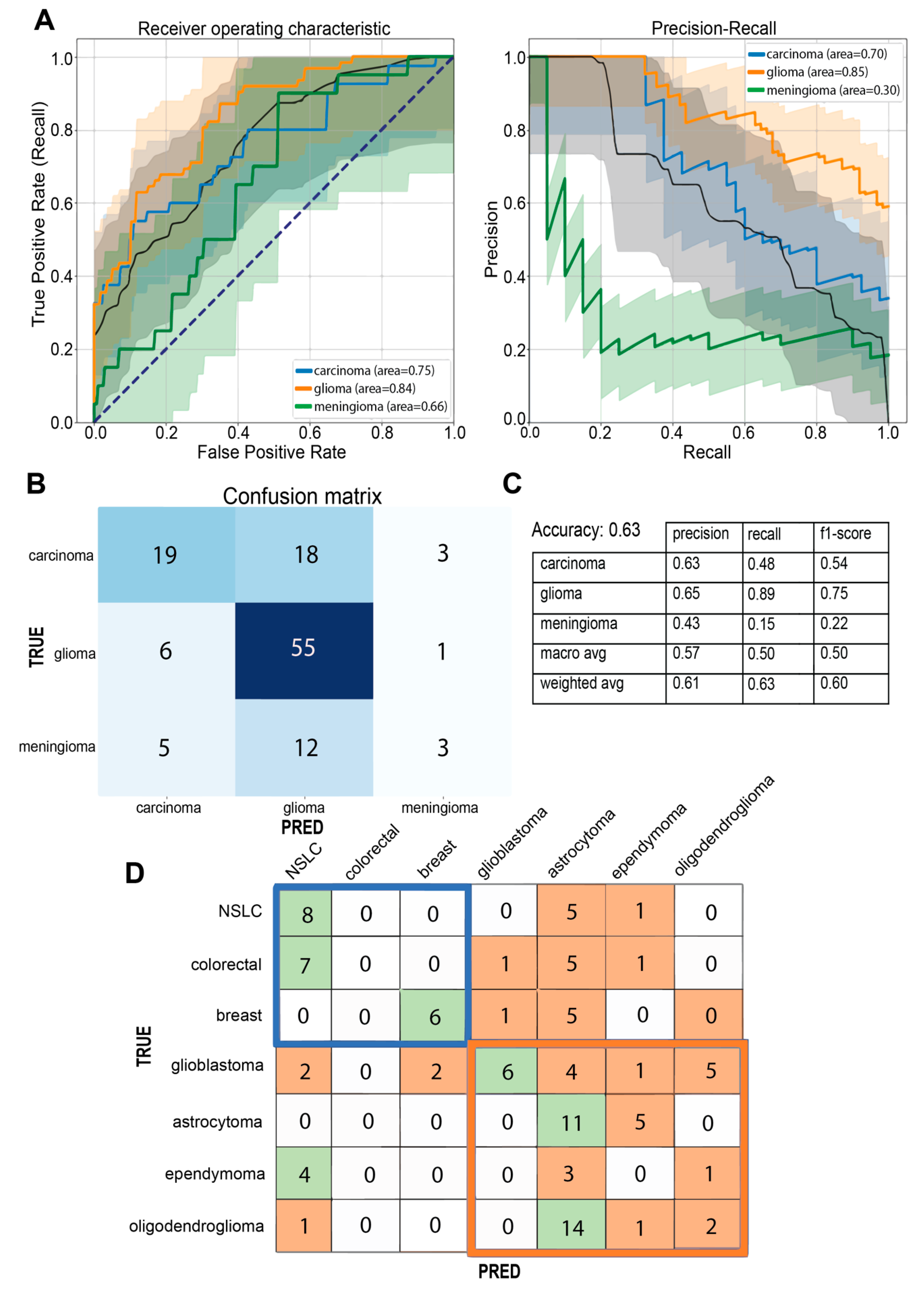
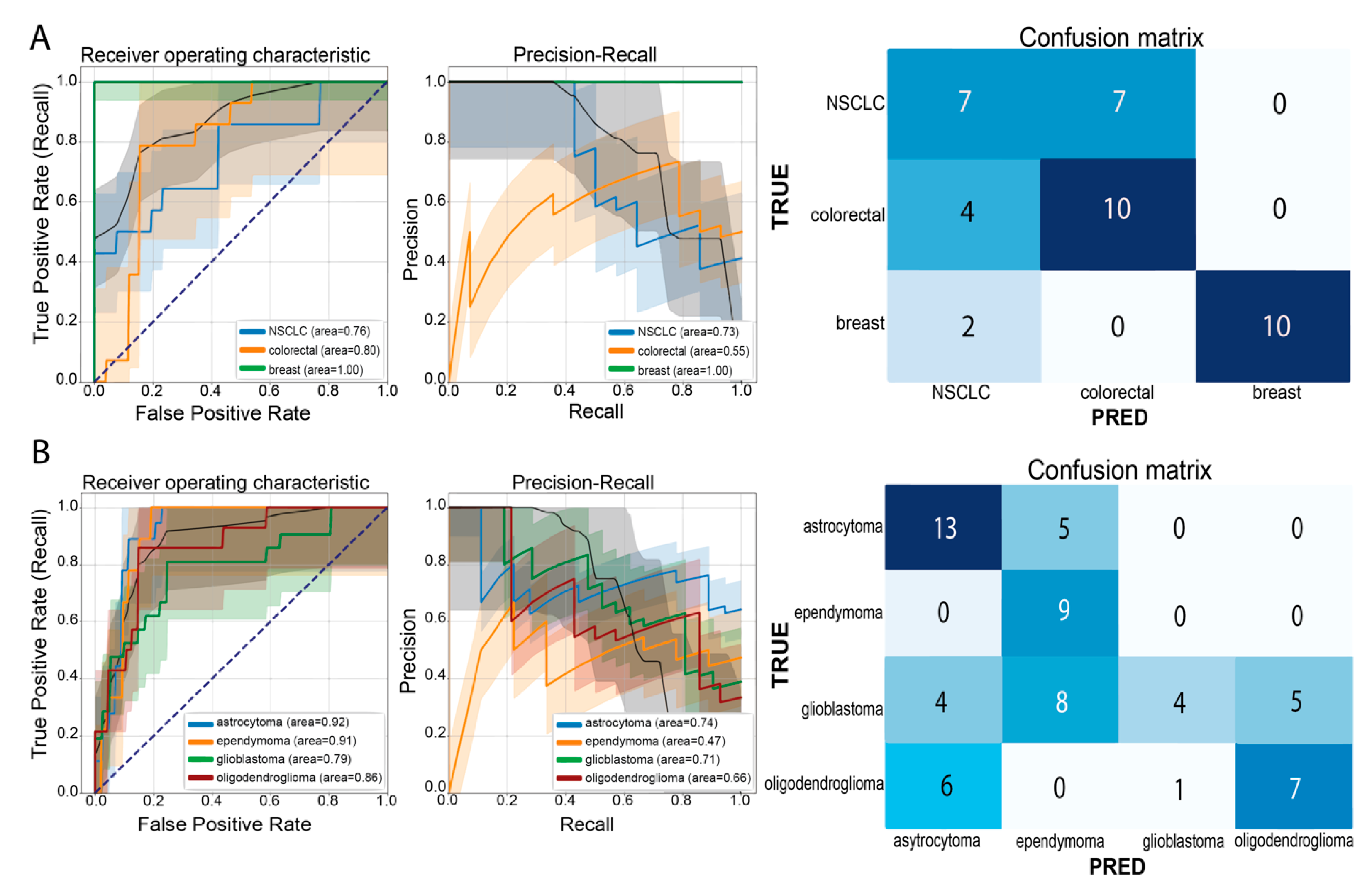
3.1. Multi-Class Classification for Discrimination of Tumor Origin
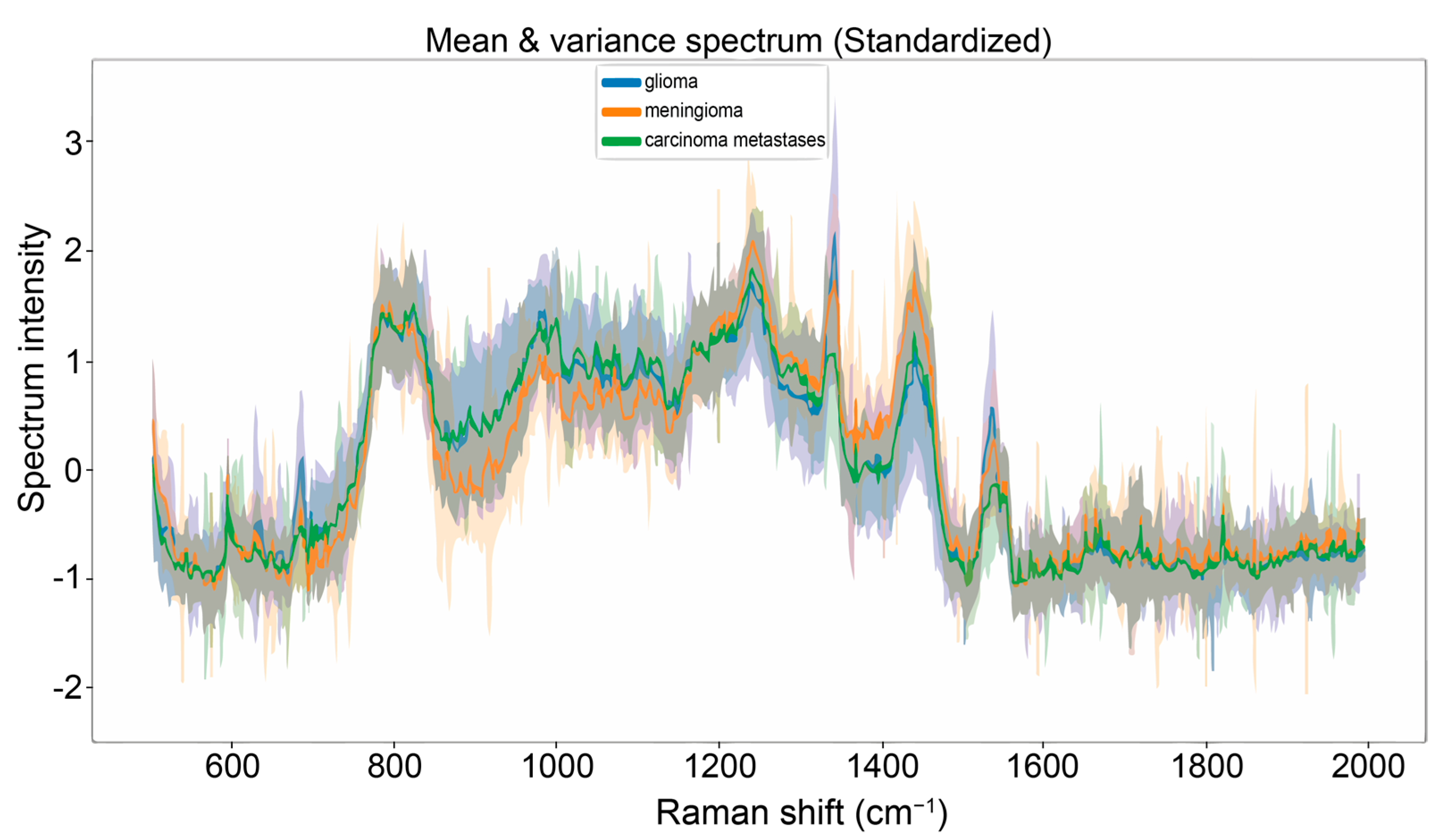
3.2. A Practical Approach: Carcinoma Metastases and Glioma Classifier
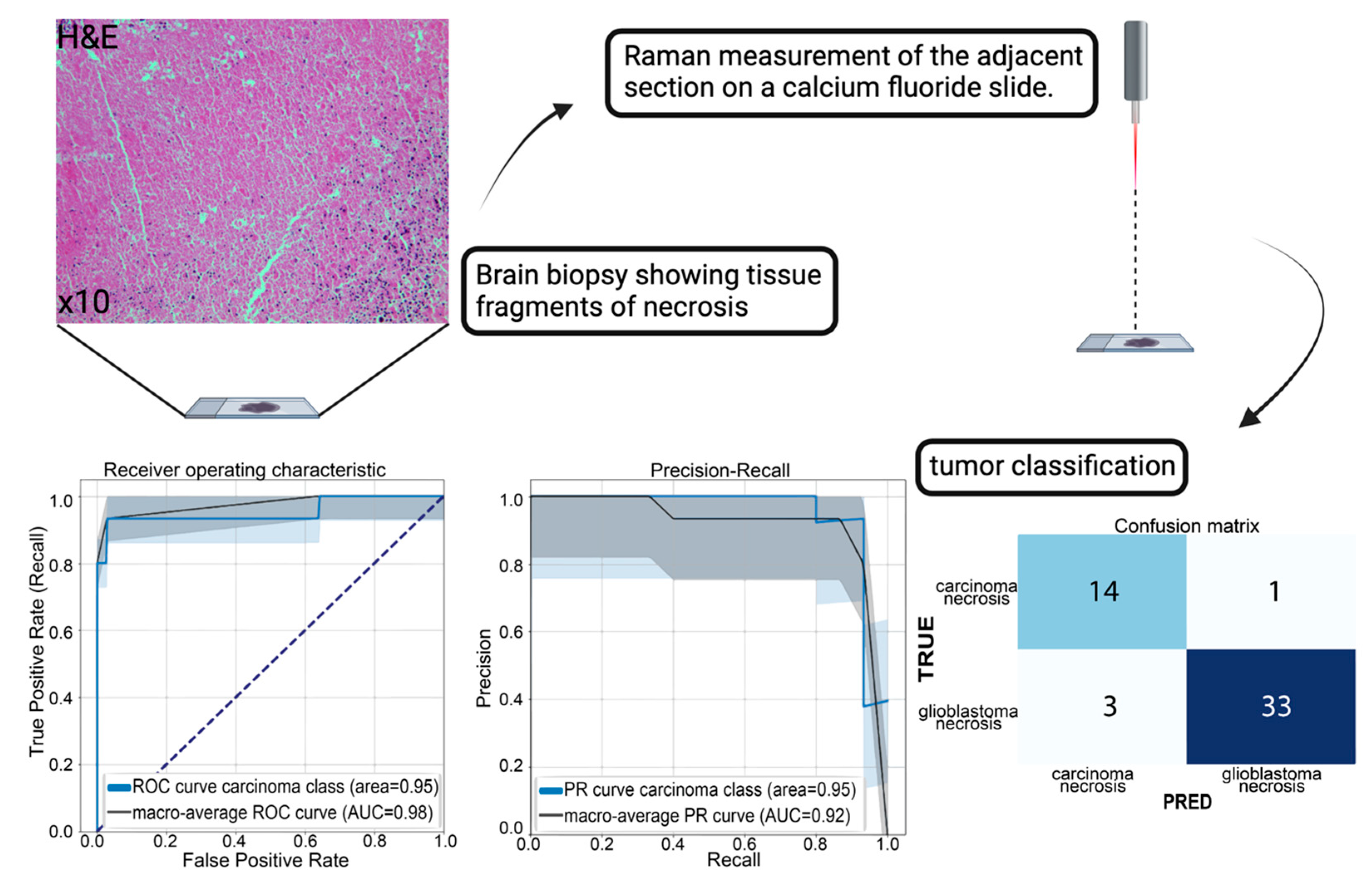
3.3. A Practical Approach: Classification of Tumor Necrosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coons, A.H.; Creech, H.J.; Jones, R.N.; Berliner, E. The Demonstration of Pneumococcal Antigen in Tissues by the Use of Fluorescent Antibody. J. Immunol. 1942, 45, 159–170. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. World Health Organization Classification of Tumours of the Central Nervous System, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2021; ISBN 978-92-832-4508-7.
- Auner, G.W.; Koya, S.K.; Huang, C.; Broadbent, B.; Trexler, M.; Auner, Z.; Elias, A.; Mehne, K.C.; Brusatori, M.A. Applications of Raman Spectroscopy in Cancer Diagnosis. Cancer Metastasis Rev. 2018, 37, 691–717. [Google Scholar] [CrossRef]
- Hollon, T.C.; Pandian, B.; Adapa, A.R.; Urias, E.; Save, A.V.; Khalsa, S.S.S.; Eichberg, D.G.; D’Amico, R.S.; Farooq, Z.U.; Lewis, S.; et al. Near Real-Time Intraoperative Brain Tumor Diagnosis Using Stimulated Raman Histology and Deep Neural Networks. Nat. Med. 2020, 26, 52–58. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, C.-H.; Wu, B.; Yu, X.; Cheng, G.; Zhu, K.; Wang, K.; Zhang, C.; Zhao, M.; Zong, R.; et al. Optical Biopsy Identification and Grading of Gliomas Using Label-Free Visible Resonance Raman Spectroscopy. J. Biomed. Opt. 2019, 24, 095001. [Google Scholar] [CrossRef]
- Pekmezci, M.; Morshed, R.A.; Chunduru, P.; Pandian, B.; Young, J.; Villanueva-Meyer, J.E.; Tihan, T.; Sloan, E.A.; Aghi, M.K.; Molinaro, A.M.; et al. Detection of Glioma Infiltration at the Tumor Margin Using Quantitative Stimulated Raman Scattering Histology. Sci. Rep. 2021, 11, 12162. [Google Scholar] [CrossRef] [PubMed]
- Kalkanis, S.N.; Kast, R.E.; Rosenblum, M.L.; Mikkelsen, T.; Yurgelevic, S.M.; Nelson, K.M.; Raghunathan, A.; Poisson, L.M.; Auner, G.W. Raman Spectroscopy to Distinguish Grey Matter, Necrosis, and Glioblastoma Multiforme in Frozen Tissue Sections. J. Neurooncol. 2014, 116, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Romanishkin, I.; Savelieva, T.; Kosyrkova, A.; Okhlopkov, V.; Shugai, S.; Orlov, A.; Kravchuk, A.; Goryaynov, S.; Golbin, D.; Pavlova, G.; et al. Differentiation of Glioblastoma Tissues Using Spontaneous Raman Scattering with Dimensionality Reduction and Data Classification. Front. Oncol. 2022, 12, 944210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, Y.; Wu, B.; Zhang, S.; Zhu, K.; Liu, C.-H.; Yu, X.; Alfano, R.R. A Handheld Visible Resonance Raman Analyzer Used in Intraoperative Detection of Human Glioma. Cancers 2023, 15, 1752. [Google Scholar] [CrossRef] [PubMed]
- Jabarkheel, R.; Ho, C.-S.; Rodrigues, A.J.; Jin, M.C.; Parker, J.J.; Mensah-Brown, K.; Yecies, D.; Grant, G.A. Rapid Intraoperative Diagnosis of Pediatric Brain Tumors Using Raman Spectroscopy: A Machine Learning Approach. Neurooncol. Adv. 2022, 4, vdac118. [Google Scholar] [CrossRef] [PubMed]
- Jermyn, M.; Mok, K.; Mercier, J.; Desroches, J.; Pichette, J.; Saint-Arnaud, K.; Bernstein, L.; Guiot, M.-C.; Petrecca, K.; Leblond, F. Intraoperative Brain Cancer Detection with Raman Spectroscopy in Humans. Sci. Transl. Med. 2015, 7, 274ra19. [Google Scholar] [CrossRef] [PubMed]
- Eichberg, D.G.; Shah, A.H.; Di, L.; Semonche, A.M.; Jimsheleishvili, G.; Luther, E.M.; Sarkiss, C.A.; Levi, A.D.; Gultekin, S.H.; Komotar, R.J.; et al. Stimulated Raman Histology for Rapid and Accurate Intraoperative Diagnosis of CNS Tumors: Prospective Blinded Study. J. Neurosurg. 2021, 134, 137–143. [Google Scholar] [CrossRef]
- Butler, H.J.; Ashton, L.; Bird, B.; Cinque, G.; Curtis, K.; Dorney, J.; Esmonde-White, K.; Fullwood, N.J.; Gardner, B.; Martin-Hirsch, P.L.; et al. Using Raman Spectroscopy to Characterize Biological Materials. Nat. Protoc. 2016, 11, 664–687. [Google Scholar] [CrossRef]
- Fairley, J.A.; Gilmour, K.; Walsh, K. Making the Most of Pathological Specimens: Molecular Diagnosis in Formalin-Fixed, Paraffin Embedded Tissue. Curr. Drug Targets 2012, 13, 1475–1487. [Google Scholar] [CrossRef] [PubMed]
- Mathieson, W.; Thomas, G.A. Why Formalin-Fixed, Paraffin-Embedded Biospecimens Must Be Used in Genomic Medicine: An Evidence-Based Review and Conclusion. J. Histochem. Cytochem. 2020, 68, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Yi, Q.; Yang, R.; Shi, J.; Zeng, N.; Liang, D.; Sha, S.; Chang, Q. Effect of Preservation Time of Formalin-Fixed Paraffin-Embedded Tissues on Extractable DNA and RNA Quantity. J. Int. Med. Res. 2020, 48, 0300060520931259. [Google Scholar] [CrossRef] [PubMed]
- Faoláin, E.Ó.; Hunter, M.B.; Byrne, J.M.; Kelehan, P.; Lambkin, H.A.; Byrne, H.J.; Lyng, F.M. Raman Spectroscopic Evaluation of Efficacy of Current Paraffin Wax Section Dewaxing Agents. J. Histochem. Cytochem. 2005, 53, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Mariani, M.M.; Lampen, P.; Popp, J.; Wood, B.R.; Deckert, V. Impact of Fixation on in Vitro Cell Culture Lines Monitored with Raman Spectroscopy. Analyst 2009, 134, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Klamminger, G.G.; Klein, K.; Mombaerts, L.; Jelke, F.; Mirizzi, G.; Slimani, R.; Husch, A.; Mittelbronn, M.; Hertel, F.; Kleine Borgmann, F.B. Differentiation of Primary CNS Lymphoma and Glioblastoma Using Raman Spectroscopy and Machine Learning Algorithms. Free Neuropathol. 2021, 2, 26. [Google Scholar] [CrossRef]
- Fullwood, L.M.; Clemens, G.; Griffiths, D.; Ashton, K.; Dawson, T.P.; Lea, R.W.; Davis, C.; Bonnier, F.; Byrne, H.J.; Baker, M.J. Investigating the Use of Raman and Immersion Raman Spectroscopy for Spectral Histopathology of Metastatic Brain Cancer and Primary Sites of Origin. Anal. Methods 2014, 6, 3948–3961. [Google Scholar] [CrossRef]
- Livermore, L.J.; Isabelle, M.; Bell, I.M.; Scott, C.; Walsby-Tickle, J.; Gannon, J.; Plaha, P.; Vallance, C.; Ansorge, O. Rapid Intraoperative Molecular Genetic Classification of Gliomas Using Raman Spectroscopy. Neurooncol. Adv. 2019, 1, vdz008. [Google Scholar] [CrossRef]
- Klamminger, G.G.; Gérardy, J.-J.; Jelke, F.; Mirizzi, G.; Slimani, R.; Klein, K.; Husch, A.; Hertel, F.; Mittelbronn, M.; Kleine-Borgmann, F.B. Application of Raman Spectroscopy for Detection of Histologically Distinct Areas in Formalin-Fixed Paraffin-Embedded Glioblastoma. Neurooncol. Adv. 2021, 3, vdab077. [Google Scholar] [CrossRef] [PubMed]
- Fullwood, L.M.; Griffiths, D.; Ashton, K.; Dawson, T.; Lea, R.W.; Davis, C.; Bonnier, F.; Byrne, H.J.; Baker, M.J. Effect of Substrate Choice and Tissue Type on Tissue Preparation for Spectral Histopathology by Raman Microspectroscopy. Analyst 2014, 139, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Crystran Ltd. Raman Substrate Materials. Available online: https://www.crystran.co.uk/raman-substrate-materials/ (accessed on 13 November 2020).
- Mian, S.A.; Colley, H.E.; Thornhill, M.H.; Rehman, I. Development of a Dewaxing Protocol for Tissue-Engineered Models of the Oral Mucosa Used for Raman Spectroscopic Analysis. Appl. Spectrosc. Rev. 2014, 49, 614–617. [Google Scholar] [CrossRef]
- Bury, D.; Morais, C.; Ashton, K.; Dawson, T.; Martin, F. Ex Vivo Raman Spectrochemical Analysis Using a Handheld Probe Demonstrates High Predictive Capability of Brain Tumour Status. Biosensors 2019, 9, 49. [Google Scholar] [CrossRef]
- Jermyn, M.; Desroches, J.; Mercier, J.; Tremblay, M.-A.; St-Arnaud, K.; Guiot, M.-C.; Petrecca, K.; Leblond, F. Neural Networks Improve Brain Cancer Detection with Raman Spectroscopy in the Presence of Operating Room Light Artifacts. J. Biomed. Opt. 2016, 21, 094002. [Google Scholar] [CrossRef]
- Barton, S.J.; Hennelly, B.M. An Algorithm for the Removal of Cosmic Ray Artifacts in Spectral Data Sets. Appl. Spectrosc. 2019, 73, 893–901. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Amharref, N.; Beljebbar, A.; Dukic, S.; Venteo, L.; Schneider, L.; Pluot, M.; Manfait, M. Discriminating Healthy from Tumor and Necrosis Tissue in Rat Brain Tissue Samples by Raman Spectral Imaging. Biochim. Biophys. Acta Biomembr. 2007, 1768, 2605–2615. [Google Scholar] [CrossRef]
- Kast, R.; Auner, G.; Yurgelevic, S.; Broadbent, B.; Raghunathan, A.; Poisson, L.M.; Mikkelsen, T.; Rosenblum, M.L.; Kalkanis, S.N. Identification of Regions of Normal Grey Matter and White Matter from Pathologic Glioblastoma and Necrosis in Frozen Sections Using Raman Imaging. J. Neurooncol. 2015, 125, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Jermyn, M.; Desroches, J.; Mercier, J.; St-Arnaud, K.; Guiot, M.-C.; Leblond, F.; Petrecca, K. Raman Spectroscopy Detects Distant Invasive Brain Cancer Cells Centimeters beyond MRI Capability in Humans. Biomed. Opt. Express 2016, 7, 5129–5137. [Google Scholar] [CrossRef] [PubMed]
- Klein, K.; Klamminger, G.G.; Mombaerts, L.; Jelke, F.; Arroteia, I.F.; Slimani, R.; Mirizzi, G.; Husch, A.; Frauenknecht, K.B.M.; Mittelbronn, M.; et al. Computational Assessment of Spectral Heterogeneity within Fresh Glioblastoma Tissue Using Raman Spectroscopy and Machine Learning Algorithms. Molecules 2024, 29, 979. [Google Scholar] [CrossRef] [PubMed]
- Quesnel, A.; Coles, N.; Angione, C.; Dey, P.; Polvikoski, T.M.; Outeiro, T.F.; Islam, M.; Khundakar, A.A.; Filippou, P.S. Glycosylation Spectral Signatures for Glioma Grade Discrimination Using Raman Spectroscopy. BMC Cancer 2023, 23, 174. [Google Scholar] [CrossRef] [PubMed]
- Koljenović, S.; Schut, T.B.; Vincent, A.; Kros, J.M.; Puppels, G.J. Detection of Meningioma in Dura Mater by Raman Spectroscopy. Anal. Chem. 2005, 77, 7958–7965. [Google Scholar] [CrossRef] [PubMed]
- Jelke, F.; Mirizzi, G.; Borgmann, F.K.; Husch, A.; Slimani, R.; Klamminger, G.G.; Klein, K.; Mombaerts, L.; Gérardy, J.-J.; Mittelbronn, M.; et al. Intraoperative Discrimination of Native Meningioma and Dura Mater by Raman Spectroscopy. Sci. Rep. 2021, 11, 23583. [Google Scholar] [CrossRef]
- Morais, C.L.M.; Lilo, T.; Ashton, K.M.; Davis, C.; Dawson, T.P.; Gurusinghe, N.; Martin, F.L. Determination of Meningioma Brain Tumour Grades Using Raman Microspectroscopy Imaging. Analyst 2019, 144, 7024–7031. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, Y.; Wu, B.; Zhang, S.; Zhu, K.; Liu, C.; Yu, X.; Alfano, R.R. Intraoperative Detection of Human Meningioma Using a Handheld Visible Resonance Raman Analyzer. Lasers Med. Sci. 2021, 37, 1311–1319. [Google Scholar] [CrossRef]
- Mirizzi, G.; Jelke, F.; Pilot, M.; Klein, K.; Klamminger, G.G.; Gérardy, J.-J.; Theodoropoulou, M.; Mombaerts, L.; Husch, A.; Mittelbronn, M.; et al. Impact of Formalin- and Cryofixation on Raman Spectra of Human Tissues and Strategies for Tumor Bank Inclusion. Molecules 2024, 29, 1167. [Google Scholar] [CrossRef]
| Tumor Group/ Tumor Type | Number of Cases n = 82 | Number of Measurements n = 679 |
|---|---|---|
| Astrocytoma of grades 2,3, IDH mutant | 9 | 74 |
| Oligodendroglioma of grades 2,3, 1p19q co-deleted | 7 | 60 |
| Ependymoma | 5 | 44 |
| Glioblastoma, IDH wildtype | 27 | 179 |
| Meningothelial meningioma | 4 | 36 |
| Transitional meningioma | 6 | 56 |
| Breast carcinoma metastases | 8 | 53 |
| Colorectal carcinoma metastases | 6 | 65 |
| Non-small cell lung carcinoma (NSCLC) metastases | 10 | 112 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klamminger, G.G.; Mombaerts, L.; Kemp, F.; Jelke, F.; Klein, K.; Slimani, R.; Mirizzi, G.; Husch, A.; Hertel, F.; Mittelbronn, M.; et al. Machine Learning-Assisted Classification of Paraffin-Embedded Brain Tumors with Raman Spectroscopy. Brain Sci. 2024, 14, 301. https://doi.org/10.3390/brainsci14040301
Klamminger GG, Mombaerts L, Kemp F, Jelke F, Klein K, Slimani R, Mirizzi G, Husch A, Hertel F, Mittelbronn M, et al. Machine Learning-Assisted Classification of Paraffin-Embedded Brain Tumors with Raman Spectroscopy. Brain Sciences. 2024; 14(4):301. https://doi.org/10.3390/brainsci14040301
Chicago/Turabian StyleKlamminger, Gilbert Georg, Laurent Mombaerts, Françoise Kemp, Finn Jelke, Karoline Klein, Rédouane Slimani, Giulia Mirizzi, Andreas Husch, Frank Hertel, Michel Mittelbronn, and et al. 2024. "Machine Learning-Assisted Classification of Paraffin-Embedded Brain Tumors with Raman Spectroscopy" Brain Sciences 14, no. 4: 301. https://doi.org/10.3390/brainsci14040301
APA StyleKlamminger, G. G., Mombaerts, L., Kemp, F., Jelke, F., Klein, K., Slimani, R., Mirizzi, G., Husch, A., Hertel, F., Mittelbronn, M., & Kleine Borgmann, F. B. (2024). Machine Learning-Assisted Classification of Paraffin-Embedded Brain Tumors with Raman Spectroscopy. Brain Sciences, 14(4), 301. https://doi.org/10.3390/brainsci14040301








