The α-7 Nicotinic Receptor Positive Allosteric Modulator Alleviates Lipopolysaccharide Induced Depressive-like Behavior by Regulating Microglial Function, Trophic Factor, and Chloride Transporters in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drugs and Treatment
2.3. Experimental Procedure
2.4. Western Blot Analysis
2.5. Immunofluorescence Assay
2.6. Quantitative Real-Time Polymerase Chain Reaction
2.7. Behavioral Tests
2.7.1. Locomotor Activity
2.7.2. Y-Maze
2.7.3. Tail Suspension Test
2.7.4. Forced Swim Test
2.8. Statistical Analysis
3. Results
3.1. Effects of PNU120596 on the Expression of BDNF and p-CREB in the DG and CA1 Regions of the Hippocampus and Medial Prefrontal Cortex
3.2. Effects of PNU120596 on BDNF Immunoreactivity in the DG and CA1 Regions of the Hippocampus and Medial Prefrontal Cortex
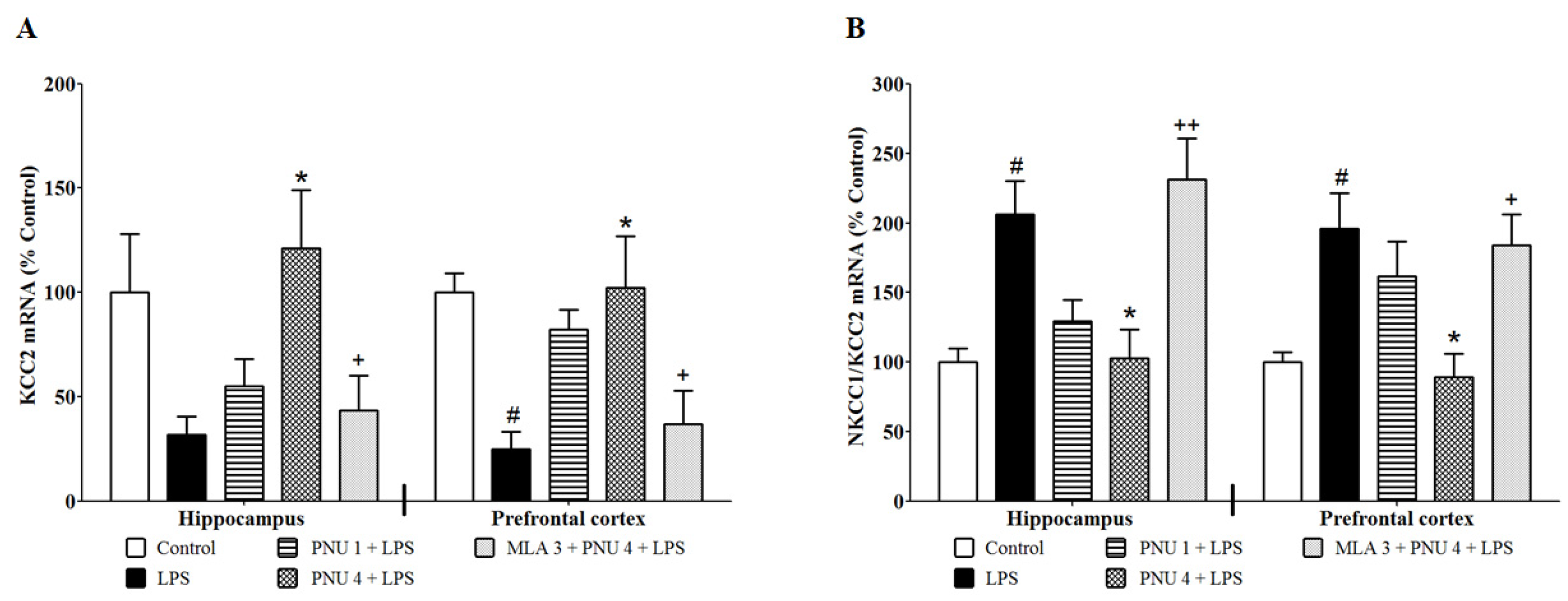
3.3. Effects of PNU120596 on the mRNA Expression of KCC2 and the NKCC1/KCC2 Ratio in the Hippocampus and Prefrontal Cortex
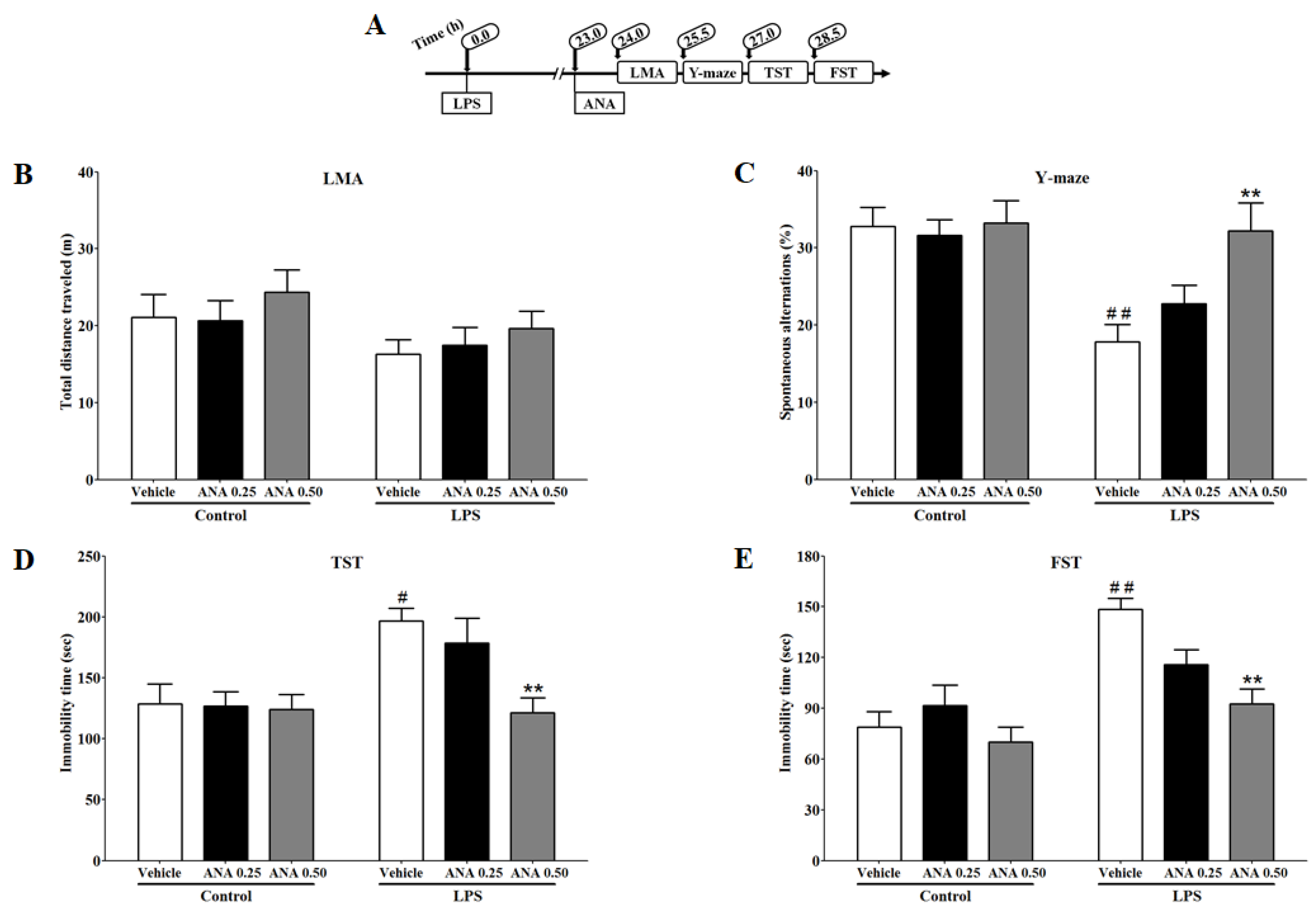
3.4. Effects of ANA12 on LPS-Induced Depressive-like Behavior
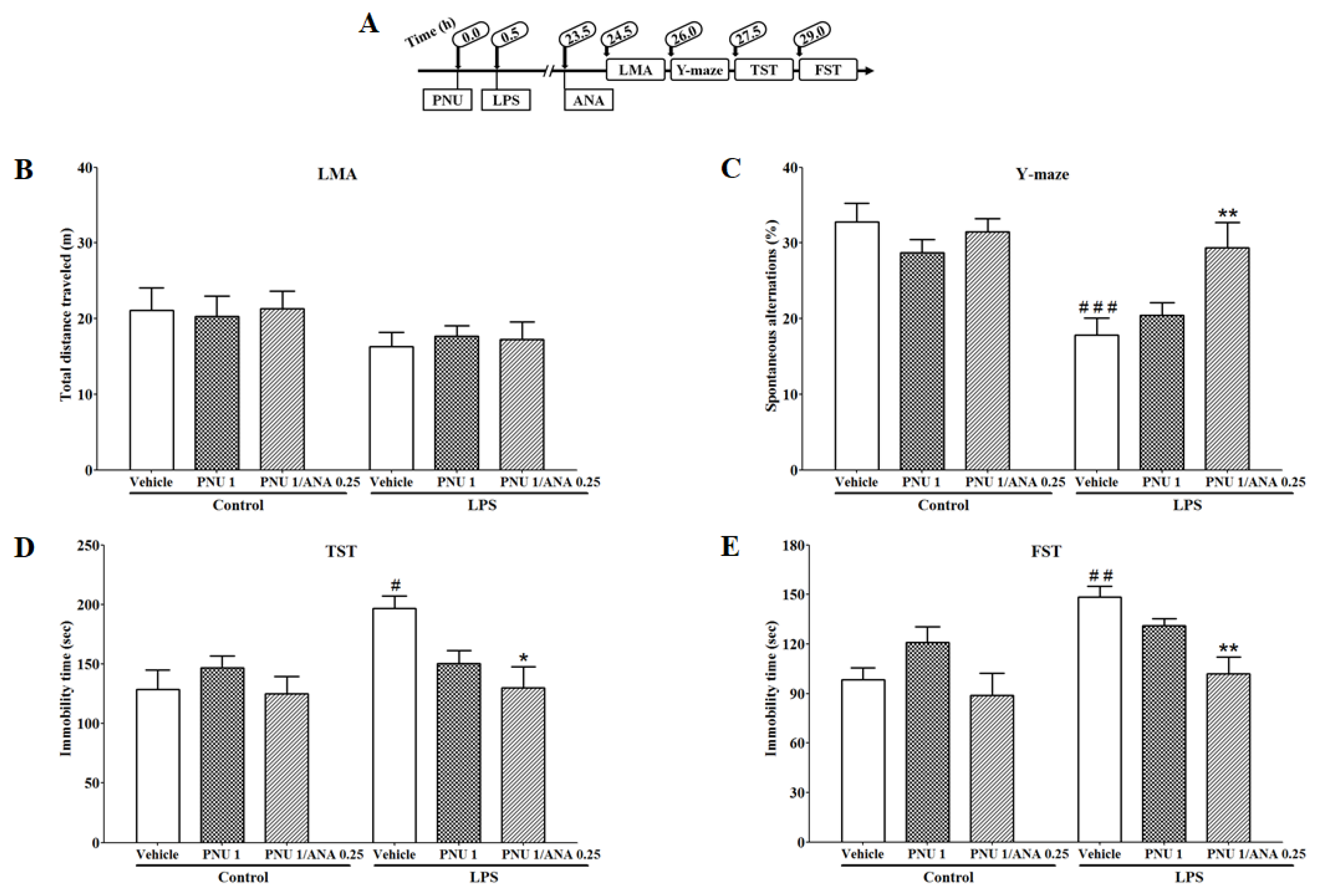
3.5. Combination Effects of PNU120596 and ANA12 on LPS-Induced Depressive-like Behavior
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vichaya, E.G.; Gross, P.S.; Estrada, D.J.; Cole, S.W.; Grossberg, A.J.; Evans, S.E.; Tuvim, M.J.; Dickey, B.F.; Dantzer, R. Lipocalin-2 is dispensable in inflammation-induced sickness and depression-like behavior. Psychopharmacology 2019, 236, 2975–2982. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E.; Toups, M.; Nemeroff, C.B. The bidirectional relationship of depression and inflammation: Double trouble. Neuron 2020, 107, 234–256. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, D.R.; Bekhbat, M.; Mehta, N.D.; Felger, J.C. Inflammation-related functional and structural dysconnectivity as a pathway to psychopathology. Biol. Psychiatry 2023, 93, 405–418. [Google Scholar] [CrossRef]
- Troubat, R.; Barone, P.; Leman, S.; Desmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; El Hage, W.; Surget, A.; Belzung, C. Neuroinflammation and depression: A review. Eur. J. Neurosci. 2021, 53, 151–171. [Google Scholar] [CrossRef]
- Liu, J.J.; Wei, Y.B.; Strawbridge, R.; Bao, Y.; Chang, S.; Shi, L.; Que, J.; Gadad, B.S.; Trivedi, M.H.; Kelsoe, J.R. Peripheral cytokine levels and response to antidepressant treatment in depression: A systematic review and meta-analysis. Mol. Psychiatry 2020, 25, 339–350. [Google Scholar] [CrossRef]
- Felger, J.C. Imaging the role of inflammation in mood and anxiety-related disorders. Curr. Neuropharmacol. 2018, 16, 533–558. [Google Scholar] [CrossRef]
- Hannestad, J.; DellaGioia, N.; Bloch, M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: A meta-analysis. Neuropsychopharmacology 2011, 36, 2452–2459. [Google Scholar] [CrossRef]
- Capuron, L.; Miller, A.H. Cytokines and psychopathology: Lessons from interferon-alpha. Biol. Psychiatry 2004, 56, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Harrison, N.A.; Brydon, L.; Walker, C.; Gray, M.A.; Steptoe, A.; Critchley, H.D. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol. Psychiatry 2009, 66, 407–414. [Google Scholar] [CrossRef]
- Brydon, L.; Harrison, N.A.; Walker, C.; Steptoe, A.; Critchley, H.D. Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biol. Psychiatry 2008, 63, 1022–1029. [Google Scholar] [CrossRef]
- Grigoleit, J.S.; Kullmann, J.S.; Wolf, O.T.; Hammes, F.; Wegner, A.; Jablonowski, S.; Engler, H.; Gizewski, E.; Oberbeck, R.; Schedlowski, M. Dose-dependent effects of endotoxin on neurobehavioral functions in humans. PLoS ONE 2011, 6, e28330. [Google Scholar] [CrossRef]
- Eisenberger, N.I.; Berkman, E.T.; Inagaki, T.K.; Rameson, L.T.; Mashal, N.M.; Irwin, M.R. Inflammation-induced anhedonia: Endotoxin reduces ventral striatum responses to reward. Biol. Psychiatry 2010, 68, 748–754. [Google Scholar] [CrossRef]
- Krabbe, K.S.; Reichenberg, A.; Yirmiya, R.; Smed, A.; Pedersen, B.K.; Bruunsgaard, H. Low-dose endotoxemia and human neuropsychological functions. Brain Behav. Immun. 2005, 19, 453–460. [Google Scholar] [CrossRef]
- Watson, D.J.; Stanton, M.E. Medial prefrontal administration of MK-801 impairs T-maze discrimination reversal learning in weanling rats. Behav. Brain Res. 2009, 205, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Parrott, J.M.; Redus, L.; Santana-Coelho, D.; Morales, J.; Gao, X.; O’Connor, J.C. Neurotoxic kynurenine metabolism is increased in the dorsal hippocampus and drives distinct depressive behaviors during inflammation. Transl. Psychiatry 2016, 6, e918. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.C.; Lawson, M.A.; Andre, C.; Moreau, M.; Lestage, J.; Castanon, N.; Kelley, K.W.; Dantzer, R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatry 2009, 14, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Chamera, K.; Trojan, E.; Szuster-Głuszczak, M.; Basta-Kaim, A. The potential role of dysfunctions in neuron-microglia communication in the pathogenesis of brain disorders. Curr. Neuropharmacol. 2020, 18, 408–430. [Google Scholar] [CrossRef]
- Onodera, J.; Nagata, H.; Nakashima, A.; Ikegaya, Y.; Koyama, R. Neuronal brain-derived neurotrophic factor manipulates microglial dynamics. Glia 2021, 69, 890–904. [Google Scholar] [CrossRef]
- Wang, H.; He, Y.; Sun, Z.; Ren, S.; Liu, M.; Wang, G.; Yang, J. Microglia in depression: An overview of microglia in the pathogenesis and treatment of depression. J. Neuroinflamm. 2022, 19, 132. [Google Scholar] [CrossRef] [PubMed]
- Alfa, R.W.; Tuszynski, M.H.; Blesch, A. A novel inducible tyrosine kinase receptor to regulate signal transduction and neurite outgrowth. J. Neurosci. Res. 2009, 87, 2624–2631. [Google Scholar] [CrossRef]
- Chan, J.P.; Unger, T.J.; Byrnes, J.; Rios, M. Examination of behavioral deficits triggered by targeting Bdnf in fetal or postnatal brains of mice. Neuroscience 2006, 142, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yang, W.; Ge, T.; Wang, Y.; Cui, R. Stress induced microglial activation contributes to depression. Pharmacol. Res. 2022, 179, 106145. [Google Scholar] [CrossRef] [PubMed]
- Prowse, N.; Hayley, S. Microglia and BDNF at the crossroads of stressor related disorders: Towards a unique trophic phenotype. Neurosci. Biobehav. Rev. 2021, 131, 135–163. [Google Scholar] [CrossRef]
- Gutierrez, H.; Hale, V.A.; Dolcet, X.; Davies, A. NF-kappaB signalling regulates the growth of neural processes in the developing PNS and CNS. Development 2005, 132, 1713–1726. [Google Scholar] [CrossRef] [PubMed]
- Marini, A.M.; Jiang, X.; Wu, X.; Tian, F.; Zhu, D.; Okagaki, P.; Lipsky, R.H. Role of brain-derived neurotrophic factor and NF-kappaB in neuronal plasticity and survival: From genes to phenotype. Restor. Neurol. Neurosci. 2004, 22, 121–130. [Google Scholar]
- Rivera, C.; Li, H.; Thomas-Crusells, J.; Lahtinen, H.; Viitanen, T.; Nanobashvili, A.; Kokaia, Z.; Airaksinen, M.S.; Voipio, J.; Kaila, K.; et al. BDNF-induced TrkB activation down-regulates the K+-Cl− cotransporter KCC2 and impairs neuronal Cl− extrusion. J. Cell Biol. 2002, 159, 747–752. [Google Scholar] [CrossRef]
- Shulga, A.; Thomas-Crusells, J.; Sigl, T.; Blaesse, A.; Mestres, P.; Meyer, M.; Yan, Q.; Kaila, K.; Saarma, M.; Rivera, C.; et al. Posttraumatic GABA(A)-mediated [Ca2+]i increase is essential for the induction of brain-derived neurotrophic factor-dependent survival of mature central neurons. J. Neurosci. 2008, 28, 6996–7005. [Google Scholar] [CrossRef]
- Luo, L.; Song, S.; Ezenwukwa, C.C.; Jalali, S.; Sun, B.; Sun, D. Ion channels and transporters in microglial function in physiology and brain diseases. Neurochem. Int. 2021, 142, 104925. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, G.; Zhao, C.; Yang, Y.; Miao, Z.; Xu, X. Interleukin-18 from neurons and microglia mediates depressive behaviors in mice with post-stroke depression. Brain Behav. Immun. 2020, 88, 411–420. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, J.-J.; Zhu, Y.; Kosten, T.; Li, D.-P. Chronic unpredictable mild stress induces loss of GABA inhibition in corticotrophin-releasing hormone-expressing neurons through NKCC1 upregulation. Neuroendocrinology 2016, 104, 194–208. [Google Scholar] [CrossRef]
- Tao, R.; Li, C.; Newburn, E.N.; Ye, T.; Lipska, B.K.; Herman, M.M.; Weinberger, D.R.; Kleinman, J.E.; Hyde, T.M. Transcript-specific associations of SLC12A5 (KCC2) in human prefrontal cortex with development, schizophrenia, and affective disorders. J. Neurosci. 2012, 32, 5216–5222. [Google Scholar] [CrossRef] [PubMed]
- Pressey, J.C.; de Saint-Rome, M.; Raveendran, V.A.; Woodin, M.A. Chloride transporters controlling neuronal excitability. Physiol. Rev. 2023, 103, 1095–1135. [Google Scholar] [CrossRef]
- Dziewczapolski, G.; Glogowski, C.M.; Masliah, E.; Heinemann, S.F. Deletion of the alpha 7 nicotinic acetylcholine receptor gene improves cognitive deficits and synaptic pathology in a mouse model of Alzheimer’s disease. J. Neurosci. 2009, 29, 8805–8815. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Hansen, H.H.; Timmerman, D.B.; Mikkelsen, J.D. Cognitive improvement by activation of alpha7 nicotinic acetylcholine receptors: From animal models to human pathophysiology. Curr. Pharm. Des. 2010, 16, 323–343. [Google Scholar] [CrossRef] [PubMed]
- Gotti, C.; Zoli, M.; Clementi, F. Brain nicotinic acetylcholine receptors: Native subtypes and their relevance. Trends Pharmacol. Sci. 2006, 27, 482–491. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, W.J.; Ulloa, L. The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br. J. Pharmacol. 2007, 151, 915–929. [Google Scholar] [CrossRef] [PubMed]
- Alzarea, S.; Rahman, S. Effects of alpha-7 nicotinic allosteric modulator PNU 120596 on depressive-like behavior after lipopolysaccharide administration in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 86, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Alzarea, S.; Papke, R.L.; Rahman, S. The alpha7 nicotinic acetylcholine receptor positive allosteric modulator attenuates lipopolysaccharide-induced activation of hippocampal IkappaB and CD11b gene expression in mice. Drug Discov. Ther. 2017, 11, 206–211. [Google Scholar] [CrossRef][Green Version]
- Rahman, S.; Alzarea, S. Glial mechanisms underlying major depressive disorder: Potential therapeutic opportunities. Prog. Mol. Biol. Transl. Sci. 2019, 167, 159–178. [Google Scholar]
- Drevets, W.C.; Price, J.L.; Furey, M.L. Brain structural and functional abnormalities in mood disorders: Implications for neurocircuitry models of depression. Brain Struct. Funct. 2008, 213, 93–118. [Google Scholar] [CrossRef]
- Picciotto, M.R.; Caldarone, B.J.; King, S.L.; Zachariou, V. Nicotinic receptors in the brain. Links between molecular biology and behavior. Neuropsychopharmacology 2000, 22, 451–465. [Google Scholar] [CrossRef]
- Alzarea, S.; Rahman, S. Alpha-7 nicotinic receptor allosteric modulator PNU120596 prevents lipopolysaccharide-induced anxiety, cognitive deficit and depression-like behaviors in mice. Behav. Brain Res. 2019, 366, 19–28. [Google Scholar] [CrossRef]
- Papke, R.L.; Kem, W.R.; Soti, F.; Lopez-Hernandez, G.Y.; Horenstein, N.A. Activation and desensitization of nicotinic alpha7-type acetylcholine receptors by benzylidene anabaseines and nicotine. J. Pharmacol. Exp. Ther. 2009, 329, 791–807. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.K.; Wang, J.; Papke, R.L. Positive allosteric modulators as an approach to nicotinic acetylcholine receptor-targeted therapeutics: Advantages and limitations. Biochem. Pharmacol. 2011, 82, 915–930. [Google Scholar] [CrossRef] [PubMed]
- Roni, M.A.; Rahman, S. The effects of lobeline on nicotine withdrawal-induced depression-like behavior in mice. Psychopharmacology 2014, 231, 2989–2998. [Google Scholar] [CrossRef] [PubMed]
- Roni, M.A.; Rahman, S. Antidepressant-like effects of lobeline in mice: Behavioral, neurochemical, and neuroendocrine evidence. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 41, 44–51. [Google Scholar] [CrossRef]
- Roni, M.A.; Rahman, S. Lobeline attenuates ethanol abstinence-induced depression-like behavior in mice. Alcohol 2017, 61, 63–70. [Google Scholar] [CrossRef]
- Lima Giacobbo, B.; Doorduin, J.; Klein, H.C.; Dierckx, R.; Bromberg, E.; de Vries, E.F.J. Brain-Derived Neurotrophic Factor in Brain Disorders: Focus on Neuroinflammation. Mol. Neurobiol. 2018, 56, 3295–3312. [Google Scholar] [CrossRef]
- Kairisalo, M.; Korhonen, L.; Sepp, M.; Pruunsild, P.; Kukkonen, J.P.; Kivinen, J.; Timmusk, T.; Blomgren, K.; Lindholm, D. NF-kappaB-dependent regulation of brain-derived neurotrophic factor in hippocampal neurons by X-linked inhibitor of apoptosis protein. Eur. J. Neurosci. 2009, 30, 958–966. [Google Scholar] [CrossRef]
- Berton, O.; McClung, C.A.; Dileone, R.J.; Krishnan, V.; Renthal, W.; Russo, S.J.; Graham, D.; Tsankova, N.M.; Bolanos, C.A.; Rios, M.; et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 2006, 311, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Eisch, A.J.; Bolanos, C.A.; de Wit, J.; Simonak, R.D.; Pudiak, C.M.; Barrot, M.; Verhaagen, J.; Nestler, E.J. Brain-derived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: A role in depression. Biol. Psychiatry 2003, 54, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Deyama, S.; Fogaça, M.V. Role of BDNF in the pathophysiology and treatment of depression: Activity-dependent effects distinguish rapid-acting antidepressants. Eur. J. Neurosci. 2021, 53, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Tomaz, V.S.; Cordeiro, R.C.; Costa, A.M.; de Lucena, D.F.; Nobre Junior, H.V.; de Sousa, F.C.; Vasconcelos, S.M.; Vale, M.L.; Quevedo, J.; Macedo, D. Antidepressant-like effect of nitric oxide synthase inhibitors and sildenafil against lipopolysaccharide-induced depressive-like behavior in mice. Neuroscience 2014, 268, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Branchi, I.; D’Andrea, I.; Sietzema, J.; Fiore, M.; Di Fausto, V.; Aloe, L.; Alleva, E. Early social enrichment augments adult hippocampal BDNF levels and survival of BrdU-positive cells while increasing anxiety- and “depression”-like behavior. J. Neurosci. Res. 2006, 83, 965–973. [Google Scholar] [CrossRef]
- Govindarajan, A.; Rao, B.S.; Nair, D.; Trinh, M.; Mawjee, N.; Tonegawa, S.; Chattarji, S. Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects. Proc. Natl. Acad. Sci. USA 2006, 103, 13208–13213. [Google Scholar] [CrossRef] [PubMed]
- Miro, X.; Perez-Torres, S.; Artigas, F.; Puigdomenech, P.; Palacios, J.M.; Mengod, G. Regulation of cAMP phosphodiesterase mRNAs expression in rat brain by acute and chronic fluoxetine treatment. An in situ hybridization study. Neuropharmacology 2002, 43, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Fang, J. Peripheral immune activation by lipopolysaccharide decreases neurotrophins in the cortex and hippocampus in rats. Brain Behav. Immun. 2006, 20, 64–71. [Google Scholar] [CrossRef]
- Öztürk, M.; Yalın Sapmaz, Ş.; Kandemir, H.; Taneli, F.; Aydemir, Ö. The role of the kynurenine pathway and quinolinic acid in adolescent major depressive disorder. Int. J. Clin. Pract. 2021, 75, e13739. [Google Scholar] [CrossRef]
- Alzarea, S.; Abbas, M.; Ronan, P.J.; Lutfy, K.; Rahman, S. The Effect of an α-7 Nicotinic Allosteric Modulator PNU120596 and NMDA Receptor Antagonist Memantine on Depressive-like Behavior Induced by LPS in Mice: The Involvement of Brain Microglia. Brain Sci. 2022, 12, 1493. [Google Scholar] [CrossRef]
- King, J.R.; Gillevet, T.C.; Kabbani, N. A G protein-coupled α7 nicotinic receptor regulates signaling and TNF-α release in microglia. FEBS Open Bio. 2017, 7, 1350–1361. [Google Scholar] [CrossRef]
- Patel, H.; McIntire, J.; Ryan, S.; Dunah, A.; Loring, R. Anti-inflammatory effects of astroglial α7 nicotinic acetylcholine receptors are mediated by inhibition of the NF-κB pathway and activation of the Nrf2 pathway. J. Neuroinflamm. 2017, 14, 192. [Google Scholar] [CrossRef]
- Ferrini, F.; De Koninck, Y. Microglia control neuronal network excitability via BDNF signalling. Neural Plast. 2013, 2013, 429815. [Google Scholar] [CrossRef]
- Luscher, B.; Shen, Q.; Sahir, N. The GABAergic deficit hypothesis of major depressive disorder. Mol. Psychiatry 2011, 16, 383–406. [Google Scholar] [CrossRef]
- Abbas, M.; Alzarea, S.; Papke, R.L.; Rahman, S. Effects of α7 Nicotinic Acetylcholine Receptor Positive Allosteric Modulator on BDNF, NKCC1 and KCC2 Expression in the Hippocampus following Lipopolysaccharide-Induced Allodynia and Hyperalgesia in a Mouse Model of Inflammatory Pain. CNS Neurol. Disord. Drug Targets (Former. Curr. Drug Targets CNS Neurol. Disord.) 2021, 20, 366–377. [Google Scholar] [CrossRef]
- Miller, S.; Maguire, J. Deficits in KCC2 and activation of the HPA axis lead to depression-like behavior following social defeat. Horm. Stud. 2014, 2, 2. [Google Scholar] [CrossRef][Green Version]
- Goubert, E.; Altvater, M.; Rovira, M.N.; Khalilov, I.; Mazzarino, M.; Sebastiani, A.; Schaefer, M.K.E.; Rivera, C.; Pellegrino, C. Bumetanide Prevents Brain Trauma-Induced Depressive-Like Behavior. Front. Mol. Neurosci. 2019, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Yirmiya, R.; Rimmerman, N.; Reshef, R. Depression as a microglial disease. Trends Neurosci. 2015, 38, 637–658. [Google Scholar] [CrossRef]
- Gomes, C.; Ferreira, R.; George, J.; Sanches, R.; Rodrigues, D.I.; Goncalves, N.; Cunha, R.A. Activation of microglial cells triggers a release of brain-derived neurotrophic factor (BDNF) inducing their proliferation in an adenosine A2A receptor-dependent manner: A2A receptor blockade prevents BDNF release and proliferation of microglia. J. Neuroinflamm. 2013, 10, 16. [Google Scholar] [CrossRef]
- Miwa, T.; Furukawa, S.; Nakajima, K.; Furukawa, Y.; Kohsaka, S. Lipopolysaccharide enhances synthesis of brain-derived neurotrophic factor in cultured rat microglia. J. Neurosci. Res. 1997, 50, 1023–1029. [Google Scholar] [CrossRef]
- Parkhurst, C.N.; Yang, G.; Ninan, I.; Savas, J.N.; Yates, J.R., 3rd; Lafaille, J.J.; Hempstead, B.L.; Littman, D.R.; Gan, W.B. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 2013, 155, 1596–1609. [Google Scholar] [CrossRef]
- Tao, X.; Finkbeiner, S.; Arnold, D.B.; Shaywitz, A.J.; Greenberg, M.E. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron 1998, 20, 709–726. [Google Scholar] [CrossRef]
- Honey, D.; Wosnitzka, E.; Klann, E.; Weinhard, L. Analysis of microglial BDNF function and expression in the motor cortex. Front. Cell. Neurosci. 2022, 16, 961276. [Google Scholar] [CrossRef]
- Corradi, J.; Bouzat, C. Understanding the Bases of Function and Modulation of alpha7 Nicotinic Receptors: Implications for Drug Discovery. Mol. Pharmacol 2016, 90, 288–299. [Google Scholar] [CrossRef]
- Kabbani, N.; Nichols, R.A. Beyond the Channel: Metabotropic Signaling by Nicotinic Receptors. Trends Pharmacol. Sci. 2018, 39, 354–366. [Google Scholar] [CrossRef]
- Zhang, J.C.; Yao, W.; Dong, C.; Yang, C.; Ren, Q.; Ma, M.; Han, M.; Hashimoto, K. Comparison of ketamine, 7,8-dihydroxyflavone, and ANA-12 antidepressant effects in the social defeat stress model of depression. Psychopharmacology 2015, 232, 4325–4335. [Google Scholar] [CrossRef]
- Zhang, J.C.; Wu, J.; Fujita, Y.; Yao, W.; Ren, Q.; Yang, C.; Li, S.X.; Shirayama, Y.; Hashimoto, K. Antidepressant effects of TrkB ligands on depression-like behavior and dendritic changes in mice after inflammation. Int. J. Neuropsychopharmacol. 2014, 18, pyu077. [Google Scholar] [CrossRef]
- Zuckerman, H.; Pan, Z.; Park, C.; Brietzke, E.; Musial, N.; Shariq, A.S.; Iacobucci, M.; Yim, S.J.; Lui, L.M.W.; Rong, C.; et al. Recognition and Treatment of Cognitive Dysfunction in Major Depressive Disorder. Front. Psychiatry 2018, 9, 655. [Google Scholar] [CrossRef]
- Sharma, S.; Rakoczy, S.; Brown-Borg, H. Assessment of spatial memory in mice. Life Sci. 2010, 87, 521–536. [Google Scholar] [CrossRef]
- Del Arco, A.; Segovia, G.; Garrido, P.; de Blas, M.; Mora, F. Stress, prefrontal cortex and environmental enrichment: Studies on dopamine and acetylcholine release and working memory performance in rats. Behav. Brain Res. 2007, 176, 267–273. [Google Scholar] [CrossRef]



| Gene | Primer Sequence (5′-3′) |
|---|---|
| KCC2 | AGCCTATGACGATGACCCA (forward) |
| CCACCTCTGCTGTCTACATC (reverse) | |
| NKCC1 | GGTATCATTAACATTGCCAGTGG (forward) |
| CAGATCCTCAGTCAGCCATAC (reverse) | |
| GAPDH | GTGGAGTCATACTGGAACATGTAG (forward) |
| AATGGTGAAGGTCGGTGTG (reverse) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alzarea, S.; Khan, A.; Ronan, P.J.; Lutfy, K.; Rahman, S. The α-7 Nicotinic Receptor Positive Allosteric Modulator Alleviates Lipopolysaccharide Induced Depressive-like Behavior by Regulating Microglial Function, Trophic Factor, and Chloride Transporters in Mice. Brain Sci. 2024, 14, 290. https://doi.org/10.3390/brainsci14030290
Alzarea S, Khan A, Ronan PJ, Lutfy K, Rahman S. The α-7 Nicotinic Receptor Positive Allosteric Modulator Alleviates Lipopolysaccharide Induced Depressive-like Behavior by Regulating Microglial Function, Trophic Factor, and Chloride Transporters in Mice. Brain Sciences. 2024; 14(3):290. https://doi.org/10.3390/brainsci14030290
Chicago/Turabian StyleAlzarea, Sami, Amna Khan, Patrick J. Ronan, Kabirullah Lutfy, and Shafiqur Rahman. 2024. "The α-7 Nicotinic Receptor Positive Allosteric Modulator Alleviates Lipopolysaccharide Induced Depressive-like Behavior by Regulating Microglial Function, Trophic Factor, and Chloride Transporters in Mice" Brain Sciences 14, no. 3: 290. https://doi.org/10.3390/brainsci14030290
APA StyleAlzarea, S., Khan, A., Ronan, P. J., Lutfy, K., & Rahman, S. (2024). The α-7 Nicotinic Receptor Positive Allosteric Modulator Alleviates Lipopolysaccharide Induced Depressive-like Behavior by Regulating Microglial Function, Trophic Factor, and Chloride Transporters in Mice. Brain Sciences, 14(3), 290. https://doi.org/10.3390/brainsci14030290







