Dopamine Concentration Changes Associated with the Retrodialysis of Methylone and 3,4-Methylenedioxypyrovalerone (MDPV) into the Caudate Putamen
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
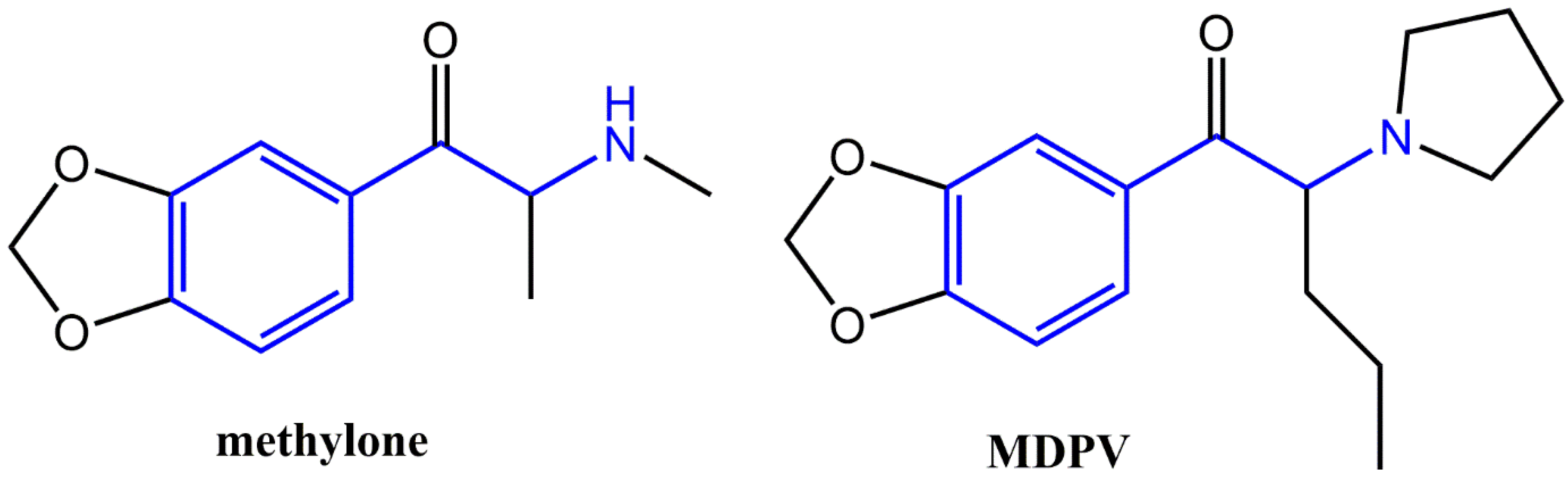
2.2. Drugs and Chemicals
2.3. In Vitro Probe Recovery Evaluation
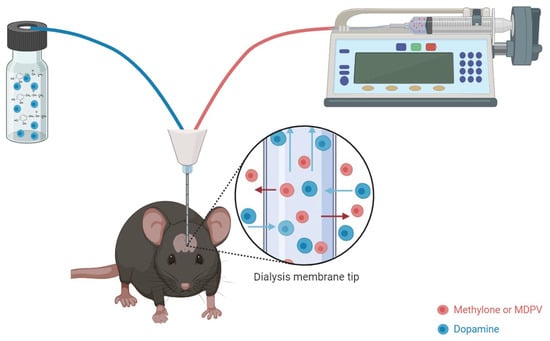
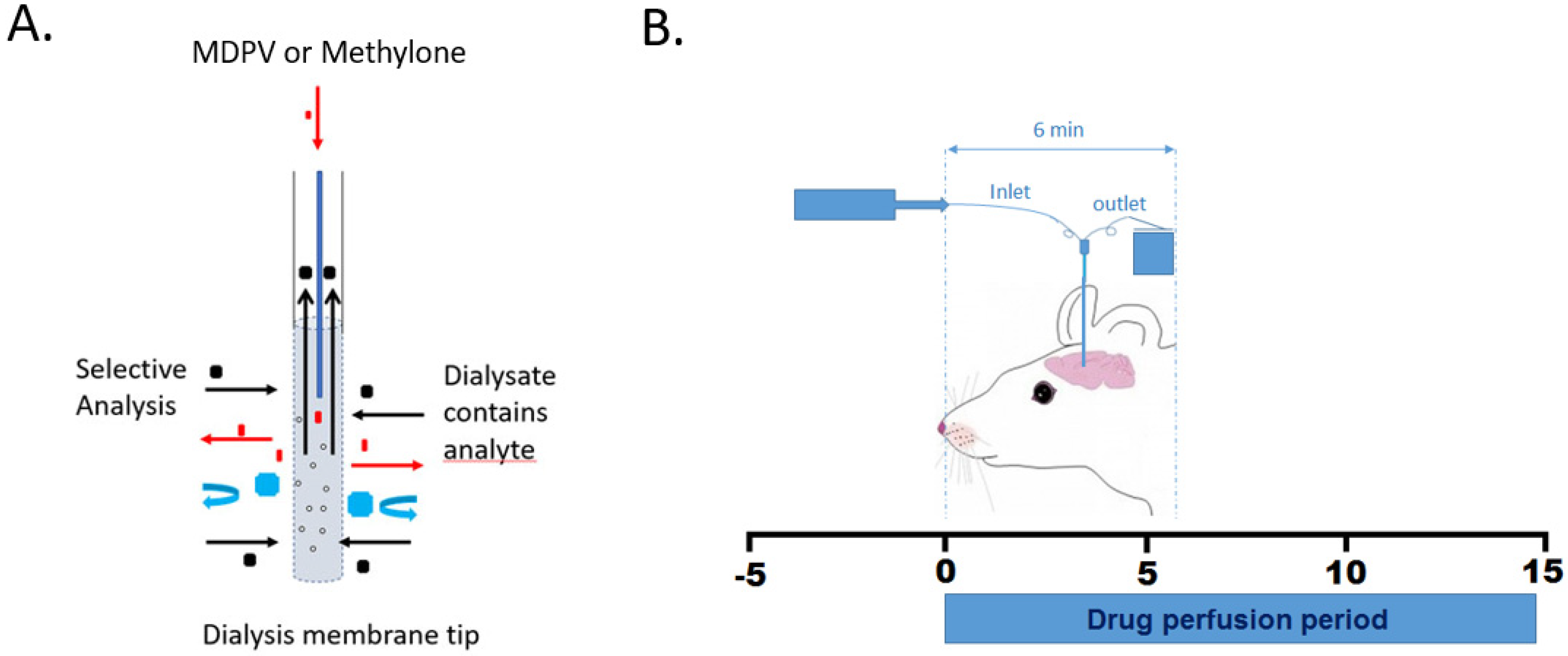
2.4. Surgical Cannula Placement and Retrodialysis
2.5. High-Performance Liquid Chromatography–Electrochemical Detection (HPLC-EC)
2.6. Statistics
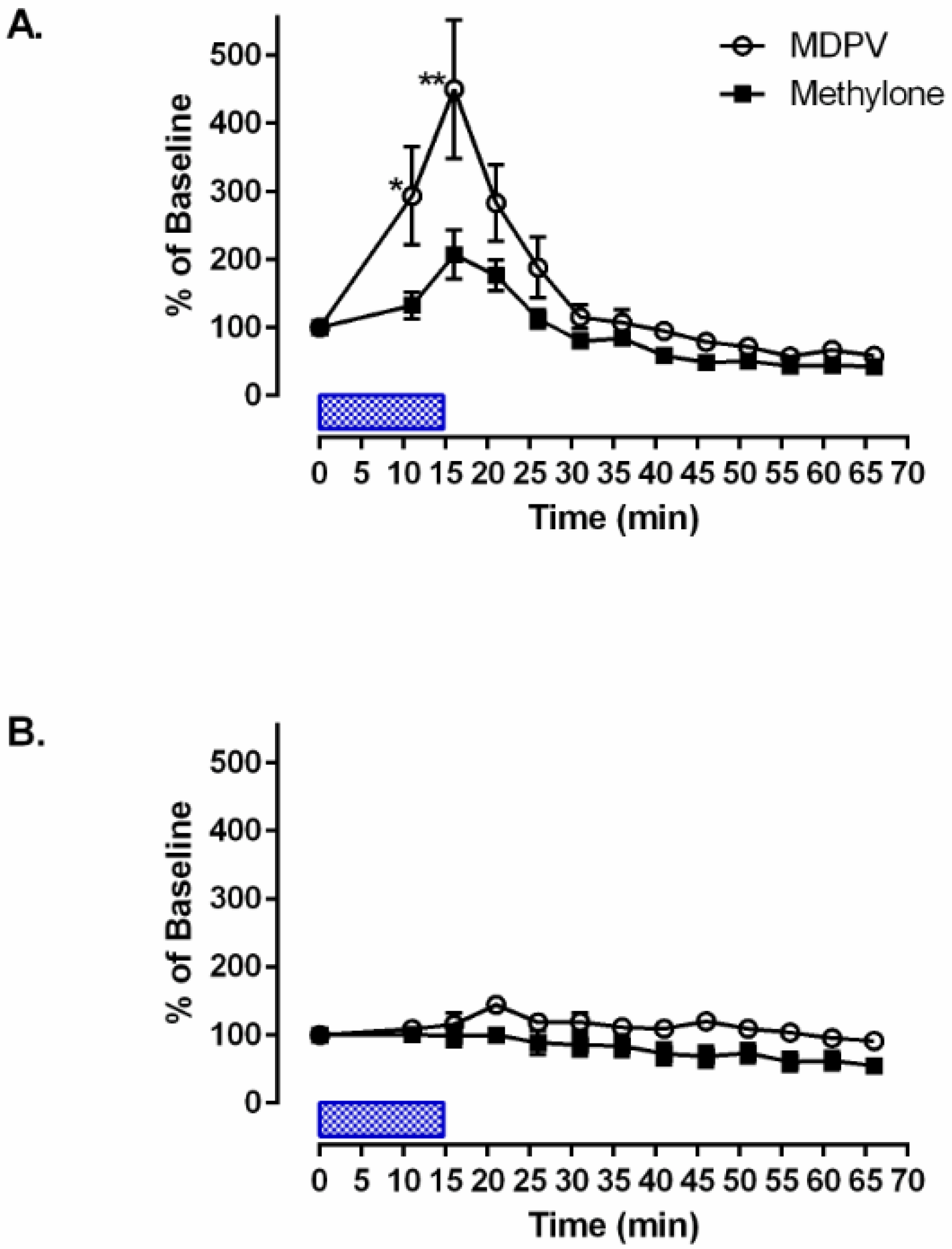
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banks, M.L.; Worst, T.J.; Rusyniak, D.E.; Sprague, J.E. The synthetic cathinones (the “bath salts”). J. Emerg. Med. 2014, 46, 632–642. [Google Scholar] [CrossRef]
- Prosser, J.; Nelson, L. The toxicology of bath salts: A review of synthetic cathinones. J. Med. Toxicol. 2012, 8, 33–42. [Google Scholar] [CrossRef]
- Calinski, D.M.; Kisor, D.F.; Sprague, J.E. A review of the influence of functional group modifications to the core scaffold of synthetic cathinones on drug pharmacokinetics. Psychopharmacology 2019, 236, 881–890. [Google Scholar] [CrossRef]
- Kolanos, R.; Partilla, J.S.; Baumann, M.H.; Hutsell, B.A.; Banks, M.L.; Negus, S.S.; Glennon, R.A. Stereoselective actions of methylenedioxypyrovalerone (MDPV) to inhibit dopamine and norepinephrine transporters and facilitate intracranial self-stimulation in rats. ACS Chem. Neurosci. 2015, 6, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Gannon, B.M.; Rice, K.C.; Collins, G.T. Reinforcing effects of abused ‘bath salts’ constituents 3,4-methylenedioxypyrovalerone and α-pyrrolidinopentiophenone and their enantiomers. Behav. Pharmacol. 2017, 28, 578–581. [Google Scholar] [CrossRef] [PubMed]
- Coppola, M.; Mondola, R. Synthetic cathinones: Chemistry, pharmacology and toxicology of a new class of designer drugs of abuse marketed as “bath salts” or “plant food”. Toxicol. Lett. 2012, 211, 144–149. [Google Scholar] [CrossRef]
- Simmler, L.; Buser, T.; Donzelli, M.; Schramm, Y.; Dieu, L.-H.; Huwyler, J.; Liechti, M. Pharmacological characterization of designer cathinones in vitro. Br. J. Pharmacol. 2013, 168, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.H.; Partilla, J.S.; Lehner, K.R.; Thorndike, E.B.; Hoffman, A.F.; Holy, M.; Rothman, R.B.; Goldberg, S.R.; Lupica, C.R.; Sitte, H.H.; et al. Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’products. Neuropsychopharmacology 2012, 38, 552–562. [Google Scholar] [CrossRef]
- Cozzi, N.V.; Sievert, M.K.; Shulgin, A.T.; Jacob, P.; Ruoho, A.E. Inhibition of plasma membrane monoamine transporters by beta-ketoamphetamines. Eur. J. Pharmacol. 1999, 381, 63–69. [Google Scholar] [CrossRef]
- Hadlock, G.C.; Webb, K.M.; McFadden, L.M.; Chu, P.W.; Ellis, J.D.; Allen, S.C.; Andrenyak, D.M.; Vieira-Brock, P.L.; German, C.L.; Conrad, K.M.; et al. 4-Methylmethcathinone (mephedrone): Neuropharmacological effects of a designer stimulant of abuse. J. Pharmacol. Exp. Ther. 2011, 339, 530–536. [Google Scholar] [CrossRef]
- Nagai, F.; Nonaka, R.; Satoh Hisashi Kamimura, K. The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain. Eur. J. Pharmacol. 2007, 559, 132–137. [Google Scholar] [CrossRef]
- Sogawa, C.; Sogawa, N.; Ohyama, K.; Kikura-Hanajiri, R.; Goda, Y.; Sora, I.; Kitayama, S. Methylone and monoamine transporters: Correlation with toxicity. Curr. Neuropharmacol. 2011, 9, 58–62. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baumann, M.H.; Ayestas, M.A.; Partilla, J.S.; Sink, J.R.; Shulgin, A.T.; Daley, P.F.; Brandt, S.D.; Rothman, R.B.; Ruoho, A.E.; Cozzi, N.V. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology 2011, 37, 1192–1203. [Google Scholar] [CrossRef] [PubMed]
- Mulvihill, K.G. Presynaptic regulation of dopamine release: Role of the DAT and VMAT2 transporters. Neurochem. Int. 2019, 122, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Suyama, J.A.; Sakloth, F.; Kolanos, R.; Glennon, R.A.; Lazenka, M.F.; Negus, S.S.; Banks, M.L. Abuse-Related Neurochemical Effects of Para-Substituted Methcathinone Analogs in Rats: Microdialysis Studies of Nucleus Accumbens Dopamine and Serotonin. J. Pharmacol. Exp. Ther. 2016, 356, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Schindler, C.W.; Thorndike, E.B.; Goldberg, S.R.; Lehner, K.R.; Cozzi, N.V.; Brandt, S.D.; Baumann, M.H. Reinforcing and neurochemical effects of the “bath salts” constituents 3,4-methylenedioxypyrovalerone (MDPV) and 3,4-methylenedioxy-N-methylcathinone (methylone) in male rats. Psychopharmacology 2015, 233, 1981–1990. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, N.V.; Brandt, S.D.; Daley, P.F.; Partilla, J.S.; Rothman, R.B.; Tulzer, A.; Sitte, H.H.; Baumann, M.H. Pharmacological examination of trifluoromethyl ring-substituted methcathione analogs. Eur. J. Pharmacol. 2013, 699, 180–187. [Google Scholar] [CrossRef]
- Johnson, A.R.; Banks, M.L.; Selley, D.E.; Negus, S.S. Amphetamine maintenance differentially modulates effects of cocaine, methylenedioxypyrovalerone (MDPV), and methamphetamine on intracranial self-stimulation and nucleus accumbens dopamine in rats. Neuropsychopharmacology 2018, 43, 1753–1762. [Google Scholar] [CrossRef]
- Zwartsen, A.; Olijhoek, M.E.; Westerink, R.H.S.; Hondebrink, L. Hazard Characterization of Synthetic Cathinones Using Viability, Monoamine Reuptake, and Neuronal Activity Assays. Front. Neurosci. 2020, 14, 9. [Google Scholar] [CrossRef]
- Bourne, J.A. Intracerebral microdialysis: 30 years as a tool for the neuroscientist. Clin. Exp. Pharmacol. Physiol. 2003, 30, 16–24. [Google Scholar] [CrossRef]
- Proctor, C.M.; Chan, C.Y.; Porcarelli, L.; Udabe, E.; Sanchez-Sanchez, A.; Del Agua, I.; Mecerreyes, D.; Malliaras, G.G. Ionic Hydrogel for Accelerated Dopamine Delivery via Retrodialysis. Chem. Mater. Publ. Am. Chem. Soc. 2019, 31, 7080–7084. [Google Scholar] [CrossRef]
- Matto, V.; Terasmaa, A.; Vasar, E.; Koks, S. Impaired striatal dopamine output of homozygous Wfs1 mutant mice in response to [K+] challenge. J. Physiol. Biochem. 2011, 67, 53–60. [Google Scholar] [CrossRef]
- Baumann, K.; Falkencrone, S.; Knudsen, N.P.H.; Woetmann, A.; Dabelsteen, S.; Skov, P.S. The skin reservoir model: A tool for evaluating microdialysis sampling of large biomarkers from human skin. Acta Derm.-Venereol. 2020, 100, adv00008. [Google Scholar] [CrossRef]
- Cheng, F.C.; Kuo, J.S.; Huang, H.M.; Yang, D.Y.; Wu, T.F.; Tsai, T.H. Determination of catecholamines in pheochromocytoma cell (PC-12) culture medium by microdialysis-microbore liquid chromatography. J. Chromatogr. A 2000, 870, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press: San Diego, CA, USA, 2007. [Google Scholar]
- Grecco, G.G.; Kisor, D.F.; Magura, J.S.; Sprague, J.E. Impact of common clandestine structural modifications on synthetic cathinone “bath salt” pharmacokinetics. Toxicol. Appl. Pharmacol. 2017, 328, 18–24. [Google Scholar] [CrossRef]
- Hitchcock, S.A.; Pennington, L.D. Structure-brain exposure relationships. J. Med. Chem. 2006, 49, 7559–7583. [Google Scholar] [CrossRef] [PubMed]
- Elmore, J.S.; Dillon-Carter, O.; Partilla, J.S.; Ellefsen, K.N.; Concheiro, M.; Suzuki, M.; Rice, K.C.; Huestis, M.A.; Baumann, M.H. Pharmacokinetic Profiles and Pharmacodynamic Effects for Methylone and Its Metabolites in Rats. Neuropsychopharmacology 2016, 42, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Bonano, J.S.; Glennon, R.A.; De Felice, L.J.; Banks, M.L.; Negus, S.S. Abuse-related and abuse-limiting effects of methcathinone and the synthetic “bath salts” cathinone analogs methylenedioxypyrovalerone (MDPV), methylone and mephedrone on intracranial self-stimulation in rats. Psychopharmacology 2013, 231, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Ferry, B.; Parrot, S.; Marien, M.; Lazarus, C.; Cassel, J.C.; McGaugh, J.L. Noradrenergic influences in the basolateral amygdala on inhibitory avoidance memory are mediated by an action on α2-adrenoceptors. Psychoneuroendocrinology 2015, 51, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Kozai, T.D.Y.; Jaquins-Gerstl, A.S.; Vazquez, A.L.; Michael, A.C.; Cui, X.T. Dexamethasone retrodialysis attenuates microglial response to implanted probes in vivo. Biomaterials 2016, 87, 157–169. [Google Scholar] [CrossRef]
- Nesbitt, K.M.; Varner, E.L.; Jaquins-Gerstl, A.; Michael, A.C. Microdialysis in the Rat Striatum: Effects of 24 h Dexamethasone Retrodialysis on Evoked Dopamine Release and Penetration Injury. ACS Chem. Neurosci. 2015, 6, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-DeRose, J.; Stauber, G.; Khroyan, T.V.; Xie, X.S.; Zaveri, N.T.; Toll, L. Retrodialysis of N/OFQ into the nucleus accumbens shell blocks cocaine-induced increases in extracellular dopamine and locomotor activity. Eur. J. Pharmacol. 2013, 699, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Hanberg, P.; Bue, M.; Öbrink-Hansen, K.; Kabel, J.; Thomassen, M.; Tøttrup, M.; Søballe, K.; Stilling, M. Simultaneous Retrodialysis by Drug for Cefuroxime Using Meropenem as an Internal Standard-A Microdialysis Validation Study. J. Pharm. Sci. 2020, 109, 1373–1379. [Google Scholar] [CrossRef]
- Yu, Y.; Chandasana, H.; Sangari, T.; Seubert, C.; Derendorf, H. Simultaneous Retrodialysis by Calibrator for Rapid In Vivo Recovery Determination in Target Site Microdialysis. J. Pharm. Sci. 2018, 107, 2259–2265. [Google Scholar] [CrossRef]
- Can, A.; Zanos, P.; Moaddel, R.; Kang, H.J.; Dossou, K.S.S.; Wainer, I.W.; Cheer, J.F.; Frost, D.O.; Huang, X.P.; Gould, T.D. Effects of ketamine and ketamine metabolites on evoked striatal dopamine release, dopamine receptors, and monoamine transporters. J. Pharmacol. Exp. Ther. 2016, 359, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Aburahma, A. Phenylethylamine Derivatives: Pharmacological and Toxicological Studies. Ph.D. Thesis, Bowling Green State University, Bowling Green, OH, USA, 2021. Available online: http://rave.ohiolink.edu/etdc/view?acc_num=bgsu1636041464110327 (accessed on 27 February 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goldsmith, R.; Aburahma, A.; Sprague, J.E. Dopamine Concentration Changes Associated with the Retrodialysis of Methylone and 3,4-Methylenedioxypyrovalerone (MDPV) into the Caudate Putamen. Brain Sci. 2024, 14, 265. https://doi.org/10.3390/brainsci14030265
Goldsmith R, Aburahma A, Sprague JE. Dopamine Concentration Changes Associated with the Retrodialysis of Methylone and 3,4-Methylenedioxypyrovalerone (MDPV) into the Caudate Putamen. Brain Sciences. 2024; 14(3):265. https://doi.org/10.3390/brainsci14030265
Chicago/Turabian StyleGoldsmith, Robert, Amal Aburahma, and Jon E. Sprague. 2024. "Dopamine Concentration Changes Associated with the Retrodialysis of Methylone and 3,4-Methylenedioxypyrovalerone (MDPV) into the Caudate Putamen" Brain Sciences 14, no. 3: 265. https://doi.org/10.3390/brainsci14030265
APA StyleGoldsmith, R., Aburahma, A., & Sprague, J. E. (2024). Dopamine Concentration Changes Associated with the Retrodialysis of Methylone and 3,4-Methylenedioxypyrovalerone (MDPV) into the Caudate Putamen. Brain Sciences, 14(3), 265. https://doi.org/10.3390/brainsci14030265