Association of the Neutrophil-to-Lymphocyte Ratio with 90-Day Functional Outcomes in Patients with Acute Ischemic Stroke
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Collection
2.3. Statistical Tests
3. Results
3.1. Demographics and Baseline Characteristics of All Participants
3.2. Comparison of Derived Blood Lymphocyte Parameters
3.3. Analysis of Risk Factors Associated with Unfavorable Prognosis in AIS Patients
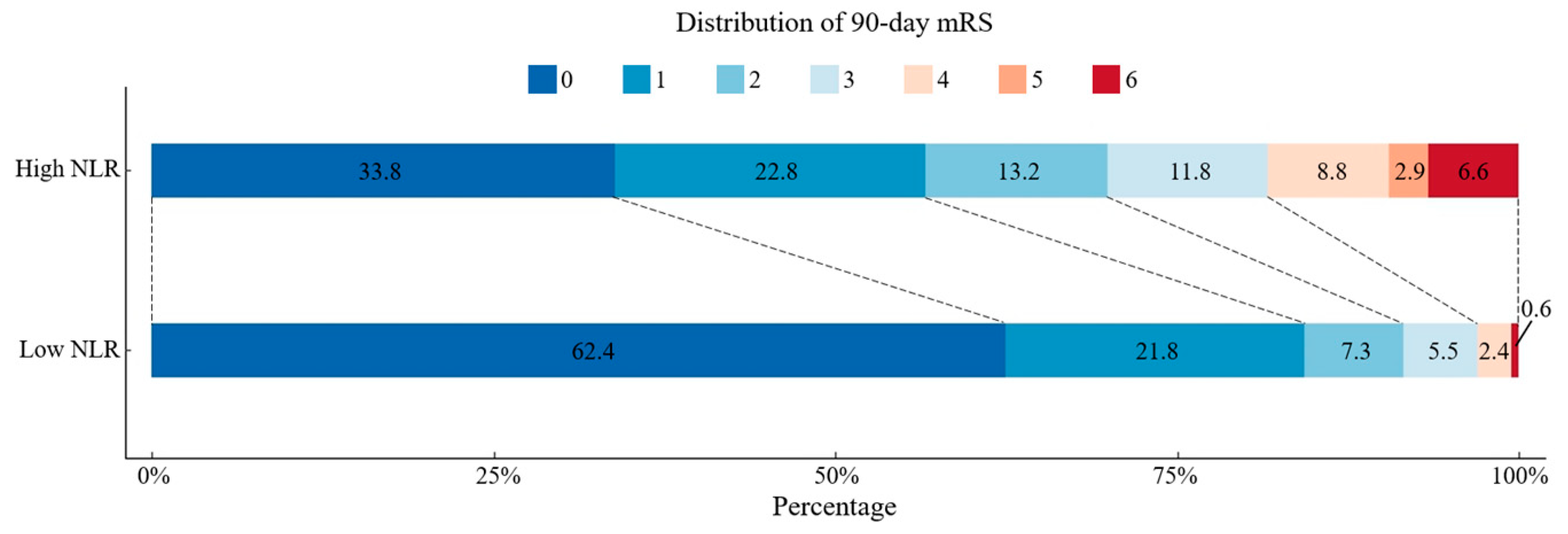
3.4. Comparison of Functional Outcomes of AIS Patients with High or Low NLR
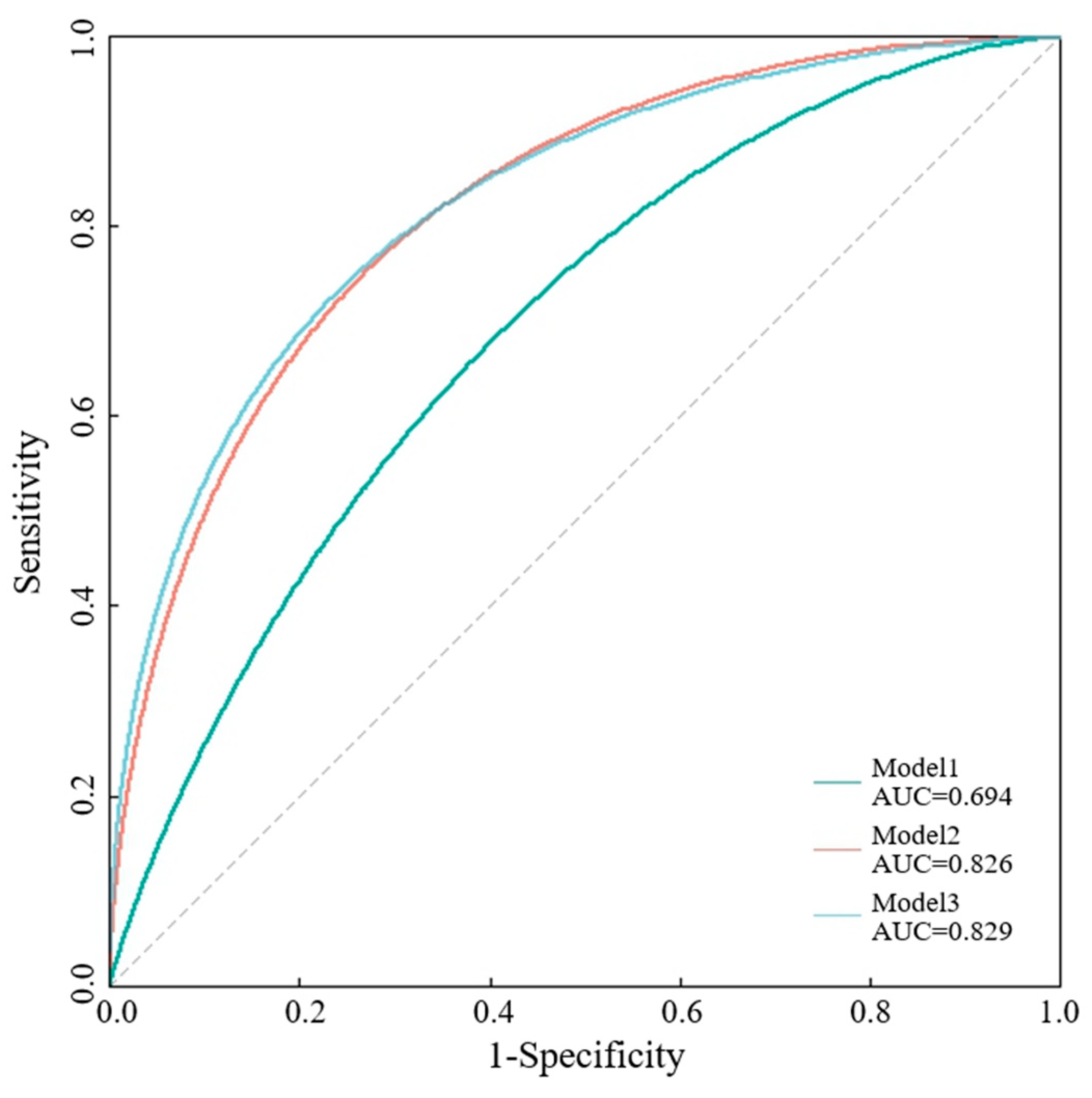
3.5. Comparison of Various Models in Predicting 90-Day Functional Outcome with AIS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Kim, J.S.; Nah, H.W.; Park, S.M.; Kim, S.K.; Cho, K.H.; Lee, J.; Lee, Y.S.; Kim, J.; Ha, S.W.; Kim, E.G.; et al. Risk factors and stroke mechanisms in atherosclerotic stroke: Intracranial compared with extracranial and anterior compared with posterior circulation disease. Stroke 2012, 43, 3313–3318. [Google Scholar] [CrossRef]
- Cui, J.; Li, H.; Chen, Z.; Dong, T.; He, X.; Wei, Y.; Li, Z.; Duan, J.; Cao, T.; Chen, Q.; et al. Thrombo-Inflammation and Immunological Response in Ischemic Stroke: Focusing on Platelet-Tregs Interaction. Front. Cell. Neurosci. 2022, 16, 955385. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Buckwalter, M.S.; Anrather, J. Immune responses to stroke: Mechanisms, modulation, and therapeutic potential. J. Clin. Investig. 2020, 130, 2777–2788. [Google Scholar] [CrossRef]
- Qiu, Y.M.; Zhang, C.L.; Chen, A.Q.; Wang, H.L.; Zhou, Y.F.; Li, Y.N.; Hu, B. Immune Cells in the BBB Disruption After Acute Ischemic Stroke: Targets for Immune Therapy? Front. Immunol. 2021, 12, 678744. [Google Scholar] [CrossRef]
- Endres, M.; Moro, M.A.; Nolte, C.H.; Dames, C.; Buckwalter, M.S.; Meisel, A. Immune Pathways in Etiology, Acute Phase, and Chronic Sequelae of Ischemic Stroke. Circ. Res. 2022, 130, 1167–1186. [Google Scholar] [CrossRef]
- Schobert, I.T.; Savic, L.J.; Chapiro, J.; Bousabarah, K.; Chen, E.; Laage-Gaupp, F.; Tefera, J.; Nezami, N.; Lin, M.; Pollak, J.; et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as predictors of tumor response in hepatocellular carcinoma after DEB-TACE. Eur. Radiol. 2020, 30, 5663–5673. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Fang, J.; Huang, X.; Liu, Y.; Yuan, Y.; Zhang, X.; Zou, C.; Xiao, K.; Wang, J. Prognostic role of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in prostate cancer: A meta-analysis of results from multivariate analysis. Int. J. Surg. 2018, 60, 216–223. [Google Scholar] [CrossRef]
- Wang, J.-H.; Chen, Y.-Y.; Kee, K.-M.; Wang, C.-C.; Tsai, M.-C.; Kuo, Y.-H.; Hung, C.-H.; Li, W.-F.; Lai, H.-L.; Chen, Y.-H. The Prognostic Value of Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio in Patients with Hepatocellular Carcinoma Receiving Atezolizumab Plus Bevacizumab. Cancers 2022, 14, 343. [Google Scholar] [CrossRef]
- Tamaki, S.; Nagai, Y.; Shutta, R.; Masuda, D.; Yamashita, S.; Seo, M.; Yamada, T.; Nakagawa, A.; Yasumura, Y.; Nakagawa, Y.; et al. Combination of Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios as a Novel Predictor of Cardiac Death in Patients with Acute Decompensated Heart Failure with Preserved Left Ventricular Ejection Fraction: A Multicenter Study. J. Am. Hear. Assoc. 2023, 12, e026326. [Google Scholar] [CrossRef]
- Mirna, M.; Schmutzler, L.; Topf, A.; Hoppe, U.C.; Lichtenauer, M. Neutrophil-to-lymphocyte ratio and monocyte-to-lymphocyte ratio predict length of hospital stay in myocarditis. Sci. Rep. 2021, 11, 18101. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y.; Li, W.; Wang, G. The Association between the Baseline and the Change in Neutrophil-to-Lymphocyte Ratio and Short-Term Mortality in Patients with Acute Respiratory Distress Syndrome. Front. Med. 2021, 8, 636869. [Google Scholar] [CrossRef]
- Wang, S.; Fu, L.; Huang, K.; Han, J.; Zhang, R.; Fu, Z. Neutrophil-to-lymphocyte ratio on admission is an independent risk factor for the severity and mortality in patients with coronavirus disease 2019. J. Infect. 2020, 82, e16–e18. [Google Scholar] [CrossRef] [PubMed]
- Pinčáková, K.; Krastev, G.; Haring, J.; Mako, M.; Mikulášková, V.; Bošák, V. Low Lymphocyte-to-Monocyte Ratio as a Possible Predictor of an Unfavourable Clinical Outcome in Patients with Acute Ischemic Stroke after Mechanical Thrombectomy. Stroke Res. Treat. 2022, 2022, 9243080. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Pan, R.; Jin, Y.; Wang, Y.; Cheng, Y.; Liu, J.; Wu, B.; Liu, M. Lymphocyte-to-monocyte ratio and risk of hemorrhagic transformation in patients with acute ischemic stroke. Neurol. Sci. 2020, 41, 2511–2520. [Google Scholar] [CrossRef]
- Gong, P.; Liu, Y.; Gong, Y.; Chen, G.; Zhang, X.; Wang, S.; Zhou, F.; Duan, R.; Chen, W.; Huang, T.; et al. The association of neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, and lymphocyte to monocyte ratio with post-thrombolysis early neurological outcomes in patients with acute ischemic stroke. J. Neuroinflammation 2021, 18, 51. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Yu, Q.; Liao, Y.; Huang, Q.; Luo, S.; Li, S.; Qiu, Y.; Wu, Y.; Zhang, J.; Chen, Q.; et al. Lymphocyte-to-Monocyte Ratio Is Independently Associated with Progressive Infarction in Patients with Acute Ischemic Stroke. BioMed Res. Int. 2022, 2022, 2290524. [Google Scholar] [CrossRef]
- Ferro, D.; Matias, M.; Neto, J.; Dias, R.; Moreira, G.; Petersen, N.; Azevedo, E.; Castro, P. Neutrophil-to-Lymphocyte Ratio Predicts Cerebral Edema and Clinical Worsening Early After Reperfusion Therapy in Stroke. Stroke 2021, 52, 859–867. [Google Scholar] [CrossRef]
- Malhotra, K.; Ahmed, N.; Filippatou, A.; Katsanos, A.H.; Goyal, N.; Tsioufis, K.; Manios, E.; Pikilidou, M.; Schellinger, P.D.; Alexandrov, A.W.; et al. Association of Elevated Blood Pressure Levels with Outcomes in Acute Ischemic Stroke Patients Treated with Intravenous Thrombolysis: A Systematic Review and Meta-Analysis. J. Stroke 2019, 21, 78–90. [Google Scholar] [CrossRef]
- Samuels, N.; van de Graaf, R.A.; Mulder, M.J.H.L.; Brown, S.; Roozenbeek, B.; van Doormaal, P.J.; Goyal, M.; Campbell, B.C.V.; Muir, K.W.; Agrinier, N.; et al. Admission systolic blood pressure and effect of endovascular treatment in patients with ischaemic stroke: An individual patient data meta-analysis. Lancet Neurol. 2023, 22, 312–319. [Google Scholar] [CrossRef]
- Malhotra, K.; Goyal, N.; Katsanos, A.H.; Filippatou, A.; Mistry, E.A.; Khatri, P.; Anadani, M.; Spiotta, A.M.; Sandset, E.C.; Sarraj, A.; et al. Association of Blood Pressure With Outcomes in Acute Stroke Thrombectomy. Hypertension 2020, 75, 730–739. [Google Scholar] [CrossRef]
- Olsen, T.S.; Christensen, R.H.; Kammersgaard, L.P.; Andersen, K.K. Higher total serum cholesterol levels are associated with less severe strokes and lower all-cause mortality: Ten-year follow-up of ischemic strokes in the Copenhagen Stroke Study. Stroke 2007, 38, 2646–2651. [Google Scholar] [CrossRef]
- Rangaraju, S.; Frankel, M.; Jovin, T.G. Prognostic Value of the 24-Hour Neurological Examination in Anterior Circulation Ischemic Stroke: A post hoc Analysis of Two Randomized Controlled Stroke Trials. Interv. Neurol. 2015, 4, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Heitsch, L.; Ibanez, L.; Carrera, C.; Binkley, M.M.; Strbian, D.; Tatlisumak, T.; Bustamante, A.; Ribó, M.; Molina, C.; Dávalos, A.; et al. Early Neurological Change After Ischemic Stroke Is Associated With 90-Day Outcome. Stroke 2021, 52, 132–141. [Google Scholar] [CrossRef]
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E., 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.C.V.; Majoie, C.B.L.M.; Albers, G.W.; Menon, B.K.; Yassi, N.; Sharma, G.; van Zwam, W.H.; van Oostenbrugge, R.J.; Demchuk, A.M.; Guillemin, F.; et al. Penumbral imaging and functional outcome in patients with anterior circulation ischaemic stroke treated with endovascular thrombectomy versus medical therapy: A meta-analysis of individual patient-level data. Lancet Neurol. 2018, 18, 46–55. [Google Scholar] [CrossRef]
- Nogueira, R.G.; Jadhav, A.P.; Haussen, D.C.; Bonafe, A.; Budzik, R.F.; Bhuva, P.; Yavagal, D.R.; Ribo, M.; Cognard, C.; Hanel, R.A.; et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N. Engl. J. Med. 2018, 378, 11–21. [Google Scholar] [CrossRef]
- Albers, G.W.; Marks, M.P.; Kemp, S.; Christensen, S.; Tsai, J.P.; Ortega-Gutierrez, S.; McTaggart, R.A.; Torbey, M.T.; Kim-Tenser, M.; Leslie-Mazwi, T.; et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N. Engl. J. Med. 2018, 378, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Stoll, G.; Nieswandt, B. Thrombo-inflammation in acute ischaemic stroke—Implications for treatment. Nat. Rev. Neurol. 2019, 15, 473–481. [Google Scholar] [CrossRef]
- Shi, K.; Tian, D.-C.; Li, Z.-G.; Ducruet, A.F.; Lawton, M.T.; Shi, F.-D. Global brain inflammation in stroke. Lancet Neurol. 2019, 18, 1058–1066. [Google Scholar] [CrossRef]
- Westendorp, W.F.; Dames, C.; Nederkoorn, P.J.; Meisel, A. Immunodepression, Infections, and Functional Outcome in Ischemic Stroke. Stroke 2022, 53, 1438–1448. [Google Scholar] [CrossRef]
- Curbelo, J.; Bueno, S.L.; Galván-Román, J.M.; Ortega-Gómez, M.; Rajas, O.; Fernández-Jiménez, G.; Vega-Piris, L.; Rodríguez-Salvanes, F.; Arnalich, B.; Díaz, A.; et al. Inflammation biomarkers in blood as mortality predictors in community-acquired pneumonia admitted patients: Importance of comparison with neutrophil count percentage or neutrophil-lymphocyte ratio. PLoS ONE 2017, 12, e0173947. [Google Scholar] [CrossRef]
- Bolton, W.S.; Gharial, P.K.; Akhunbay-Fudge, C.; Chumas, P.; Mathew, R.K.; Anderson, I.A. Day 2 neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios for prediction of delayed cerebral ischemia in subarachnoid hemorrhage. Neurosurg. Focus 2022, 52, E4. [Google Scholar] [CrossRef]
- Chen, C.; Gu, L.; Chen, L.; Hu, W.; Feng, X.; Qiu, F.; Fan, Z.; Chen, Q.; Qiu, J.; Shao, B. Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio as Potential Predictors of Prognosis in Acute Ischemic Stroke. Front. Neurol. 2021, 11, 525621. [Google Scholar] [CrossRef]
- Giede-Jeppe, A.; Madžar, D.; Sembill, J.A.; Sprügel, M.I.; Atay, S.; Hoelter, P.; Lücking, H.; Huttner, H.B.; Bobinger, T. Increased Neutrophil-to-Lymphocyte Ratio is Associated with Unfavorable Functional Outcome in Acute Ischemic Stroke. Neurocritical Care 2019, 33, 97–104. [Google Scholar] [CrossRef]
- Tokgoz, S.; Kayrak, M.; Akpinar, Z.; Seyithanoğlu, A.; Güney, F.; Yürüten, B. Neutrophil Lymphocyte Ratio as a Predictor of Stroke. J. Stroke Cerebrovasc. Dis. 2013, 22, 1169–1174. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-S.; Heo, M.Y.; Joo, H.J.; Shim, G.Y.; Chon, J.; Chung, S.J.; Soh, Y.; Yoo, M.C. Neutrophil-to-Lymphocyte Ratio as a Predictor of Short-Term Functional Outcomes in Acute Ischemic Stroke Patients. Int. J. Environ. Res. Public Health 2023, 20, 898. [Google Scholar] [CrossRef] [PubMed]
- Tokgoz, S.; Keskin, S.; Kayrak, M.; Seyithanoglu, A.; Ogmegul, A. Is neutrophil/lymphocyte ratio predict to short-term mortality in acute cerebral infarct independently from infarct volume? J. Stroke Cerebrovasc. Dis. 2014, 23, 2163–2168. [Google Scholar] [CrossRef] [PubMed]
- Lattanzi, S.; Norata, D.; Broggi, S.; Meletti, S.; Świtońska, M.; Słomka, A.; Silvestrini, M. Neutrophil-to-Lymphocyte Ratio Predicts Early Neurological Deterioration after Endovascular Treatment in Patients with Ischemic Stroke. Life 2022, 12, 1415. [Google Scholar] [CrossRef] [PubMed]
- Goyal, N.; Tsivgoulis, G.; Chang, J.J.; Malhotra, K.; Pandhi, A.; Ishfaq, M.F.; Alsbrook, D.; Arthur, A.S.; Elijovich, L.; Alexandrov, A.V. Admission Neutrophil-to-Lymphocyte Ratio as a Prognostic Biomarker of Outcomes in Large Vessel Occlusion Strokes. Stroke 2018, 49, 1985–1987. [Google Scholar] [CrossRef] [PubMed]
- Świtońska, M.; Piekuś-Słomka, N.; Słomka, A.; Sokal, P.; Żekanowska, E.; Lattanzi, S. Neutrophil-to-Lymphocyte Ratio and Symptomatic Hemorrhagic Transformation in Ischemic Stroke Patients Undergoing Revascularization. Brain Sci. 2020, 10, 771. [Google Scholar] [CrossRef]
- Pikija, S.; Sztriha, L.K.; Killer-Oberpfalzer, M.; Weymayr, F.; Hecker, C.; Ramesmayer, C.; Hauer, L.; Sellner, J. Neutrophil to lymphocyte ratio predicts intracranial hemorrhage after endovascular thrombectomy in acute ischemic stroke. J. Neuroinflammation 2018, 15, 319. [Google Scholar] [CrossRef]
- Chen, H.; Luan, X.; Zhao, K.; Qiu, H.; Liu, Y.; Tu, X.; Tang, W.; He, J. The association between neutrophil-to-lymphocyte ratio and post-stroke depression. Clin. Chim. Acta 2018, 486, 298–302. [Google Scholar] [CrossRef]
- Chen, L.-Z.; Luan, X.-Q.; Wu, S.-Z.; Xia, H.-W.; Lin, Y.-S.; Zhan, L.-Q.; He, J.-C. Optimal time point for neutrophil-to-lymphocyte ratio to predict stroke-associated pneumonia. Neurol. Sci. 2023, 44, 2431–2442. [Google Scholar] [CrossRef]
- Nam, K.-W.; Kim, T.J.; Lee, J.S.; Kwon, H.-M.; Lee, Y.-S.; Ko, S.-B.; Yoon, B.-W. High Neutrophil-to-Lymphocyte Ratio Predicts Stroke-Associated Pneumonia. Stroke 2018, 49, 1886–1892. [Google Scholar] [CrossRef] [PubMed]
- Kotfis, K.; Bott-Olejnik, M.; Szylińska, A.; Rotter, I. Could Neutrophil-to-Lymphocyte Ratio (NLR) Serve as a Potential Marker for Delirium Prediction in Patients with Acute Ischemic Stroke? A Prospective Observational Study. J. Clin. Med. 2019, 8, 1075. [Google Scholar] [CrossRef] [PubMed]
- Zha, F.; Zhao, J.; Chen, C.; Ji, X.; Li, M.; Wu, Y.; Yao, L. A High Neutrophil-to-Lymphocyte Ratio Predicts Higher Risk of Poststroke Cognitive Impairment: Development and Validation of a Clinical Prediction Model. Front. Neurol. 2022, 12, 755011. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Lim, J.-S.; Kim, C.-H.; Lee, S.-H.; Kim, Y.; Lee, J.H.; Jang, M.U.; Oh, M.S.; Lee, B.-C.; Yu, K.-H. High Neutrophil–Lymphocyte Ratio Predicts Post-stroke Cognitive Impairment in Acute Ischemic Stroke Patients. Front. Neurol. 2021, 12, 693318. [Google Scholar] [CrossRef] [PubMed]

| Demographic and Clinical Data | Excellent Functional Outcome (n = 217) | Poor Functional Outcome (n = 86) | t/Z/χ2 | p |
|---|---|---|---|---|
| Age (years) | 63.91 ± 13.02 | 67.30 ± 11.89 | t = 2.10 | 0.037 * |
| Sex male/female | 149/68 | 56/30 | χ2 = 0.35 | 0.552 |
| SBP † (mmHg) | 146.66 ± 19.88 | 154.51 ± 22.68 | t = 2.97 | 0.003 * |
| DBP † (mmHg) | 83.52 ± 11.86 | 84.64 ± 12.83 | t = 0.72 | 0.470 |
| History of hypertension yes/no | 146/71 | 56/30 | χ2 = 0.13 | 0.719 |
| History of diabetes yes/no | 63/154 | 23/63 | χ2 = 0.16 | 0.690 |
| Alcoholic habit yes/no | 42/175 | 14/72 | χ2 = 0.39 | 0.534 |
| Smoking yes/no | 55/162 | 19/67 | χ2 = 0.35 | 0.552 |
| History of AF † yes/no | 20/197 | 8/78 | χ2 = 0.00 | 0.981 |
| Other heart diseases yes/no | 12/205 | 4/82 | χ2 = 0.10 | 0.758 |
| Previous stroke yes/no | 38/179 | 14/72 | χ2 = 0.07 | 0.798 |
| TG † (mmol/L) | [1.37 (1.03, 1.76)] | [1.36 (0.99, 2.04)] | Z = 0.13 | 0.900 |
| TC † (mmol/L) | 4.40 ± 1.05 | 4.69 ± 1.14 | t = 2.15 | 0.032 * |
| LDL-C † (mmol/L) | 2.73 ± 0.98 | 2.89 ± 1.09 | t = 1.27 | 0.205 |
| Albumin (g/L) Prealbumin (g/L) | 39.11 ± 3.44 238.31 ± 50.48 | 39.68 ± 3.72 234.57 ± 63.79 | t = 1.28 t = 0.49 | 0.202 0.628 |
| Blood platelet (109/L) | [208 (172, 249)] | [199 (165, 256)] | Z = 0.65 | 0.514 |
| Creatinine (µmol/L) | [69.1 (58.8, 77.6)] | [71.6 (57.8, 83.9)] | Z = 1.45 | 0.149 |
| Uric acid (µmol/L) | 329.39 ± 96.65 | 347.08 ± 122.05 | t = 1.20 | 0.231 |
| FBG † (µmol/L) | [5.38 (4.77, 6.74)] | [5.75 (4.98, 7.44)] | Z = 1.93 | 0.054 |
| CRP † (mg/L) | [2.46 (0.95, 8.05)] | [7.49 (2.58, 15.36)] | Z = 4.518 | 0.000 * |
| CLR † HCY † (µmol/L) WBC † (109/L) | [1.38 (0.58, 4.90)] [9.90 (8.20, 12.10)] [6.97 (5.61, 8.54)] | [5.11 (1.61, 10.64)] [11.75 (9.60, 13.75)] [8.18 (6.60, 10.00)] | Z = 5.216 Z = 3.314 Z = 3.371 | 0.000 * 0.001 * 0.001 * |
| NLR † | [2.62 (1.94, 3.99)] | [3.93 (2.83, 6.01)] | Z = 5.43 | 0.000 * |
| SII † PLR † FIB † (g/L) | [529.74 (373.78, 841.03)] [124.64 (92.75, 171.93)] [2.80 (2.30, 3.32)] | [847.39 (531.30, 1375.46)] [149.77 (113.44, 202.28)] [3.22 (2.69, 3.95)] | Z = 4.555 Z = 3.140 Z = 3.940 | 0.000 * 0.002 * 0.000 * |
| Hemoglobin A1c ‡ (%) | [6.1 (5.6, 7.3)] | [6.1(5.7,7.2)] | Z = 0.38 | 0.703 |
| NIHSS † score | [2 (1, 5)] | [7 (4, 11)] | Z = 8.47 | 0.000 * |
| TOAST classification † | 98/23/80/16/0 | 59/10/12/5/0 | χ2 = 17.46 | 0.001 * |
| CTP † -positive yes/no # | 102/64 | 55/11 | χ2 = 10.36 | 0.006 * |
| Thrombolytic yes/no | 53/164 | 28/58 | χ2 = 2.08 | 0.149 |
| HT † yes/no † | 5/212 | 2/84 | χ2 = 0.88 | 0.831 |
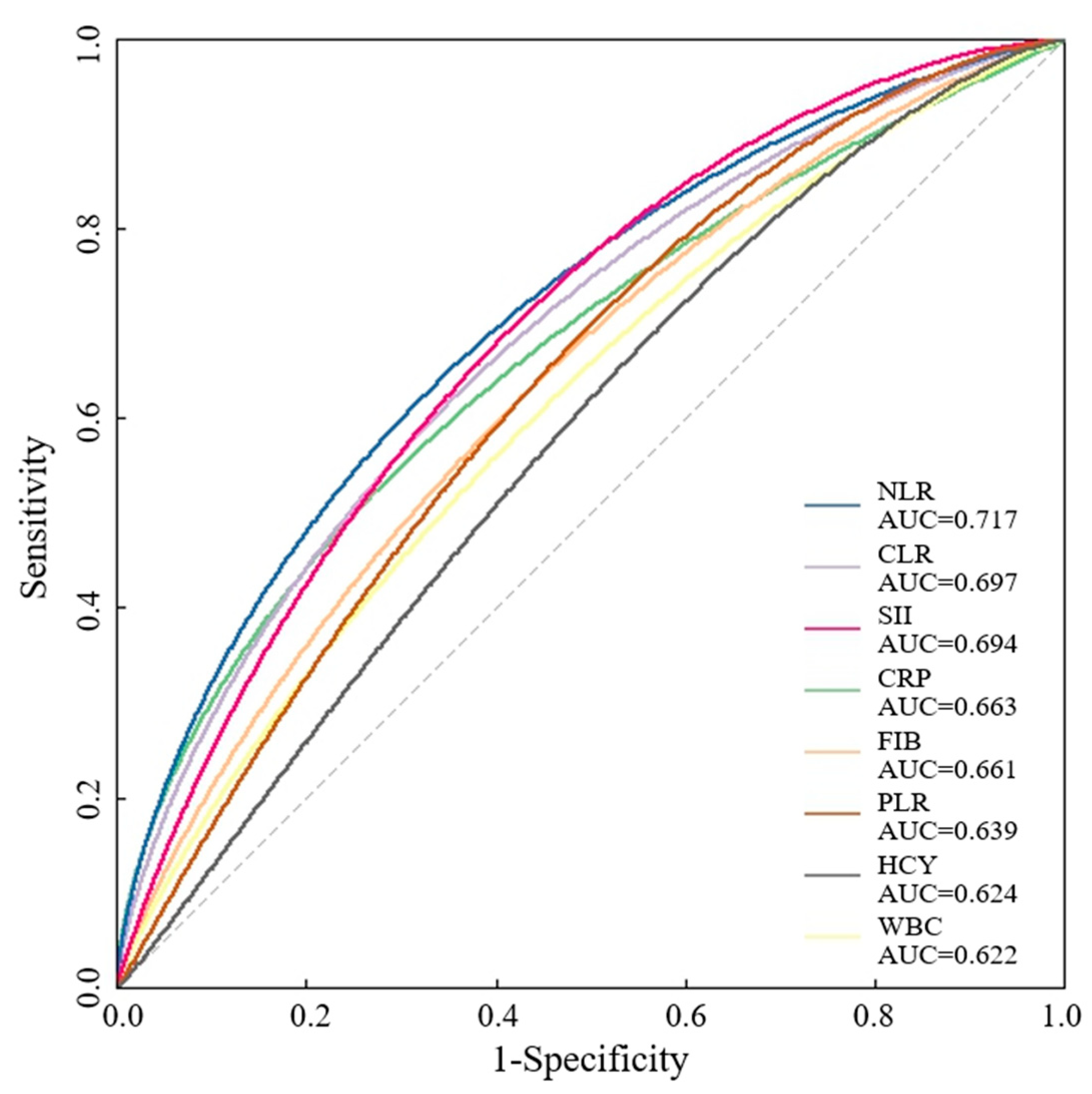
| AUC-ROC | 95% CI | Cut-Off Level | p Value | |
|---|---|---|---|---|
| NLR | 0.717 | 0.648, 0786 | 3.06 | <0.001 |
| CLR | 0.697 | 0.625, 0.768 | 3.87 | <0.001 |
| SII | 0.694 | 0.626, 0.763 | 769.83 | <0.001 |
| CRP | 0.663 | 0.587, 0.738 | 4.4 | <0.001 |
| FIB | 0.661 | 0.589, 0.734 | 3.03 | <0.001 |
| PLR | 0.639 | 0.567, 0.710 | 125.09 | <0.001 |
| HCY | 0.624 | 0.550, 0.698 | 11.15 | 0.002 |
| WBC | 0.622 | 0.547, 0.697 | 7.43 | 0.002 |
| Variables | Odd Ratio | 95% CI | p Value |
|---|---|---|---|
| Age | 1.016 | 0.99, 1.05 | 0.313 |
| Sex | 0.903 | 0.46, 1.77 | 0.766 |
| SBP (mmHg) | 1.015 | 1.00, 1.03 | 0.043 |
| NLR | 1.148 | 1.04, 1.27 | 0.008 |
| NIHSS score | 1.255 | 1.16, 1.36 | 0.000 |
| Large-artery atherosclerosis | 1.458 | 0.36, 5.96 | 0.599 |
| Cardio-embolism | 0.524 | 0.10, 2.90 | 0.459 |
| Small-vessel occlusion | 0.467 | 0.10, 2.10 | 0.320 |
| TC | 1.260 | 0.95, 1.67 | 0.104 |
| CTP-positive | 0.550 | 0.31, 1.43 | 0.221 |
| CTP-negative | 0.775 | 0.35, 1.74 | 0.536 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Zhang, L.; Li, Y.; Zhang, Q.; Fang, Q.; Tang, X. Association of the Neutrophil-to-Lymphocyte Ratio with 90-Day Functional Outcomes in Patients with Acute Ischemic Stroke. Brain Sci. 2024, 14, 250. https://doi.org/10.3390/brainsci14030250
Chen L, Zhang L, Li Y, Zhang Q, Fang Q, Tang X. Association of the Neutrophil-to-Lymphocyte Ratio with 90-Day Functional Outcomes in Patients with Acute Ischemic Stroke. Brain Sciences. 2024; 14(3):250. https://doi.org/10.3390/brainsci14030250
Chicago/Turabian StyleChen, Licong, Lulu Zhang, Yidan Li, Quanquan Zhang, Qi Fang, and Xiang Tang. 2024. "Association of the Neutrophil-to-Lymphocyte Ratio with 90-Day Functional Outcomes in Patients with Acute Ischemic Stroke" Brain Sciences 14, no. 3: 250. https://doi.org/10.3390/brainsci14030250
APA StyleChen, L., Zhang, L., Li, Y., Zhang, Q., Fang, Q., & Tang, X. (2024). Association of the Neutrophil-to-Lymphocyte Ratio with 90-Day Functional Outcomes in Patients with Acute Ischemic Stroke. Brain Sciences, 14(3), 250. https://doi.org/10.3390/brainsci14030250







