Short-Term Effect of Auditory Stimulation on Neural Activities: A Scoping Review of Longitudinal Electroencephalography and Magnetoencephalography Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
- Inclusion criteria:
- Exclusion criteria:
| Conditions | Measures of Interest | Inclusion | Exclusion |
|---|---|---|---|
| Intervention, stimuli | Sound exposure | Pure tones Music White noise | Syllables Sentences Phonemes Crossmodal stimuli |
| Intervention, period | Short-term | Training over a few minutes, hours, days | Training over several months or years |
| Study design | Longitudinal | Monitoring a population over a certain period | Cross-sectional comparisons (musicians vs. non-musicians, different age groups, healthy vs. diseased) |
| Participants, subjects | Healthy people | People irrespective of age, diseases or musical skills | Patients |
| Participants, state | Awake and listening | Awake condition Attentive listening Passive listening | Playing instruments Vocalization Stimuli during sleep Musical imagery Listening combined with transcranial magnetic stimulation |
| Recording | Electrophysiological measures | MEG EEG | fMRI ECoG |
3. Results
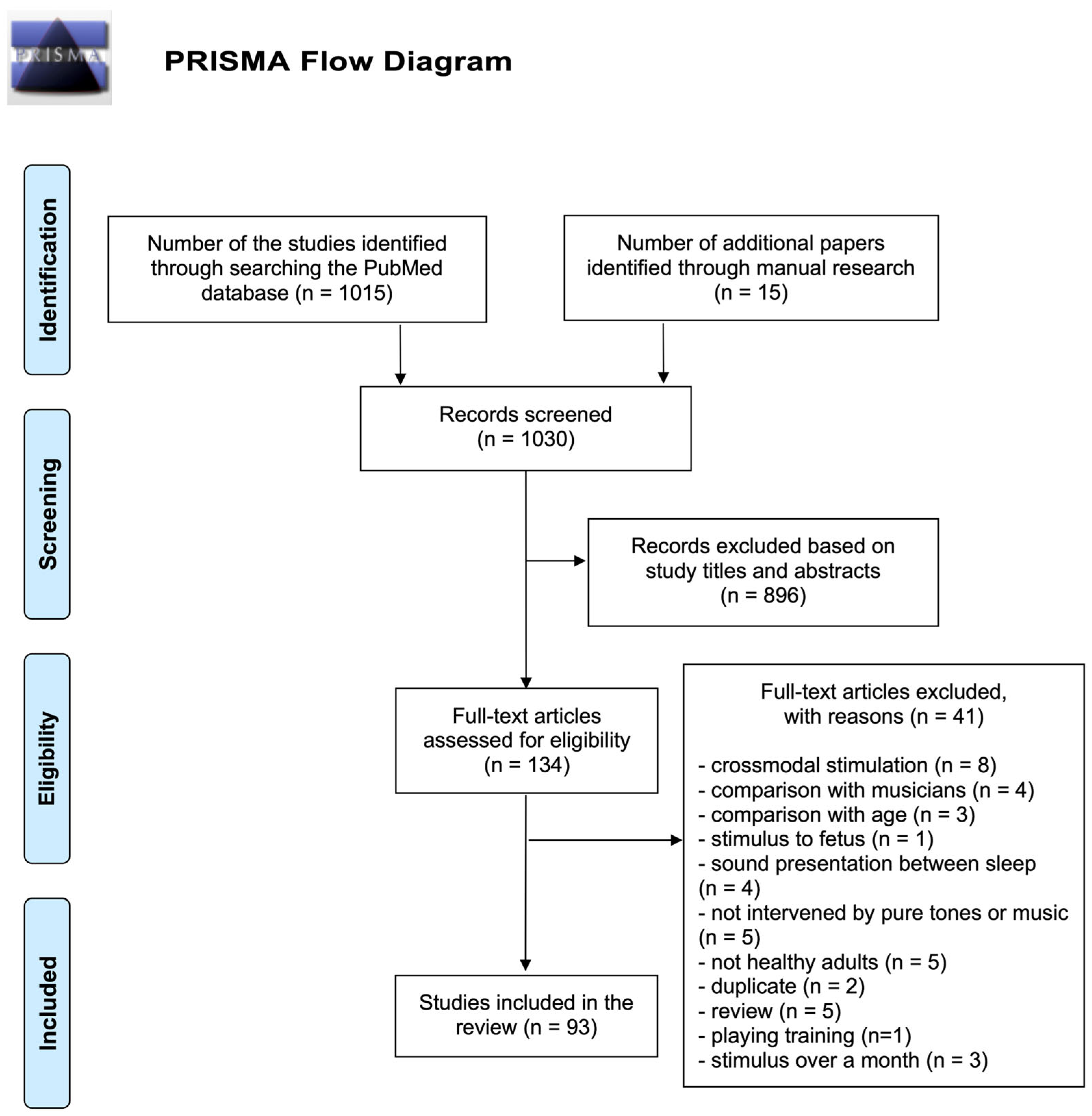
3.1. Overview of Studies
3.1.1. Screening of Articles
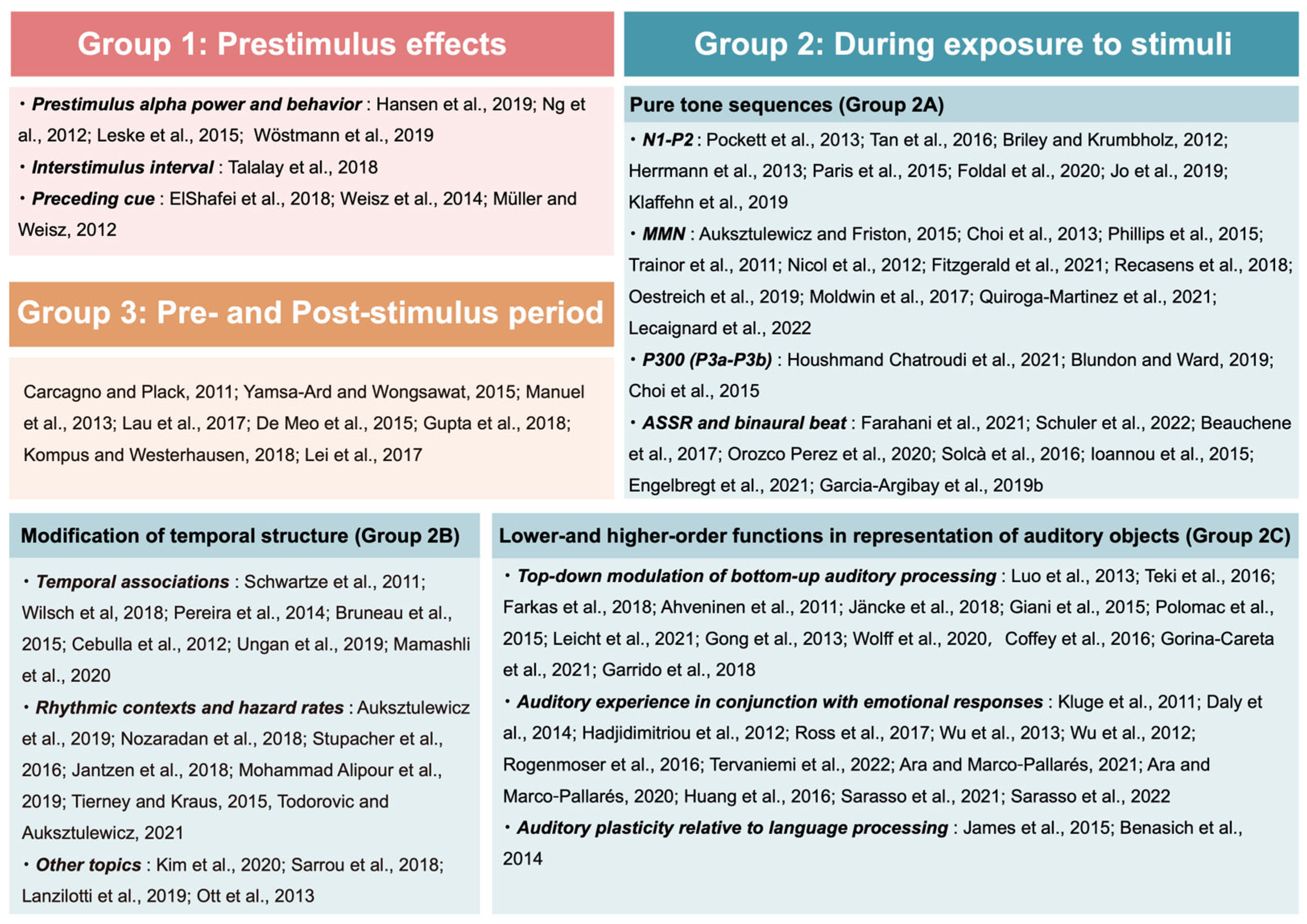
3.1.2. Classification of Selected Articles
3.1.3. Characteristics of the Interventions in the Selected Articles
3.2. Individual Study Results and Synthesis
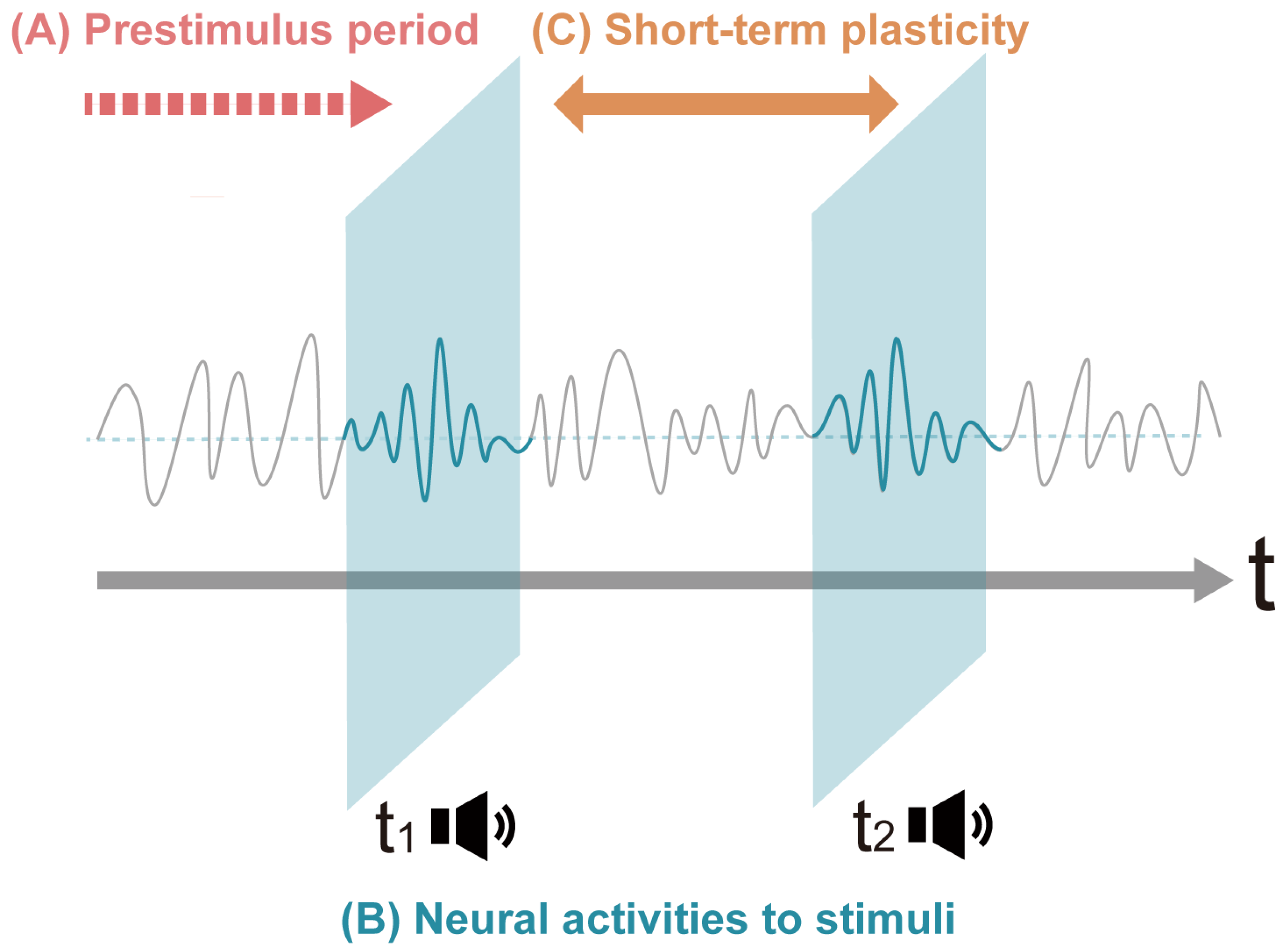
3.2.1. Prestimulus Effects 1. Prestimulus Alpha Power and Behavior
- Interstimulus Interval
- 2.
- Preceding Cue
3.2.2. During Exposure to Stimuli
- Pure Tone Sequences
- N1-P2
- MMN
- P300 (P3a-P3b)
- ASSR and binaural beat
- 2.
- Modification of Temporal Structure
- Temporal associations
- Rhythmic contexts and hazard rates
- Other topics
- 3.
- Lower- and Higher-Order Functions in Representation of Auditory Objects
- Top-down modulation of bottom-up auditory processing
- Auditory experience in conjunction with emotional responses
- Auditory plasticity relative to language processing
3.2.3. Pre- and Post-Stimulus Period
4. Discussion
4.1. Inhibitory Role of Prestimulus Alpha
4.2. Dilemma about Alpha Lateralization
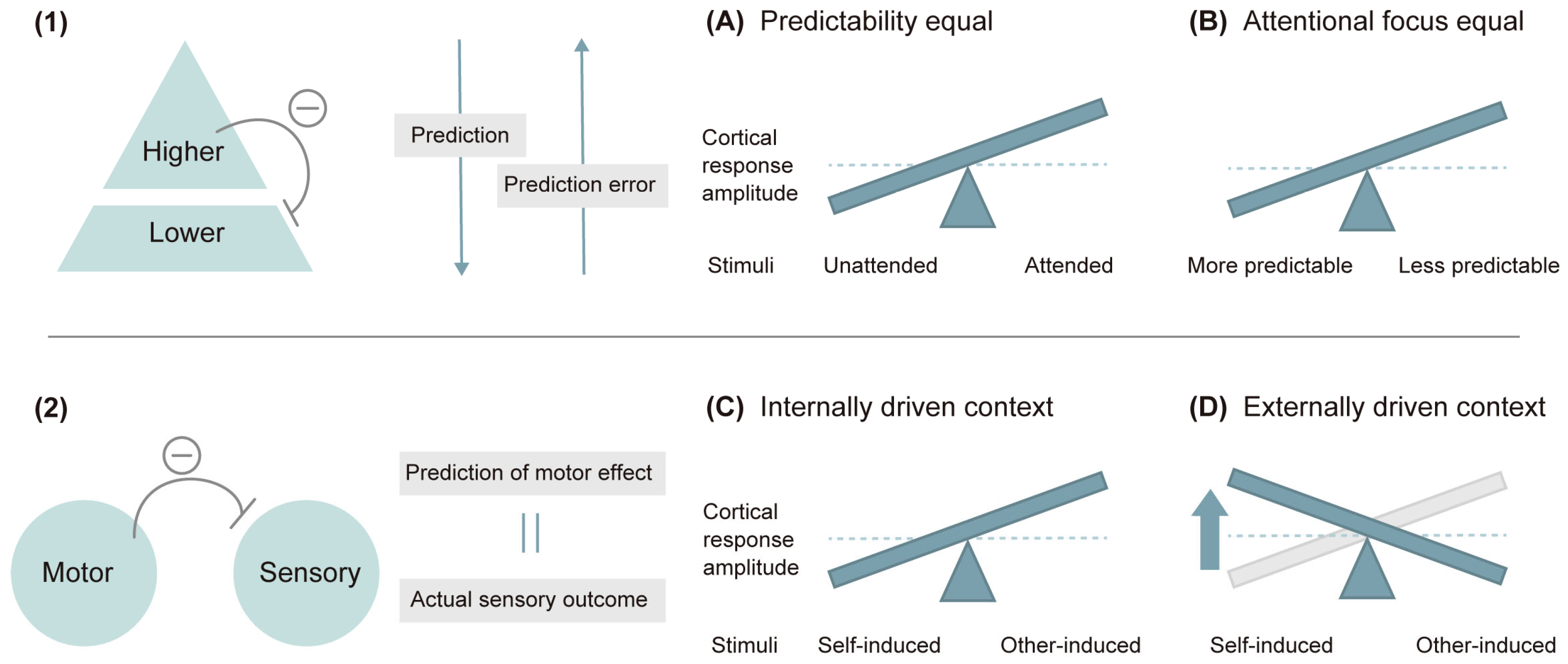
4.3. Modulation of N1 by Prediction and Attention
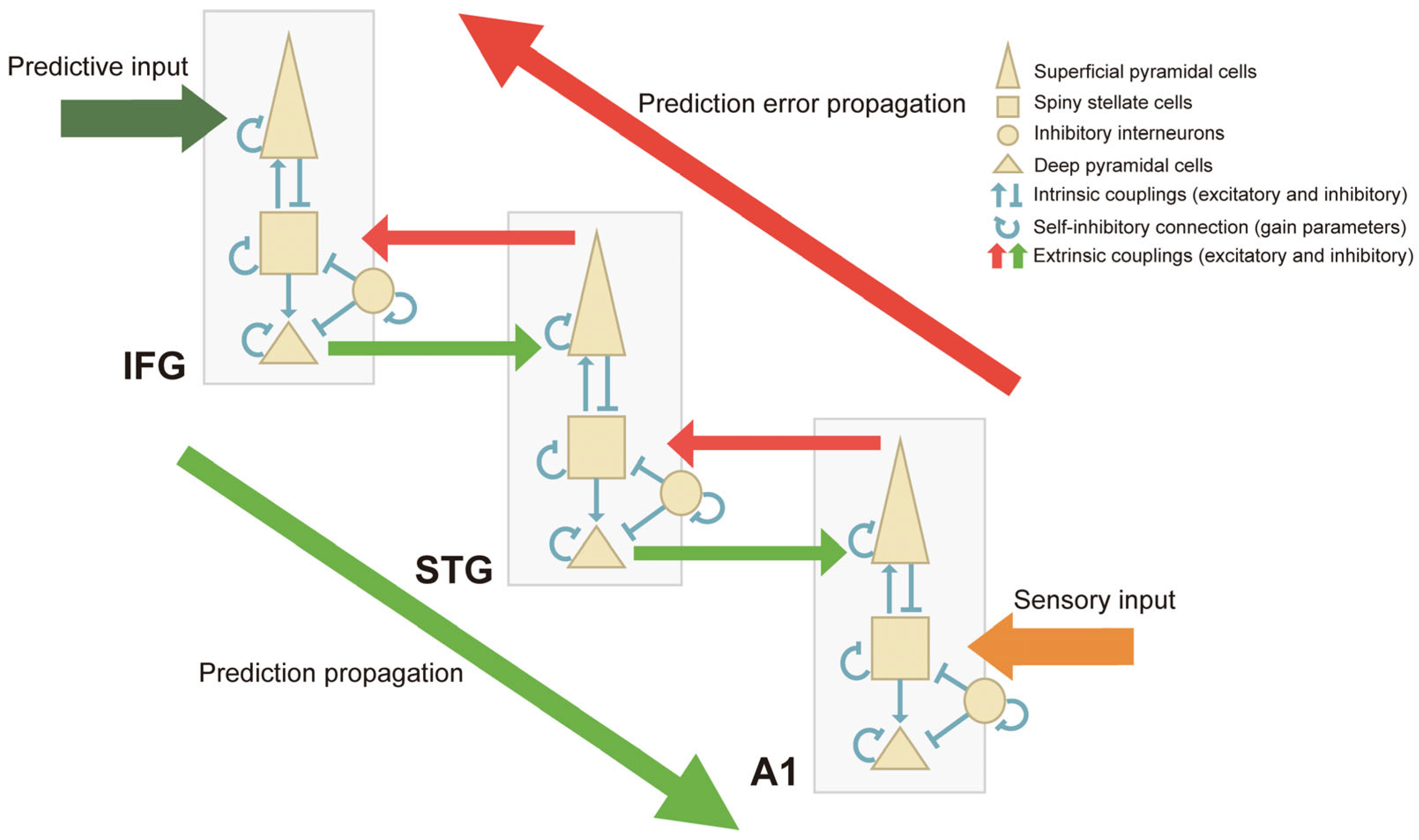
4.4. The Generation of Prediction Error Responses
4.5. Contradiction about Cortical Response Dynamics and Its Solution
4.6. Oscillatory Synchronization to the Presented Stimuli
4.7. The Interplay of Bottom-Up Processing and Top-Down Modulations
4.8. Confusion of the Terminology: Attention
4.9. Dissociation of Attention, Awareness and Consciousness
4.10. The Benefit of Auditory Plasticity for Language Development
4.11. Confounds of Auditory Factors
4.12. Sustained Post-Exposure Effects in Longitudinal Studies
4.13. Dynamism of Short-Term Neural Oscillations Influenced by Various Factors
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Item | Item | Prisma-ScR Checklist Item | Section of This Review |
|---|---|---|---|
| Title | 1 | Identify the report as a scoping review. | Title |
| Structured summary | 2 | Provide a structured summary that includes (as applicable): background, objectives, eligibility criteria, sources of evidence, charting methods, results and conclusions that relate to the review questions and objectives. | Abstract |
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. Explain why the review questions/objectives lend themselves to a scoping review approach. | Introduction |
| Objectives | 4 | Provide an explicit statement of the questions and objectives being addressed with reference to their key elements (e.g., population or participants, concepts and context) or other relevant key elements used to conceptualize the review questions and/or objectives. | Introduction |
| Protocol and registration | 5 | Indicate whether a review protocol exists; state if and where it can be accessed (e.g., a Web address); and if available, provide registration information, including the registration number. | Search strategy |
| Eligibility criteria | 6 | Specify characteristics of the sources of evidence used as eligibility criteria (e.g., years considered, language and publication status), and provide a rationale. | Selection criteria |
| Information sources | 7 | Describe all information sources in the search (e.g., databases with dates of coverage and contact with authors to identify additional sources), as well as the date the most recent search was executed. | Search strategy |
| Search | 8 | Present the full electronic search strategy for at least 1 database, including any limits used, such that it could be repeated. | Search strategy |
| Selection of sources of evidence | 9 | State the process for selecting sources of evidence (i.e., screening and eligibility) included in the scoping review. | Selection criteria |
| Data charting process | 10 | Describe the methods of charting data from the included sources of evidence (e.g., calibrated forms or forms that have been tested by the team before their use and whether data charting was done independently or in duplicate) and any processes for obtaining and confirming data from investigators. | Classification of selected articles |
| Data items | 11 | List and define all variables for which data were sought and any assumptions and simplifications made. | Characteristics of the interventions in the selected articles |
| Critical appraisal of individual sources of evidence | 12 | If done, provide a rationale for conducting a critical appraisal of included sources of evidence; describe the methods used and how this information was used in any data synthesis (if appropriate). | Screening of articles |
| Synthesis of results | 13 | Describe the methods of handling and summarizing the data that were charted. | Classification of selected articles |
| Selection of sources of evidence | 14 | Give numbers of sources of evidence screened, assessed for eligibility and included in the review, with reasons for exclusions at each stage, ideally using a flow diagram. | Screening of articles |
| Characteristics of sources of evidence | 15 | For each source of evidence, present characteristics for which data were charted and provide the citations. | Screening of articles |
| Critical appraisal within sources of evidence | 16 | If done, present data on critical appraisal of included sources of evidence (see item 12). | Screening of articles |
| Results of individual sources of evidence | 17 | For each included source of evidence, present the relevant data that were charted that relate to the review questions and objectives. | Individual study results and synthesis |
| Synthesis of results | 18 | Summarize and/or present the charting results as they relate to the review questions and objectives. | Individual study results and synthesis |
| Summary of evidence | 19 | Summarize the main results (including an overview of concepts, themes and types of evidence available), link to the review questions and objectives and consider the relevance to key groups. | Discussion |
| Limitations | 20 | Discuss the limitations of the scoping review process. | Discussion |
| Conclusions | 21 | Provide a general interpretation of the results with respect to the review questions and objectives, as well as potential implications and/or next steps. | Conclusion |
| Funding | 22 | Describe sources of funding for the included sources of evidence, as well as sources of funding for the scoping review. Describe the role of the funders of the scoping review. | Acknowledgments |
| References | Selection of Participants | Confounding Variables | Measurement of Exposure | Blinding of Outcome Assessments | Incomplete Outcome Data | Selective Outcome Reporting |
|---|---|---|---|---|---|---|
| [56] | ● | ● | ● | ● | ● | ● |
| [57] | ● | ● | ● | ● | ● | ● |
| [58] | ● | ● | ● | ● | ● | ● |
| [59] | ● | ● | ● | ● | ● | ● |
| [60] | ● | ● | ● | ● | ● | ● |
| [61] | ● | ● | ● | ● | ● | ● |
| [62] | ● | ● | ● | ● | ● | ● |
| [63] | ● | ● | ● | ● | ● | ● |
| [64] | ● | ● | ● | ● | ● | ● |
| [65] | ● | ● | ● | ● | ● | ● |
| [66] | ● | ● | ● | ● | ● | ● |
| [67] | ● | ● | ● | ● | ● | ● |
| [68] | ● | ● | ● | ● | ● | ● |
| [69] | ● | ● | ● | ● | ● | ● |
| [70] | ● | ● | ● | ● | ● | ● |
| [71] | ● | ● | ● | ● | ● | ● |
| [72] | ● | ● | ● | ● | ● | ● |
| [73] | ● | ● | ● | ● | ● | ● |
| [74] | ● | ● | ● | ● | ● | ● |
| [75] | ● | ● | ● | ● | ● | ● |
| [76] | ● | ● | ● | ● | ● | ● |
| [77] | ● | ● | ● | ● | ● | ● |
| [78] | ● | ● | ● | ● | ● | ● |
| [79] | ● | ● | ● | ● | ● | ● |
| [42] | ● | ● | ● | ● | ● | ● |
| [38] | ● | ● | ● | ● | ● | ● |
| [80] | ● | ● | ● | ● | ● | ● |
| [81] | ● | ● | ● | ● | ● | ● |
| [82] | ● | ● | ● | ● | ● | ● |
| [83] | ● | ● | ● | ● | ● | ● |
| [84] | ● | ● | ● | ● | ● | ● |
| [85] | ● | ● | ● | ● | ● | ● |
| [86] | ● | ● | ● | ● | ● | ● |
| [87] | ● | ● | ● | ● | ● | ● |
| [88] | ● | ● | ● | ● | ● | ● |
| [89] | ● | ● | ● | ● | ● | ● |
| [90] | ● | ● | ● | ● | ● | ● |
| [91] | ● | ● | ● | ● | ● | ● |
| [92] | ● | ● | ● | ● | ● | ● |
| [93] | ● | ● | ● | ● | ● | ● |
| [94] | ● | ● | ● | ● | ● | ● |
| [95] | ● | ● | ● | ● | ● | ● |
| [96] | ● | ● | ● | ● | ● | ● |
| [97] | ● | ● | ● | ● | ● | ● |
| [98] | ● | ● | ● | ● | ● | ● |
| [103] | ● | ● | ● | ● | ● | ● |
| [99] | ● | ● | ● | ● | ● | ● |
| [54] | ● | ● | ● | ● | ● | ● |
| [55] | ● | ● | ● | ● | ● | ● |
| [100] | ● | ● | ● | ● | ● | ● |
| [101] | ● | ● | ● | ● | ● | ● |
| [102] | ● | ● | ● | ● | ● | ● |
| [104] | ● | ● | ● | ● | ● | ● |
| [105] | ● | ● | ● | ● | ● | ● |
| [106] | ● | ● | ● | ● | ● | ● |
| [107] | ● | ● | ● | ● | ● | ● |
| [108] | ● | ● | ● | ● | ● | ● |
| [109] | ● | ● | ● | ● | ● | ● |
| [110] | ● | ● | ● | ● | ● | ● |
| [111] | ● | ● | ● | ● | ● | ● |
| [112] | ● | ● | ● | ● | ● | ● |
| [113] | ● | ● | ● | ● | ● | ● |
| [114] | ● | ● | ● | ● | ● | ● |
| [115] | ● | ● | ● | ● | ● | ● |
| [116] | ● | ● | ● | ● | ● | ● |
| [117] | ● | ● | ● | ● | ● | ● |
| [118] | ● | ● | ● | ● | ● | ● |
| [119] | ● | ● | ● | ● | ● | ● |
| [53] | ● | ● | ● | ● | ● | ● |
| [120] | ● | ● | ● | ● | ● | ● |
| [121] | ● | ● | ● | ● | ● | ● |
| [122] | ● | ● | ● | ● | ● | ● |
| [123] | ● | ● | ● | ● | ● | ● |
| [124] | ● | ● | ● | ● | ● | ● |
| [125] | ● | ● | ● | ● | ● | ● |
| [126] | ● | ● | ● | ● | ● | ● |
| [127] | ● | ● | ● | ● | ● | ● |
| [128] | ● | ● | ● | ● | ● | ● |
| [129] | ● | ● | ● | ● | ● | ● |
| [130] | ● | ● | ● | ● | ● | ● |
| [131] | ● | ● | ● | ● | ● | ● |
| [132] | ● | ● | ● | ● | ● | ● |
| [133] | ● | ● | ● | ● | ● | ● |
| [134] | ● | ● | ● | ● | ● | ● |
| [135] | ● | ● | ● | ● | ● | ● |
| [136] | ● | ● | ● | ● | ● | ● |
| [137] | ● | ● | ● | ● | ● | ● |
| [138] | ● | ● | ● | ● | ● | ● |
| [139] | ● | ● | ● | ● | ● | ● |
| [140] | ● | ● | ● | ● | ● | ● |
| [141] | ● | ● | ● | ● | ● | ● |
| [142] | ● | ● | ● | ● | ● | ● |
| [143] | ● | ● | ● | ● | ● | ● |
References
- Palva, S.; Palva, J.M. Discovering oscillatory interaction networks with M/EEG: Challenges and breakthroughs. Trends Cogn. Sci. 2012, 16, 219–230. [Google Scholar] [CrossRef]
- Rimmele, J.M.; Morillon, B.; Poeppel, D.; Arnal, L.H. Proactive Sensing of Periodic and Aperiodic Auditory Patterns. Trends Cogn. Sci. 2018, 22, 870–882. [Google Scholar] [CrossRef] [PubMed]
- Uhlhaas, P.; Pipa, G.; Lima, B.; Melloni, L.; Neuenschwander, S.; Nikolić, D.; Singer, W. Neural synchrony in cortical networks: History, concept and current status. Front. Integr. Neurosci. 2009, 3, 543. [Google Scholar] [CrossRef] [PubMed]
- Lakatos, P.; Gross, J.; Thut, G. A New Unifying Account of the Roles of Neuronal Entrainment. Curr. Biol. 2019, 29, R890–R905. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, C.E.; Lakatos, P. Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci. 2009, 32, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.J.; Obleser, J. Frequency modulation entrains slow neural oscillations and optimizes human listening behavior. Proc. Natl. Acad. Sci. USA 2012, 109, 20095–20100. [Google Scholar] [CrossRef]
- Obleser, J.; Kayser, C. Neural Entrainment and Attentional Selection in the Listening Brain. Trends Cogn. Sci. 2019, 23, 913–926. [Google Scholar] [CrossRef] [PubMed]
- Llinás, R.R. The Intrinsic Electrophysiological Properties of Mammalian Neurons: Insights into Central Nervous System Function. Science 1988, 242, 1654–1664. [Google Scholar] [CrossRef]
- Haegens, S.; Zion Golumbic, E. Rhythmic facilitation of sensory processing: A critical review. Neurosci. Biobehav. Rev. 2018, 86, 150–165. [Google Scholar] [CrossRef]
- Haenschel, C.; Baldeweg, T.; Croft, R.J.; Whittington, M.; Gruzelier, J. Gamma and beta frequency oscillations in response to novel auditory stimuli: A comparison of human electroencephalogram (EEG) data with in vitro models. Proc. Natl. Acad. Sci. USA 2000, 97, 7645–7650. [Google Scholar] [CrossRef]
- Chang, A.; Bosnyak, D.J.; Trainor, L.J. Rhythmicity facilitates pitch discrimination: Differential roles of low and high frequency neural oscillations. NeuroImage 2019, 198, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Eickhoff, S.B.; Müller, V.I. Functional Connectivity. In Brain Mapping; Toga, A.W., Ed.; Academic Press: Waltham, MA, USA, 2015; pp. 187–201. [Google Scholar]
- Pantev, C.; Oostenveld, R.; Engelien, A.; Ross, B.; Roberts, L.E.; Hoke, M. Increased auditory cortical representation in musicians. Nature 1998, 392, 811–814. [Google Scholar] [CrossRef]
- Shahin, A.J.; Roberts, L.E.; Pantev, C.; Aziz, M.; Picton, T.W. Enhanced anterior-temporal processing for complex tones in musicians. Clin. Neurophysiol. 2007, 118, 209–220. [Google Scholar] [CrossRef]
- Pantev, C.; Herholz, S.C. Plasticity of the human auditory cortex related to musical training. Neurosci. Biobehav. Rev. 2011, 35, 2140–2154. [Google Scholar] [CrossRef] [PubMed]
- Baumann, S.; Meyer, M.; Jäncke, L. Enhancement of auditory-evoked potentials in musicians reflects an influence of expertise but not selective attention. J. Cogn. Neurosci. 2008, 20, 2238–2249. [Google Scholar] [CrossRef] [PubMed]
- Paraskevopoulos, E.; Chalas, N.; Kartsidis, P.; Wollbrink, A.; Bamidis, P. Statistical learning of multisensory regularities is enhanced in musicians: An MEG study. Neuroimage 2018, 175, 150–160. [Google Scholar] [CrossRef]
- Jenni, R.; Oechslin, M.S.; James, C.E. Impact of major and minor mode on EEG frequency range activities of music processing as a function of expertise. Neurosci. Lett. 2017, 647, 159–164. [Google Scholar] [CrossRef]
- Schneider, P.; Scherg, M.; Dosch, H.G.; Specht, H.J.; Gutschalk, A.; Rupp, A. Morphology of Heschl’s gyrus reflects enhanced activation in the auditory cortex of musicians. Nat. Neurosci. 2002, 5, 688–694. [Google Scholar] [CrossRef]
- Münte, T.F.; Altenmüller, E.; Jäncke, L. The musician’s brain as a model of neuroplasticity. Nat. Rev. Neurosci. 2002, 3, 473–478. [Google Scholar] [CrossRef]
- Moreno, S.; Bidelman, G.M. Examining neural plasticity and cognitive benefit through the unique lens of musical training. Hear. Res. 2014, 308, 84–97. [Google Scholar] [CrossRef]
- Honing, H.; ten Cate, C.; Peretz, I.; Trehub, S.E. Without it no music: Cognition, biology and evolution of musicality. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140088. [Google Scholar] [CrossRef]
- Hyde, K.L.; Lerch, J.; Norton, A.; Forgeard, M.; Winner, E.; Evans, A.C.; Schlaug, G. Musical training shapes structural brain development. J. Neurosci. 2009, 29, 3019–3025. [Google Scholar] [CrossRef] [PubMed]
- Habibi, A.; Damasio, A.; Ilari, B.; Veiga, R.; Joshi, A.A.; Leahy, R.M.; Haldar, J.P.; Varadarajan, D.; Bhushan, C.; Damasio, H. Childhood Music Training Induces Change in Micro and Macroscopic Brain Structure: Results from a Longitudinal Study. Cereb. Cortex 2018, 28, 4336–4347. [Google Scholar] [CrossRef] [PubMed]
- Moreno, S.; Marques, C.; Santos, A.; Santos, M.; Castro, S.L.; Besson, M. Musical Training Influences Linguistic Abilities in 8-Year-Old Children: More Evidence for Brain Plasticity. Cereb. Cortex 2008, 19, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Olszewska, A.M.; Gaca, M.; Herman, A.M.; Jednorog, K.; Marchewka, A. How Musical Training Shapes the Adult Brain: Predispositions and Neuroplasticity. Front. Neurosci. 2021, 15, 630829. [Google Scholar] [CrossRef] [PubMed]
- Herholz, S.C.; Zatorre, R.J. Musical training as a framework for brain plasticity: Behavior, function, and structure. Neuron 2012, 76, 486–502. [Google Scholar] [CrossRef] [PubMed]
- Pantev, C.; Wollbrink, A.; Roberts, L.E.; Engelien, A.; Lütkenhöner, B. Short-term plasticity of the human auditory cortex. Brain Res. 1999, 842, 192–199. [Google Scholar] [CrossRef]
- Bangert, M.; Haeusler, U.; Altenmuller, E. On practice: How the brain connects piano keys and piano sounds. Ann. N. Y. Acad. Sci. 2001, 930, 425–428. [Google Scholar] [CrossRef]
- Kral, A.; Eggermont, J.J. What’s to lose and what’s to learn: Development under auditory deprivation, cochlear implants and limits of cortical plasticity. Brain Res. Rev. 2007, 56, 259–269. [Google Scholar] [CrossRef]
- Lakatos, P.; Karmos, G.; Mehta, A.D.; Ulbert, I.; Schroeder, C.E. Entrainment of neuronal oscillations as a mechanism of attentional selection. Science 2008, 320, 110–113. [Google Scholar] [CrossRef]
- Nobre, A.C.; Correa, A.; Coull, J.T. The hazards of time. Curr. Opin. Neurobiol. 2007, 17, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Stefanics, G.; Hangya, B.; Hernádi, I.; Winkler, I.; Lakatos, P.; Ulbert, I. Phase entrainment of human delta oscillations can mediate the effects of expectation on reaction speed. J. Neurosci. 2010, 30, 13578–13585. [Google Scholar] [CrossRef] [PubMed]
- Vuust, P.; Heggli, O.A.; Friston, K.J.; Kringelbach, M.L. Music in the brain. Nat. Rev. Neurosci. 2022, 23, 287–305. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W. alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn. Sci. 2012, 16, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Zoefel, B.; Ten Oever, S.; Sack, A.T. The Involvement of Endogenous Neural Oscillations in the Processing of Rhythmic Input: More Than a Regular Repetition of Evoked Neural Responses. Front. Neurosci. 2018, 12, 95. [Google Scholar] [CrossRef] [PubMed]
- Koelsch, S.; Gunter, T.; Friederici, A.D.; Schröger, E. Brain Indices of Music Processing: “Nonmusicians” are Musical. J. Cogn. Neurosci. 2000, 12, 520–541. [Google Scholar] [CrossRef]
- Quiroga-Martinez, D.R.; Hansen, N.C.; Hojlund, A.; Pearce, M.; Brattico, E.; Holmes, E.; Friston, K.; Vuust, P. Musicianship and melodic predictability enhance neural gain in auditory cortex during pitch deviance detection. Hum. Brain Mapp. 2021, 42, 5595–5608. [Google Scholar] [CrossRef]
- Müllensiefen, D.; Gingras, B.; Musil, J.; Stewart, L. The musicality of non-musicians: An index for assessing musical sophistication in the general population. PLoS ONE 2014, 9, e89642. [Google Scholar] [CrossRef]
- Koelsch, S.; Gunter, T.; Schroger, E.; Friederici, A.D. Processing tonal modulations: An ERP study. J. Cogn. Neurosci. 2003, 15, 1149–1159. [Google Scholar] [CrossRef]
- Hannon, E.E.; Snyder, J.S.; Eerola, T.; Krumhansl, C.L. The role of melodic and temporal cues in perceiving musical meter. J. Exp. Psychol. Hum. Percept. Perform. 2004, 30, 956. [Google Scholar] [CrossRef]
- Moldwin, T.; Schwartz, O.; Sussman, E.S. Statistical Learning of Melodic Patterns Influences the Brain’s Response to Wrong Notes. J. Cogn. Neurosci. 2017, 29, 2114–2122. [Google Scholar] [CrossRef]
- Van Diepen, R.M.; Foxe, J.J.; Mazaheri, A. The functional role of alpha-band activity in attentional processing: The current zeitgeist and future outlook. Curr. Opin. Psychol. 2019, 29, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Jensen, O.; Mazaheri, A. Shaping functional architecture by oscillatory alpha activity: Gating by inhibition. Front. Hum. Neurosci. 2010, 4, 186. [Google Scholar] [CrossRef]
- Nobre, A.C.; van Ede, F. Anticipated moments: Temporal structure in attention. Nat. Rev. Neurosci. 2018, 19, 34–48. [Google Scholar] [CrossRef]
- Frey, J.N.; Mainy, N.; Lachaux, J.P.; Muller, N.; Bertrand, O.; Weisz, N. Selective modulation of auditory cortical alpha activity in an audiovisual spatial attention task. J. Neurosci. 2014, 34, 6634–6639. [Google Scholar] [CrossRef] [PubMed]
- Wilsch, A.; Mercier, M.R.; Obleser, J.; Schroeder, C.E.; Haegens, S. Spatial Attention and Temporal Expectation Exert Differential Effects on Visual and Auditory Discrimination. J. Cogn. Neurosci. 2020, 32, 1562–1576. [Google Scholar] [CrossRef] [PubMed]
- Rimmele, J.; Jolsvai, H.; Sussman, E. Auditory Target Detection Is Affected by Implicit Temporal and Spatial Expectations. J. Cogn. Neurosci. 2011, 23, 1136–1147. [Google Scholar] [CrossRef] [PubMed]
- Denison, R.N.; Heeger, D.J.; Carrasco, M. Attention flexibly trades off across points in time. Psychon. Bull. Rev. 2017, 24, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Morillon, B.; Schroeder, C.E.; Wyart, V.; Arnal, L.H. Temporal prediction in lieu of periodic stimulation. J. Neurosci. 2016, 36, 2342–2347. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Kim, S.Y.; Park, J.E.; Lee, Y.J.; Seo, H.J.; Sheen, S.S.; Hahn, S.; Jang, B.H.; Son, H.J. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J. Clin. Epidemiol. 2013, 66, 408–414. [Google Scholar] [CrossRef]
- Gorina-Careta, N.; Kurkela, J.L.O.; Hamalainen, J.; Astikainen, P.; Escera, C. Neural generators of the frequency-following response elicited to stimuli of low and high frequency: A magnetoencephalographic (MEG) study. Neuroimage 2021, 231, 117866. [Google Scholar] [CrossRef]
- Nozaradan, S.; Schonwiesner, M.; Keller, P.E.; Lenc, T.; Lehmann, A. Neural bases of rhythmic entrainment in humans: Critical transformation between cortical and lower-level representations of auditory rhythm. Eur. J. Neurosci. 2018, 47, 321–332. [Google Scholar] [CrossRef]
- Stupacher, J.; Witte, M.; Hove, M.J.; Wood, G. Neural Entrainment in Drum Rhythms with Silent Breaks: Evidence from Steady-state Evoked and Event-related Potentials. J. Cogn. Neurosci. 2016, 28, 1865–1877. [Google Scholar] [CrossRef] [PubMed]
- Hansen, N.E.; Harel, A.; Iyer, N.; Simpson, B.D.; Wisniewski, M.G. Pre-stimulus brain state predicts auditory pattern identification accuracy. Neuroimage 2019, 199, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Ng, B.S.; Schroeder, T.; Kayser, C. A precluding but not ensuring role of entrained low-frequency oscillations for auditory perception. J. Neurosci. 2012, 32, 12268–12276. [Google Scholar] [CrossRef] [PubMed]
- Leske, S.; Ruhnau, P.; Frey, J.; Lithari, C.; Muller, N.; Hartmann, T.; Weisz, N. Prestimulus Network Integration of Auditory Cortex Predisposes Near-Threshold Perception Independently of Local Excitability. Cereb. Cortex 2015, 25, 4898–4907. [Google Scholar] [CrossRef] [PubMed]
- Wostmann, M.; Waschke, L.; Obleser, J. Prestimulus neural alpha power predicts confidence in discriminating identical auditory stimuli. Eur. J. Neurosci. 2019, 49, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Talalay, I.V.; Kurgansky, A.V.; Machinskaya, R.I. Alpha-band functional connectivity during cued versus implicit modality-specific anticipatory attention: EEG-source coherence analysis. Psychophysiology 2018, 55, e13269. [Google Scholar] [CrossRef]
- ElShafei, H.A.; Bouet, R.; Bertrand, O.; Bidet-Caulet, A. Two Sides of the Same Coin: Distinct Sub-Bands in the alpha Rhythm Reflect Facilitation and Suppression Mechanisms during Auditory Anticipatory Attention. eNeuro 2018, 5, ENEURO.0141-0118.2018. [Google Scholar] [CrossRef]
- Weisz, N.; Muller, N.; Jatzev, S.; Bertrand, O. Oscillatory alpha modulations in right auditory regions reflect the validity of acoustic cues in an auditory spatial attention task. Cereb. Cortex 2014, 24, 2579–2590. [Google Scholar] [CrossRef]
- Muller, N.; Weisz, N. Lateralized auditory cortical alpha band activity and interregional connectivity pattern reflect anticipation of target sounds. Cereb. Cortex 2012, 22, 1604–1613. [Google Scholar] [CrossRef]
- Pockett, S.; Purdy, S.C.; Brennan, B.J.; Holmes, M.D. Auditory click stimuli evoke event-related potentials in the visual cortex. Neuroreport 2013, 24, 837–840. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.; Hu, L.; Tu, Y.; Chen, R.; Hung, Y.S.; Zhang, Z. N1 Magnitude of Auditory Evoked Potentials and Spontaneous Functional Connectivity Between Bilateral Heschl’s Gyrus Are Coupled at Interindividual Level. Brain Connect. 2016, 6, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Briley, P.M.; Krumbholz, K. The specificity of stimulus-specific adaptation in human auditory cortex increases with repeated exposure to the adapting stimulus. J. Neurophysiol. 2013, 110, 2679–2688. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, B.; Henry, M.J.; Obleser, J. Frequency-specific adaptation in human auditory cortex depends on the spectral variance in the acoustic stimulation. J. Neurophysiol. 2013, 109, 2086–2096. [Google Scholar] [CrossRef] [PubMed]
- Paris, T.; Kim, J.; Davis, C. The processing of attended and predicted sounds in time. J. Cogn. Neurosci. 2016, 28, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Foldal, M.D.; Blenkmann, A.O.; Llorens, A.; Knight, R.T.; Solbakk, A.K.; Endestad, T. The brain tracks auditory rhythm predictability independent of selective attention. Sci. Rep. 2020, 10, 7975. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.G.; Habel, U.; Schmidt, S. Role of the supplementary motor area in auditory sensory attenuation. Brain Struct. Funct. 2019, 224, 2577–2586. [Google Scholar] [CrossRef] [PubMed]
- Klaffehn, A.L.; Baess, P.; Kunde, W.; Pfister, R. Sensory attenuation prevails when controlling for temporal predictability of self- and externally generated tones. Neuropsychologia 2019, 132, 107145. [Google Scholar] [CrossRef]
- Auksztulewicz, R.; Friston, K. Attentional Enhancement of Auditory Mismatch Responses: A DCM/MEG Study. Cereb. Cortex 2015, 25, 4273–4283. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Lee, J.K.; Ko, D.; Lee, G.T.; Jung, K.Y.; Kim, K.H. Fronto-temporal interactions in the theta-band during auditory deviant processing. Neurosci. Lett. 2013, 548, 120–125. [Google Scholar] [CrossRef]
- Phillips, H.N.; Blenkmann, A.; Hughes, L.E.; Bekinschtein, T.A.; Rowe, J.B. Hierarchical Organization of Frontotemporal Networks for the Prediction of Stimuli across Multiple Dimensions. J. Neurosci. 2015, 35, 9255–9264. [Google Scholar] [CrossRef] [PubMed]
- Trainor, L.J.; Lee, K.; Bosnyak, D.J. Cortical plasticity in 4-month-old infants: Specific effects of experience with musical timbres. Brain Topogr. 2011, 24, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Nicol, R.M.; Chapman, S.C.; Vertes, P.E.; Nathan, P.J.; Smith, M.L.; Shtyrov, Y.; Bullmore, E.T. Fast reconfiguration of high-frequency brain networks in response to surprising changes in auditory input. J. Neurophysiol. 2012, 107, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.; Auksztulewicz, R.; Provost, A.; Paton, B.; Howard, Z.; Todd, J. Hierarchical Learning of Statistical Regularities over Multiple Timescales of Sound Sequence Processing: A Dynamic Causal Modeling Study. J. Cogn. Neurosci. 2021, 33, 1549–1562. [Google Scholar] [CrossRef] [PubMed]
- Recasens, M.; Gross, J.; Uhlhaas, P.J. Low-Frequency Oscillatory Correlates of Auditory Predictive Processing in Cortical-Subcortical Networks: A MEG-Study. Sci. Rep. 2018, 8, 14007. [Google Scholar] [CrossRef]
- Oestreich, L.K.L.; Randeniya, R.; Garrido, M.I. Auditory white matter pathways are associated with effective connectivity of auditory prediction errors within a fronto-temporal network. Neuroimage 2019, 195, 454–462. [Google Scholar] [CrossRef]
- Lecaignard, F.; Bertrand, O.; Caclin, A.; Mattout, J. Neurocomputational Underpinnings of Expected Surprise. J. Neurosci. 2022, 42, 474–486. [Google Scholar] [CrossRef]
- Houshmand Chatroudi, A.; Rostami, R.; Nasrabadi, A.M.; Yotsumoto, Y. Effect of inhibition indexed by auditory P300 on transmission of visual sensory information. PLoS ONE 2021, 16, e0247416. [Google Scholar] [CrossRef]
- Blundon, E.G.; Ward, L.M. Search asymmetry in a serial auditory task: Neural source analyses of EEG implicate attention strategies. Neuropsychologia 2019, 134, 107204. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Cha, K.S.; Choi, J.D.; Jung, K.Y.; Kim, K.H. Difficulty-related changes in inter-regional neural synchrony are dissociated between target and non-target processing. Brain Res. 2015, 1603, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Farahani, E.D.; Wouters, J.; van Wieringen, A. Brain mapping of auditory steady-state responses: A broad view of cortical and subcortical sources. Hum. Brain Mapp. 2021, 42, 780–796. [Google Scholar] [CrossRef] [PubMed]
- Schuler, A.L.; Ferrazzi, G.; Colenbier, N.; Arcara, G.; Piccione, F.; Ferreri, F.; Marinazzo, D.; Pellegrino, G. Auditory driven gamma synchrony is associated with cortical thickness in widespread cortical areas. Neuroimage 2022, 255, 119175. [Google Scholar] [CrossRef]
- Beauchene, C.; Abaid, N.; Moran, R.; Diana, R.A.; Leonessa, A. The effect of binaural beats on verbal working memory and cortical connectivity. J. Neural Eng. 2017, 14, 026014. [Google Scholar] [CrossRef]
- Orozco Perez, H.D.; Dumas, G.; Lehmann, A. Binaural Beats through the Auditory Pathway: From Brainstem to Connectivity Patterns. eNeuro 2020, 7, ENEURO.0232-19.2020. [Google Scholar] [CrossRef]
- Solcà, M.; Mottaz, A.; Guggisberg, A.G. Binaural beats increase interhemispheric alpha-band coherence between auditory cortices. Hear. Res. 2016, 332, 233–237. [Google Scholar] [CrossRef]
- Ioannou, C.I.; Pereda, E.; Lindsen, J.P.; Bhattacharya, J. Electrical Brain Responses to an Auditory Illusion and the Impact of Musical Expertise. PLoS ONE 2015, 10, e0129486. [Google Scholar] [CrossRef]
- Engelbregt, H.; Barmentlo, M.; Keeser, D.; Pogarell, O.; Deijen, J.B. Effects of binaural and monaural beat stimulation on attention and EEG. Exp. Brain Res. 2021, 239, 2781–2791. [Google Scholar] [CrossRef]
- Garcia-Argibay, M.; Santed, M.A.; Reales, J.M. Binaural auditory beats affect long-term memory. Psychol. Res. 2019, 83, 1124–1136. [Google Scholar] [CrossRef]
- Schwartze, M.; Rothermich, K.; Schmidt-Kassow, M.; Kotz, S.A. Temporal regularity effects on pre-attentive and attentive processing of deviance. Biol. Psychol. 2011, 87, 146–151. [Google Scholar] [CrossRef]
- Wilsch, A.; Henry, M.J.; Herrmann, B.; Herrmann, C.S.; Obleser, J. Temporal Expectation Modulates the Cortical Dynamics of Short-Term Memory. J. Neurosci. 2018, 38, 7428–7439. [Google Scholar] [CrossRef]
- Pereira, D.R.; Cardoso, S.; Ferreira-Santos, F.; Fernandes, C.; Cunha-Reis, C.; Paiva, T.O.; Almeida, P.R.; Silveira, C.; Barbosa, F.; Marques-Teixeira, J. Effects of inter-stimulus interval (ISI) duration on the N1 and P2 components of the auditory event-related potential. Int. J. Psychophysiol. 2014, 94, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Bruneau, N.; Bidet-Caulet, A.; Roux, S.; Bonnet-Brilhault, F.; Gomot, M. Asymmetry of temporal auditory T-complex: Right ear-left hemisphere advantage in Tb timing in children. Int. J. Psychophysiol. 2015, 95, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Cebulla, M.; Stürzebecher, E.; Don, M.; Müller-Mazzotta, J. Auditory brainstem response recording to multiple interleaved broadband chirps. Ear Hear. 2012, 33, 466–479. [Google Scholar] [CrossRef] [PubMed]
- Ungan, P.; Karsilar, H.; Yagcioglu, S. Pre-attentive Mismatch Response and Involuntary Attention Switching to a Deviance in an Earlier-Than-Usual Auditory Stimulus: An ERP Study. Front. Hum. Neurosci. 2019, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Mamashli, F.; Huang, S.; Khan, S.; Hamalainen, M.S.; Ahlfors, S.P.; Ahveninen, J. Distinct Regional Oscillatory Connectivity Patterns During Auditory Target and Novelty Processing. Brain Topogr. 2020, 33, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Auksztulewicz, R.; Myers, N.E.; Schnupp, J.W.; Nobre, A.C. Rhythmic Temporal Expectation Boosts Neural Activity by Increasing Neural Gain. J. Neurosci. 2019, 39, 9806–9817. [Google Scholar] [CrossRef]
- Jantzen, K.J.; Ratcliff, B.R.; Jantzen, M.G. Cortical Networks for Correcting Errors in Sensorimotor Synchronization Depend on the Direction of Asynchrony. J. Mot. Behav. 2018, 50, 235–248. [Google Scholar] [CrossRef]
- Mohammad Alipour, Z.; Mohammadkhani, S.; Khosrowabadi, R. Alteration of perceived emotion and brain functional connectivity by changing the musical rhythmic pattern. Exp. Brain Res. 2019, 237, 2607–2619. [Google Scholar] [CrossRef]
- Tierney, A.; Kraus, N. Neural entrainment to the rhythmic structure of music. J. Cogn. Neurosci. 2015, 27, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Todorovic, A.; Auksztulewicz, R. Dissociable neural effects of temporal expectations due to passage of time and contextual probability. Hear. Res. 2021, 399, 107871. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Seol, J.; Jin, S.H.; Kim, J.S.; Kim, Y.; Yi, S.W.; Chung, C.K. Increased fronto-temporal connectivity by modified melody in real music. PLoS ONE 2020, 15, e0235770. [Google Scholar] [CrossRef] [PubMed]
- Sarrou, M.; Schmitz, P.M.; Hamm, N.; Rubsamen, R. Sound frequency affects the auditory motion-onset response in humans. Exp. Brain Res. 2018, 236, 2713–2726. [Google Scholar] [CrossRef] [PubMed]
- Lanzilotti, C.; Dumas, R.; Grassi, M.; Schon, D. Prolonged exposure to highly rhythmic music affects brain dynamics and perception. Neuropsychologia 2019, 129, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Ott, C.G.; Stier, C.; Herrmann, C.S.; Jancke, L. Musical expertise affects attention as reflected by auditory-evoked gamma-band activity in human EEG. Neuroreport 2013, 24, 445–450. [Google Scholar] [CrossRef]
- Luo, H.; Tian, X.; Song, K.; Zhou, K.; Poeppel, D. Neural response phase tracks how listeners learn new acoustic representations. Curr. Biol. 2013, 23, 968–974. [Google Scholar] [CrossRef][Green Version]
- Teki, S.; Barascud, N.; Picard, S.; Payne, C.; Griffiths, T.D.; Chait, M. Neural Correlates of Auditory Figure-Ground Segregation Based on Temporal Coherence. Cereb. Cortex 2016, 26, 3669–3680. [Google Scholar] [CrossRef]
- Farkas, D.; Denham, S.L.; Winkler, I. Functional brain networks underlying idiosyncratic switching patterns in multi-stable auditory perception. Neuropsychologia 2018, 108, 82–91. [Google Scholar] [CrossRef]
- Ahveninen, J.; Hamalainen, M.; Jaaskelainen, I.P.; Ahlfors, S.P.; Huang, S.; Lin, F.H.; Raij, T.; Sams, M.; Vasios, C.E.; Belliveau, J.W. Attention-driven auditory cortex short-term plasticity helps segregate relevant sounds from noise. Proc. Natl. Acad. Sci. USA 2011, 108, 4182–4187. [Google Scholar] [CrossRef]
- Jäncke, L.; Leipold, S.; Burkhard, A. The neural underpinnings of music listening under different attention conditions. Neuroreport 2018, 29, 594–604. [Google Scholar] [CrossRef]
- Giani, A.S.; Belardinelli, P.; Ortiz, E.; Kleiner, M.; Noppeney, U. Detecting tones in complex auditory scenes. Neuroimage 2015, 122, 203–213. [Google Scholar] [CrossRef]
- Polomac, N.; Leicht, G.; Nolte, G.; Andreou, C.; Schneider, T.R.; Steinmann, S.; Engel, A.K.; Mulert, C. Generators and Connectivity of the Early Auditory Evoked Gamma Band Response. Brain Topogr. 2015, 28, 865–878. [Google Scholar] [CrossRef]
- Leicht, G.; Bjorklund, J.; Vauth, S.; Mussmann, M.; Haaf, M.; Steinmann, S.; Rauh, J.; Mulert, C. Gamma-band synchronisation in a frontotemporal auditory information processing network. Neuroimage 2021, 239, 118307. [Google Scholar] [CrossRef]
- Gong, D.; Ma, W.; Kendrick, K.M.; Hu, Q.; Yao, D. How cognitive plasticity resolves the brain’s information processing dilemma. Sci. Rep. 2013, 3, 2860. [Google Scholar] [CrossRef]
- Wolff, M.J.; Kandemir, G.; Stokes, M.G.; Akyurek, E.G. Unimodal and Bimodal Access to Sensory Working Memories by Auditory and Visual Impulses. J. Neurosci. 2020, 40, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Mamashli, F.; Khan, S.; Hamalainen, M.; Jas, M.; Raij, T.; Stufflebeam, S.M.; Nummenmaa, A.; Ahveninen, J. Synchronization patterns reveal neuronal coding of working memory content. Cell Rep. 2021, 36, 109566. [Google Scholar] [CrossRef] [PubMed]
- Coffey, E.B.; Herholz, S.C.; Chepesiuk, A.M.; Baillet, S.; Zatorre, R.J. Cortical contributions to the auditory frequency-following response revealed by MEG. Nat. Commun. 2016, 7, 11070. [Google Scholar] [CrossRef] [PubMed]
- Garrido, M.I.; Rowe, E.G.; Halasz, V.; Mattingley, J.B. Bayesian Mapping Reveals That Attention Boosts Neural Responses to Predicted and Unpredicted Stimuli. Cereb. Cortex 2018, 28, 1771–1782. [Google Scholar] [CrossRef]
- Kluge, C.; Bauer, M.; Leff, A.P.; Heinze, H.J.; Dolan, R.J.; Driver, J. Plasticity of human auditory-evoked fields induced by shock conditioning and contingency reversal. Proc. Natl. Acad. Sci. USA 2011, 108, 12545–12550. [Google Scholar] [CrossRef]
- Daly, I.; Malik, A.; Hwang, F.; Roesch, E.; Weaver, J.; Kirke, A.; Williams, D.; Miranda, E.; Nasuto, S.J. Neural correlates of emotional responses to music: An EEG study. Neurosci. Lett. 2014, 573, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Hadjidimitriou, S.K.; Hadjileontiadis, L.J. Toward an EEG-based recognition of music liking using time-frequency analysis. IEEE Trans. Biomed. Eng. 2012, 59, 3498–3510. [Google Scholar] [CrossRef] [PubMed]
- Ross, B.; Barat, M.; Fujioka, T. Sound-Making Actions Lead to Immediate Plastic Changes of Neuromagnetic Evoked Responses and Induced beta-Band Oscillations during Perception. J. Neurosci. 2017, 37, 5948–5959. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, J.; Ding, X.; Li, R.; Zhou, C. The effects of music on brain functional networks: A network analysis. Neuroscience 2013, 250, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, J.; Liu, C.; Liu, D.; Ding, X.; Zhou, C. Graph theoretical analysis of EEG functional connectivity during music perception. Brain Res. 2012, 1483, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Rogenmoser, L.; Zollinger, N.; Elmer, S.; Jancke, L. Independent component processes underlying emotions during natural music listening. Soc. Cogn. Affect. Neurosci. 2016, 11, 1428–1439. [Google Scholar] [CrossRef] [PubMed]
- Tervaniemi, M.; Pousi, S.; Seppala, M.; Makkonen, T. Brain oscillation recordings of the audience in a live concert-like setting. Cogn. Process 2022, 23, 329–337. [Google Scholar] [CrossRef]
- Ara, A.; Marco-Pallares, J. Different theta connectivity patterns underlie pleasantness evoked by familiar and unfamiliar music. Sci. Rep. 2021, 11, 18523. [Google Scholar] [CrossRef]
- Ara, A.; Marco-Pallares, J. Fronto-temporal theta phase-synchronization underlies music-evoked pleasantness. Neuroimage 2020, 212, 116665. [Google Scholar] [CrossRef]
- Huang, R.; Wang, J.; Wu, D.; Long, H.; Yang, X.; Liu, H.; Gao, X.; Zhao, R.; Lai, W. The effects of customised brainwave music on orofacial pain induced by orthodontic tooth movement. Oral. Dis. 2016, 22, 766–774. [Google Scholar] [CrossRef]
- Sarasso, P.; Perna, P.; Barbieri, P.; Neppi-Modona, M.; Sacco, K.; Ronga, I. Memorisation and implicit perceptual learning are enhanced for preferred musical intervals and chords. Psychon. Bull. Rev. 2021, 28, 1623–1637. [Google Scholar] [CrossRef] [PubMed]
- Sarasso, P.; Barbieri, P.; Del Fante, E.; Bechis, L.; Neppi-Modona, M.; Sacco, K.; Ronga, I. Preferred music listening is associated with perceptual learning enhancement at the expense of self-focused attention. Psychon. Bull. Rev. 2022, 29, 2108–2121. [Google Scholar] [CrossRef] [PubMed]
- James, C.E.; Cereghetti, D.M.; Roullet Tribes, E.; Oechslin, M.S. Electrophysiological evidence for a specific neural correlate of musical violation expectation in primary-school children. Neuroimage 2015, 104, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Benasich, A.A.; Choudhury, N.A.; Realpe-Bonilla, T.; Roesler, C.P. Plasticity in developing brain: Active auditory exposure impacts prelinguistic acoustic mapping. J. Neurosci. 2014, 34, 13349–13363. [Google Scholar] [CrossRef] [PubMed]
- Carcagno, S.; Plack, C.J. Subcortical plasticity following perceptual learning in a pitch discrimination task. J. Assoc. Res. Otolaryngol. 2011, 12, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Yamsa-Ard, T.; Wongsawat, Y. The observation of theta wave modulation on brain training by 5 Hz-binaural beat stimulation in seven days. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 6667–6670. [Google Scholar]
- Manuel, A.L.; Bernasconi, F.; Spierer, L. Plastic modifications within inhibitory control networks induced by practicing a stop-signal task: An electrical neuroimaging study. Cortex 2013, 49, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Lau, B.K.; Ruggles, D.R.; Katyal, S.; Engel, S.A.; Oxenham, A.J. Sustained Cortical and Subcortical Measures of Auditory and Visual Plasticity following Short-Term Perceptual Learning. PLoS ONE 2017, 12, e0168858. [Google Scholar] [CrossRef]
- De Meo, R.; Bourquin, N.M.; Knebel, J.F.; Murray, M.M.; Clarke, S. From bird to sparrow: Learning-induced modulations in fine-grained semantic discrimination. Neuroimage 2015, 118, 163–173. [Google Scholar] [CrossRef]
- Gupta, A.; Bhushan, B.; Behera, L. Short-term enhancement of cognitive functions and music: A three-channel model. Sci. Rep. 2018, 8, 15528. [Google Scholar] [CrossRef]
- Kompus, K.; Westerhausen, R. Increased MMN amplitude following passive perceptual learning with LTP-like rapid stimulation. Neurosci. Lett. 2018, 666, 28–31. [Google Scholar] [CrossRef]
- Lei, G.; Zhao, Z.; Li, Y.; Yu, L.; Zhang, X.; Yan, Y.; Ma, X.; Wang, Q.; Wang, K.; Zhang, D.; et al. A method to induce human cortical long-term potentiation by acoustic stimulation. Acta Otolaryngol. 2017, 137, 1069–1076. [Google Scholar] [CrossRef]
- Woodward, S.H.; Brown, W.S.; Marsh, J.T.; Dawson, M.E. Probing the time-course of the auditory oddball P3 with secondary reaction time. Psychophysiology 1991, 28, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Nash, A.J.; Fernandez, M. P300 and allocation of attention in dual-tasks. Int. J. Psychophysiol. 1996, 23, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Polich, J. Task difficulty, probability, and inter-stimulus interval as determinants of P300 from auditory stimuli. Electroencephalogr. Clin. Neurophysiol. Evoked Potentials Sect. 1987, 68, 311–320. [Google Scholar] [CrossRef] [PubMed]
- van Dinteren, R.; Arns, M.; Jongsma, M.L.; Kessels, R.P. P300 development across the lifespan: A systematic review and meta-analysis. PLoS ONE 2014, 9, e87347. [Google Scholar] [CrossRef] [PubMed]
- Polich, J. Updating P300: An integrative theory of P3a and P3b. Clin. Neurophysiol. 2007, 118, 2128–2148. [Google Scholar] [CrossRef] [PubMed]
- Slugocki, C.; Bosnyak, D.; Trainor, L.J. Simultaneously-evoked auditory potentials (SEAP): A new method for concurrent measurement of cortical and subcortical auditory-evoked activity. Hear. Res. 2017, 345, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Blundon, E.G.; Rumak, S.P.; Ward, L.M. Sequential search asymmetry: Behavioral and psychophysiological evidence from a dual oddball task. PLoS ONE 2017, 12, e0173237. [Google Scholar] [CrossRef]
- Choi, J.W.; Jung, K.Y.; Kim, C.H.; Kim, K.H. Changes in gamma- and theta-band phase synchronization patterns due to the difficulty of auditory oddball task. Neurosci. Lett. 2010, 468, 156–160. [Google Scholar] [CrossRef]
- Tada, M.; Kirihara, K.; Ishishita, Y.; Takasago, M.; Kunii, N.; Uka, T.; Shimada, S.; Ibayashi, K.; Kawai, K.; Saito, N.; et al. Global and Parallel Cortical Processing Based on Auditory Gamma Oscillatory Responses in Humans. Cereb. Cortex 2021, 31, 4518–4532. [Google Scholar] [CrossRef]
- Herrmann, C.S.; Fründ, I.; Lenz, D. Human gamma-band activity: A review on cognitive and behavioral correlates and network models. Neurosci. Biobehav. Rev. 2010, 34, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.L.; Cobb, P.R.; Balasubramaniam, R. Recruitment of the motor system during music listening: An ALE meta-analysis of fMRI data. PLoS ONE 2018, 13, e0207213. [Google Scholar] [CrossRef] [PubMed]
- Oster, G. Auditory beats in the brain. Sci. Am. 1973, 229, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Bastos, A.M.; Vezoli, J.; Bosman, C.A.; Schoffelen, J.-M.; Oostenveld, R.; Dowdall, J.R.; De Weerd, P.; Kennedy, H.; Fries, P. Visual areas exert feedforward and feedback influences through distinct frequency channels. Neuron 2015, 85, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Grahn, J.A.; Brett, M. Rhythm and beat perception in motor areas of the brain. J. Cogn. Neurosci. 2007, 19, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Jäncke, L.; Shah, N.; Posse, S.; Grosse-Ryuken, M.; Müller-Gärtner, H.-W. Intensity coding of auditory stimuli: An fMRI study. Neuropsychologia 1998, 36, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Sowman, P.F.; Kuusik, A.; Johnson, B.W. Self-initiation and temporal cueing of monaural tones reduce the auditory N1 and P2. Exp. Brain Res. 2012, 222, 149–157. [Google Scholar] [CrossRef]
- Recanzone, G.H.; Schreiner, C.E.; Merzenich, M.M. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J. Neurosci. 1993, 13, 87–103. [Google Scholar] [CrossRef]
- Zarate, J.M.; Delhommeau, K.; Wood, S.; Zatorre, R.J. Vocal accuracy and neural plasticity following micromelody-discrimination training. PLoS ONE 2010, 5, e11181. [Google Scholar] [CrossRef]
- Lappe, C.; Herholz, S.C.; Trainor, L.J.; Pantev, C. Cortical plasticity induced by short-term unimodal and multimodal musical training. J. Neurosci. 2008, 28, 9632–9639. [Google Scholar] [CrossRef]
- Schulte, M.; Knief, A.; Seither-Preisler, A.; Pantev, C. Different modes of pitch perception and learning-induced neuronal plasticity of the human auditory cortex. Neural Plast. 2002, 9, 161–175. [Google Scholar] [CrossRef]
- Musacchia, G.; Ortiz-Mantilla, S.; Choudhury, N.; Realpe-Bonilla, T.; Roesler, C.; Benasich, A.A. Active auditory experience in infancy promotes brain plasticity in Theta and Gamma oscillations. Dev. Cogn. Neurosci. 2017, 26, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Haegens, S.; Handel, B.F.; Jensen, O. Top-down controlled alpha band activity in somatosensory areas determines behavioral performance in a discrimination task. J. Neurosci. 2011, 31, 5197–5204. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.P.; Lalor, E.C.; Reilly, R.B.; Foxe, J.J. Increases in alpha oscillatory power reflect an active retinotopic mechanism for distracter suppression during sustained visuospatial attention. J. Neurophysiol. 2006, 95, 3844–3851. [Google Scholar] [CrossRef] [PubMed]
- Weisz, N.; Obleser, J. Synchronisation signatures in the listening brain: A perspective from non-invasive neuroelectrophysiology. Hear. Res. 2014, 307, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Samaha, J.; Iemi, L.; Postle, B.R. Prestimulus alpha-band power biases visual discrimination confidence, but not accuracy. Conscious. Cogn. 2017, 54, 47–55. [Google Scholar] [CrossRef]
- Samaha, J.; Iemi, L.; Haegens, S.; Busch, N.A. Spontaneous Brain Oscillations and Perceptual Decision-Making. Trends Cogn. Sci. 2020, 24, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Iemi, L.; Chaumon, M.; Crouzet, S.M.; Busch, N.A. Spontaneous Neural Oscillations Bias Perception by Modulating Baseline Excitability. J. Neurosci. 2017, 37, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Kloosterman, N.A.; de Gee, J.W.; Werkle-Bergner, M.; Lindenberger, U.; Garrett, D.D.; Fahrenfort, J.J. Humans strategically shift decision bias by flexibly adjusting sensory evidence accumulation. eLife 2019, 8, e37321. [Google Scholar] [CrossRef]
- Thomaschke, R.; Kiesel, A.; Hoffmann, J. Response specific temporal expectancy: Evidence from a variable foreperiod paradigm. Atten. Percept. Psychophys. 2011, 73, 2309–2322. [Google Scholar] [CrossRef]
- Capotosto, P.; Corbetta, M.; Romani, G.L.; Babiloni, C. Electrophysiological correlates of stimulus-driven reorienting deficits after interference with right parietal cortex during a spatial attention task: A TMS-EEG study. J. Cogn. Neurosci. 2012, 24, 2363–2371. [Google Scholar] [CrossRef]
- Wostmann, M.; Maess, B.; Obleser, J. Orienting auditory attention in time: Lateralized alpha power reflects spatio-temporal filtering. Neuroimage 2021, 228, 117711. [Google Scholar] [CrossRef] [PubMed]
- Worden, M.S.; Foxe, J.J.; Wang, N.; Simpson, G.V. Anticipatory biasing of visuospatial attention indexed by retinotopically specific alpha-band electroencephalography increases over occipital cortex. J. Neurosci. Off. J. Soc. Neurosci. 2000, 20, RC63. [Google Scholar] [CrossRef] [PubMed]
- Ahveninen, J.; Huang, S.; Belliveau, J.W.; Chang, W.T.; Hamalainen, M. Dynamic oscillatory processes governing cued orienting and allocation of auditory attention. J. Cogn. Neurosci. 2013, 25, 1926–1943. [Google Scholar] [CrossRef] [PubMed]
- Wostmann, M.; Herrmann, B.; Maess, B.; Obleser, J. Spatiotemporal dynamics of auditory attention synchronize with speech. Proc. Natl. Acad. Sci. USA 2016, 113, 3873–3878. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D.; Herbst, S.K.; Klatt, L.I.; Wostmann, M. Target enhancement or distractor suppression? Functionally distinct alpha oscillations form the basis of attention. Eur. J. Neurosci. 2022, 55, 3256–3265. [Google Scholar] [CrossRef] [PubMed]
- Lange, K. The reduced N1 to self-generated tones: An effect of temporal predictability? Psychophysiology 2011, 48, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Lange, K. Brain correlates of early auditory processing are attenuated by expectations for time and pitch. Brain Cogn. 2009, 69, 127–137. [Google Scholar] [CrossRef]
- Lange, K. The ups and downs of temporal orienting: A review of auditory temporal orienting studies and a model associating the heterogeneous findings on the auditory N1 with opposite effects of attention and prediction. Front. Hum. Neurosci. 2013, 7, 263. [Google Scholar] [CrossRef]
- Friston, K.; Kiebel, S. Predictive coding under the free-energy principle. Philos. Trans. R. Soc. B: Biol. Sci. 2009, 364, 1211–1221. [Google Scholar] [CrossRef]
- Friston, K. The free-energy principle: A unified brain theory? Nat. Rev. Neurosci. 2010, 11, 127–138. [Google Scholar] [CrossRef]
- Feldman, H.; Friston, K.J. Attention, uncertainty, and free-energy. Front. Hum. Neurosci. 2010, 4, 215. [Google Scholar] [CrossRef] [PubMed]
- Baldeweg, T. ERP repetition effects and mismatch negativity generation: A predictive coding perspective. J. Psychophysiol. 2007, 21, 204–213. [Google Scholar] [CrossRef]
- Knolle, F.; Schwartze, M.; Schroger, E.; Kotz, S.A. Auditory Predictions and Prediction Errors in Response to Self-Initiated Vowels. Front. Neurosci. 2019, 13, 1146. [Google Scholar] [CrossRef] [PubMed]
- Hillyard, S.A.; Hink, R.F.; Schwent, V.L.; Picton, T.W. Electrical signs of selective attention in the human brain. Science 1973, 182, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Horváth, J. Action-related auditory ERP attenuation: Paradigms and hypotheses. Brain Res. 2015, 1626, 54–65. [Google Scholar] [CrossRef]
- Kok, P.; Rahnev, D.; Jehee, J.F.; Lau, H.C.; de Lange, F.P. Attention reverses the effect of prediction in silencing sensory signals. Cereb. Cortex 2012, 22, 2197–2206. [Google Scholar] [CrossRef] [PubMed]
- Bäß, P.; Jacobsen, T.; Schroger, E. Suppression of the auditory N1 event-related potential component with unpredictable self-initiated tones: Evidence for internal forward models with dynamic stimulation. Int. J. Psychophysiol. 2008, 70, 137–143. [Google Scholar] [CrossRef]
- Sato, A. Both motor prediction and conceptual congruency between preview and action-effect contribute to explicit judgment of agency. Cognition 2009, 110, 74–83. [Google Scholar] [CrossRef]
- Weiss, C.; Herwig, A.; Schütz-Bosbach, S. The self in action effects: Selective attenuation of self-generated sounds. Cognition 2011, 121, 207–218. [Google Scholar] [CrossRef]
- Miall, R.C.; Wolpert, D.M. Forward models for physiological motor control. Neural Netw. 1996, 9, 1265–1279. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-M.A.; Mathalon, D.H.; Roach, B.J.; Cavus, I.; Spencer, D.D.; Ford, J.M. The corollary discharge in humans is related to synchronous neural oscillations. J. Cogn. Neurosci. 2011, 23, 2892–2904. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, J.; Schutz-Bosbach, S. Sensory attenuation of self-produced signals does not rely on self-specific motor predictions. Eur. J. Neurosci. 2018, 47, 1303–1310. [Google Scholar] [CrossRef]
- Näätänen, R. Attention and Brain Function; Lawrence Erlbaum Associates, Inc.: Hillsdale, NJ, USA, 1992. [Google Scholar]
- Garrido, M.I.; Kilner, J.M.; Stephan, K.E.; Friston, K.J. The mismatch negativity: A review of underlying mechanisms. Clin. Neurophysiol. 2009, 120, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Lui, T.K.; Shum, Y.H.; Xiao, X.Z.; Wang, Y.; Cheung, A.T.; Chan, S.S.; Neggers, S.F.W.; Tse, C.Y. The critical role of the inferior frontal cortex in establishing a prediction model for generating subsequent mismatch negativity (MMN): A TMS-EEG study. Brain Stimul. 2021, 14, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Auksztulewicz, R.; Barascud, N.; Cooray, G.; Nobre, A.C.; Chait, M.; Friston, K. The Cumulative Effects of Predictability on Synaptic Gain in the Auditory Processing Stream. J. Neurosci. 2017, 37, 6751–6760. [Google Scholar] [CrossRef]
- Desimone, R. Neural mechanisms for visual memory and their role in attention. Proc. Natl. Acad. Sci. USA 1996, 93, 13494–13499. [Google Scholar] [CrossRef]
- Parras, G.G.; Nieto-Diego, J.; Carbajal, G.V.; Valdes-Baizabal, C.; Escera, C.; Malmierca, M.S. Neurons along the auditory pathway exhibit a hierarchical organization of prediction error. Nat. Commun. 2017, 8, 2148. [Google Scholar] [CrossRef]
- Summerfield, C.; Egner, T. Expectation (and attention) in visual cognition. Trends Cogn. Sci. 2009, 13, 403–409. [Google Scholar] [CrossRef]
- Friston, K. A theory of cortical responses. Philos. Trans. R. Soc. B: Biol. Sci. 2005, 360, 815–836. [Google Scholar] [CrossRef]
- Auksztulewicz, R.; Friston, K. Repetition suppression and its contextual determinants in predictive coding. Cortex 2016, 80, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Skosnik, P.D.; Krishnan, G.P.; O’Donnell, B.F. The effect of selective attention on the gamma-band auditory steady-state response. Neurosci. Lett. 2007, 420, 223–228. [Google Scholar] [CrossRef]
- Galambos, R.; Makeig, S.; Talmachoff, P.J. A 40-Hz auditory potential recorded from the human scalp. Proc. Natl. Acad. Sci. USA 1981, 78, 2643–2647. [Google Scholar] [CrossRef] [PubMed]
- Parciauskaite, V.; Bjekic, J.; Griskova-Bulanova, I. Gamma-Range Auditory Steady-State Responses and Cognitive Performance: A Systematic Review. Brain Sci. 2021, 11, 217. [Google Scholar] [CrossRef] [PubMed]
- Pratt, H.; Starr, A.; Michalewski, H.J.; Dimitrijevic, A.; Bleich, N.; Mittelman, N. A comparison of auditory evoked potentials to acoustic beats and to binaural beats. Hear. Res. 2010, 262, 34–44. [Google Scholar] [CrossRef]
- Kasprzak, C. Influence of binaural beats on EEG signal. Acta Phys. Pol. A 2011, 119, 986–990. [Google Scholar] [CrossRef]
- Garcia-Argibay, M.; Santed, M.A.; Reales, J.M. Efficacy of binaural auditory beats in cognition, anxiety, and pain perception: A meta-analysis. Psychol. Res. 2019, 83, 357–372. [Google Scholar] [CrossRef]
- Jones, M.R.; Boltz, M. Dynamic attending and responses to time. Psychol. Rev. 1989, 96, 459. [Google Scholar] [CrossRef]
- Jones, M.R. Time, our lost dimension: Toward a new theory of perception, attention, and memory. Psychol. Rev. 1976, 83, 323. [Google Scholar] [CrossRef]
- Large, E.W.; Jones, M.R. The dynamics of attending: How people track time-varying events. Psychol. Rev. 1999, 106, 119. [Google Scholar] [CrossRef]
- Vuust, P.; Witek, M.A. Rhythmic complexity and predictive coding: A novel approach to modeling rhythm and meter perception in music. Front. Psychol. 2014, 5, 1111. [Google Scholar] [CrossRef] [PubMed]
- Lakatos, P.; Musacchia, G.; O’Connel, M.N.; Falchier, A.Y.; Javitt, D.C.; Schroeder, C.E. The spectrotemporal filter mechanism of auditory selective attention. Neuron 2013, 77, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Spaak, E.; de Lange, F.P.; Jensen, O. Local entrainment of alpha oscillations by visual stimuli causes cyclic modulation of perception. J. Neurosci. 2014, 34, 3536–3544. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.; Kilner, J.; Harrison, L. A free energy principle for the brain. J. Physiol. 2006, 100, 70–87. [Google Scholar] [CrossRef] [PubMed]
- Proksch, S.; Comstock, D.C.; Mede, B.; Pabst, A.; Balasubramaniam, R. Motor and Predictive Processes in Auditory Beat and Rhythm Perception. Front. Hum. Neurosci. 2020, 14, 578546. [Google Scholar] [CrossRef] [PubMed]
- Kotz, S.A.; Ravignani, A.; Fitch, W.T. The Evolution of Rhythm Processing. Trends Cogn. Sci. 2018, 22, 896–910. [Google Scholar] [CrossRef] [PubMed]
- Bavassi, L.; Kamienkowski, J.E.; Sigman, M.; Laje, R. Sensorimotor synchronization: Neurophysiological markers of the asynchrony in a finger-tapping task. Psychol. Res. 2017, 81, 143–156. [Google Scholar] [CrossRef]
- Bijsterbosch, J.D.; Lee, K.-H.; Hunter, M.D.; Tsoi, D.T.; Lankappa, S.; Wilkinson, I.D.; Barker, A.T.; Woodruff, P.W. The role of the cerebellum in sub-and supraliminal error correction during sensorimotor synchronization: Evidence from fMRI and TMS. J. Cogn. Neurosci. 2011, 23, 1100–1112. [Google Scholar] [CrossRef]
- Repp, B.H.; Su, Y.-H. Sensorimotor synchronization: A review of recent research (2006–2012). Psychon. Bull. Rev. 2013, 20, 403–452. [Google Scholar] [CrossRef]
- Repp, B.H. Sensorimotor synchronization: A review of the tapping literature. Psychon. Bull. Rev. 2005, 12, 969–992. [Google Scholar] [CrossRef]
- Miyata, K.; Yamamoto, T.; Fukunaga, M.; Sugawara, S.; Sadato, N. Neural correlates with individual differences in temporal prediction during auditory-motor synchronization. Cereb. Cortex Commun. 2022, 3, tgac014. [Google Scholar] [CrossRef] [PubMed]
- Bavassi, M.L.; Tagliazucchi, E.; Laje, R. Small perturbations in a finger-tapping task reveal inherent nonlinearities of the underlying error correction mechanism. Hum. Mov. Sci. 2013, 32, 21–47. [Google Scholar] [CrossRef] [PubMed]
- VanRullen, R.; Busch, N.A.; Drewes, J.; Dubois, J. Ongoing EEG phase as a trial-by-trial predictor of perceptual and attentional variability. Front. Psychol. 2011, 2, 60. [Google Scholar] [CrossRef] [PubMed]
- VanRullen, R. How to Evaluate Phase Differences between Trial Groups in Ongoing Electrophysiological Signals. Front. Neurosci. 2016, 10, 426. [Google Scholar] [CrossRef]
- Mathias, B.; Zamm, A.; Gianferrara, P.G.; Ross, B.; Palmer, C. Rhythm Complexity Modulates Behavioral and Neural Dynamics During Auditory-Motor Synchronization. J. Cogn. Neurosci. 2020, 32, 1864–1880. [Google Scholar] [CrossRef] [PubMed]
- Itti, L.; Koch, C. Computational modelling of visual attention. Nat. Rev. Neurosci. 2001, 2, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, H.R.; Nyberg, L. Common fronto-parietal activity in attention, memory, and consciousness: Shared demands on integration? Conscious. Cogn. 2005, 14, 390–425. [Google Scholar] [CrossRef]
- Katsuki, F.; Constantinidis, C. Bottom-up and top-down attention: Different processes and overlapping neural systems. Neurosci. 2014, 20, 509–521. [Google Scholar] [CrossRef]
- Nani, A.; Manuello, J.; Mancuso, L.; Liloia, D.; Costa, T.; Cauda, F. The Neural Correlates of Consciousness and Attention: Two Sister Processes of the Brain. Front. Neurosci. 2019, 13, 1169. [Google Scholar] [CrossRef] [PubMed]
- Friederici, A.D. Towards a neural basis of auditory sentence processing. Trends Cogn. Sci. 2002, 6, 78–84. [Google Scholar] [CrossRef]
- Werker, J.F.; Tees, R.C. Speech perception as a window for understanding plasticity and commitment in language systems of the brain. Dev. Psychobiol. J. Int. Soc. Dev. Psychobiol. 2005, 46, 233–251. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, P.K. Brain mechanisms in early language acquisition. Neuron 2010, 67, 713–727. [Google Scholar] [CrossRef]
- Reed, A.; Riley, J.; Carraway, R.; Carrasco, A.; Perez, C.; Jakkamsetti, V.; Kilgard, M.P. Cortical map plasticity improves learning but is not necessary for improved performance. Neuron 2011, 70, 121–131. [Google Scholar] [CrossRef]
- Ranasinghe, K.G.; Carraway, R.S.; Borland, M.S.; Moreno, N.A.; Hanacik, E.A.; Miller, R.S.; Kilgard, M.P. Speech discrimination after early exposure to pulsed-noise or speech. Hear. Res. 2012, 289, 1–12. [Google Scholar] [CrossRef]
- Choudhury, N.; Benasich, A.A. Maturation of auditory evoked potentials from 6 to 48 months: Prediction to 3 and 4 year language and cognitive abilities. Clin. Neurophysiol. 2011, 122, 320–338. [Google Scholar] [CrossRef] [PubMed]
- Potes, C.; Brunner, P.; Gunduz, A.; Knight, R.T.; Schalk, G. Spatial and temporal relationships of electrocorticographic alpha and gamma activity during auditory processing. Neuroimage 2014, 97, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Cheung, V.K.M.; Harrison, P.M.C.; Meyer, L.; Pearce, M.T.; Haynes, J.D.; Koelsch, S. Uncertainty and Surprise Jointly Predict Musical Pleasure and Amygdala, Hippocampus, and Auditory Cortex Activity. Curr. Biol. 2019, 29, 4084–4092 e4084. [Google Scholar] [CrossRef]
- de la Cruz, F.; Schumann, A.; Köhler, S.; Reichenbach, J.R.; Wagner, G.; Bär, K.-J. The relationship between heart rate and functional connectivity of brain regions involved in autonomic control. Neuroimage 2019, 196, 318–328. [Google Scholar] [CrossRef]
- Chen, J.E.; Lewis, L.D.; Chang, C.; Tian, Q.; Fultz, N.E.; Ohringer, N.A.; Rosen, B.R.; Polimeni, J.R. Resting-state “physiological networks”. NeuroImage 2020, 213, 116707. [Google Scholar] [CrossRef]

| Reference Number | Content of the Auditory Stimuli | Tasks during the Experiment and Paradigms | Number of Participants | Stimulus More Than a Day | Recording | Major Findings |
|---|---|---|---|---|---|---|
| 3.2.1. Prestimulus effects | ||||||
| 1. Prestimulus alpha power and behavior | ||||||
| [56] | Sequence patterns comprising pure tones | Tone pattern indication task | 17 | - | EEG | Different prestimulus EEG phase between correct and incorrect trials |
| [57] | A short target sound within the background sound | Target sound detection task | 12 | - | EEG | The dependence of the chance of target detection on power and phase of theta-band oscillations before target |
| [58] | White noise bursts presented near hearing threshold with various inter-trial intervals | Near-threshold detection task | 19 | - | MEG | A decrease in alpha power in the auditory cortex prior to conscious percepts |
| [59] | Two identical sine tones | Pitch discrimination and confidence rating | 17 | - | EEG | A negative link between prestimulus alpha power and decision confidence |
| 2. Interstimulus interval | ||||||
| [60] | Presentation of two frequencies, whose temporal order was explicit through a cue or learned implicitly | Temporal order judgment task | 24 | - | EEG | Enhanced functional links in implicit anticipation |
| 3. Preceding cue | ||||||
| [61] | Target sounds with two different frequencies preceded by a visual cue as to the spatial location | Spatial attention task | 14 | - | MEG | An asymmetrical modulation of alpha power within the right AC1, depending on the cued side |
| [62] | A target sound and a distractor sound presented simultaneously on opposite ears, preceded by an auditory cue on either ear | Spatial attention task | 11 | - | MEG | Alpha lateralization in a right-lateralized network in response to cue validity and side-related acoustic stimulation |
| [63] | Standard tones and target tones that changed the modulation frequency, preceded by a visual cue to shift the focus of either ear | Spatial attention task | 15 | - | MEG | A stronger alpha power increase for the attend-right condition in the right AC1 |
| 3.2.2. During exposure to stimuli | ||||||
| 1. Pure tone sequences | ||||||
| ● N1-P2 | ||||||
| [64] | Auditory click stimuli | Listening | 6 | - | EEG | Evoked ERPs over both the auditory and visual cortex by unimodal click stimuli |
| [65] | Identical auditory stimuli consisting of brief pure tones | Listening | 19 | - | EEG and fMRI | Positive correlation with N1 magnitude of spontaneous functional connectivity between bilateral Heschl’s gyruses |
| [66] | Pure tones with varying frequency separation and stimulus onset asynchrony | Oddball-like paradigm | 15 | - | EEG | Decrease in stimulus-specific adaptation with the increase in stimulus onset asynchrony |
| [67] | Random tone sequences varying in spectral variance | Detecting deviants vs. ignoring stimuli | 20 | - | EEG | Largest frequency-specific neural responses on the N1 component |
| [68] | Sounds with onsets that were either predicted by a visual cue or unpredicted | Attending or unattending intervals | 37 | - | EEG | An N1 enhancement effect for attended sounds and an N1 suppression effect for predicted sounds |
| [69] | Regular and irregular rhythmic sequences of tones | Responding to deviants in the attended ear | 34 | - | EEG | Attenuated N1 for tones when rhythm predictability was high and was enhanced by attention to tones |
| [70] | A self-generated or externally generated tone | Indicating onset of the motion or tone | 39 | - | EEG | Suppressed N1–P2 complex when the tone was self-generated compared to externally generated |
| [71] | A single marimba tone | Self-generation of tones vs. listening | 24 | - | EEG | An attenuated N1 component for self-generated tones as compared to externally generated tones |
| ● MMN | ||||||
| [72] | Sine wave tones delivered at six possible carrier frequencies | Mismatch paradigm | 20 | - | MEG | Mismatch responses to frequency deviants being modulated by temporal attention strongly |
| [73] | Randomly ordered sequences of two tones | Oddball paradigm | 13 | - | EEG | Strong theta-band phase synchrony between the frontal and temporal areas during deviant processing |
| [74] | Standard sinusoidal tones and deviant tones that differed in duration, frequency, intensity, location or a silent gap | Multiple mismatch paradigm | 11 | - | MEG | Prediction error responses in bilateral AC1, STG and lateral prefrontal cortex for deviations |
| [75] | Melodies in either guitar or marimba timbre | Passive listening of oddball paradigm | 38 | A total of a few hours over a week | EEG | A larger negative response in auditory areas for tones previously experienced during exposure |
| [76] | Standard frequency tones interspersed randomly with deviant frequency trials | Passive listening of mismatch paradigm | 16 | - | MEG | Increased interlobar, long-distance synchronization during the MMN time epoch for deviants |
| [77] | Two different tones each becoming deviants in different blocks | Automatic sequential learning | 19 | - | EEG | Errors within the first block type exerting influence on the updating of longer timescale predictions |
| [78] | Sound sequences containing predictable repetitions and order manipulations | Orthogonal auditory one-back task | 17 | - | MEG | Involvement of theta-band oscillations for prediction-error generation in cortical–subcortical networks |
| [79] | A stream of sounds with log-frequencies and different standard deviations | Auditory frequency oddball paradigm and a simultaneous visual n-back task | 89 | - | EEG and MRI | The dynamics of auditory mismatch responses being interconnected by auditory white-matter pathways |
| [42] | Eight tones presented in two different four-tone patterns | Passive listening of statistical learning of melodic patterns | 10 | - | EEG | Stronger signal strength when cohesive patterns were violated |
| [38] | Simple melodies consisting of a repeated pitch pattern and novel melodies with less repetitive structure | Listening | 40 | - | MEG and MRI | Larger MMNm responses for pitch deviants in highly predictable compared to less predictable melodies |
| [80] | Repeating 42-tone pattern following the deterministic incrementing rule or pseudo-randomly assigned tones | Passive listening of oddball paradigm with predictability manipulation | 20 | - | EEG, MEG and MRI | Adaptive learning of surprise with larger integration of past information in the context of expected surprises |
| ● P300 (P3a-P3b) | ||||||
| [81] | Two sinusoidal tones assigned as target and standard stimuli | Auditory followed by visual oddball tasks | 24 | - | EEG | Inhibitory effect of auditory P300 influencing second target processing |
| [82] | Two types of runs consisted of two tones with different frequency | Target detection in an oddball paradigm | 17 | - | EEG | Ventral Attention Network and Dorsal Attention Network as the neural generators of P3a and P3b, respectively |
| [83] | Three tones with different frequencies | Target discrimination in an oddball paradigm | 15 | - | EEG | Difficulty-related changes in inter-regional gamma-band synchrony in target/non-target processing |
| ● ASSR and binaural beat | ||||||
| [84] | Amplitude modulated white noise on either ear | Passive listening | 19 | - | EEG | Successful location of subcortical and cortical sources of ASSR |
| [85] | Binaural exposure of 40 Hz amplitude modulated auditory tones | Auditory-driven gamma synchronization paradigm | 52 | - | MEG and MRI | Gamma synchrony of the entire cortical mantle driven by auditory stimulation in the gamma range |
| [86] | Acoustic stimulation conditions (none, pure tones, classical music, 5 Hz BBs, 10 Hz BBs and 15 Hz BBs) | Passive listening and N-back verbal working memory task | 34 | - | EEG | 15 Hz BBs affecting cortical network properties |
| [87] | 7 Hz and 40 Hz BBs and monaural beats | Passive listening and mood self-report | 16 | - | EEG | Cross-frequency activity elicited by BBs |
| [88] | 10 Hz and 4 Hz BBs and corresponding monaural beats | Listening (expt. 1) | 9 (expt. 1) | - | EEG | Enhanced alpha-band synchrony between auditory cortices during auditory stimulation by BBs |
| [89] | Non-binaural beats and BBs with frequency varying from 1 Hz to 48 Hz | Passive listening and rating pleasantness after exposure | 32 | - | EEG | Enhanced alpha-phase synchronization after listening to BBs in the delta and alpha bands |
| [90] | Pink noise, 40 Hz BBs and 40 Hz monaural beats | Selective attentional task | 25 | - | EEG | No occurrence of neural entrainment by 40 Hz BBs |
| [91] | White noise and 20 Hz BBs or 5 Hz BBs | Free recall task and recognition task | 32 | - | EEG | Improved free recall and recognition by beta-frequency BBs |
| 2. Modification of temporal structure | ||||||
| ● Temporal associations | ||||||
| [92] | An isochronous sequence and a random oddball sequence, varying the ISI duration | Deviant counting | 24 | - | EEG | Smaller P3b for deviant tones embedded in irregular temporal structure |
| [93] | A standard stimulus and a deviant stimulus consisting of 5 pure-tone sequences with various ISIs | Delayed matching-to-sample task | 20 (Expt. 2) | - | MEG | Increased alpha power in temporal auditory regions with longer delay durations |
| [94] | Identical pure tones or standard and deviant pure tones | Single-tone task and an auditory oddball task | 22 | - | EEG | Enhanced N1 and P2 amplitudes with longer ISIs |
| [95] | Pure tones delivered monaurally at four different levels of stimulus onset asynchrony | Passive listening | 20 | - | EEG | Increased amplitude and decreased peak latency with increasing stimulus onset asynchrony |
| [96] | Two chirp trains applied concurrently at different repetition rates | An analog to forward-masking paradigm | 11 | - | EEG | Decreased amplitudes with decreasing distance to the preceding stimulus of the other stimulus train |
| [97] | Standard tones and deviant tones which differed in pitch and/or onset timing | Passive listening of mismatch paradigm | 10 | - | EEG | Larger P3a for pitch deviations with shorter ISIs |
| [98] | A buzzer cue, a target harmonic sound, which were sometimes replaced with task-irrelevant novel sounds | Cued auditory attention task | 13 | - | MEG | Stronger beta-band functional connectivity in response to the target stimuli than to the novel stimuli |
| ● Rhythmic contexts and hazard rates | ||||||
| [99] | A pure-tone acoustic stream interleaved with chords presented in a rhythmic or jittered way | Auditory discrimination task | 23 | - | EEG and MEG | Improved neural decoding of targets and distractors by rhythmic expectation |
| [54] | Rhythmically regular or syncopated sequences of a repeated non-harmonic chord with three partials | Tapping task | 20 | - | EEG | Increased amplitudes at meter-related frequencies compared to meter-unrelated frequencies |
| [55] | Drum clips with different rhythmic structures interrupted by silent breaks | Tapping task or passive listening | 14 | - | EEG | More negative N1 amplitude for metronome than for rhythmic sequences |
| [100] | Auditory metronome with delayed or advanced phase shift and with large or small perturbations | Sensorimotor synchronization task | 16 | - | EEG | Theta coupling between pre-SMA and ACC increases in response to a large positive tap-tone asynchrony |
| [101] | Multiple musical rhythmic patterns by manipulating note values in beats while keeping time signature | Reporting experienced arousal and valence | 18 | - | EEG | Emotional changes associated with the alpha-band connectivity alterations in the fronto-central connections |
| [102] | A single pop song with a super-imposed bassoon sound either lined up or shifted away from the beat | Passive listening | 98 | - | EEG | A clear neural response elicited at the first harmonic of the beat only for the on-the-beat condition |
| [103] | Two standard pure tones with various ISIs and a deviant stimulus which replaced either of a standard stimulus | Deviant detection in a two-tone paradigm with various ISIs | 25 | - | MEG | The asymmetric effect of the passage of time on descending connections |
| ● Other topics | ||||||
| [104] | A theme with an original melody of Mozart and its significant variations | Passive listening | 25 | - | MEG | Increased beta connectivity with modified melody compared to the original melody |
| [105] | Combinations of two sounds with a low to moderate and a high frequency range, either stationary or moving | Modality-change detection in a delayed motion-onset sound paradigm | 14 | - | EEG | Larger amplitudes of motion responses elicited by stimuli with high frequency range |
| [106] | Rhythmically regular and an irregular music presented with an intermittent and continuous type of stimulation | Target detection in an auditory monitoring task | 22 | - | EEG | Smaller P300 amplitude during the continuous and regular compared to the intermittent condition |
| [107] | Pure 1000 Hz sine tones presented at three systematically varied sound intensities | A forced-choice discrimination task or passive listening condition | 22 | - | EEG | Stronger GBRs and enhanced phase locking under the active condition compared with passive listening |
| 3. Lower- and higher-order functions in representation of auditory objects | ||||||
| ● Top-down modulation of bottom-up auditory processing | ||||||
| [108] | A noise sample generated by concatenating three identical noise segments or a running noise | Noise type detection in an unsupervised noise memory paradigm | 13 | - | MEG | The establishment of low-frequency oscillatory phase patterns in auditory neuronal responses during learning new acoustic representations |
| [109] | Signals comprised of a sequence of brief broadband chords containing random pure tone components | Performing auditory figure-ground segregation during a visual task | 16 | - | MEG | Neural sources underlying bottom-up-driven figure-ground segregation |
| [110] | Auditory streaming stimuli with cyclically repeating patterns | Reporting perception of four categories of auditory patterns | 60 | - | EEG | Functional brain networks underlying idiosyncratic switching patterns in multi-stable auditory perception |
| [111] | Two asynchronous standard-tone streams presented to different ears, in separate blocks with or without notch-filtered white-noise masking | Performing a selective attention task | 10 | - | MRI, fMRI, MEG and EEG | Short-term tuning changes in neurons that support segregation of relevant sounds from noise |
| [112] | An electronic pop song and a classical musical piece | Attentive and passive listening of musical pieces | 30 | - | EEG | Different neural activations depending on the direction of attention |
| [113] | A pair of target tones embedded within a multi-tone mask | Detecting a pair of tones embedded within a multi-tone background | 21 | - | MEG | Recurrent processing between auditory and higher-order parietal cortices in complex auditory scenes |
| [114] | Tones with timbres of three different pitches | Performing a choice reaction task | 13 | - | MEG | The involvement of dACC in the effortful processing of auditory stimuli |
| [115] | Tones of three different pitches | Performing a choice reaction task | 28 | - | EEG and fMRI | Top-down influence of the ACC on the AC executed by means of gamma synchronization |
| [116] | Four pure-tone stimuli with different pitches and intensities | Performing pitch and intensity go/no-go assignments | 24 | - | EEG | Cognitive plasticity during learning that involves transformation of asynchronous/synchronous processing pattern |
| [117] | Structured visual stimuli and pure tones | Performing a visual and auditory working memory task | 47 | - | EEG | The extent to which sensory processing areas are essential for the maintenance of information in working memory |
| [118] | Six ripple velocities separated by their just-noticeable differences | Performing a working memory task in a retro-cueing paradigm | 20 | - | MEG | Synchronization patterns across auditory sensory and association areas that support neuronal coding of auditory WM content |
| [119] | Pure tones with different frequencies | Fine pitch discrimination | 20 | - | MEG | The neural origins of the FFR |
| [53] | Two pure tones with a frequency of 89 and 333 Hz | Watching a silent movie whilst ignoring auditory stimulation | 21 | - | MEG and EEG | Neural generators of the frequency-following response elicited to stimuli of low and high frequencies |
| [120] | Independent streams of white noise concurrently in each of the two ears | Detecting brief gaps in noise streams | 21 | - | EEG | Opposing effects of attention and expectations within a fronto-temporal network engaged in sensory prediction errors |
| ● Auditory experience in conjunction with emotional responses | ||||||
| [121] | Random sequences of high or low tones | Listening auditory stimuli with classical conditioning and contingency reversal | 19 | - | MEG | Plasticity of auditory cortex responses when sounds are paired with shock in a classical contingency |
| [122] | Excerpts from film scores spanning a variety of styles | Reporting music-evoked emotional responses | 31 | - | EEG | Neural correlates of musical stimuli-induced emotion, such as pre-frontal cortex asymmetry |
| [123] | Musical excerpts from four common musical genres | Reporting liking of music | 9 | - | EEG | Larger amplitudes of motion responses elicited by stimuli with high frequency range |
| [124] | Sounds of a Tibetan singing bowl | Action–perception cycle of sound making | 32 | - | MEG | Brain processes underlying perception after learning a new association between a sound and the action for making that sound |
| [125] | Three pieces of Guqin music | Listening in varying auditory surroundings | 16 | - | EEG | Increase in functional connectivity as well as a more random network structure in the alpha2 band during music perception |
| [126] | Guqin music and pink noise | Listening to auditory stimuli in various conditions | 20 | - | EEG | Increased connectivity and topological change in functional networks with an enhancement of small-world attributes |
| [127] | A pool of 40 various musical excerpts | Reporting induced emotional responses | 22 | - | EEG | Independent component processes underlying emotions during natural music listening |
| [128] | Trio live performance | Rating improvisation, attractiveness and emotion in concert-like auditory surroundings | 16 | - | EEG | Theta activity reflecting the presence of improvisation in the performances |
| [129] | Experimental excerpts taken from sixty musical fragments | Reporting familiarity of music | 22 | - | EEG | Different theta connectivity patterns underlying pleasantness evoked by familiar and unfamiliar music |
| [130] | Experimental excerpts taken from sixty musical fragments | Reporting music-evoked pleasantness | 25 | - | EEG | Fronto-temporal theta phase synchronization underlying music-evoked pleasantness |
| [131] | Brainwave music | Psychotherapy in pain management | 36 | - | EEG | Improved functional connectivity among different brain regions and brain regularity induced by listening to brainwave music |
| [132] | More and less consonant chords and intervals | Memorizing chords and evaluating the beauty of the intervals | 60 (Expt. 1), 22 (Expt. 2) | - | EEG | A relationship between aesthetic appreciation and implicit learning dynamics, as well as memorization |
| [133] | More and less consonant fifths and dissonant tritones with two different frequencies | Performing aesthetic judgment and detection tasks | 26 | - | EEG | A positive correlation between aesthetic appreciation and perceptual learning |
| ● Auditory plasticity relative to language processing | ||||||
| [134] | Musical pieces with a regular ending or a harmonic transgression at closure | Musical violation discrimination | 16 | - | EEG | A specific neural correlate of musical violation expectation in primary-school children |
| [135] | Modulated nonspeech stimuli | Performing a go/no-go looking task | 49 | - | EEG | Prelinguistic acoustic mapping affected by active auditory exposure |
| 3.2.3. Pre- and Post-stimulus period | ||||||
| [136] | Different complex tone stimuli | Pitch discrimination | 27 | An hour for 10 days | EEG | Subcortical plasticity induced by pitch discrimination training |
| [137] | Piano music mixed with a 5 Hz (theta band enhancement) BB | Listening | 7 | 5 min a day for a week | EEG | After seven days of training, modulation of the absolute power, relative power and coherence |
| [138] | Band-pass noise bursts | Performing a stop-signal task | 13 | - | EEG | Plastic modifications within inhibitory control networks |
| [139] | Band-pass-filtered harmonic complexes | Discriminating auditory fundamental frequency, amplitude modulation rate or visual orientation | 40 | 30 min a day for 6 days | EEG | Sustained cortical and subcortical measures of auditory and visual plasticity following short-term perceptual learning |
| [140] | Songs of different bird species | Auditory semantic categorization | 19 | - | EEG | The cortical representation of birdsongs modulated by brief training to recognize individual bird species |
| [141] | Indian classical music | Mood assessment before and after listening | 20 | - | EEG | On exposure to music, reduced information flow in long-distance connections |
| [142] | A standard sinusoidal tone alternating with two tones before/after a stimulation with a deviant tone continuously at 13 Hz | Mismatch paradigm and LTP-like stimulation | 21 | - | EEG | Increased amplitude of the negative-going MMN wave led by the LTP-like stimulation |
| [143] | Probe blocks of pure-tones, narrow-band noises and white noises or their tetanic presentation | A tetanic-stimulation paradigm | 10 | One day rest between conditions | EEG | Higher post-tetanus amplitude of the N1 component in the tetanus condition than the pre-tetanus state |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, K.; Shiba, Y.; Honda, S.; Nakajima, S.; Fujii, S.; Mimura, M.; Noda, Y. Short-Term Effect of Auditory Stimulation on Neural Activities: A Scoping Review of Longitudinal Electroencephalography and Magnetoencephalography Studies. Brain Sci. 2024, 14, 131. https://doi.org/10.3390/brainsci14020131
Kobayashi K, Shiba Y, Honda S, Nakajima S, Fujii S, Mimura M, Noda Y. Short-Term Effect of Auditory Stimulation on Neural Activities: A Scoping Review of Longitudinal Electroencephalography and Magnetoencephalography Studies. Brain Sciences. 2024; 14(2):131. https://doi.org/10.3390/brainsci14020131
Chicago/Turabian StyleKobayashi, Kanon, Yasushi Shiba, Shiori Honda, Shinichiro Nakajima, Shinya Fujii, Masaru Mimura, and Yoshihiro Noda. 2024. "Short-Term Effect of Auditory Stimulation on Neural Activities: A Scoping Review of Longitudinal Electroencephalography and Magnetoencephalography Studies" Brain Sciences 14, no. 2: 131. https://doi.org/10.3390/brainsci14020131
APA StyleKobayashi, K., Shiba, Y., Honda, S., Nakajima, S., Fujii, S., Mimura, M., & Noda, Y. (2024). Short-Term Effect of Auditory Stimulation on Neural Activities: A Scoping Review of Longitudinal Electroencephalography and Magnetoencephalography Studies. Brain Sciences, 14(2), 131. https://doi.org/10.3390/brainsci14020131








