Abstract
Background and objective: Staging Parkinson’s disease (PD) with a novel simple classification called MNCD, based on four axes (Motor; Non-motor; Cognition; Dependency) and five stages, correlated with disease severity, patients’ quality of life and caregivers’ strain and burden. Our aim was to apply the MNCD classification in advanced PD patients treated with device-aided therapy (DAT). Patients and Methods: A multicenter observational retrospective study of the first patients to start the levodopa-entacapone-carbidopa intestinal gel (LECIG) in Spain was performed (LECIPARK study). The MNCD total score (from 0 to 12) and MNCD stages (from 1 to 5) were collected by the neurologist at V0 (before starting LECIG) and V2 (follow-up visit). Wilcoxon’s signed rank and Marginal Homogeneity tests were applied to compare changes from V0 to V2. Results: Sixty-seven PD patients (58.2% males; 69.9 ± 9.3 years old) with a mean disease duration of 14.4 ± 6.5 years were included. The mean treatment duration (V2) was 172.9 ± 105.2 days. At V0, patients were classified as in stage 2 (35.8%), 3 (46.3%) or 4 (17.9%). The frequency of patients in stage 4 decreased to 9% at V2 (p = 0.001). The MNCD total score decreased from 6.27 ± 1.94 at V0 to 5.21 ± 2.23 (p < 0.0001). From V0 to V2, the motor (M; p < 0.0001) and non-motor symptom (N; p < 0.0001) burden decreased, and autonomy for the activities of daily living (D; p = 0.005) improved. Conclusions: The MNCD classification could be useful to classify advanced PD patients and to monitor the response to a DAT.
1. Introduction
Parkinson’s disease (PD) is a complex and very heterogeneous progressive neurodegenerative disorder causing not only motor but also non-motor symptoms (NMS) that result in loss of patient autonomy for activities of daily living (ADL) and a worse quality of life (QoL) [1,2,3]. From a clinical point of view and given the great variability of outcomes in PD, it is essential to have an easy-to-use tool that allows the staging of PD. Recently, a new classification called “MNCD” has been proposed [4]. The MNCD is based on four major axes (M, Motor; N, Non-motor; C, Cognition; D, Dependency) and proposes five stages (MNCDst), from MNCD stage 1, no relevant symptoms, to MNCD stage 5, dementia and dependency for basic activities of daily living (ADL), and a total score (MNCDsc) from 0 (0 + 0 + 0 + 0 = 0; the best possible status) to 12 (4 + 4 + 2 + 2 = 12; the worst possible status). We demonstrated using data from the Spanish PD cohort COPPADIS [5,6] that PD staging applying the MNCD classification correlated with disease severity, patients’ QoL [7], and caregiver’ burden [8]. Moreover, a group from China observed in a cohort of 357 PD patients that the correlation of the MNCD staging with the QoL was more statistically significant than to the Hoehn&Yahr staging [9]. Originally, the MNCD was proposed as a tool to monitor the progression of PD, from the first moment (at diagnosis) to the end of the follow-up of the patient. It could be even used in cohort studies or clinical trials, especially in those with a long follow-up (e.g., disease-modifying therapies) [10]. Interestingly, the MNCD stage could change from a higher to a lower stage after treatment in some cases (e.g., the third example case in the original description of the classification [4]). Regarding this aspect, we suggested that the MNCD could be useful to be applied in advanced PD patients treated with a device-aided therapy (DAT), both to classify the patient according to all information collected with the MNCD but also to monitor the response to the DAT.
The aim of this study was to apply, for the first time, the MNCD classification in advanced PD patients treated with a DAT. Specifically, the change in the characteristics, MNCDst and the MNCDsc from before to after levodopa-entacapone-carbidopa intestinal gel (LECIG) infusion was analyzed in advanced PD patients from the very recently reported LECIPARK study [11].
2. Material and Methods
A multicenter, longitudinal, retrospective, observational study of the first patients to start LECIG in Spain was performed (LECIPARK [descriptive analysis about the use of LECIgon in patients with PARKnson’s disease in Spain]) [11]. All centers from Spain with an experience of at least 2 PD patients treated with LECIG until 31 March 2024, were invited to participate. The data were collected from three different time points: V0, an indication of therapy (LECIG) by the neurologist; V1, initiation of LECIG; V2, a follow-up visit. The data for visits V0 and V1 were collected from the medical records whereas the data for visit V2 were collected from a specific data report registry assessed in the clinic. The period for collecting the data was 6 months, from December 2023 to May 2024. Information on sociodemographic aspects, comorbidity, factors related to PD, and treatment including LECIG and the levodopa equivalent daily dose (LEDD) [12] was collected [11].
The MNCD classification was applied by the neurologist at V0 and at V2. This classification [4] is based on four axes: (1) Motor symptoms; (2) Non-motor symptoms; (3) Cognition; and (4) Dependency for ADL. The first axis (Motor symptoms) is subdivided into four defined sub axes: (1) motor fluctuations; (2) dyskinesia; (3) axial symptoms; and (4) tremor. The second axis (Non-motor symptoms) is subdivided into four defined sub axes: (1) neuropsychiatric symptoms; (2) autonomic dysfunction; (3) sleep disturbances and fatigue; and (4) pain and sensory disorders. Regarding the third axis (Cognition), patients are classified as having normal cognition, with mild cognitive impairment or dementia. Finally, patients are classified according to the fourth axis (Dependency) as having independence for activities of daily living, with dependency for instrumental or with dependency for basic activities. Patients were classified into five groups according to the MNCDst: Stage 1 (the patient has no relevant symptoms); Stage 2 (there is at least 1 motor symptom or 1 NMS scoring in the MNCD classification); Stage 3 (there is mild cognitive impairment and/or dependency for instrumental ADL); Stage 4 (there is dependency for basic ADL but no dementia); and Stage 5 (there is dementia and dependency for ADL). Moreover, the MNCDsc (from 0 to 12) was calculated according to the sum of the score of all axes of the MNCD classification: M (from 0 to 4) + N (from 0 to 4) + C (from 0 to 2) + D (from 0 to 2).
2.1. Statistical Analysis
Data were processed using SPSS 20.0 for Windows. Continuous variables were presented as mean ± standard deviation (SD) or median (interquartile range), while categorical variables were expressed as n (%). The distribution for variables was verified by a one-sample Kolmogorov–Smirnov test. Wilcoxon’s signed rank and Marginal Homogeneity tests were applied to compare changes from V0 to V2. Spearman’s or Pearson’s correlation coefficient, as appropriate, was used to analyze the relationship between the change in both the MNCDst and MNCDsc and the clinical global impression of change (CGI-C; 1, very much worse; 2, much worse; 3, minimally worse; 4, no change; 5, minimally improved; 6, much improved; 7, very much improved) and visual analogue scale global improvement (VAS-GI; from 0, the worst, to 10, the best improvement) according to the opinion of the neurologist, patient and principal caregiver. Correlations were considered weak for coefficient values ≤ 0.29, moderate for values between 0.30 and 0.59, and strong for values ≥ 0.60. The value of p was considered significant when it was <0.05.
2.2. Standard Protocol Approvals, Registrations, and Patient Consents
For this study, we received approval from the Comité de Ética de Investigación de medicamentos de Galicia (CEImG) from Spain (2023/527; 19 November 2023). Written informed consents from all participants in this study was obtained.
2.3. Data Availability
The protocol, statistical analysis plan and data are available on request.
3. Results
A total of 67 out of 73 PD patients (58.2% males; 69.9 ± 9.3 years old) from the LECIPARK study were included in the analysis (91.8% of the sample; 6 patients without data collected about the MNCD). The mean disease duration at baseline was 14.4 ± 6.5 years (range, 5–31). At V0, the mean OFF time (N = 65) was 5.2 ± 3 h (range, 1–15) and 74.6% and 80.3% of the patients had non-motor fluctuations and dyskinesia, respectively. Other characteristics related to PD and treatment at the baseline are shown in Table 1. The mean exposure to LECIG was 172.9 ± 105.2 days (range, 7–476). The follow-up time was ≥3 months and ≥6 months in 76.2% and 47.6% of the patients, respectively.

Table 1.
Data about sociodemographic aspects, comorbidities, PD, antiparkinsonian drugs and other therapies at baseline (V0).
The results represent %, mean ± SD or median [p25, p75]. BMI, body mass index; COMT, catechol-O-methyl transferase; DA, dopamine agonist; DAT, device-aided therapy; DBS, deep brain stimulation; H&Y, Hoehn&Yahr; LCIG, levodopa-carbidopa infusion gel; LECGI, levodopa-entacapone-carbidopa infusion gel; LEDD, levodopa equivalent daily dose; MAO, monoamine oxidase; MCI, mild cognitive impairment; UPDRS, Unified Parkinson’s Disease Rating Scale.
At V0, patients were classified as in stage 2 (35.8%), 3 (46.3%) or 4 (17.9%). From V0 to V2, a significant change was detected in the MNCDst with a decrease in the percentage of stage 4 (from 17.9% to 9%) and an increase in stage 2 (from 35.8% to 44.8%) (p = 0.001) (Figure 1A). Two patients were even classified as in MNCD stage 1 at V2. The number of patients with a greater score (from 0 to 4) decreased significantly from V0 to V2 regarding motor symptoms (M; p < 0.0001) and NMS (N; p < 0.0001) (Figure 1B). Independence for ADL was observed in 33 patients at V2 compared to 27 at baseline (p = 0.005) (Figure 1B). No differences were detected in cognition (p = 1.000). Regarding the MNCDsc, a significant decrease from V0 to V2 was detected in the total (from 6.27 ± 1.94 at V0 to 5.21 ± 2.23; p < 0.0001), M (from 2.73 ± 0.71 at V0 to 2.24 ± 0.8; p < 0.0001), N (from 2.51 ± 1.01 at V0 to 2.1 ± 1.11; p < 0.0001) and D (from 0.78 ± 0.73 at V0 to 0.59 ± 0.65; p = 0.005) scores (Figure 2). No correlation was found between the change from V0 to V2 in the MNCDst and the CGI-C or VAS-GI but there was between the change in the MNCDsc and the CGI-C and the MNCDsc and the VAS-GI (Table 2). Regarding the motor stage, no significant changes were detected in this sample (N = 67) in the H&Y during the ON state from before (2 [2, 3]); 2.5 ± 0.6) to after (2 [2, 3]); 2.2 ± 0.7) LECIG (p = 0.649).
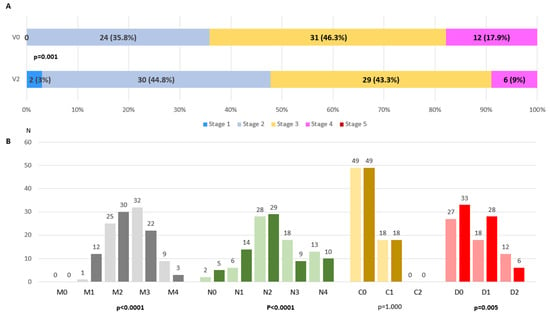
Figure 1.
(A). Frequency of different MNCD stages (from 1 to 5) at V0 (V0; pre-LECIG) compared to at V2 (follow-up visit; 172.9 ± 105.2 days after starting LECIG) (p < 0.0001). Marginal Homogeneity test applied. (B). Number of patients with each score of the MNCD classification (M, N, C, and D) at V0 (on the left for each score, in light color) compared to at V2 (on the right for each score, in dark color). Marginal Homogeneity test applied.
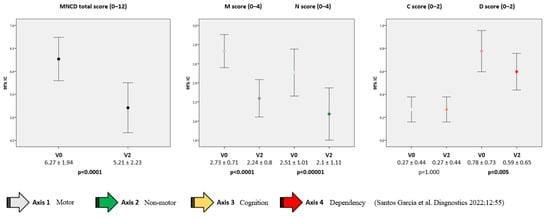
Figure 2.
Change from the baseline (V0; pre-LECIG) to the follow-up visit (V2) in the MNCD total score (from 0 to 12), M and N scores (from 0 to 4), and C and D scores (from 0 to 2). Wilcoxon’s signed rank tests were applied. The bars represent mean ± standard deviation.

Table 2.
Correlation (r) between the change from V0 to V2 (Δ = value at V0–value at V2) and the CGI-C and the VAS-GI.
4. Discussion
The MNCD is a new proposed classification for PD based on four axes [4] that has been demonstrated to correlate with disease severity, patients’ QoL, and caregivers’ strain and burden [7,8,9]. It has been designed to monitor the progression of PD and hypothetically, even the response to a therapy [4]. Here, we applied, for the first time, the MNCD in patients with advanced PD treated with a DAT. Very interestingly, a significant improvement in the MNCD stage and the MNCD score was detected in 67 PD patients treated with LECIG infusion therapy. Moreover, the improvement in the MNCD score correlated with the clinical global impression of change (improvement) according to the neurologist, the patient, and the principal caregiver’s opinion. From a clinical point of view and based on these findings, we suggest that the MNCD classification could be useful to apply in advanced PD patients in daily clinical practical.
A critical factor selecting a patient for a DAT is the proper indication of the therapy. Differences in access to care, referral pattern (timing and frequency), as well as physician biases (unconscious/implicit or conscious/explicit bias) and patients’ preferences or health-seeking behavior are to be considered [13]. However, a critical factor is the necessity to conduct a very complete evaluation collecting a lot of information about different aspect of the disease and other comorbidities [14]. In this context, some tools have been developed with the aim of helping the physician to select an advanced PD patient for a DAT such as the CDEPA questionnaire, 5-2-1 criteria, MANAGE-PD, D-DATS, FLASQ-PD, and Stimulus 1 or Stimulus 2 [15]. In a recent narrative review, Moes et al. [15] briefly included the “MNCD tool” in the “Other screening tools and testing methods” section. Although the MNCD classification was not specifically designed to apply in advanced PD patients as a tool to help in the selection of a DAT as Moes et al. commented, our novel findings can be interpreted as it could be useful due to the amount of information with this tool being collected (motor fluctuations; dyskinesias; axial symptoms; refractory tremor; neuropsychiatric symptoms; sleep/fatigue; dysautonomic symptoms; pain and sensory symptoms; cognitive status; disability or dependency for ADL). Importantly, the classification shows the information in such a way that it can be visually interpreted very quickly and the stage and score can also help to classify the patient as more advanced or with a greater burden of symptoms that generate disability. As expected, of 67 advanced PD patients selected to be treated with LECIG, there were no cases with a MNCD stage 5 (dementia and dependency for basic ADL) due to the fact that a patient in stage 5 would not be a good candidate for a DAT. On the other side, only one out of three patients were advanced PD patients with normal cognition and independence for instrumental and basic ADL. Moreover, only two patients at baseline had no relevant NMS (N0) whereas nearly one out five patients had at least one relevant NMS of each sub-axis (N4).
A very interesting finding is that the improvement obtained by patients with LECIG, recently reported [11], was reflected in the MNCD classification, both in stage and score. After starting with LECIG, the number of patients in stage 4 was reduced to half (from 12 to 6), and 2 patients were even classified as stage 1 (without relevant motor and NMS). Moreover, the motor and non-motor burden decreased as it was reflected in the total, M and N MNCD scores, and the number of patients reporting symptoms (M and N). In example 3 of the original description of the MNCD classification [4], we suggested that a patient could hypothetically pass from a higher to a lower MNCD stage and decrease the symptoms burden and global score after effective specific treatment (e.g., refractory tremor improvement after ultrasound therapy and impulse control disorder remission after dopamine agonist withdrawal passing from stage 2 to 1 and from score 2 [0001/1000/0/0] to 0 [0000/0000/0/0]). Here, in advanced PD patients, a critical factor explaining the changes in the MNCDst was the improvement in disability, again reflected in the score (D score). Curiously, although gaining autonomy and independence is one of the objectives of treating symptomatically with a DAT, it is an aspect that is often not properly evaluated in advanced PD patients in favor of others such as QoL or the OFF-time reduction [16]. The MNCD classification is simple but could be used to monitor the response to a DAT (motor symptoms; NMS; cognition; dependency) not only in the short- (i.e., a possible improvement in some cases reflected in the MNCDst and MNCDsc due to the DAT effect) but also in the long-term (i.e., impairment reflected in the MNCDst and MNCDsc due to the progression of the disease). Finally, a correlation was observed between the improvement in the MNCDsc and the improvement perception reported by the neurologist, the patient, and the principal caregiver. This agrees with known improvement very frequently reported by patients and physicians after being treated with a DAT [17,18].
The present study has some limitations. Firstly, limitations related to the retrospective observational design. However, much information was available and collected from the medical records due to patients being exhaustively evaluated in centers with experience from Spain in the management of DATs. Secondly, only patients treated with LECIG were included in this study but not with other DATs. Thirdly, the sample was small (N = 67) and the mean follow-up was short (mean of about 6 months), making it necessary to conduct a study applying the MNCD in a big cohort of PD patients treated with different DATs with a short- and long-term follow-up. Fourthly, the changes in the MNCD classification were not directly compared to other scales. However, significant changes were detected in M, N and D scores and also in the MNCD total score from pre to post-LECIG but not in the H&Y during the ON state. More information is collected with the MNCD and it is also more sensible to change the H&Y. Fifthly, non-parametric tests were applied to analyze the change in different variables from V0 to V2 according to the observational design, so the influence of covariates was not taken into consideration. On the other hand, this is the first time that the MNCD classification is used in advanced PD patients to classify and monitor the response to a DAT.
5. Conclusions
In conclusion, the MNCD classification was applied here for the first time in advanced PD patients treated with a DAT. Our findings suggest that it could be useful to classify advanced PD patients and to monitor the response to a DAT. More data are needed to know the possible role of the MNCD classification as a tool to use to monitor PD progression.
Author Contributions
D.S.-G.: conception, organization, and execution of the project; statistical analysis; writing of the first draft of the manuscript; recruitment and/or evaluation of participants. L.L.-M.: review and critique; recruitment and/or evaluation of participants and/or data entry into CRF. I.M.: review and critique; recruitment and/or evaluation of participants and/or data entry into CRF. P.L.-B.: review and critique; recruitment and/or evaluation of participants and/or data entry into CRF. E.C.P.: review and critique; recruitment and/or evaluation of participants and/or data entry into CRF. R.G.-R.: review and critique; recruitment and/or evaluation of participants and/or data entry into CRF. T.F.V.: review and critique; recruitment and/or evaluation of participants and/or data entry into CRF. C.M.-M.: review and critique; recruitment and/or evaluation of participants and/or data entry into CRF. R.B.-M.: review and critique; recruitment and/or evaluation of participants and/or data entry into CRF. I.M.-T.: review and critique; recruitment and/or evaluation of participants and/or data entry into CRF. M.Á.-S.: review and critique; recruitment and/or evaluation of participants and/or data entry into CRF. D.A.-M.: review and critique; recruitment and/or evaluation of participants and/or data entry into CRF. I.L.: review and critique; recruitment and/or evaluation of participants and/or data entry into CRF. M.F.V.-G.: review and critique; recruitment and/or evaluation of participants and/or data entry into CRF. J.A.S.-M.: review and critique; recruitment and/or evaluation of participants and/or data entry into CRF. J.C.M.-C.: review and critique; recruitment and/or evaluation of participants and/or data entry into CRF. A.B.P.: review and critique; recruitment and/or evaluation of participants and/or data entry into CRF. J.M.S.: review and critique; recruitment and/or evaluation of participants and/or data entry into CRF. E.C.: review and critique; recruitment and/or evaluation of participants and/or data entry into CRF. C.V.-M.: review and critique; recruitment and/or evaluation of participants and/or data entry into CRF. N.L.-A.: review and critique; recruitment and/or evaluation of participants and/or data entry into CRF. P.S.A.: review and critique; recruitment and/or evaluation of participants and/or data entry into CRF. S.N.P.: review and critique; recruitment and/or evaluation of participants and/or data entry into CRF. E.G.G.: review and critique; recruitment and/or evaluation of participants and/or data entry into CRF. R.M.G.: review and critique; recruitment and/or evaluation of participants and/or data entry into CRF. R.E.: review and critique; recruitment and/or evaluation of participants and/or data entry into CRF. M.C.: review and critique; recruitment and/or evaluation of participants and/or data entry into CRF. C.E.F.: review and critique; recruitment and/or evaluation of participants and/or data entry into CRF. P.G.R.: review and critique; recruitment and/or evaluation of participants and/or data entry into CRF. T.M.R.: review and critique; recruitment and/or evaluation of participants and/or data entry into CRF. B.F.R.: review and critique; recruitment and/or evaluation of participants and/or data entry into CRF. M.M.A.-S.: review and critique; recruitment and/or evaluation of participants and/or data entry into CRF. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Comité de Ética de Investigación de medicamentos de Galicia (CEImG) from Spain (2023/527; 19 November 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The protocol, statistical analysis plan, and data are available on request. This can be requested from the promoter of the study, Diego Santos García, by sending an email to the following address: diegosangar@yahoo.es.
Acknowledgments
We would like to thank all patients and their caregivers who collaborated in this study. Many thanks also to Fundación Española de Ayuda a la Investigación en Enfermedades Neurodegenerativas y/o de Origen Genético (Fundación Degen) (https://fundaciondegen.org/, accessed on 14 November 2024) and Stada Spain.
Conflicts of Interest
Diego Santos-García has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Lundbeck, KRKA, Zambon, Bial, Italfarmaco, Teva, Archímedes, Esteve, Stada, Merz, and grants from the Spanish Ministry of Economy and Competitiveness [PI16/01575] co-founded by ISCIII (Concesión de subvenciones de Proyectos de Investigación en Salud de la convocatoria 2020 de la Acción Estratégica en Salud 2017-2020 por el proyecto “PROGRESIÓN NO MOTORA E IMPACTO EN LA CALIDAD DE VIDA EN LA ENFERMEDAD DE PARKINSON”; Concesión de Contrato para la intensificación de la actividad investigadora en el Sistema Nacional de Salud, Convocatoria 2021, Instituto de Salud Carlos III). Lydia López-Manzanares compensated advisory services, consulting, research grant support, or speaker honoraria: AbbVie, Acorda, Bial, Intec Pharma, Italfarmaco, Pfizer, Roche, Teva, UCB, and Zambon. Inés Muro has received honoraria for lectures with educational purposes and advisory from ABBVIE, BIAL, Lundbeck, Zambon, and STADA. Pablo Lorenzo-Barreto received honoraria for educational presentations and advice service by Zambon and Orion Pharma. Elena Casas Peña has received honoraria for educational presentations and advice service by Bial. Rocío García-Ramos has received honoraria and grants for lecturing and advisory services from Abbvie, Zambón, Bial, Merk, and Stada. Tamara Fernández Valle: None. Carlos Morata-Martínez: None. Raquel Baviera-Muñoz is supported by a Rio Hortega contract (CM22/00099) from Instituto de Salud Carlos III. Irene Martínez-Torres has received honoraria from Abbvie, Bial, Ipsen, Medtronic, Merz, and Pallex for lecturing. María Álvarez-Sauco has received honoraria for educational presentations and advice services by Abbvie, UCB Pharma, Zambon, Bial, and Teva. Déborah Alonso-Modino: None. Inés Legarda has received honoraria for educational presentations and advice services by Abbvie, UCB Pharma, Zambon, Bial, and Teva. María Fuensanta Valero-García has received honoraria for educational presentations by Bial. José Andrés Suárez-Muñoz: None. Juan Carlos Martínez-Castrillo has received research support from Lundbeck, Italfarmaco, Allergan, Zambon, Merz, and Abbvie. He has received speaking honoraria from AbbVie, Bial, Italfarmaco, Lundbeck, Krka, TEVA, UCB, Zambon, Allergan, Ipsen, and Merz. Ana Belén Perona has received honoraria from Abbvie, Bial, Stada, and Merz for lecturing. Jose María Salom: None. Esther Cubo has received travel grants from Abbvie, Allergan, Boston, and lecturing honoraria from Abbvie, International Parkinson’s Disease Movement Disorder Society. Caridad Valero-Merino has received honoraria for educational services from Zambon, Abbvie, and UCB. Nuria López-Ariztegui compensated advisory services, consulting, research grant support, or speaker honoraria from Abbvie, Italfarmaco, Stada, Bial, Zambon, UCB, Lundbeck. Pilar Sánchez Alonso has received honoraria for educational presentations and advice services from Abbvie, UCB Pharma, Lundbeck, KRKA, Zambon, Bial, and Teva. Sabela Novo Ponte has received honoraria for educational presentations from Abbvie, Alter, and Schwabe Pharma Iberica and travel grants from Bial, Zambón, and Esteve. Elisa Gamo Gónzález has received honoraria for educational presentations and advice service by Italfarmaco, Bial, and Healthincode. Raquel Martín García has received honoraria for educational presentations and advice service by Almirall. Raúl Espinosa has received honoraria for educational presentations, advice service, and travel grants by AbbVie, Stada, Orion, Merz, Bial, Italfarmaco, Novartis, Pfizer, and Lundbeck. Mar Carmona has received honoraria from Abbvie, Bial, and Esteve and italfarmaco for lecturing and from Bial and Stada as consultant/scientific advisor. Cici Esmerali Feliz has received honoraria for educational presentations from Abbvie, Bial, Steve, Zambom, and Alter. Pedro García Ruíz has received personal compensation as a consultant/scientific advisory board from Italfarmaco, Britannia, Bial, Stada, and Esteve and speaking honoraria from Italfarmaco, Bial, Stada, and Esteve. Teresa Muñoz Ruíz: None. Beatriz Fernández Rodríguez: None. Marina Mata has recived honoraria for educational presentations or adviced services from Stada, Abbvie, Orion Pharma, Italfarmaco, Bial, Zambon, and Esteve.
Abbreviations
ADL, activities of daily living; CGI-C, clinical global impression of change; DAT, device-aided therapy; LECIG, levodopa-entacapone-carbidopa intestinal gel; MNCDsc, MNCD score; MNCDst, MNCD stage; NMS, non-motor symptoms; PD, Parkinson’s disease; QoL, quality of life; VAS-GI, visual analog scale global improvement.
References
- Bock, M.A.; Brown, E.G.; Zhang, L.; Tanner, C. Association of Motor and Nonmotor Symptoms with Health-Related Quality of Life in a Large Online Cohort of People with Parkinson Disease. Neurology 2022, 98, e2194–e2203. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Yang, Y.; Zhang, L.; Zhang, Q.; Balbuena, L.; Ungvari, G.S.; Zang, Y.F.; Xiang, Y.T. Quality of life in Parkinson’s disease: A systematic review and meta-analysis of comparative studies. CNS Neurosci. Ther. 2021, 27, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Santos García, D.; de Deus Fonticoba, T.; Cores, C.; Muñoz, G.; Paz González, J.M.; Martínez Miró, C.; Suárez, E.; Jesús, S.; Aguilar, M.; Pastor, P.; et al. Predictors of clinically significant quality of life impairment in Parkinson’s disease. NPJ Park. Dis. 2021, 7, 118. [Google Scholar] [CrossRef] [PubMed]
- Santos García, D.; Álvarez Sauco, M.; Calopa, M.; Carrillo, F.; Escamilla Sevilla, F.; Freire, E.; García Ramos, R.; Kulisevsky, J.; Gómez Esteban, J.C.; Legarda, I.; et al. MNCD: A New Tool for Classifying Parkinson’s Disease in Daily Clinical Practice. Diagnostics 2021, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Santos-García, D.; Mir, P.; Cubo, E.; Vela, L.; Rodríguez-Oroz, M.C.; Martí, M.J.; Arbelo, J.M.; Infante, J.; Kulisevsky, J.; Martínez-Martín, P.; et al. COPPADIS-2015 (Cohort of Patients with Parkinson’s Disease in Spain, 2015), a global—Clinical evaluations, serum biomarkers, genetic studies and neuroimaging--prospective, multicenter, non-interventional, long-term study on Parkinson’s disease progression. BMC Neurol. 2016, 16, 26. [Google Scholar]
- Santos García, D.; Jesús, S.; Aguilar, M.; Planellas, L.L.; García Caldentey, J.; Caballol, N.; Legarda, I.; Hernández Vara, J.; Cabo, I.; López Manzanares, L.; et al. COPPADIS-2015 (COhort of Patients with PArkinson’s DIsease in Spain, 2015): An ongoing global Parkinson’s disease project about disease progression with more than 1000 subjects included. Results from the baseline evaluation. Eur. J. Neurol. 2019, 26, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Santos-García, D.; de Deus Fonticoba, T.; Cores Bartolomé, C.; Feal Painceiras, M.J.; Íñiguez-Alvarado, M.C.; García Díaz, I.; Jesús, S.; Buongiorno, M.T.; Planellas, L.; Cosgaya, M.; et al. Staging Parkinson’s Disease According to the MNCD (Motor/Non-Motor/Cognition/Dependency) Classification Correlates with Disease Severity and Quality of Life. J. Park. Dis. 2023, 13, 379–402. [Google Scholar] [CrossRef] [PubMed]
- Santos-García, D.; de Deus Fonticoba, T.; Cores Bartolomé, C.; Feal Painceiras, M.J.; García Díaz, I.; Alvarado, M.C.Í.; Paz, J.M.; Jesús, S.; Cosgaya, M.; Caldentey, J.G.; et al. Staging Parkinson’s disease according to the MNCD classification correlates with caregiver burden. Brain Behav. 2023, 13, e3295. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Ren, J.; Shi, H.; Liu, W.; Lu, M. Correlation between the MNCD classification-based staging of Parkinson’s disease and quality of life: A cross-sectional study. J. Neural Transm. 2024, 131, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Escamilla-Sevilla, F.; Freire-Alvarez, E.; Martínez Castrillo, J.C.; Sánchez Ferro, Á.; Santos-García, D. Concerns with the new biological research criteria for synucleinopathy. Lancet Neurol. 2024, 23, 662. [Google Scholar] [CrossRef] [PubMed]
- Santos-García, D.; López-Manzanares, L.; Muro, I.; Lorenzo-Barreto, P.; Casas Peña, E.; García-Ramos, R.; Fernández Valle, T.; Morata-Martínez, C.; Baviera-Muñoz, R.; Martínez-Torres, I.; et al. Effectiveness and safety of levodopa-entacapone-carbidopa infusion in Parkinson’s disease. A real-world data study. Eur. J. Neurol. 2024, e16535. [Google Scholar] [CrossRef] [PubMed]
- Jost, S.T.; Kaldenbach, M.A.; Antonini, A.; Martinez-Martin, P.; Timmermann, L.; Odin, P.; Katzenschlager, R.; Borgohain, R.; Fasano, A.; Stocchi, F.; et al. Levodopa Dose Equivalency in Parkinson’s Disease: Updated Systematic Review and Proposals. Mov. Disord. 2023, 38, 1236–1252. [Google Scholar] [CrossRef] [PubMed]
- Auffret, M.; Weiss, D.; Stocchi, F.; Vérin, M.; Jost, W.H. Access to device-aided therapies in advanced Parkinson’s disease: Navigating clinician biases, patient preference, and prognostic uncertainty. J. Neural Transm. 2023, 130, 1411–1432. [Google Scholar] [CrossRef] [PubMed]
- Phokaewvarangkul, O.; Auffret, M.; Groppa, S.; Markovic, V.; Petrovic, I.; Bhidayasiri, R. What was first and what is next in selecting device-aided therapy in Parkinson’s disease? Balancing evidence and experience. J. Neural Transm. 2024, 131, 1307–1320. [Google Scholar] [CrossRef] [PubMed]
- Moes, H.R.; Henriksen, T.; Sławek, J.; Phokaewvarangkul, O.; Buskens, E.; van Laar, T. Tools and criteria to select patients with advanced Parkinson’s disease for device-aided therapies: A narrative review. J. Neural Transm. 2023, 130, 1359–1377. [Google Scholar] [CrossRef] [PubMed]
- Antonini, A.; Pahwa, R.; Odin, P.; Isaacson, S.H.; Merola, A.; Wang, L.; Kandukuri, P.L.; Alobaidi, A.; Yan, C.H.; Bao, Y.; et al. Comparative Effectiveness of Device-Aided Therapies on Quality of Life and Off-Time in Advanced Parkinson’s Disease: A Systematic Review and Bayesian Network Meta-analysis. CNS Drugs 2022, 36, 1269–1283. [Google Scholar] [CrossRef] [PubMed]
- van Laar, T.; Chaudhuri, K.R.; Antonini, A.; Henriksen, T.; Trošt, M. Infusion Therapies in the Treatment of Parkinson’s Disease. J. Park. Dis. 2023, 13, 641–657. [Google Scholar] [CrossRef] [PubMed]
- Perestelo-Pérez, L.; Rivero-Santana, A.; Pérez-Ramos, J.; Serrano-Pérez, P.; Panetta, J.; Hilarion, P. Deep brain stimulation in Parkinson’s disease: Meta-analysis of randomized controlled trials. J. Neurol. 2014, 261, 2051–2060. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).