Reactive Astrocytosis—A Potential Contributor to Increased Suicide in Long COVID-19 Patients?
Abstract
1. Introduction
2. Astrocytes and CNS Homeostasis
3. Astrocyte Dysfunction in Suicide Development
3.1. Astrocyte-Related Abnormality in Depressed Subjects
3.2. Astrocyte-Related Abnormality in Suicidal Subjects
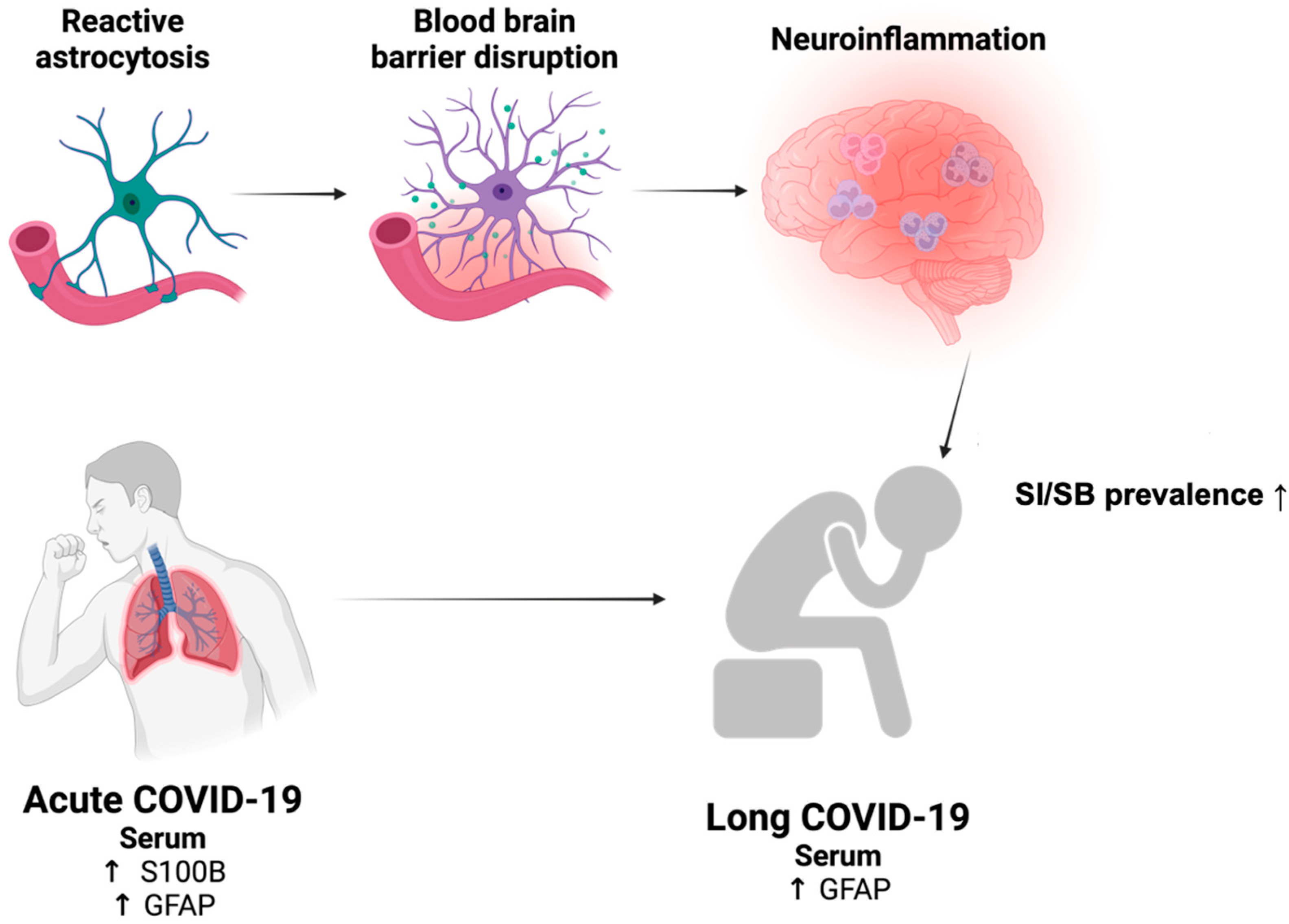
4. Astrocyte Dysfunction in COVID-19
4.1. Astrocyte-Related Abnormality in Acute COVID-19
4.2. Astrocyte-Related Abnormality in Long COVID-19
5. Reactive Astrocytosis as a Precipitating Factor for SI/SB Development in Long COVID-19?
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- WHO. COVID-19 Cases. COVID-19 Dashboard 2024. Available online: https://data.who.int/dashboards/covid19/cases (accessed on 6 February 2024).
- Russell, C.D.; Lone, N.I.; Baillie, J.K. Comorbidities, multimorbidity and COVID-19. Nat. Med. 2023, 29, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Premraj, L.; Kannapadi, N.V.; Briggs, J.; Seal, S.M.; Battaglini, D.; Fanning, J.; Suen, J.; Robba, C.; Fraser, J.; Cho, S.M. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: A meta-analysis. J. Neurol. Sci. 2022, 434, 120162. [Google Scholar] [CrossRef]
- Nalleballe, K.; Reddy Onteddu, S.; Sharma, R.; Dandu, V.; Brown, A.; Jasti, M.; Yadala, S.; Veerapaneni, K.; Siddamreddy, S.; Avula, A. Spectrum of neuropsychiatric manifestations in COVID-19. Brain Behav. Immun. 2020, 88, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Barlattani, T.; D’Amelio, C.; Capelli, F.; Mantenuto, S.; Rossi, R.; Socci, V.; Stratta, P.; Di Stefano, R.; Rossi, A.; Pacitti, F. Suicide and COVID-19: A rapid scoping review. Ann. Gen. Psychiatry 2023, 22, 10. [Google Scholar] [CrossRef]
- Matić, T.; Pregelj, P.; Sadikov, A.; Rus Prelog, P. Depression, Anxiety, Stress, and Suicidality Levels in Young Adults Increased Two Years into the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2022, 20, 339. [Google Scholar] [CrossRef]
- Wang, M.; Hu, C.; Zhao, Q.; Feng, R.; Wang, Q.; Cai, H.; Guo, Z.; Xu, K.; Luo, W.; Guo, C.; et al. Acute psychological impact on COVID-19 patients in Hubei: A multicenter observational study. Transl. Psychiatry 2021, 11, 133. [Google Scholar] [CrossRef]
- Erlangsen, A.; Qin, P.; Madsen, T.; Hawton, K.; Osler, M.; Hjorthøj, C.; Benros, M.E.; Ethelberg, S.; Mølbak, K.; Laursen, T.M.; et al. Association between SARS-CoV-2 infection and self-harm: Danish nationwide register-based cohort study. Br. J. Psychiatry J. Ment. Sci. 2023, 222, 167–174. [Google Scholar] [CrossRef]
- Sher, L. Long COVID and the risk of suicide. Gen. Hosp. Psychiatry 2023, 80, 66–67. [Google Scholar] [CrossRef]
- Costanza, A.; Amerio, A.; Aguglia, A.; Serafini, G.; Amore, M.; Hasler, R.; Ambrosetti, J.; Bondolfi, G.; Sampogna, G.; Berardelli, I.; et al. Hyper/neuroinflammation in COVID-19 and suicide etiopathogenesis: Hypothesis for a nefarious collision? Neurosci. Biobehav. Rev. 2022, 136, 104606. [Google Scholar] [CrossRef]
- Steardo, L.; Steardo, L., Jr.; Scuderi, C. Astrocytes and the Psychiatric Sequelae of COVID-19: What We Learned from the Pandemic. Neurochem. Res. 2023, 48, 1015–1025. [Google Scholar] [CrossRef]
- Ma, D.K.; Bonaguidi, M.A.; Ming, G.-L.; Song, H. Adult neural stem cells in the mammalian central nervous system. Cell Res. 2009, 19, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, A.; Avci, H.X.; Leist, M.; Kobolák, J.; Dinnyés, A. Astrocyte Differentiation of Human Pluripotent Stem Cells: New Tools for Neurological Disorder Research. Front. Cell. Neurosci. 2016, 10, 215. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Nedergaard, M. Physiology of Astroglia. Physiol. Rev. 2018, 98, 239–389. [Google Scholar] [CrossRef]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Magistretti, P.J.; Pellerin, L.; Rothman, D.L.; Shulman, R.G. Energy on Demand. Science 1999, 283, 496–497. [Google Scholar] [CrossRef]
- Sonnewald, U.; Westergaard, N.; Schousboe, A. Glutamate transport and metabolism in astrocytes. Glia 1997, 21, 56–63. [Google Scholar] [CrossRef]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Matteoli, M.; Parpura, V.; Mothet, J.-P.; Zorec, R. Astrocytes as secretory cells of the central nervous system: Idiosyncrasies of vesicular secretion. EMBO J. 2016, 35, 239–257. [Google Scholar] [CrossRef]
- Takata, F.; Nakagawa, S.; Matsumoto, J.; Dohgu, S. Blood-Brain Barrier Dysfunction Amplifies the Development of Neuroinflammation: Understanding of Cellular Events in Brain Microvascular Endothelial Cells for Prevention and Treatment of BBB Dysfunction. Front. Cell. Neurosci. 2021, 15, 661838. [Google Scholar] [CrossRef]
- Yue, Q.; Hoi, M.P.M. Emerging roles of astrocytes in blood-brain barrier disruption upon amyloid-beta insults in Alzheimer’s disease. Neural Regen. Res. 2023, 18, 1890–1902. [Google Scholar] [CrossRef]
- Pekny, M.; Pekna, M. Reactive gliosis in the pathogenesis of CNS diseases. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2016, 1862, 483–491. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, L.A.; Mechawar, N. Implication of cerebral astrocytes in major depression: A review of fine neuroanatomical evidence in humans. Glia 2021, 69, 2077–2099. [Google Scholar] [CrossRef]
- Yamamoto, M.; Sakai, M.; Yu, Z.; Nakanishi, M.; Yoshii, H. Glial Markers of Suicidal Behavior in the Human Brain-A Systematic Review of Postmortem Studies. Int. J. Mol. Sci. 2024, 25, 5750. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, H.-G.; Meyer-Lotz, G.; Dobrowolny, H.; Bannier, J.; Steiner, J.; Walter, M.; Bogerts, B. Reduced density of glutamine synthetase immunoreactive astrocytes in different cortical areas in major depression but not in bipolar I disorder. Front. Cell. Neurosci. 2015, 9, 273. [Google Scholar] [CrossRef]
- Gos, T.; Schroeter, M.L.; Lessel, W.; Bernstein, H.-G.; Dobrowolny, H.; Schiltz, K.; Bogerts, B.; Steiner, J. S100B-immunopositive astrocytes and oligodendrocytes in the hippocampus are differentially afflicted in unipolar and bipolar depression: A postmortem study. J. Psychiatr. Res. 2013, 47, 1694–1699. [Google Scholar] [CrossRef]
- Cobb, J.A.; O’Neill, K.; Milner, J.; Mahajan, G.J.; Lawrence, T.J.; May, W.L.; Miguel-Hidalgo, J.; Rajkowska, G.; Stockmeier, C.A. Density of GFAP-immunoreactive astrocytes is decreased in left hippocampi in major depressive disorder. Neuroscience 2016, 316, 209–220. [Google Scholar] [CrossRef]
- Davis, S.; Thomas, A.; Perry, R.; Oakley, A.; Kalaria, R.N.; O’Brien, J.T. Glial fibrillary acidic protein in late life major depressive disorder: An immunocytochemical study. J. Neurol. Neurosurg. Psychiatry 2002, 73, 556–560. [Google Scholar] [CrossRef]
- Oh, D.H.; Son, H.; Hwang, S.; Kim, S.H. Neuropathological abnormalities of astrocytes, GABAergic neurons, and pyramidal neurons in the dorsolateral prefrontal cortices of patients with major depressive disorder. Eur. Neuropsychopharmacol. 2012, 22, 330–338. [Google Scholar] [CrossRef]
- Rodnight, R.; Gonçalves, C.A.; Wofchuk, S.T.; Leal, R. Control of the phosphorylation of the astrocyte marker glial fibrillary acidic protein (GFAP) in the immature rat hippocampus by glutamate and calcium ions: Possible key factor in astrocytic plasticity. Braz. J. Med. Biol. Res. 1997, 30, 325–338. [Google Scholar] [CrossRef]
- Webster, M.J.; Knable, M.B.; Johnston-Wilson, N.; Nagata, K.; Inagaki, M.; Yolken, R.H. Immunohistochemical Localization of Phosphorylated Glial Fibrillary Acidic Protein in the Prefrontal Cortex and Hippocampus from Patients with Schizophrenia, Bipolar Disorder, and Depression. Brain Behav. Immun. 2001, 15, 388–400. [Google Scholar] [CrossRef]
- Chandley, M.J.; Szebeni, K.; Szebeni, A.; Crawford, J.; Stockmeier, C.A.; Turecki, G.; Miguel-Hidalgo, J.J.; Ordway, G.A. Gene expression deficits in pontine locus coeruleus astrocytes in men with major depressive disorder. J. Psychiatry Neurosci. JPN 2013, 38, 276–284. [Google Scholar] [CrossRef]
- Medina, A.; Watson, S.J.; Bunney, W., Jr.; Myers, R.M.; Schatzberg, A.; Barchas, J.; Akil, H.; Thompson, R.C. Evidence for alterations of the glial syncytial function in major depressive disorder. J. Psychiatr. Res. 2016, 72, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Miguel-Hidalgo, J.J.; Wilson, B.A.; Hussain, S.; Meshram, A.; Rajkowska, G.; Stockmeier, C.A. Reduced connexin 43 immunolabeling in the orbitofrontal cortex in alcohol dependence and depression. J. Psychiatr. Res. 2014, 55, 101–109. [Google Scholar] [CrossRef]
- Yang, K.; Xie, G.-R.; Hu, Y.-Q.; Mao, F.-Q.; Su, L.-Y. Association Study of Astrocyte-Derived Protein S100B Gene Polymorphisms with Major Depressive Disorder in Chinese People. Can. J. Psychiatry 2009, 54, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-K.; Kim, S.G.; Kim, H.J.; Song, Y.R. Serum S100B protein is associated with depressive symptoms in patients with end-stage renal disease. Clin. Biochem. 2012, 45, 1573–1577. [Google Scholar] [CrossRef]
- Tulner, D.M.; Smith, O.R.F.; de Jonge, P.; van Melle, J.P.; Slomp, J.; Storm, H.; Quere, M.; den Boer, J.A.; Honig, A.; Korf, J. Circulating Cerebral S100B Protein Is Associated with Depressive Symptoms following Myocardial Infarction. Neuropsychobiology 2009, 59, 87–95. [Google Scholar] [CrossRef]
- Benitez, A.; Gunstad, J.; Hughes, J.; Glickman, E.; Alexander, T.; Spitznagel, M.B.; Juvancic-Heltzel, J.; Murray, L. Troponin and S100β are associated with depression in healthy older adults. Aging Ment. Health 2009, 13, 894–898. [Google Scholar] [CrossRef]
- Gulen, B.; Serinken, M.; Eken, C.; Karcıoglu, Ö.; Kucukdagli, O.T.; Kilic, E.; Akpinar, G.; Nogay, S.; Kuh, M. Serum S100B as a Surrogate Biomarker in the Diagnoses of Burnout and Depression in Emergency Medicine Residents. Acad. Emerg. Med. 2016, 23, 786–789. [Google Scholar] [CrossRef]
- Li, X.; Wilder-Smith, C.H.; Kan, M.E.; Lu, J.; Cao, Y.; Wong, R.K. Combat-training stress in soldiers increases S100B, a marker of increased blood-brain-barrier permeability, and induces immune activation. Neuro Endocrinol. Lett. 2014, 35, 58–63. [Google Scholar]
- Tural, U.; Irvin, M.K.; Iosifescu, D.V. Correlation between S100B and severity of depression in MDD: A meta-analysis. World J. Biol. Psychiatry 2021, 23, 456–463. [Google Scholar] [CrossRef]
- Schroeter, M.L.; Abdul-Khaliq, H.; Krebs, M.; Diefenbacher, A.; Blasig, I.E. Serum markers support disease-specific glial pathology in major depression. J. Affect. Disord. 2008, 111, 271–280. [Google Scholar] [CrossRef]
- Arolt, V.; Peters, M.; Erfurth, A.; Wiesmann, M.; Missler, U.; Rudolf, S.; Kirchner, H.; Rothermundt, M. S100B and response to treatment in major depression: A pilot study. Eur. Neuropsychopharmacol. 2003, 13, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Navinés, R.; Oriolo, G.; Horrillo, I.; Cavero, M.; Aouizerate, B.; Schaefer, M.; Capuron, L.; Meana, J.J.; Martin-Santos, R. High S100B Levels Predict Antidepressant Response in Patients with Major Depression Even When Considering Inflammatory and Metabolic Markers. Int. J. Neuropsychopharmacol. 2022, 25, 468–478. [Google Scholar] [CrossRef]
- Michel, M.; Fiebich, B.L.; Kuzior, H.; Meixensberger, S.; Berger, B.; Maier, S.; Nickel, K.; Runge, K.; Denzel, D.; Pankratz, B.; et al. Increased GFAP concentrations in the cerebrospinal fluid of patients with unipolar depression. Transl. Psychiatry 2021, 11, 308. [Google Scholar] [CrossRef] [PubMed]
- Endres, D.; Lerchenmüller, V.; Runge, K.; von Zedtwitz, K.; Nickel, K.; Urbach, H.; Domschke, K.; Prüss, H.; Tebartz van Elst, L. Anti-astrocytic autoantibody patterns in the cerebrospinal fluid of patients with depression and psychosis. Psychiatry Res. 2022, 317, 114905. [Google Scholar] [CrossRef]
- Steinacker, P.; Al Shweiki, M.H.D.R.; Oeckl, P.; Graf, H.; Ludolph, A.C.; Schönfeldt-Lecuona, C.; Otto, M. Glial fibrillary acidic protein as blood biomarker for differential diagnosis and severity of major depressive disorder. J. Psychiatr. Res. 2021, 144, 54–58. [Google Scholar] [CrossRef]
- Wallensten, J.; Nager, A.; Åsberg, M.; Borg, K.; Beser, A.; Wilczek, A.; Mobarrez, F. Leakage of astrocyte-derived extracellular vesicles in stress-induced exhaustion disorder: A cross-sectional study. Sci. Rep. 2021, 11, 2009. [Google Scholar] [CrossRef]
- Qi, X.-R.; Kamphuis, W.; Shan, L. Astrocyte Changes in the Prefrontal Cortex From Aged Non-suicidal Depressed Patients. Front. Cell. Neurosci. 2019, 13, 503. [Google Scholar] [CrossRef]
- Tanaka, M.; Yamaguchi, K.; Tatsukawa, T.; Nishioka, C.; Nishiyama, H.; Theis, M.; Willecke, K.; Itohara, S. Lack of Connexin43-mediated bergmann glial gap junctional coupling does not affect cerebellar long-term depression, motor coordination, or eyeblink conditioning. Front. Behav. Neurosci. 2008, 2, 1. [Google Scholar] [CrossRef]
- Torres-Platas, S.G.; Hercher, C.; Davoli, M.A.; Maussion, G.; Labonté, B.; Turecki, G.; Mechawar, N. Astrocytic hypertrophy in anterior cingulate white matter of depressed suicides. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2011, 36, 2650–2658. [Google Scholar] [CrossRef] [PubMed]
- Schlicht, K.; Büttner, A.; Siedler, F.; Scheffer, B.; Zill, P.; Eisenmenger, W.; Ackenheil, M.; Bondy, B. Comparative proteomic analysis with postmortem prefrontal cortex tissues of suicide victims versus controls. J. Psychiatr. Res. 2007, 41, 493–501. [Google Scholar] [CrossRef]
- Torres-Platas, S.G.; Nagy, C.; Wakid, M.; Turecki, G.; Mechawar, N. Glial fibrillary acidic protein is differentially expressed across cortical and subcortical regions in healthy brains and downregulated in the thalamus and caudate nucleus of depressed suicides. Mol. Psychiatry 2015, 21, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Nagy, C.; Suderman, M.; Yang, J.; Szyf, M.; Mechawar, N.; Ernst, C.; Turecki, G. Astrocytic abnormalities and global DNA methylation patterns in depression and suicide. Mol. Psychiatry 2015, 20, 320–328. [Google Scholar] [CrossRef]
- O’Leary, L.A.; Belliveau, C.; Davoli, M.A.; Ma, J.C.; Tanti, A.; Turecki, G.; Mechawar, N. Widespread Decrease of Cerebral Vimentin-Immunoreactive Astrocytes in Depressed Suicides. Front. Psychiatry 2021, 12, 640963. [Google Scholar] [CrossRef]
- Zhang, L.; Verwer, R.W.H.; Zhao, J.; Huitinga, I.; Lucassen, P.J.; Swaab, D.F. Changes in glial gene expression in the prefrontal cortex in relation to major depressive disorder, suicide and psychotic features. J. Affect. Disord. 2021, 295, 893–903. [Google Scholar] [CrossRef]
- Zhang, L.; Verwer, R.W.H.; Lucassen, P.J.; Huitinga, I.; Swaab, D.F. Prefrontal cortex alterations in glia gene expression in schizophrenia with and without suicide. J. Psychiatr. Res. 2020, 121, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Maussion, G.; Yang, J.; Suderman, M.; Diallo, A.; Nagy, C.; Arnovitz, M.; Mechawar, N.; Turecki, G. Functional DNA methylation in a transcript specific 3’UTR region of TrkB associates with suicide. Epigenetics 2014, 9, 1061–1070. [Google Scholar] [CrossRef]
- Maussion, G.; Yang, J.; Yerko, V.; Barker, P.; Mechawar, N.; Ernst, C.; Turecki, G. Regulation of a truncated form of tropomyosin-related kinase B (TrkB) by Hsa-miR-185* in frontal cortex of suicide completers. PLoS ONE 2012, 7, e39301. [Google Scholar] [CrossRef]
- Pantazatos, S.P.; Huang, Y.Y.; Rosoklija, G.B.; Dwork, A.J.; Arango, V.; Mann, J.J. Whole-transcriptome brain expression and exon-usage profiling in major depression and suicide: Evidence for altered glial, endothelial and ATPase activity. Mol. Psychiatry 2017, 22, 760–773. [Google Scholar] [CrossRef]
- Ernst, C.; Nagy, C.; Kim, S.; Yang, J.P.; Deng, X.; Hellstrom, I.C.; Choi, K.H.; Gershenfeld, H.; Meaney, M.J.; Turecki, G. Dysfunction of Astrocyte Connexins 30 and 43 in Dorsal Lateral Prefrontal Cortex of Suicide Completers. Biol. Psychiatry 2011, 70, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Nagy, C.; Torres-Platas, S.G.; Mechawar, N.; Turecki, G. Repression of Astrocytic Connexins in Cortical and Subcortical Brain Regions and Prefrontal Enrichment of H3K9me3 in Depression and Suicide. Int. J. Neuropsychopharmacol. 2017, 20, 50–57. [Google Scholar] [CrossRef]
- Tanti, A.; Lutz, P.-E.; Kim, J.; O’Leary, L.; Théroux, J.-F.; Turecki, G.; Mechawar, N. Evidence of decreased gap junction coupling between astrocytes and oligodendrocytes in the anterior cingulate cortex of depressed suicides. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2019, 44, 2099–2111. [Google Scholar] [CrossRef] [PubMed]
- Ventorp, F.; Barzilay, R.; Erhardt, S.; Samuelsson, M.; Träskman-Bendz, L.; Janelidze, S.; Weizman, A.; Offen, D.; Brundin, L. The CD44 ligand hyaluronic acid is elevated in the cerebrospinal fluid of suicide attempters and is associated with increased blood–brain barrier permeability. J. Affect. Disord. 2016, 193, 349–354. [Google Scholar] [CrossRef]
- Dogan, K.H.; Unaldi, M.; Demirci, S. Evaluation of Postmortem Cerebrospinal Fluid S100B Protein and Serotonin Levels: Comparison of Suicidal Versus Nonsuicidal Deaths in Konya, Turkey. J. Forensic Sci. 2016, 61, 1285–1291. [Google Scholar] [CrossRef]
- Falcone, T.; Fazio, V.; Lee, C.; Simon, B.; Franco, K.; Marchi, N.; Janigro, D. Serum S100B: A potential biomarker for suicidality in adolescents? PLoS ONE 2010, 5, e11089. [Google Scholar] [CrossRef]
- Lu, C.L.; Ren, J.; Cao, X. An astroglial basis of major depressive disorder: Molecular, Cellular, and Circuit Features. Biol. Psychiatry 2024. online ahead of print. [Google Scholar] [CrossRef]
- Boroujeni, M.E.; Simani, L.; Bluyssen, H.A.R.; Samadikhah, H.R.; Zamanlui Benisi, S.; Hassani, S.; Akbari Dilmaghani, N.; Fathi, M.; Vakili, K.; Mahmoudiasl, G.R.; et al. Inflammatory Response Leads to Neuronal Death in Human Post-Mortem Cerebral Cortex in Patients with COVID-19. ACS Chem. Neurosci. 2021, 12, 2143–2150. [Google Scholar] [CrossRef]
- Reichard, R.R.; Kashani, K.B.; Boire, N.A.; Constantopoulos, E.; Guo, Y.; Lucchinetti, C.F. Neuropathology of COVID-19: A spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020, 140, 1–6. [Google Scholar] [CrossRef]
- Lee, M.-H.; Perl, D.P.; Nair, G.; Li, W.; Maric, D.; Murray, H.; Dodd, S.J.; Koretsky, A.P.; Watts, J.A.; Cheung, V.; et al. Microvascular Injury in the Brains of Patients with Covid-19. N. Engl. J. Med. 2021, 384, 481–483. [Google Scholar] [CrossRef]
- Aceti, A.; Margarucci, L.M.; Scaramucci, E.; Orsini, M.; Salerno, G.; Di Sante, G.; Gianfranceschi, G.; Di Liddo, R.; Valeriani, F.; Ria, F.; et al. Serum S100B protein as a marker of severity in Covid-19 patients. Sci. Rep. 2020, 10, 18665. [Google Scholar] [CrossRef] [PubMed]
- Mete, E.; Sabirli, R.; Goren, T.; Turkcuer, I.; Kurt, Ö.; Koseler, A. Association between S100b Levels and COVID-19 Pneumonia: A Case Control Study. Vivo 2021, 35, 2923–2928. [Google Scholar] [CrossRef]
- Silva, R.C.; da Rosa, M.M.; Leão, H.I.; Silva, E.D.L.; Ferreira, N.T.; Albuquerque, A.P.B.; Duarte, G.S.; Siqueira, A.M.; Pereira, M.C.; Rêgo, M.J.B.M.; et al. Brain damage serum biomarkers induced by COVID-19 in patients from northeast Brazil. J. Neurovirology 2023, 29, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Kokkoris, S.; Stamataki, E.; Emmanouil, G.; Psachoulia, C.; Ntaidou, T.; Maragouti, A.; Kanavou, A.; Malachias, S.; Christodouli, F.; Papachatzakis, I.; et al. Serum inflammatory and brain injury biomarkers in COVID-19 patients admitted to intensive care unit: A pilot study. eNeurologicalSci 2022, 29, 100434. [Google Scholar] [CrossRef]
- Sahin, B.E.; Celikbilek, A.; Kocak, Y.; Saltoglu, G.T.; Konar, N.M.; Hizmali, L. Plasma biomarkers of brain injury in COVID-19 patients with neurological symptoms. J. Neurol. Sci. 2022, 439, 120324. [Google Scholar] [CrossRef]
- Passos, F.R.S.; Heimfarth, L.; Monteiro, B.S.; Corrêa, C.B.; Moura TRd Araújo, A.A.d.S.; Martins-Filho, P.R.; Quintans-Júnior, L.J.; Quintans, J.S.S. Oxidative stress and inflammatory markers in patients with COVID-19: Potential role of RAGE, HMGB1, GFAP and COX-2 in disease severity. Int. Immunopharmacol. 2022, 104, 108502. [Google Scholar] [CrossRef]
- Frithiof, R.; Rostami, E.; Kumlien, E.; Virhammar, J.; Fällmar, D.; Hultström, M.; Lipcsey, M.; Ashton, N.; Blennow, K.; Zetterberg, H.; et al. Critical illness polyneuropathy, myopathy and neuronal biomarkers in COVID-19 patients: A prospective study. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2021, 132, 1733–1740. [Google Scholar] [CrossRef] [PubMed]
- Tokic, D.; Mikacic, M.; Kumric, M.; Ticinovic Kurir, T.; Rancic, I.; Martinovic, D.; Bukic, J.; Vrdoljak, J.; Lizatovic, I.K.; Stipic, S.S.; et al. Association between Brain Injury Markers and Testosterone in Critically-Ill COVID-19 Male Patients. Microorganisms 2022, 10, 2095. [Google Scholar] [CrossRef]
- Plantone, D.; Locci, S.; Bergantini, L.; Manco, C.; Cortese, R.; Meocci, M.; Cavallaro, D.; d’Alessandro, M.; Bargagli, E.; De Stefano, N. Brain neuronal and glial damage during acute COVID-19 infection in absence of clinical neurological manifestations. J. Neurol. Neurosurg. Psychiatry 2022, 93, 1343–1348. [Google Scholar] [CrossRef]
- Lennol, M.P.; Ashton, N.J.; Moreno-Pérez, O.; García-Ayllón, M.-S.; Ramos-Rincon, J.-M.; Andrés, M.; León-Ramírez, J.M.; Boix, V.; Gil, J.; Blennow, K.; et al. Transient Changes in the Plasma of Astrocytic and Neuronal Injury Biomarkers in COVID-19 Patients without Neurological Syndromes. Int. J. Mol. Sci. 2023, 24, 2715. [Google Scholar] [CrossRef]
- Bonetto, V.; Pasetto, L.; Lisi, I.; Carbonara, M.; Zangari, R.; Ferrari, E.; Punzi, V.; Luotti, S.; Bottino, N.; Biagianti, B.; et al. Markers of blood-brain barrier disruption increase early and persistently in COVID-19 patients with neurological manifestations. Front. Immunol. 2022, 13, 1070379. [Google Scholar] [CrossRef] [PubMed]
- Savarraj, J.; Park, E.S.; Colpo, G.D.; Hinds, S.N.; Morales, D.; Ahnstedt, H.; Paz, A.S.; Assing, A.; Liu, F.; Juneja, S.; et al. Brain injury, endothelial injury and inflammatory markers are elevated and express sex-specific alterations after COVID-19. J. Neuroinflamm. 2021, 18, 277. [Google Scholar] [CrossRef] [PubMed]
- de Boni, L.; Odainic, A.; Gancarczyk, N.; Kaluza, L.; Strassburg, C.P.; Kersting, X.A.K.; Johnson, J.M.; Wüllner, U.; Schmidt, S.V.; Nattermann, J.; et al. No serological evidence for neuronal damage or reactive gliosis in neuro-COVID-19 patients with long-term persistent headache. Neurol. Res. Pract. 2022, 4, 53. [Google Scholar] [CrossRef] [PubMed]
- Kanberg, N.; Simrén, J.; Edén, A.; Andersson, L.-M.; Nilsson, S.; Ashton, N.J.; Sundvall, P.D.; Nellgård, B.; Blennow, K.; Zetterberg, H.; et al. Neurochemical signs of astrocytic and neuronal injury in acute COVID-19 normalizes during long-term follow-up. EBioMedicine 2021, 70, 103512. [Google Scholar] [CrossRef]
- Spanos, M.; Shachar, S.; Sweeney, T.; Lehmann, H.I.; Gokulnath, P.; Li, G.; Sigal, G.B.; Nagaraj, R.; Bathala, P.; Rana, F.; et al. Elevation of neural injury markers in patients with neurologic sequelae after hospitalization for SARS-CoV-2 infection. iScience 2022, 25, 104833. [Google Scholar] [CrossRef]
- Hanson, B.A.; Visvabharathy, L.; Ali, S.T.; Kang, A.K.; Patel, T.R.; Clark, J.R.; Lim, P.H.; Orban, Z.S.; Hwang, S.S.; Mattoon, D.; et al. Plasma Biomarkers of Neuropathogenesis in Hospitalized Patients with COVID-19 and Those with Postacute Sequelae of SARS-CoV-2 Infection. Neurol. (R) Neuroimmunol. Neuroinflamm. 2022, 9, e1151. [Google Scholar] [CrossRef]
- Peluso, M.J.; Sans, H.M.; Forman, C.A.; Nylander, A.N.; Ho, H.-E.; Lu, S.; Goldberg, S.A.; Hoh, R.; Tai, V.; Munter, S.E.; et al. Plasma Markers of Neurologic Injury and Inflammation in People with Self-Reported Neurologic Postacute Sequelae of SARS-CoV-2 Infection. Neurol. (R) Neuroimmunol. Neuroinflamm. 2022, 9, e200003. [Google Scholar] [CrossRef]
- Thaweethai, T.; Jolley, S.E.; Karlson, E.W.; Levitan, E.B.; Levy, B.; McComsey, G.A.; McCorkell, L.; Nadkarni, G.N.; Parthasarathy, S.; Singh, U.; et al. Development of a Definition of Postacute Sequelae of SARS-CoV-2 Infection. JAMA 2023, 329, 1934–1946. [Google Scholar] [CrossRef]
- Yang, A.C.; Kern, F.; Losada, P.M.; Agam, M.R.; Maat, C.A.; Schmartz, G.P.; Fehlmann, T.; Stein, J.A.; Schaum, N.; Lee, D.P.; et al. Dysregulation of brain and choroid plexus cell types in severe COVID-19. Nature 2021, 595, 565–571. [Google Scholar] [CrossRef]
- Bussani, R.; Zentilin, L.; Correa, R.; Colliva, A.; Silvestri, F.; Zacchigna, S.; Collesi, C.; Giacca, M. Persistent SARS-CoV-2 infection in patients seemingly recovered from COVID-19. J. Pathol. 2023, 259, 254–263. [Google Scholar] [CrossRef]
- Virhammar, J.; Kumlien, E.; Fällmar, D.; Frithiof, R.; Jackmann, S.; Sköld, M.K.; Kadir, M.; Frick, J.; Lindeberg, J.; Olivero-Reinius, H.; et al. Acute necrotizing encephalopathy with SARS-CoV-2 RNA confirmed in cerebrospinal fluid. Neurology 2020, 95, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Andrews, M.G.; Mukhtar, T.; Eze, U.C.; Simoneau, C.R.; Ross, J.; Parikshak, N.; Wang, S.; Zhou, L.; Koontz, M.; Velmeshev, D.; et al. Tropism of SARS-CoV-2 for human cortical astrocytes. Proc. Natl. Acad. Sci. USA 2022, 119, e2122236119. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Fishell, G. In SARS-CoV-2, astrocytes are in it for the long haul. Proc. Natl. Acad. Sci. USA 2022, 119, e2209130119. [Google Scholar] [CrossRef]
- Rosu, G.C.; Mateescu, V.O.; Simionescu, A.; Istrate-Ofiteru, A.-M.; Curcă, G.C.; Pirici, I.; Mogoanta, L.; Mindrila, I.; Kumar-Singh, S.; Hostiuc, S.; et al. Subtle vascular and astrocytic changes in the brain of coronavirus disease 2019 (COVID-19) patients. Eur. J. Neurol. 2022, 29, 3676–3692. [Google Scholar] [CrossRef]
- Etter, M.M.; Martins, T.A.; Kulsvehagen, L.; Pössnecker, E.; Duchemin, W.; Hogan, S.; Sanabria-Diaz, G.; Müller, J.; Chiappini, A.; Rychen, J.; et al. Severe Neuro-COVID is associated with peripheral immune signatures, autoimmunity and neurodegeneration: A prospective cross-sectional study. Nat. Commun. 2022, 13, 6777. [Google Scholar] [CrossRef]
- Serafini, G.; Costanza, A.; Aguglia, A.; Amerio, A.; Trabucco, A.; Escelsior, A.; Sher, L.; Amore, M. The Role of Inflammation in the Pathophysiology of Depression and Suicidal Behavior. Med. Clin. N. Am. 2023, 107, 1–29. [Google Scholar] [CrossRef]
- Pompili, M.; Gentile, G.; Scassellati, C.; Bonvicini, C.; Innamorati, M.; Erbuto, D.; Montebovi, F.; Ducci, G.; Forte, A.; De Pisa, E.; et al. Genetic association analysis of serotonin and signal transduction pathways in suicide attempters from an Italian sample of psychiatric patients. Neurosci. Lett. 2017, 656, 94–102. [Google Scholar] [CrossRef]
- Patel, U.K.; Mehta, N.; Patel, A.; Patel, N.; Ortiz, J.F.; Khurana, M.; Urhoghide, E.; Parulekar, A.; Bhriguvanshi, A.; Patel, N.; et al. Long-Term Neurological Sequelae Among Severe COVID-19 Patients: A Systematic Review and Meta-Analysis. Cureus 2022, 14, e29694. [Google Scholar] [CrossRef]
- Gasnier, M.; Choucha, W.; Radiguer, F.; Faulet, T.; Chappell, K.; Bougarel, A.; Kondarjian, C.; Thorey, P.; Baldacci, A.; Ballerini, M.; et al. Comorbidity of long COVID and psychiatric disorders after a hospitalisation for COVID-19: A cross-sectional study. J. Neurol. Neurosurg. Psychiatry 2022, 93, 1091–1098. [Google Scholar] [CrossRef]
- Woodward, S.F.; Bari, S.; Vike, N.; Lalvani, S.; Stetsiv, K.; Kim, B.W.; Stefanopoulos, L.; Maglaveras, N.; Breiter, H.; Katsaggelos, A.K. Anxiety, Post-COVID-19 Syndrome-Related Depression, and Suicidal Thoughts and Behaviors in COVID-19 Survivors: Cross-sectional Study. JMIR Form. Res. 2022, 6, e36656. [Google Scholar] [CrossRef]
- Chaumont, H.; Meppiel, E.; Roze, E.; Tressières, B.; de Broucker, T.; Lannuzel, A.; contributors to the French NeuroCOVID registry. Long-term outcomes after NeuroCOVID: A 6-month follow-up study on 60 patients. Rev. Neurol. 2022, 178, 137–143. [Google Scholar] [CrossRef]
- Damiano, R.F.; Caruso, M.J.G.; Cincoto, A.V.; de Almeida Rocca, C.C.; de Pádua Serafim, A.; Bacchi, P.; Guedes, B.F.; Brunoni, A.R.; Pan, P.M.; Nitrini, R.; et al. Post-COVID-19 psychiatric and cognitive morbidity: Preliminary findings from a Brazilian cohort study. Gen. Hosp. Psychiatry 2022, 75, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Paradowska-Nowakowska, E.; Łoboda, D.; Gołba, K.S.; Sarecka-Hujar, B. Long COVID-19 Syndrome Severity According to Sex, Time from the Onset of the Disease, and Exercise Capacity-The Results of a Cross-Sectional Study. Life 2023, 13, 508. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, H.Y.; Alrashed, A.M.; Jawhari, A.M.; Abdel-Moneim, A.S. Neuropsychiatric symptoms in post-COVID-19 long haulers. Acta Neuropsychiatr. 2022, 34, 318–329. [Google Scholar] [CrossRef]
- Magnúsdóttir, I.; Lovik, A.; Unnarsdóttir, A.B.; McCartney, D.; Ask, H.; Kõiv, K.; Christoffersen, L.A.N.; Johnson, S.U.; Hauksdóttir, A.; Fawns-Ritchie, C.; et al. Acute COVID-19 severity and mental health morbidity trajectories in patient populations of six nations: An observational study. Lancet. Public Health 2022, 7, e406–e416. [Google Scholar] [CrossRef]
- Su, W.; Ju, J.; Gu, M.; Wang, X.; Liu, S.; Yu, J.; Mu, D. SARS-CoV-2 envelope protein triggers depression-like behaviors and dysosmia via TLR2-mediated neuroinflammation in mice. J. Neuroinflamm. 2023, 20, 110. [Google Scholar] [CrossRef]
- Paidas, M.J.; Cosio, D.S.; Ali, S.; Kenyon, N.S.; Jayakumar, A.R. Long-Term Sequelae of COVID-19 in Experimental Mice. Mol. Neurobiol. 2022, 59, 5970–5986. [Google Scholar] [CrossRef]
- Nasirpour, N.; Esmailzadehha, N.; Hajebi, A.; Savari, E.; Ghanbari, B.; Motevalian, A. Preexisting depression and COVID-19: A cohort study on the risk of susceptibility and hospitalization. BMC Psychiatry 2023, 23, 942. [Google Scholar] [CrossRef]
- Greenblatt-Kimron, L.; Shinan-Altman, S.; Alperin, M.; Levkovich, I. Depression and Medicine Use among Older Adults during the COVID-19 Pandemic: The Role of Psychosocial Resources and COVID-19 Perceived Susceptibility. Int. J. Env. Res. Public Health 2023, 20, 3398. [Google Scholar] [CrossRef]
- Delgado-Alonso, C.; Valles-Salgado, M.; Delgado-Álvarez, A.; Gómez-Ruiz, N.; Yus, M.; Polidura, C.; Pérez-Izquierdo, C.; Marcos, A.; Gil, M.J.; Matías-Guiu, J.; et al. Examining Association of Personality Characteristics and Neuropsychiatric Symptoms in Post-COVID Syndrome. Brain Sci. 2022, 12, 265. [Google Scholar] [CrossRef]
- Kazemi Arababadi, M.; Abdollahi, S.H.; Ramezani, M.; Zare-Bidaki, M. A Review of Immunological and Neuropsychobehavioral Effects of Latent Toxoplasmosis on Humans. Parasite Immunol. 2024, 46, e13060. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.J.; Lim, S.S.; Yoon, J.H. Fluctuations in influenza-like illness epidemics and suicide mortality: A time-series regression of 13-year mortality data in South Korea. PLoS ONE 2021, 16, e0244596. [Google Scholar] [CrossRef] [PubMed]
- Yip, P.S.; Cheung, Y.T.; Chau, P.H.; Law, Y.W. The impact of epidemic outbreak: The case of severe acute respiratorysyndrome (SARS) and suicide among older adults in Hong Kong. Crisis 2010, 31, 86–92. [Google Scholar] [CrossRef]
- Ahn, S.H.; Kim, J.L.; Kim, J.R.; Lee, S.H.; Yim, H.W.; Jeong, H.; Chae, J.H.; Park, H.Y.; Lee, J.J.; Lee, H. Association between chronic fatigue syndrome and suicidality among survivors of Middle East respiratory syndrome over a 2-year follow-up period. J. Psychiatr. Res. 2021, 137, 1–6. [Google Scholar] [CrossRef]
- Ahn, S.H.; Kim, J.L.; Lee, S.H.; Park, H.Y.; Lee, J.J.; Lee, H. Associations of health-related quality of life with depression and stigma in MERS-CoV survivors during the recovery period. Medicine 2022 2022, 101, e29440. [Google Scholar] [CrossRef]
- Boden, M.; Cohen, N.; Froelich, J.M.; Hoggatt, K.J.; Abdel Magid, H.S.; Mushiana, S.S. Mental disorder prevalence among populations impacted by coronavirus pandemics: A multilevel meta-analytic study of COVID-19, MERS & SARS. Gen. Hosp. Psychiatry 2021, 70, 124–133. [Google Scholar] [CrossRef]
- Kahil, K.; Cheaito, M.A.; El Hayek, R.; Nofal, M.; El Halabi, S.; Pereira-Sanchez, V.; El Hayek, S. Suicide during COVID-19 and other major international respiratory outbreaks: A systematic review. Asian J. Psychiatr. 2021, 56, 102509. [Google Scholar] [CrossRef]
- Rogers, J.P.; Chesney, E.; Oliver, D.; Begum, N.; Saini, A.; Wang, S.; McGuire, P.; Fusar-Poli, P.; Lewis, G.; David, A.S. Suicide, self-harm and thoughts of suicide or self-harm in infectious disease epidemics: A systematic review and meta-analysis. Epidemiol. Psychiatr. Sci. 2021, 30, e32. [Google Scholar] [CrossRef]
- Pagonabarraga, J.; Álamo, C.; Castellanos, M.; Díaz, S.; Manzano, S. Depression in Major Neurodegenerative Diseases and Strokes: A Critical Review of Similarities and Differences among Neurological Disorders. Brain Sci. 2023, 13, 318. [Google Scholar] [CrossRef]
- Erlangsen, A.; Stenager, E.; Conwell, Y.; Andersen, P.K.; Hawton, K.; Benros, M.E.; Nordentoft, M.; Stenager, E. Association between Neurological Disorders and Death by Suicide in Denmark. JAMA 2020, 323, 444–454. [Google Scholar] [CrossRef]
- Song, H.; Lei, N.; Zeng, L.; Li, X.; Li, X.; Liu, Y.; Liu, J.; Wu, W.; Mu, J.; Feng, Q. Genetic predisposition to subjective well-being, depression, and suicide in relation to COVID-19 susceptibility and severity. J. Affect. Disord. 2023, 335, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.C.; Devason, A.S.; Umana, I.C.; Cox, T.O.; Dohnalová, L.; Litichevskiy, L.; Perla, J.; Lundgren, P.; Etwebi, Z.; Izzo, L.T.; et al. Serotonin reduction in post-acute sequelae of viral infection. Cell 2023, 186, 4851–4867. [Google Scholar] [CrossRef] [PubMed]
- Yohn, C.N.; Gergues, M.M.; Samuels, B.A. The role of 5-HT receptors in depression. Mol. Brain 2017, 10, 28. [Google Scholar] [CrossRef] [PubMed]
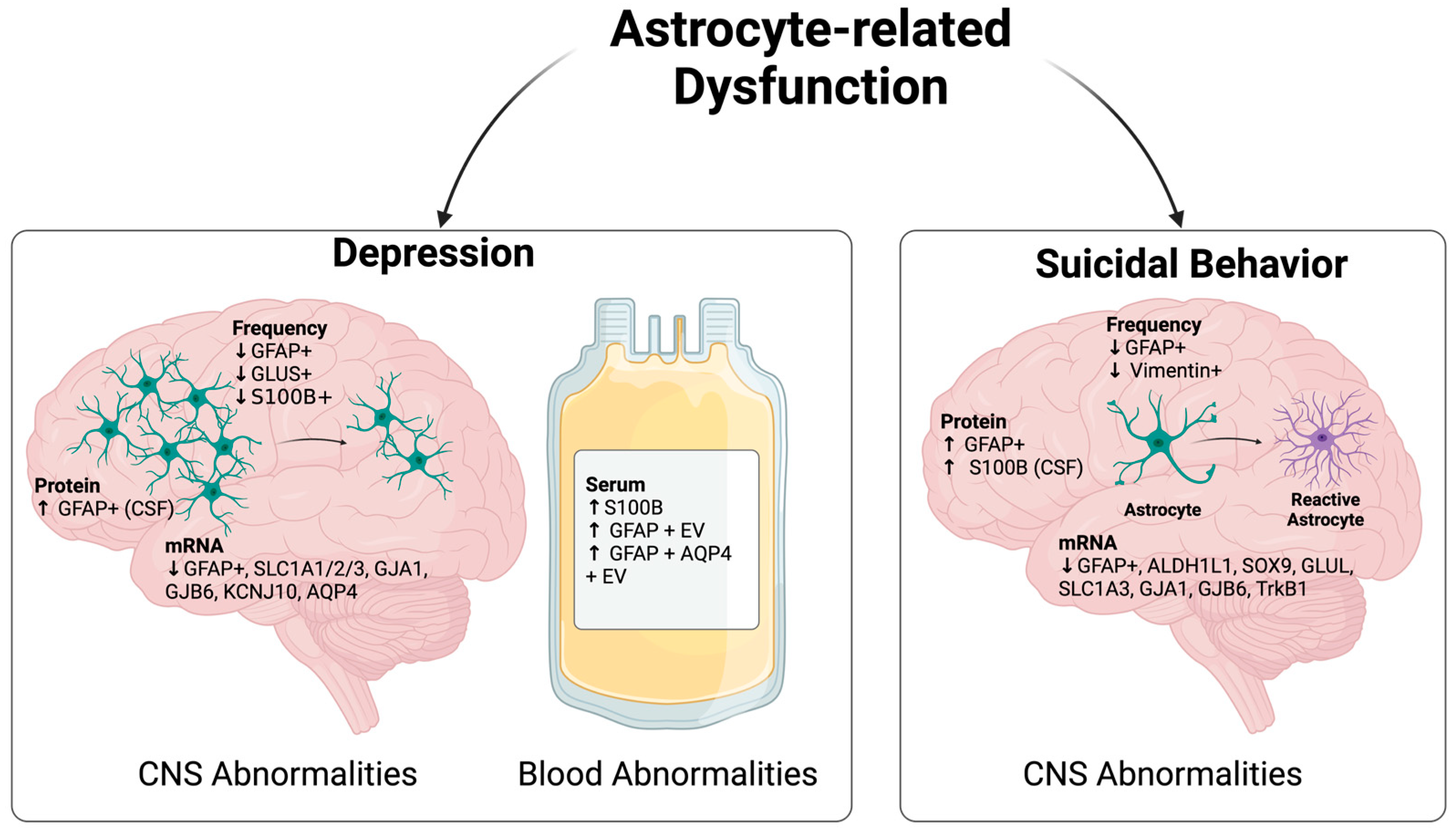
| Study Type and Tissue | Study Methods | Study Participants | Major Findings | |
|---|---|---|---|---|
| Bernstein et al. [26] | Post-mortem brain biopsy | Immunohistochemistry | MDD (n = 14) vs. HC (n = 16) | Reduced GLUS+ astrocytes in DLPFC, sACC, and AiC in MDD compared to HC |
| Gos et al. [27] | Post-mortem brain biopsy | Immunohistochemistry | MDD (n = 9) vs. HC (n = 13) | Reduced S100B+ astrocytes in pyramidal CA1 in MDD compared to HC |
| Cobb et al. [28] | Post-mortem brain biopsy | Immunohistochemistry | MDD (n = 17) vs. HC (n = 17) | Reduced GFAP+ astrocytes in hippocampal CA1/2/3 and dentate gyrus in MDD compared to HC |
| Davis et al. [29] | Post-mortem brain biopsy | Immunohistochemistry | MDD (n = 20) vs. HC (n = 20) | Reduced GFAP+ astrocytes in cortical gray matter and increased GFAP+ astrocytes in PFC white matter in MDD compared to HC |
| Oh et al. [30] | Post-mortem brain biopsy | Immunohistochemistry | MDD (n = 15) vs. HC (n = 15) | Reduced GFAP correlated positively with GABA+ neuron density and negatively with glutamate concentration in PFC of MDD patients. |
| Webster et al. [32] | Post-mortem brain biopsy | Immunohistochemistry | MDD (n = 15) vs. HC (n = 15) | Reduced phosphorylated GFAP+ astrocytes in PFC in MDD compared to HC |
| Chandley et al. [33] | Post-mortem brain biopsy | Immunohistochemistry, gene expression | MDD (n = 19) vs. HC (n = 20) | Reduced GFAP and SLCA1/2/3 mRNA as well as GFAP protein in locus coeruleus and/or hippocampus in MDD compared to HC |
| Medina et al. [34] | Post-mortem brain biopsy | Gene expression | MDD (n = 13) vs. HC (n = 10) | Decreased KCNJ10, AQP4, GJA1, and SLC1A2/3 mRNA in hippocampus in MDD compared to HC |
| Miguel-Hidalgo et al. [35] | Post-mortem brain biopsy | Immunohistochemistry | MDD (n = 23) vs. HC (n = 20) | Decreased GJA1 and GJB6 protein in OFC in MDD compared to HC |
| Yang et al. [36] | Blood | Genetic polymorphism | MDD (n = 152) vs. HC (n = 150) | No association between S100B polymorphism and MDD |
| Kim et al. [37] | Serum | ELISA | End-stage renal disease (n = 78) | Higher S100B was linked to depressive symptoms. |
| Tulner et al. [38] | Serum | ELISA | Myocardial infarction (n = 48) | Higher S100B was linked to depressive symptoms. |
| Benitez et al. [39] | Serum | ELISA | Elderly HC (n = 35) | Higher S100B was linked to depressive symptoms. |
| Gulen et al. [40] | Serum | ELISA | Burned-out medical residents (n = 48) | Higher S100B was linked to depressive symptoms. |
| Li et al. [41] | Serum | ELISA | Combat trainees (n = 37) | Higher S100B was linked to depressive symptoms. |
| Tural et al. [42] | Serum | Meta-analysis | MDD (n = 658) | Higher S100B was linked to depression severity, age of MDD onset, and female sex. |
| Schroeter et al. [43] | Serum | ELISA | MDD (n = 193) vs. HC (n = 131) | Higher S100B in MDD compared to HC; S100B levels decrease after antidepressant treatment correlated with clinical improvement. |
| Arolt et al. [44] | Serum | ELISA | MDD (n = 25) vs. HC (n = 25) | Higher S100B in MDD compared to HC; S100B levels correlated positively with treatment response. |
| Navines et al. [45] | Serum | ELISA | MDD (n = 27) | S100B levels correlated positively with treatment response. |
| Michel et al. [46] | CSF | ELISA | Unipolar depression (n = 102) vs. HC (n = 39) | Higher GFAP in patients compared to HC |
| Endres et al. [47] | CSF | ELISA | MDD with psychosis (n = 2) | Higher GFAP autoantibodies in patients compared to HC |
| Steinacker et al. [48] | Serum | ELISA | MDD (n = 81) vs. HC (n = 81) | Higher GFAP in MDD compared to HC; GFAP levels positively correlated with disease severity. |
| Wallensten et al. [49] | Plasma | ELISA | MDD (n = 31) vs. HC (n = 61) | Increased extracellular vesicles co-expressing AQP4/GFAP in MDD compared to HC |
| Qi et al. [50] | Post-mortem brain biopsy | Immunohistochemistry, gene expression | MDD (n = 5) vs. HC (n = 12) | No differences in GFAP mRNA and protein expression in DLPFC of MDD compared to HC |
| Study Type and Tissue | Study Methods | Study Participants | Major Findings | |
|---|---|---|---|---|
| Torres-Platas et al. [52] | Post-mortem brain biopsy | Immunohistochemistry | Depressed suicide (n = 10) vs. Sudden-death control (n = 10) | Astrocyte hypertrophy with increased cell body and ramifications in ACC white matter, caudate nucleus, and thalamus of depressed suicides compared to controls |
| Schlicht et al. [53] | Post-mortem brain biopsy | Mass spectrometry | Suicide (n = 17) vs. Accident or heart-disease control (n = 9) | Increased GFAP in PFC of suicide completers compared to controls |
| Torres-Platas et al. 2015 [54] | Post-mortem brain biopsy | Immunohistochemistry, gene expression | Depressed suicide (n = 22) vs. Accident or sudden-death control (n = 22) | GFAP mRNA and protein were reduced in mediodorsal thalamus and caudate nucleus of depressed suicides compared to controls. |
| Nagy et al. [55] | Post-mortem brain biopsy | Gene expression (methylation pattern) | Suicide (n = 22) vs. Sudden-death control (n = 17) | Decreased expression of GFAP, ALDH1L1, SLC1A3, GJA1, GJB6, GLUL, and SOX9 in DLPFC of suicides compared to controls |
| O’Leary et al. [56] | Post-mortem brain biopsy | Immunohistochemistry | Depressed suicide (n = 10) vs. Sudden-death control (n = 10) | Decreased abundance of Vimentin+ and GFAP+ astrocytes in DLPFC, dorsal caudate nucleus, and mediodorsal thalamus of depressed suicides compared to controls |
| Zhang et al. [57] | Post-mortem brain biopsy | Gene expression | Depressed suicide (n = 17) vs. Non-suicidal depressed (n = 17) | Decreased ALDHL1 in the DLPFC and ACC regions of depressed suicides compared to non-suicidal depressed |
| Zhang et al. [58] | Post-mortem brain biopsy | Gene expression | Non-schizophrenic suicide (n = 7) vs. non-suicidal schizophrenic (n = 28) | Decreased ALDHL1 in the DLPFC of non-schizophrenic suicides compared to non-suicidal schizophrenics |
| Maussion et al. [59] | Post-mortem brain biopsy | Gene expression (methylation pattern) | Suicide (n = 13) vs. Control (n = 11) | Decreased TrkB.T1 expression in the frontal cortex of suicides compared to controls |
| Maussion et al. [60] | Post-mortem brain biopsy | Gene expression (miRNA profile) | Suicide (n = 38) vs. Control (n = 17) | Increased Hsa-miR-185 expression in the frontal cortex of suicides compared to controls |
| Pantazatos et al. [61] | Post-mortem brain biopsy | Gene expression | Depressed suicide (n = 21) vs. Sudden-death control (n = 29) | Lower expression of genes associated with astrocyte migration in DLPFC of depressed suicides compared to controls |
| Ernst et al. [62] | Post-mortem brain biopsy | Gene expression | Suicide (n = 95) vs. Sudden-death control (n = 81) | Decreased expression of GJA1 and GJB6 in DLPFC of depressed suicides compared to controls |
| Nagy et al. [63] | Post-mortem brain biopsy | Gene expression (methylation pattern) | Depressed suicide (n = 22) vs. Sudden-death control (n = 22) | Decreased expression of GJA1 and GJB6 as well as increased H3K9me3 in these two gene loci in the neocortex (Brodmann areas 4 and 17), mediodorsal thalamus, and caudate of depressed suicides compared to controls |
| Tanti et al. [64] | Post-mortem brain biopsy | Immunohistochemistry | Depressed suicide (n = 48) vs. Sudden-death control (n = 23) | Decreased GJA1 in astrocytes adjacent to oligodendrocytes in ACC of depressed suicides compared to controls |
| Dogan et al. [65] | Post-mortem CSF | ELISA | Suicide (n = 32) vs. Heart-disease or other-cause control (n = 56) | Higher S100B in suicides compared to controls |
| Falcone et al. [66] | Serum | ELISA | Suicide with psychosis (n = 40) or affective disorders (n = 24) vs. Control (n = 20) | Increased S100B correlates positively with SI severity in suicides compared to controls. |
| Study Type and Tissue | Study Methods | Study Participants | Major Findings | |
|---|---|---|---|---|
| Acute COVID-19 | ||||
| Boroujeni et al. [69] | Post-mortem brain biopsy | Immunohistochemistry | Acute COVID-19 (n = 3) vs. HC (n = 3) | Increased astrocyte abundance in cerebral cortex of COVID-19 patients compared to controls |
| Reichard et al. [70] | Post-mortem brain biopsy | Immunohistochemistry | Acute COVID-19 with disseminated encephalomyelitis (n = 1) | Increased GFAP expression in cerebral white matter in a COVID-19 patient |
| Lee et al. [71] | Post-mortem brain biopsy | Immunohistochemistry | Acute COVID-19 (n = 13) | Hypertrophic astrocytes with lower branching complexity and GFAP/collagen IV and AQP4 co-localization in close proximity to microglia/dying neurons in COVID-19 patients |
| Aceti et al. [72] | Serum | ELISA | Acute COVID-19 (n = 74) vs. HC (n = 5) | Increased S100B in COVID-19 compared to controls upon hospitalization; S100B correlated positively with inflammation, organ damage markers, and disease severity. |
| Mete et al. [73] | Serum | ELISA | Acute COVID-19 (n = 64) vs. HC (n = 30) | Increased S100B in COVID-19 compared to controls upon hospitalization; S100B correlated positively with disease severity. |
| Silva et al. [74] | Serum | ELISA | Acute COVID-19 (n = 141) vs. HC (n = 36) | Increased S100B in COVID-19 compared to controls at 14 days after disease onset; S100B correlated positively with disease severity. |
| Kokkoris et al. [75] | Serum | ELISA | Acute COVID-19 (n = 50) | Higher S100B in non-survivors compared to survivors among ICU patients upon hospitalization; S100B positively correlated with IL-6, low lymphocyte count, hypoperfusion indices, disease severity, and short-term outcome. |
| Sahin et al. [76] | Serum | ELISA | Acute COVID-19 (n = 58) vs. HC (n = 20) | No difference in S100B between two groups; higher GFAP in severe COVID-19 compared to controls (unspecified time during acute disease). |
| Passos et al. [77] | Serum | ELISA | Acute COVID-19 (n = 42) vs. HC (n = 34) | Higher GFAP in severe COVID-19 compared to controls upon hospitalization; GFAP positively correlated with RAGE and HMGB1 levels. |
| Frithiof et al. [78] | Serum | ELISA | Acute COVID-19 (n = 111) | Higher GFAP correlated with polyneuropathy and myopathy (unspecified time during acute disease). |
| Tokic et al. [79] | Serum | ELISA | Acute COVID-19 (n = 65) | Higher UCHL1, not GFAP, correlated with the presence of neurological symptoms upon admission in male patients. |
| Plantone et al. [80] | Serum | ELISA | Acute COVID-19 (n = 148) vs. HC (n = 108) | Higher GFAP in COVID-19 compared to controls (unspecified time during acute disease) |
| Lennol et al. [81] | Serum | ELISA | Acute COVID-19 (n = 45) | Higher GFAP correlated with the absence of neurological symptoms; GFAP normalized during recovery (2 months afterwards). |
| Bonetto et al. [82] | Serum | ELISA | Acute COVID-19 (n = 157) vs. HC (n = 20) | Higher GFAP in COVID-19 compared to controls (unspecified time during acute disease) regardless of the presence of neurological symptoms |
| Savarraj et al. [83] | Serum | ELISA | Acute COVID-19 (n = 57) vs. HC (n = 20) | No differences in GFAP between two groups upon hospitalization |
| Long COVID-19 | ||||
| de Boni et al. [84] | Serum | ELISA | Long COVID-19 (n = 6) vs. Severe Acute COVID-19 (n = 11) | Reduced GFAP in long COVID-19 (>12 weeks of headache) compared to severe acute COVID-19 |
| Kanberg et al. [85] | Serum | ELISA | Long COVID-19 (n = 50) vs. Acute COVID-19 (n = 50) | GFAP normalized after 6 months despite persistent long COVID-19 symptoms (fatigue, brain fog, and impaired cognition). |
| Spanos et al. [86] | Serum | ELISA | Long COVID-19 (n = 21) vs. Acute COVID-19 (n = 32) | Higher GFAP was linked to long COVID-19 (neurological complications at 1-year follow-up). |
| Hanson et al. [87] | Serum | ELISA | Long COVID-19 (n = 47) vs. Severe Acute COVID-19 (n = 9) | Anxiety and depression correlated positively with higher serum neuroglial GFAP in long COVID-19 (post-acute neurological complications). |
| Peluso et al. [88] | Serum | ELISA | Long COVID-19 (n = 52) vs. Acute COVID-19 (n = 69) | Higher GFAP during early (<90–120 days), but not late, recovery in long COVID-19 compared to acute COVID-19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costanza, A.; Amerio, A.; Aguglia, A.; Rossi, M.; Parise, A.; Magnani, L.; Serafini, G.; Amore, M.; Martins, D.; Nguyen, K.D. Reactive Astrocytosis—A Potential Contributor to Increased Suicide in Long COVID-19 Patients? Brain Sci. 2024, 14, 973. https://doi.org/10.3390/brainsci14100973
Costanza A, Amerio A, Aguglia A, Rossi M, Parise A, Magnani L, Serafini G, Amore M, Martins D, Nguyen KD. Reactive Astrocytosis—A Potential Contributor to Increased Suicide in Long COVID-19 Patients? Brain Sciences. 2024; 14(10):973. https://doi.org/10.3390/brainsci14100973
Chicago/Turabian StyleCostanza, Alessandra, Andrea Amerio, Andrea Aguglia, Martina Rossi, Alberto Parise, Luca Magnani, Gianluca Serafini, Mario Amore, Daniel Martins, and Khoa D. Nguyen. 2024. "Reactive Astrocytosis—A Potential Contributor to Increased Suicide in Long COVID-19 Patients?" Brain Sciences 14, no. 10: 973. https://doi.org/10.3390/brainsci14100973
APA StyleCostanza, A., Amerio, A., Aguglia, A., Rossi, M., Parise, A., Magnani, L., Serafini, G., Amore, M., Martins, D., & Nguyen, K. D. (2024). Reactive Astrocytosis—A Potential Contributor to Increased Suicide in Long COVID-19 Patients? Brain Sciences, 14(10), 973. https://doi.org/10.3390/brainsci14100973









