Post-Movement Beta Synchrony Inhibits Cortical Excitability
Abstract
1. Introduction
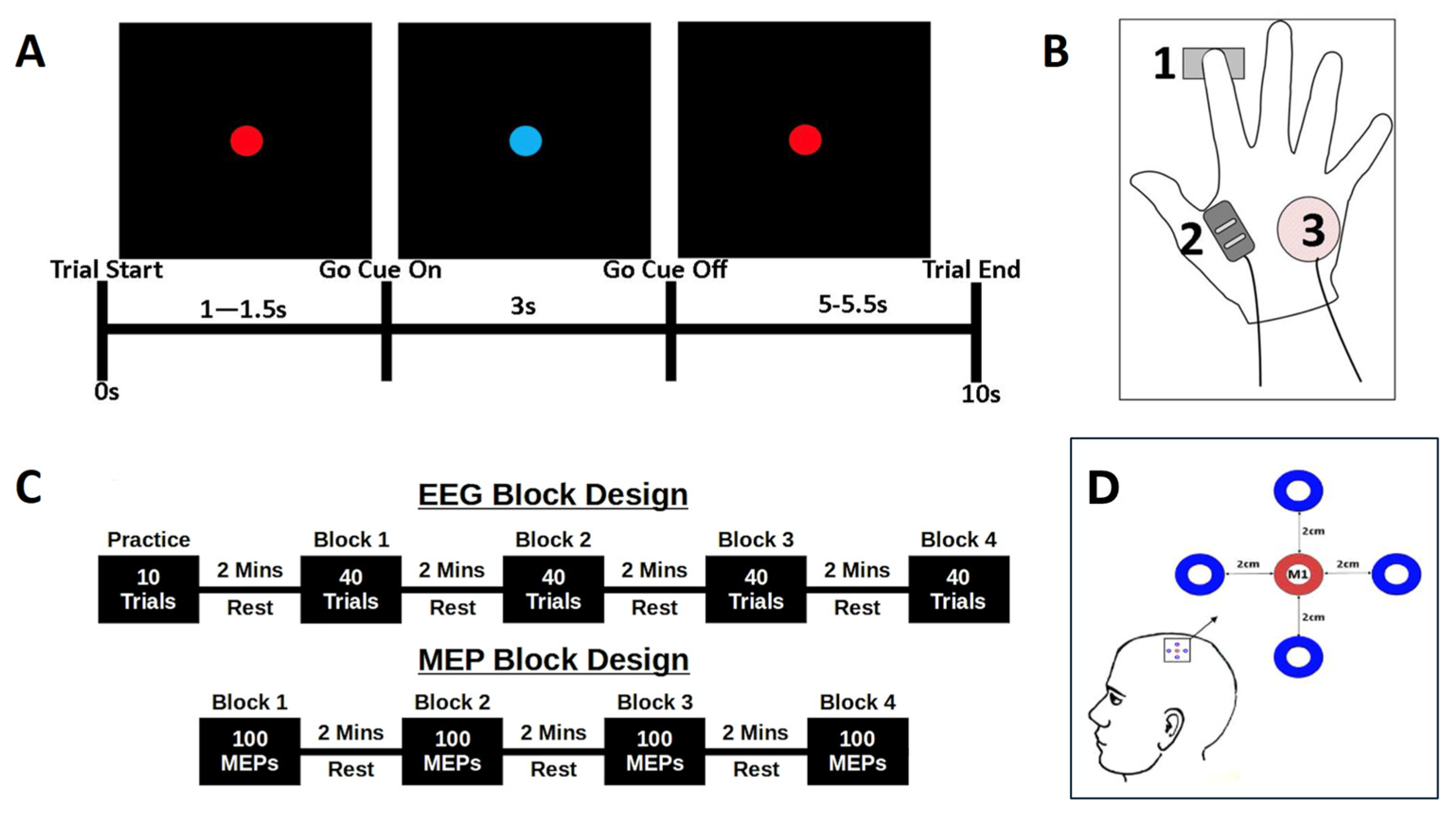
2. Methods
2.1. Data Acquisition
2.1.1. Electromyography Recording
2.1.2. TMS Localisation of M1 and MEP Recording
2.1.3. Electroencephalography (EEG) Recording
2.2. Task Design
2.2.1. Reaction Time Task
2.2.2. Experiment 1: EEG
2.3. Analysis
2.3.1. EEG Analysis: Computing the Beta Power Envelope
2.3.2. Experiment 2: TMS-Evoked MEP
2.3.3. MEP Analysis
3. Results
3.1. Experiment 1: EEG
3.2. Experiment 2: TMS-Evoked EMG
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pfurtscheller, G.; Berghold, A. Patterns of cortical activation during planning of voluntary movement. Electroencephalogr. Clin. Neurophysiol. 1989, 72, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Leocani, L.; Toro, C.; Manganotti, P.; Zhuang, P.; Hallett, M. Event-related coherence and event-related desynchronization/synchronization in the 10 Hz and 20 Hz EEG during self-paced movements. Electroencephalogr. Clin. Neurophysiol. Potentials Sect. 1997, 104, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, G.; Lopes Da Silva, F.H. Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clin. Neurophysiol. 1999, 110, 1842–1857. [Google Scholar] [CrossRef]
- Formaggio, E.; Storti, S.F.; Cerini, R.; Fiaschi, A.; Manganotti, P. Brain oscillatory activity during motor imagery in EEG-fMRI coregistration. Magn. Reson. Imaging 2010, 28, 1403–1412. [Google Scholar] [CrossRef]
- Miller, K.J.; Schalk, G.; Fetz, E.E.; Den Nijs, M.; Ojemann, J.G.; Rao, R.P.N. Cortical activity during motor execution, motor imagery, and imagery-based online feedback. Proc. Natl. Acad. Sci. USA 2010, 107, 4430–4435. [Google Scholar] [CrossRef]
- Salmelin, R.; Hámáaláinen, M.; Kajola, M.; Hari, R. Functional segregation of movement-related rhythmic activity in the human brain. NeuroImage 1995, 2, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Stancák, A.; Pfurtscheller, G. Desynchronization and recovery of β rhythms during brisk and slow self-paced finger movements in man. Neurosci. Lett. 1995, 196, 21–24. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Stancak, A.; Neuper, C. Post-movement beta synchronization. A correlate of an idling motor area? Electroencephalogr. Clin. Neurophysiol. 1996, 98, 281. [Google Scholar] [CrossRef]
- Cassim, F.; Monaca, C.; Szurhaj, W.; Bourriez, J.-L.; Defebvre, L.; Derambure, P.; Guieu, J.-D. Does post-movement beta synchronization reflect an idling motor cortex? Neuroreport 2001, 12, 3859–3863. [Google Scholar] [CrossRef]
- Jurkiewicz, M.T.; Gaetz, W.C.; Bostan, A.C.; Cheyne, D. Post-movement beta rebound is generated in motor cortex: Evidence from neuromagnetic recordings. NeuroImage 2006, 32, 1281–1289. [Google Scholar] [CrossRef]
- Gross, J.; Pollok, B.; Dirks, M.; Timmermann, L.; Butz, M.; Schnitzler, A. Task-dependent oscillations during unimanual and bimanual movements in the human primary motor cortex and SMA studied with magnetoencephalography. NeuroImage 2005, 26, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Cheyne, D.; Bakhtazad, L.; Gaetz, W. Spatiotemporal mapping of cortical activity accompanying voluntary movements using an event-related beamforming approach. Hum. Brain Mapp. 2006, 27, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Pollok, B.; Gross, J.; Kamp, D.; Schnitzler, A. Evidence for anticipatory motor control within a cerebello-diencephalic-parietal network. J. Cogn. Neurosci. 2008, 20, 828–840. [Google Scholar] [CrossRef]
- Pollok, B.; Krause, V.; Butz, M.; Schnitzler, A. Modality specific functional interaction in sensorimotor synchronization. Hum. Brain Mapp. 2009, 30, 1783–1790. [Google Scholar] [CrossRef]
- Wilson, T.W.; Slason, E.; Asherin, R.; Kronberg, E.; Reite, M.L.; Teale, P.D.; Rojas, D.C. An extended motor network generates beta and gamma oscillatory perturbations during development. Brain Cogn. 2010, 73, 75–84. [Google Scholar] [CrossRef]
- Heinrichs-Graham, E.; Wilson, T.W. Coding complexity in the human motor circuit. Hum. Brain Mapp. 2015, 36, 5155–5167. [Google Scholar] [CrossRef]
- Ohara, S.; Ikeda, A.; Kunieda, T.; Yazawa, S.; Baba, K.; Nagamine, T.; Taki, W.; Hashimoto, N.; Mihara, T.; Shibasaki, H. Movement-related change of electrocorticographic activity in human supplementary motor area proper. Brain 2000, 123, 1203–1215. [Google Scholar] [CrossRef]
- Gaetz, W.; MacDonald, M.; Cheyne, D.; Snead, O. Neuromagnetic imaging of movement-related cortical oscillations in children and adults: Age predicts post-movement beta rebound. NeuroImage 2010, 51, 792–807. [Google Scholar] [CrossRef]
- Pfurtscheller, G. Event-related synchronization (ERS): An electrophysiological correlate of cortical areas at rest. Electroencephalogr. Clin. Neurophysiol. 1992, 83, 62–69. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Stancák, A.; Edlinger, G. On the existence of different types of central beta rhythms below 30 Hz. Electroencephalogr. Clin. Neurophysiol. 1997, 102, 316–325. [Google Scholar] [CrossRef]
- Gilbertson, T.; Lalo, E.; Doyle, L.; Di Lazzaro, V.; Cioni, B.; Brown, P. Existing motor state is favored at the expense of new movement during 13-35 hz oscillatory synchrony in the human corticospinal system. J. Neurosci. 2005, 25, 7771–7779. [Google Scholar] [CrossRef] [PubMed]
- Swann, N.; Tandon, N.; Canolty, R.; Ellmore, T.M.; McEvoy, L.K.; Dreyer, S.; DiSano, M.; Aron, A.R. Intracranial EEG reveals a time- and frequency-specific role for the right inferior frontal gyrus and primary motor cortex in stopping initiated responses. J. Neurosci. 2009, 29, 12675–12685. [Google Scholar] [CrossRef] [PubMed]
- Engel, A.K.; Fries, P. Beta-band oscillations—Signalling the status quo? Curr. Opin. Neurobiol. 2010, 20, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, N.; Brown, P. New insights into the relationship between dopamine, beta oscillations and motor function. Trends Neurosci. 2011, 34, 611–618. [Google Scholar] [CrossRef]
- Pastotter, B.; Hanslmayr, S.; Bauml, K.H. Inhibition of return arises from inhibition of response processes: An analysis of oscillatory beta activity. J. Cogn. Neurosci. 2008, 20, 65–75. [Google Scholar] [CrossRef]
- Van Wijk, B.C.M.; Daffertshofer, A.; Roach, N.; Praamstra, P. A role of beta oscillatory synchrony in biasing response competition? Cerebral. Cortex 2008, 19, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Alegre, M.; Labarga, A.; Gurtubay, I.; Iriarte, J.; Malanda, A.; Artieda, J. Beta electroencephalograph changes during passive movements: Sensory afferences contribute to beta event-related desynchronization in humans. Neurosci. Lett. 2002, 331, 29–32. [Google Scholar] [CrossRef]
- Koelewijn, T.; van Schie, H.T.; Bekkering, H.; Oostenveld, R.; Jensen, O. Motor-cortical beta oscillations are modulated by correctness of observed action. NeuroImage 2008, 40, 767–775. [Google Scholar] [CrossRef]
- Tan, H.; Jenkinson, N.; Brown, P. Dynamic neural correlates of motor error monitoring and adaptation during trial-to-trial learning. J. Neurosci. 2014, 34, 5678–5688. [Google Scholar] [CrossRef]
- Tan, H.; Wade, C.; Brown, P. Post-Movement Beta Activity in Sensorimotor Cortex Indexes Confidence in the Estimations from Internal Models. J. Neurosci. 2016, 36, 1516–1528. [Google Scholar] [CrossRef]
- Barone, J.; Rossiter, H.E. Understanding the Role of Sensorimotor Beta Oscillations. Front. Syst. Neurosci. 2021, 15, 655886. [Google Scholar] [CrossRef] [PubMed]
- Alegre, M.; Gurtubay, I.G.; Labarga, A.; Iriarte, J.; Valencia, M.; Artieda, J. Frontal and central oscillatory changes related to different aspects of the motor process: A study in go/no-go paradigms. Exp. Brain Res. 2004, 159, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Coxon, J.P.; Stinear, C.M.; Byblow, W.D. Intracortical inhibition during volitional inhibition of prepared action. J. Neurophysiol. 2006, 95, 3371–3383. [Google Scholar] [CrossRef]
- Pogosyan, A.; Gaynor, L.D.; Eusebio, A.; Brown, P. Boosting cortical activity at beta-band frequencies slows movement in humans. Curr. Biol. 2009, 19, 1637–1641. [Google Scholar] [CrossRef]
- Zarkowski, P.; Shin, C.; Dang, T.; Russo, J.; Avery, D. EEG and the variance of motor evoked potential amplitude. Clin. EEG Neurosci. 2006, 37, 247–251. [Google Scholar] [CrossRef]
- Sauseng, P.; Klimesch, W.; Gerloff, C.; Hummel, F. Spontaneous locally restricted EEG alpha activity determines cortical excitability in the motor cortex. Neuropsychologia 2009, 47, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Lepage, J.F.; Saint-Amour, D.; Théoret, H. EEG and neuronavigated single-pulse TMS in the study of the observation/execution matching system: Are both techniques measuring the same process? J. Neurosci. Methods 2008, 175, 17–24. [Google Scholar] [CrossRef]
- Chen, R.; Yaseen, Z.; Cohen, L.G.; Hallett, M. Time course of corticospinal excitability in reaction time and self-paced movements. Ann. Neurol. 1998, 44, 317–325. [Google Scholar] [CrossRef]
- Leocani, L.; Cohen, L.G.; Wassermann, E.M.; Ikoma, K.; Hallett, M. Human corticospinal excitability evaluated with transcranial magnetic stimulation during different reaction time paradigms. Brain 2000, 123, 1161–1173. [Google Scholar] [CrossRef]
- Gaetz, W.; Rhodes, E.; Bloy, L.; Blaskey, L.; Jackel, C.R.; Brodkin, E.S.; Waldman, A.; Embick, D.; Hall, S.; Roberts, T.P. Evaluating motor cortical oscillations and age-related change in autism spectrum disorder. NeuroImage 2020, 207, 116349. [Google Scholar] [CrossRef]
- Espenhahn, S.; de Berker, A.O.; van Wijk, B.C.; Rossiter, H.E.; Ward, N.S. Movement-related beta oscillations show high intra-individual reliability. NeuroImage 2017, 147, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Ugawa, Y.; Terao, Y.; Hanajima, R.; Furubayashi, T.; Kanazawa, I. Preferential activation of different I waves by transcranial magnetic stimulation with a figure-of-eight-shaped coil. Exp. Brain Res. 1997, 113, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Opitz, A.; Legon, W.; Rowlands, A.; Bickel, W.K.; Paulus, W.; Tyler, W.J. Physiological observations validate finite element models for estimating subject-specific electric field distributions induced by transcranial magnetic stimulation of the human motor cortex. NeuroImage 2013, 81, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Rossini, P.M.; Rossi, S. Clinical applications of motor evoked potentials. Electroencephalogr. Clin. Neurophysiol. 1998, 106, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Groppa, S.; Oliviero, A.; Eisen, A.; Quartarone, A.; Cohen, L.; Mall, V.; Kaelin-Lang, A.; Mima, T.; Rossi, S.; Thickbroom, G.; et al. A practical guide to diagnostic transcranial magnetic stimulation: Report of an IFCN committee. Clin. Neurophysiol. 2012, 123, 858–882. [Google Scholar] [CrossRef]
- Rhodes, E.; Gaetz, W.C.; Marsden, J.; Hall, S.D. Transient Alpha and Beta Synchrony Underlies Preparatory Recruitment of Directional Motor Networks. J. Cogn. Neurosci. 2018, 30, 867–875. [Google Scholar] [CrossRef]
- Oostenveld, R.; Fries, P.; Maris, E.; Schoffelen, J.-M. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011, 2011, 156869. [Google Scholar] [CrossRef]
- Maki, H.; Ilmoniemi, R.J. EEG oscillations and magnetically evoked motor potentials reflect motor system excitability in overlapping neuronal populations. Clin. Neurophysiol. 2010, 121, 492–501. [Google Scholar] [CrossRef]
- Takemi, M.; Masakado, Y.; Liu, M.; Ushiba, J. Event-related desynchronization reflects downregulation of intracortical inhibition in human primary motor cortex. J. Neurophysiol. 2013, 110, 1158–1166. [Google Scholar] [CrossRef]
- Kim, H.; Yoshimura, N.; Koike, Y. Classification of Movement Intention Using Independent Components of Premovement EEG. Front. Hum. Neurosci. 2019, 13, 63. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Yoshimura, N.; Koike, Y. Characteristics of Kinematic Parameters in Decoding Intended Reaching Movements Using Electroencephalography (EEG). Front. Neurosci. 2019, 13, 1148. [Google Scholar] [CrossRef] [PubMed]
- Lew, E.; Chavarriaga, R.; Silvoni, S.; Millán, J.D.R. Detection of Self-Paced Reaching Movement Intention from EEG Signals. Front. Neuroeng. 2012, 5, 13. [Google Scholar] [CrossRef]
- Jensen, O.; Goel, P.; Kopell, N.; Pohja, M.; Hari, R.; Ermentrout, B. On the human sensorimotor-cortex beta rhythm: Sources and modeling. NeuroImage 2005, 26, 347–355. [Google Scholar] [CrossRef]
- Hall, S.D.; Barnes, G.R.; Furlong, P.L.; Seri, S.; Hillebrand, A. Neuronal network pharmacodynamics of GABAergic modulation in the human cortex determined using pharmaco-magnetoencephalography. Hum. Brain Mapp. 2010, 31, 581–594. [Google Scholar] [CrossRef]
- Gaetz, W.; Edgar, J.; Wang, D.; Roberts, T. Relating MEG measured motor cortical oscillations to resting γ-Aminobutyric acid (GABA) concentration. NeuroImage 2011, 55, 616–621. [Google Scholar] [CrossRef]
- Hall, S.D.; Stanford, I.M.; Yamawaki, N.; McAllister, C.J.; Rönnqvist, K.C.; Woodhall, G.L.; Furlong, P.L. The role of GABAergic modulation in motor function related neuronal network activity. NeuroImage 2011, 56, 1506–1510. [Google Scholar] [CrossRef]
- Hall, S.; Prokic, E.; McAllister, C.; Ronnqvist, K.; Williams, A.; Yamawaki, N.; Witton, C.; Woodhall, G.; Stanford, I. GABA-mediated changes in inter-hemispheric beta frequency activity in early-stage Parkinson’s disease. Neuroscience 2014, 281, 68–76. [Google Scholar] [CrossRef]
- Lacey, M.G.; Gooding-Williams, G.; Prokic, E.J.; Yamawaki, N.; Hall, S.D.; Stanford, I.M.; Woodhall, G.L. Spike firing and IPSPs in layer V pyramidal neurons during beta oscillations in rat primary motor cortex (M1) in vitro. PLoS ONE 2014, 9, e85109. [Google Scholar] [CrossRef] [PubMed]
- Schönle, P.; Isenberg, C.; Crozier, T.; Dressler, D.; Machetanz, J.; Conrad, B. Changes of transcranially evoked motor responses in man by midazolam, a short acting benzodiazepine. Neurosci. Lett. 1989, 101, 321–324. [Google Scholar] [CrossRef]
- Boroojerdi, B.; Battaglia, F.; Muellbacher, W.; Cohen, L. Mechanisms influencing stimulus-response properties of the human corticospinal system. Clin. Neurophysiol. 2001, 112, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Kimiskidis, V.K.; Papagiannopoulos, S.; Kazis, D.A.; Sotirakoglou, K.; Vasiliadis, G.; Zara, F.; Kazis, A.; Mills, K.R. Lorazepam-induced effects on silent period and corticomotor excitability. Exp. Brain Res. 2006, 173, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Heidegger, T.; Krakow, K.; Ziemann, U. Effects of antiepileptic drugs on associative LTP-like plasticity in human motor cortex. Eur. J. Neurosci. 2010, 32, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Ziemann, U.; Reis, J.; Schwenkreis, P.; Rosanova, M.; Strafella, A.; Badawy, R.; Muller-Dahlhaus, F. TMS and drugs revisited 2014. Clin. Neurophysiol. 2015, 126, 1847–1868. [Google Scholar] [CrossRef] [PubMed]
- Barratt, E.L.; Tewarie, P.K.; Clarke, M.A.; Hall, E.L.; Gowland, P.A.; Morris, P.G.; Francis, S.T.; Evangelou, N.; Brookes, M.J. Abnormal task driven neural oscillations in multiple sclerosis: A visuomotor MEG study. Hum. Brain Mapp. 2017, 38, 2441–2453. [Google Scholar] [CrossRef]
- Buard, I.; Kronberg, E.; Steinmetz, S.; Hepburn, S.; Rojas, D.C. Neuromagnetic Beta-Band Oscillations during Motor Imitation in Youth with Autism. Autism Res. Treat. 2018, 2018, 9035793. [Google Scholar] [CrossRef]



| Stimulation Point | Time-Point (ms): Mean (SD) | Definition |
|---|---|---|
| Response termination | 565.7 (±66.5) | During beta ERD, following response termination and after EMG activity returns to within 0.5 SD of baseline. |
| Early PMBR | 1025.9 (±264.1) | Ascending slope at half the maximal amplitude of the PMBR. |
| Peak PMBR | 1476.8 (±445.5) | Time-point of maximal PMBR amplitude. |
| Late PMBR | 4186.4 (±674.9) | Descending slope immediately prior to return to mean baseline. |
| Active rest period | 9500 (0) | 500 ms prior to the end of each trial. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rhodes, E.; Gaetz, W.; Marsden, J.; Hall, S.D. Post-Movement Beta Synchrony Inhibits Cortical Excitability. Brain Sci. 2024, 14, 970. https://doi.org/10.3390/brainsci14100970
Rhodes E, Gaetz W, Marsden J, Hall SD. Post-Movement Beta Synchrony Inhibits Cortical Excitability. Brain Sciences. 2024; 14(10):970. https://doi.org/10.3390/brainsci14100970
Chicago/Turabian StyleRhodes, Edward, William Gaetz, Jonathan Marsden, and Stephen D. Hall. 2024. "Post-Movement Beta Synchrony Inhibits Cortical Excitability" Brain Sciences 14, no. 10: 970. https://doi.org/10.3390/brainsci14100970
APA StyleRhodes, E., Gaetz, W., Marsden, J., & Hall, S. D. (2024). Post-Movement Beta Synchrony Inhibits Cortical Excitability. Brain Sciences, 14(10), 970. https://doi.org/10.3390/brainsci14100970






