Abstract
The predictive coding theory is currently widely accepted as the theoretical basis of perception and chronic perceptual disorders are explained as the maladaptive compensation of the brain to a prediction error. Although this gives us a general framework to work with, it is still not clear who may be more susceptible and/or vulnerable to aberrations in this system. In this paper, we study changes in predictive coding through the lens of tinnitus and pain. We take a step back to understand how the predictive coding system develops from infancy, what are the different neural and bio markers that characterise this system in the acute, transition and chronic phases and what may be the factors that pose a risk to the aberration of this system. Through this paper, we aim to identify people who may be at a higher risk of developing chronic perceptual disorders as a reflection of aberrant predictive coding, thereby giving future studies more facets to incorporate in their investigation of early markers of tinnitus, pain and other disorders of predictive coding. We therefore view this paper to encourage the thinking behind the development of preclinical biomarkers to maladaptive predictive coding.
1. Introduction
Perception is hypothesised to be a controlled hallucination, where the brain attempts to make sense of the incoming information in different contexts. This is widely agreed to follow the predictive coding framework [1,2]. According to this framework, the brain is considered to maintain an internal model of the external environment [1,2]. The predictions of this internal model (or priors) are compared with the sensory input (or likelihood) to produce a prediction error [1,2]. The priors and likelihood are associated with a precision (weighting), which determines the precision of the prediction error. Precise (or large) prediction errors can change the internal model or the perceptual inference of the world [1,2].
Such a change in perceptual inference is observed in healthy young adults during the perception of different types of illusions [3,4]. It is also observed as a natural phenomenon when exposed to a harmful stimulus—i.e., perceiving a ringing in the ears (tinnitus) after leaving a loud concert owing to a temporary shift in hearing thresholds [5], or perceiving pain following tissue damage [6]. Both of these perceptions go away when the respective sensory receptors revert back to normal.
However, in the chronic state, tinnitus and pain are accompanied by a myriad of biopsychosocial challenges. From a predictive coding perspective, we can hypothesise phantom perception as a maladaptive perceptual inference to minimise a prediction error owing to the overweighting of the sensory input or prior [7,8]. Permanent sensory nerve damage (deafferentation) can lead to an overweighting of the sensory input [9]. If this is not reduced through top–down inhibitory connections [10,11], this noise is hypothesised to reach consciousness resulting in a phantom perception—tinnitus or pain—depending on the modality of deafferentation [7,12]. Both in the auditory and somatosensory domains, phantom perceptions may be produced even in the absence of a sensory deafferentation. Although less understood why, we may attribute this to an overweighting of priors [8] or a change in the context of processing the same input. This is seen in patients with chronic tinnitus [13,14] and psychosomatic pain [15] where stress, anxiety and other traumatic incidents play a significant role in the generation of the phantom percept.
Both these chronic perceptual disorders affect a vast majority of the population (up to 15% for chronic tinnitus [16] and up to 40% for chronic pain [17]). Furthermore, in the U.S alone, tinnitus and chronic pain, respectively, cost the government USD 3.3 billion [18] and between USD 560 and USD 635 billion [19] annually, thereby imposing a huge socio-economic burden. Therefore, there is an urgent need to not only invest in the treatment of these disorders, but also in the identification of markers and prevention strategies for subsets of the population who may be at a higher risk of developing a chronic condition.
In this perspective, we take a life-course approach to (i) track how the predictive coding system develops from infancy, use phantom perceptions as a model to understand (ii) what are the changes in the neural and biomarkers of the predictive coding system in the acute and chronic phase, (iii) what are the neural and biomarkers that may indicate the transition from an acute to chronic phase and, finally, (iv) to explore developmental, environmental and genetic risk factors that may affect the maladaptive compensation of prediction errors, thereby identifying subsets of the population who may be at a higher risk of developing chronic perceptual disorders.
To build this perspective, we reviewed the literature in terms of the neural and biomarkers of predictive coding. We also reviewed the tinnitus and chronic pain literature in the acute, transition and chronic phase from the perspective of predictive coding to best capture the manuscripts that cover these neural and biomarkers of predictive coding. To highlight the factors that could predispose someone to maladaptive changes in predictive coding, we reviewed the biopsychosocial models for tinnitus and chronic pain combined with our experience and discretion to capture as many factors as possible.
2. Development of the Predictive Coding System through the Life Course
Predictive learning is shown to be the most efficient form of learning even outside the neuroscience realm. In humans, the development of higher-level cognition is proposed to originate from the predictive learning of sensorimotor signals in two ways: (i) updating an immature prediction error to refine one’s own sensorimotor abilities and (ii) executing an action estimated by the predictor’s response to others’ actions [20]. In her paper, Yuki Nagai describes how the predictor in the brain learns from early sensory input, such as reflexes, to build the proprioceptive sense of self and that of others. She states that the prediction error generated by sensory/motor input from the self is more predictable than that from an external source which is less predictable. The brain constantly works on reducing this prediction error and updating the model and learning to expand its abilities to higher levels of cognition, such as hand–eye co-ordination and understanding social and emotional cues.
Empirically, changes to this predictive coding system can be measured using different neuroimaging techniques. Particularly, sensory oddball tasks probe different features of the predictive coding network and characterise them in different components of the neural response. Oddball paradigms usually consist of a standard stimulus that is randomly interspersed with a deviant stimulus which acts as a surprise [21]. One of the theories behind this is that the brain records a temporary memory trace of the standard (which can be hypothesised to be the prediction) and the difference between the response of the deviant and the standard is the prediction error [22]. Through the life-course, oddball paradigms produce early and late components in infants and adults that record changes in sensory and cognitive processes.
2.1. Neural Markers
2.1.1. Mismatch Negativity
Mismatch negativity (MMN) is a fronto-central negativity occurring around 100–200 ms post-stimulus presentation [21] that is known to encode prediction errors. In infants, pitch [23,24], timing [25,26] and deviants in melodies [27] create mismatch responses showing a very early sign of encoding prediction errors. Furthermore, MMN components change after only a few hours of exposure, reflecting learning [28]. For a long time, it was debated that MMN responses represented neural adaptation patterns given that they were recorded in response to changes in stimulus characteristics [29]. However, more recent studies in both adult humans and monkeys show that MMNs generated by the omission of a stimulus in a sequence cannot be explained by neural adaptations and are a reliable marker to prediction errors [22,30].
Using functional near-infrared spectroscopy (fNIRS), the authors demonstrated that infants showed the activation of the visual cortex during the unexpected and not during expected omission of a visual stimulus showing the early presence of prediction errors in the visual cortex [31]. Further evidence for the presence of predictions in infants was given using a cross-modal cueing paradigm where infants formed associations between auditory and visual cues that depended on the prior knowledge acquired [32]. In this study, predicted events produced a higher amplitude than unpredicted events in the early components of the event response potential and vice-versa during the later components.
2.1.2. Late Positive Potential
MMNs record sensory prediction errors. Changes in cognitive components and more complex processes such as the updating of sensory memory occur later in time. In infants, an increase in a late positive component between 700–1000 ms, called the positive slow wave (PSW) [32], is traditionally considered as a marker representing the updating of memory specifically in response to partially encoded infrequent stimuli [33,34]. The PSW is also considered an early precursor of the P300 component observed in adults in response to oddball paradigms [33,35]. In adults, the P300 component is further divided into P3a and P3b depending on the paradigm and the task. In an active oddball paradigm with a standard, a target (rare stimulus that is attended to) and a novelty (surprise) stimulus, the target produces a posterior P3b component usually originating from the parietal cortex and the novelty produces an anterior P3a component that originates in the anterior cingulate [36]. The P3b is elicited on violating one’s predictions by the current input calls for updating internal models. Although the P300 is usually recorded as the target and novelty stimulus, its credibility to be a marker for prediction error is gaining traction [14,22].
2.2. Molecular Markers
The different components of the predictive coding system are guided by different neurotransmitters in the brain. Although there is no clear evidence of a one-on-one relationship between the neurotransmitter and component of the predictive coding system, pharmacological interventions and advanced neuroimaging techniques explore their dependence. Different drugs affecting glutaminergic transmission have been shown to affect the MMN. Ketamine, an N-methyl-D-aspartate receptor (NMDAR) antagonist, reduces the amplitude and delays the peak latency of the MMN [37,38]. The theory is that MMN, whether explained using neural adaptation models or memory-based predictive coding, is influenced by the NMDAR receptor [37].
Unlike the MMN, there is much discussion surrounding the neurotransmitters influencing the P300. An early review talked about the influence of the different neurotransmitter systems on the P300 [39]. They claimed that the P300 potential was directly influenced by the glutaminergic system since they are a direct result of excitatory post-synaptic potentials and indirectly by the GABA-ergic system through their inhibitory influence on excitatory post-synaptic potentials. In a seminal paper by Polich, he states that the P3a may be driven by the dopaminergic system and the P3b by the noradrenergic system [36]. This is backed up pharmacological and neuromodulatory interventions, showing the possible role of dopamine and noradrenaline in encoding the late positive potential [40,41]. Specifically, using an active inference model, a recent study shows how prediction errors trigger noradrenaline activity in the locus coeruleus which subsequently alters the rate of learning about the environment [42]. At a systems level, the noradrenergic activity of the locus coeruleus has been correlated with the amplitude of P3b [43].
However, there is still a lot of debate regarding the role of dopamine. Dopamine has been proposed to be involved in forming priors through associative learning [3]. Although there is some evidence, it is still not very clear if it is also involved in encoding the precision of the priors [44]. Some other authors suggest that dopamine is involved in the low-level precision weighted prediction error in trying to predict an outcome based on a given cue [45,46]. With respect to the precision of the prediction errors dominated by the likelihood, researchers associate this with the cholinergic activity in the basal forebrain [45]. This is backed up with the pharmacological modulation, neuroimaging and mathematical modelling of a system that constantly learns how to adapt to the changes in its environment [46,47]. Although the above studies try to somewhat explain a mutually exclusive relationship between different neurotransmitters and the different components of the predictive coding system, it is still a challenge to disentangle this relationship completely. A summary of the different neural and biomarkers of predictive coding is shown in Figure 1.
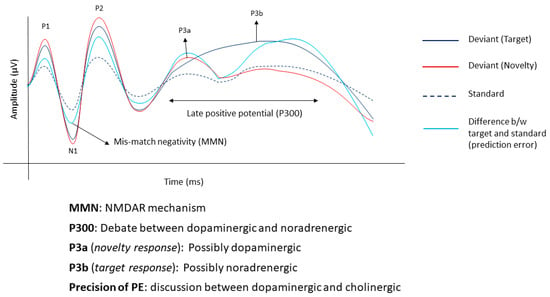
Figure 1.
Summary of event-related potentials generated by a three-stimulus active oddball paradigm. The dashed black line shows the response to standard stimuli, the solid black line shows the response to the target (usually paid attention to) stimuli, the solid red line shows the response to a novel distractor stimulus and the solid blue line shows the difference in response to the standard and target.
3. Changes in Predictive Coding in the Acute Phase of Phantom Perceptions
As introduced above, phantom perceptions may be a compensation of the brain to minimise a prediction error that has been brought on by a change in the incoming input or a change in the context in which the same input is presented. Therefore, it may be a natural phenomenon of the brain to perceive a phantom perception in an acute manner. In both acute tinnitus and pain, there are a myriad of temporary peripheral inconsistencies that potentially change the precision of the input. In the auditory domain, increased spontaneous emissions of the outer hair cells due to temporary damage following exposure to loud sounds and changing activity in the dorsal cochlear nucleus owing to stimulation through the somatosensory neurons, etc., may be some reasons [48]. These inconsistencies change the excitability of the peripheral nervous system which is relayed to the primary auditory cortex through the thalamus and perceived as phantom sound.
In the pain literature, the peripheral pathways are more comprehensively understood. The Aβ and Aδ fibres carry the “primary” pain signals, whereas the C fibres carry the “secondary” pain signals, both of which enter the spinal cord through the dorsal horn [49]. These are projected to the periaqueductal gray and on to the thalamus which relays this information to the somatosensory cortices through the lateral pain pathway and to the dorsal anterior cingulate cortex (dACC) and anterior insula (aI) through the medial pathways [49,50]. The lateral pathway records the sensory component of pain and the medial pathway records the emotional discomfort of pain [50]. Both pain and tinnitus are proposed to be modulated by a descending pathway—respectively, the first starting from the pregenual anterior cingulate cortex (pgACC) and going down through the periaqueductal gray [50], the other from the dlPFC and vmPFC to the thalamus [10,51]—that, from a predictive coding perspective, would reduce the precision of the incoming stimulus.
3.1. Neural Markers
Studies directly examining changes in predictive coding markers are sparse both in tinnitus and pain. However one study shows the increase in auditory evoked potential in the MMN time frame in the acute phase in both tinnitus [52] and pain [53]. Indirectly, ERPs examining the oddballs using nociceptive stimuli to generate acute pain showed that rare noxious stimuli produced a larger P300 amplitude compared to the standard, indicating an increase in the attention-related prediction error [54]. It was also seen that strong novel stimuli showed a bigger P300 response than weak novel stimuli when compared to the standard. Furthermore, this amplitude was enhanced with attention [54]. The role of acute pain on attention in relation to concurrent cognitive tasks showed that the amplitude of the late positive potential to a visual stimulus following a novel noxious stimulus was lower [54]. Unlike pain, tinnitus will have to be simulated through different proxies and illusions. Intermittent auditory ringing generated neural patterns that were similar to chronic tinnitus, showing a similar mechanism in the acute phase [4,55,56]. Further studies are need to understand brain dynamics in the acute phase to understand the transition to the chronic phase.
3.2. Molecular Markers
At the molecular level, the mechanisms that generate tinnitus and pain are slightly different, but overall involve an increase in the excitatory post-synaptic potential and increased spontaneous activity in neurons, which is interpreted as either perception. Following an acute injury, inflammatory mediators are released which act on G-coupled receptors to cascade a change in the threshold and kinetics of nociceptors to allow for a “soup” of molecules to bind with the nociceptors to regulate pain [57]. In tinnitus, acoustic trauma or ototoxic drugs could use one of the protein kinase pathways to increase opening calcium channels, or salicylate could increase the membrane conductance of the outer hair cells by either decreasing GABA or increasing NMDA to accelerate outer hair cell damage [58]. Salicylate and acoustic trauma can also change molecular events at the level of the auditory nerve synapse, cochlear efferents and at the level of the spiral ganglion [58] to cause an imbalance between excitatory and inhibitory dynamics to increase spontaneous activity in the peripheral nervous system. Theoretically, it would affect the input to the predictive coding system, but this has not been shown in the acute phase.
4. Changes in Predictive Coding in the Chronic Phase of Phantom Perceptions
In the chronic state, tinnitus and pain are more complex. In 2–5% of the population, the continuous awareness of the sound is associated with emotional distress, cognitive dysfunction and/or autonomic arousal leading to behavioural changes and functional disability. This is defined as tinnitus disorder [59]. Similarly, the International Association for the Study of Pain (IASP) defines chronic pain as “an unpleasant sensory and emotional experience associated with, or resembling that associated with actual or potential tissue damage” [60]. Tinnitus and pain are regarded as chronic when the perception lasts continuously for more than three months [61,62]. From a predictive coding perspective, in a chronic state, the brain learns that perceiving a phantom perception becomes the new norm, thereby changing the prediction of the brain to perceive a sound [9] (or pain).
4.1. Psychosocial Components
Both conditions in the chronic state are associated with sensory impairments that are specific to the auditory or somatosensory domains. About 90% of people with tinnitus have hearing loss that may or may not (hidden) be detected by an audiogram [63]. Although in chronic pain, the tissue damage itself may have resolved, there may be lasting changes in nerve sensitivity and inflammation [64]. Both conditions in the chronic state show changes in the central sensitisation [65,66] of the nervous system and a change in central gain [67,68] that arises because of peripheral nerve damage. Furthermore, increased sensitivity to sounds (hyperacusis) [69] and tactile stimuli (hyperalgesia [70]) is observed in patients with chronic tinnitus and pain, respectively.
The commonality between the two conditions is also seen in comorbid cognitive, mood and behavioural disorders. Dysfunctional attention in tinnitus is seen as slower reaction times on attention-oriented tasks and is mediated by tinnitus-related distress [71]. A similar observation has been made in patients with chronic pain and attention switching tasks [72]. Particularly, induced and chronic pain is shown to affect different aspects of attention [73]. Furthermore, in patients with chronic pain, an attentional bias is observed towards negative information on the Emotional Stroop task which is in turn related to higher levels of pain [74].
Emotionally, chronic tinnitus and pain are both accompanied by anxiety and depression. Among the 21.4 ± 0.69 million tinnitus sufferers in a cross sectional analysis of the 2007 Integrated Health Interview Series, 26.1% and 25% reported severe anxiety and depression, respectively, while only 9.2% and 9.1% of people without tinnitus reported the same [75]. Furthermore, people who reported tinnitus as a “big” or “very big” problem were significantly more likely to report anxiety and depression (OR = 5.7 and OR = 4.8) [75]. Similarly, an epidemiological survey of adults in the U.S. showed that those who reported chronic pain had an elevated severity of depression (PHQ-8 categories: none/minimal: 57.6%, mild: 22.3%, moderate: 11.4%, severe: 8.7%) and anxiety (GAD-7 categories: none/minimal: 66.4%, mild: 17.1%, moderate: 8.5%, severe: 8.0%) compared to those without chronic pain [depression (none/minimal: 87.6%, mild: 8.8%, moderate: 2.3%, severe: 1.2%; p < 0.001) and anxiety (none/minimal: 89.0%, mild: 7.5%, moderate: 2.1%, severe: 1.4%; p < 0.001)] [76]. Furthermore, 22.4% and 24.5% of chronic pain sufferers were taking medication for depression and anxiety, respectively, compared to 6.6% and 8.5% of those without chronic pain [76].
Finally, sleep disturbance is one of the most commonly noted behavioural effects of chronic tinnitus [77] and pain [78]. A cross-sectional assessment of the Mini Sleep Questionnaire (MSQ) in military personnel with tinnitus showed that MSQ scores were higher in 77% of patients compared to controls [79]. It was also noted that the regions involved in tinnitus were also the ones that were involved in REM sleep, posing a systems-level hypothesis of the relationship between the two [80]. A meta-analysis of sleep disturbances and sleep disorders in people with chronic pain showed that it was related with subjective and diagnosed sleep disorders [81].
4.2. Neural Markers
From a neurophysiological perspective, tinnitus and chronic pain show changes in widespread regions of the brain. Given that both disorders have a sensory and an affective component, they affect domain-specific and domain-general regions of the brain. The sensory component is encoded by an auditory network in tinnitus, consisting of primary and secondary auditory regions [82], and by the somatosensory network in chronic pain, consisting of the primary and somatosensory regions [83]. The broader domain-general network consists of regions that overlap with regions noted in the predictive coding framework of chronic tinnitus [9,84] and pain [85,86]. These are the dorsolateral prefrontal cortex (dlPFC), dorsal anterior cingulate cortex (dACC), ventromedial prefrontal cortex/pregenual anterior cingulate cortex (vmPFC/pgACC), subgenual anterior cingulate cortex (sgACC), posterior cingulate cortex (PCC), precuneus, inferior parietal cortex (IPC), anterior insula (aI), amygdala (AMG) and parahippocampus (PHC). These regions are divided among the default mode network [87] (DMN), the salience network [88] (SN) and a domain-general distress network [89,90] that is impacted both by chronic tinnitus and pain. Particularly, hyperactivity in the SN has been attributed to suffering related to pain [50] or tinnitus [91] and connectivity between the DMN and auditory networks has been attributed to the processing of internally directed perceptions and a change with hearing loss [87].
The predictive coding network was empirically tested in both chronic tinnitus and pain using oddball paradigms. In chronic pain, early [92,93,94,95] (MMN) and late [96,97] (P300) responses to auditory and visual oddball paradigms report a change in amplitude and latency. A reduced MMN amplitude is suggested to be correlated with the sensory component of the McGill Pain Questionnaire [92]. However, when subjected to somatosensory-evoked potentials, it seems like there is an increase in attention-related ERPs in the P300 timeframe showing an attentional bias to somatosensory stimuli in chronic pain patients [98,99].
In chronic tinnitus, changes to the predictive coding system were mostly recorded in the auditory domain with mixed results. While some studies show a decrease in the MMN amplitude [100,101,102], others show an increase in the MMN amplitude depending on the frequency and probability of the deviant [14,103]. Empirically, tinnitus becoming the new prediction has been shown as a reduction in the MMN amplitude to the tinnitus tone when presented as a deviant [53]. A meta-analysis of different ERP-based studies showed a reduction in the P300 amplitude [104]; however, in our recent study, we showed an increase in the P300 amplitude [14]. This difference may be attributed to the stimuli and the paradigm itself, where we used a local–global paradigm as opposed to the classic oddball paradigm, showing the hierarchical nature of the predictive coding system.
4.3. Molecular Markers
Tinnitus is often referred to as auditory pain and shares a lot of commonalities in terms of mechanisms [105]. Low glutamate and GABA levels were shown in a group with tinnitus [106] and chronic pain [107]. However, NMDA receptor activation by salicylate has shown to induce tinnitus [108], whereas NMDA receptor antagonists such as memantine and GABA agonist gabapentin [109] have shown to improve tinnitus. In patients with chronic pain, the level of glutamate and GABA changed with different types of pain [110]. Changes in GABA are also associated with changes in cortical inhibition and the disruption of the balance between excitation and inhibition [111]. Tinnitus is also considered to be maladaptive learning between the loudness of the sound and the distress because of the sound through the modulation of dopaminergic pathways [112]. Similarly, the dopaminergic modulation of different pain symptoms are also shown [113]. There are also changes in the predictive coding when transitioning from the acute to chronic phase.
5. Changes in Predictive Coding when Transitioning from Acute to Chronic Phase
5.1. Psychosocial Components
One of the most important differences between the acute and the chronic phases of change in perceptual disorders is the complex interplay of psychosocial factors in the chronic phase. This has been thoroughly examined in people with chronic pain rather than with tinnitus. A systematic review of the psychosocial aspects of the transition into chronic pain showed that the association with depression, fear avoidance, pain catastrophising, high traumatic exposure, emotional distress and the feelings of helplessness and hopelessness were key factors [114]. In tinnitus, preliminary evidence shows that between 6 weeks and 6 months of onset, the factors involved in the transition to a chronic condition include depression and anxiety levels, hyperacusis and the severity and frequency of hearing loss [115,116]. Another study showed that the subjective perception, moderate hearing loss and score on the Tinnitus Handicap Inventory were negatively associated with tinnitus chronicity [117]. However, it should be noted that this was a cross-sectional study.
5.2. Neural Markers
One of the hallmarks of chronic perceptual disorders is the central sensitisation and increase in central gain. This is seen as increased activity in the sensory regions of the predictive coding network, both in tinnitus and chronic pain. There are not many studies that show a direct relationship between the change in neural markers of predictive coding in the transition from the acute to chronic phase of perceptual disorders. In chronic pain, it is shown that the markers of prediction errors in the MMN timeframe are significantly larger in the acute phase compared to the chronic phase owing to this central sensitisation [53]. A similar observation is also made in tinnitus [52]. A cross-sectional comparison of changes in the activity and connectivity of predictive coding regions between acute and chronic tinnitus show increased activity in regions involved in predictive coding and connectivity between the PHC and PCC [118]. From a theoretical perspective, Sedley and colleagues proposed that in transition, tinnitus may involve the change of the prediction of the brain from perceiving silence in the absence of external stimuli to perceiving a ringing [9].
A longitudinal follow-up of people who recovered or did not recover from subacute back pain over a period of one year showed that people who did not recover from the back pain (chronic state) showed a decrease in the grey matter volume in the insula, nucleus accumbens (NAc), striatum and the sensorimotor cortex [119]. Furthermore, they showed decreased connectivity between the hippocampus and medial prefrontal cortex (mPFC) compared to those who recovered [120]. Particularly, the connectivity between the mPFC and NAc determined the accuracy of the transition from the acute to chronic phase with >80% accuracy [121]. The NAc is part of the limbic system that is involved in the reinforcement learning of a positive or negative affect. This is in line with the hypothesis that chronic perceptual disorders may be a learned maladaptive association of the sensory component with the affective component leading to a shift in the brain’s prediction of the new norm [9,84]. The neural markers of the acute, chronic and transition phases are shown in Figure 2. The summary of these findings are summarised in Table 1.
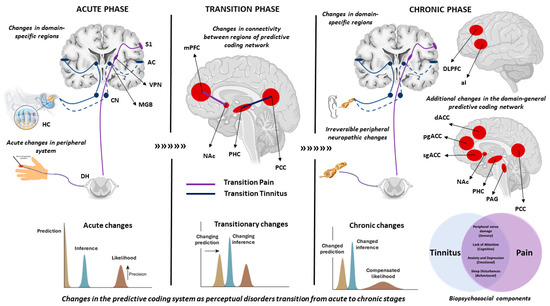
Figure 2.
This figure summarises the neural markers and changes in the predictive coding system involved in the acute, transition and chronic phase of tinnitus and pain. In the acute phase, damage to the cochlear hair cells or an acute injury increase spontaneous activity in the peripheral system which is brought to consciousness by the domain-specific lateral pathway (blue = auditory, purple = somatosensory). Specific to predictive coding, there is an increase in the precision of the likelihood. In the transition phase, there are specific changes to connectivity between regions of the predictive coding network. In pain, the connectivity between mPFC and NAc predicts the transition from acute to chronic with over 80% accuracy. In tinnitus, a change in the connectivity between the PHC and PCC was noted in a cross-sectional study comparing acute and chronic patients. From a predictive coding perspective, there is a change in the prediction (from silence to perceiving a phantom) accompanied by a change in inference owing to a possible reinforcement learning facilitated by the limbic system. In the chronic phase, damaged peripheral nerves in the auditory and somatosensory systems cause a domain-specific central sensitisation, which together with a malfunctioning inhibitory pathway from the DLPFC or the pgACC for tinnitus and pain, respectively, bring the increased gain to consciousness. It is accompanied by a myriad of biopsychosocial components and a domain-general distress network (shown in red) whose regions also overlap with the predictive coding network. From a predictive coding perspective, the current prediction has now fully transitioned to expect the perception of the phantom as the new norm, thereby compensating for the increased precision of the likelihood and changing the inference as well. The abbreviations involved are HC = hair cells, DH = dorsal horn, S1 = primary somatosensory cortex, AC = primary auditory cortex, VPN = ventral posterior nucleus, MGB = medial geniculate body, CN = cochlear nucleus, mPFC = medial prefrontal cortex, NAc = nucleus accumbens, DLPFC = dorsolateral pre-frontal cortex, aI = anterior insula, dACC = dorsal anterior cingulate cortex, pgACC = pregenual anterior cingulate cortex, sgACC = subgenual anterior cingulate cortex, PHC = parahippocampal cortex, PCC = posterior cingulate cortex, PAG = periaqueductal gray.

Table 1.
Summary of findings of neural and molecular markers in the acute, chronic and transition phase of tinnitus and pain.
5.3. Molecular Markers
Several markers that are neuronal, immune and glial are related to the chronification of pain through central sensitisation. One is through the recruitment of NMDA receptors and their activation in the dorsal horn through the increase in the post-synaptic glutamate receptor [122]. They increase calcium permeability causing long term plasticity and neuronal damage. The long-term maintenance of chronic pain involves the release of BDNF by modifying molecular transcriptional processes. It has been shown that the transcription of the genes c-Fos, cyclooxyfensae, neurokinin, TrkB and prodynorphin contribute to long-term central sensitisation [123]. On the other hand, BDNF released from microglia also has been shown in the development of chronic pain. The BDNF leads to a depolarising shift in the dorsal horn causing GABA-related signals to become excitatory, leading to a cascade of molecular shifts, ending in hyperalgesia [123]. The increase in excitation at the peripheral level is seen in the change between acute and chronic ERP markers where the amplitude in the MMN time frame is larger than in the chronic state. This indicates an increase in the peripheral sensitisation process leading to a more stable central sensitisation pattern. From our search of the literature, there are not a lot of studies that look into which molecular markers are involved in the transition from acute to chronic tinnitus. This presents a gap in the literature that can filled by future studies.
6. Factors Predisposing Children and Adults through the Life-Course to Maladaptive Changes to Predictive Coding and Perceptual Disorders
6.1. Developmental Insults
Infants are exposed to several kinds of insults pre- and postnatally that may directly or indirectly affect the development of the predictive coding system. In utero, foetuses exposed to drugs consumed orally or administered intravenously by pregnant mothers are born with withdrawal symptoms to the drugs called Foetal Alcohol Syndrome (FAS) or Neonatal Abstinence Syndrome (NAS). Children born with FAS experience physical birth deformations, growth retardation, facial dysmorphism, cognitive delay and behavioural abnormalities [124]. Studies show that FAS can also influence the morphology of the brain, subcortical structures, such as the hippocampus, olfactory bulb, cerebellum, pituitary and septal regions, and the ventricles, depending on the gestational time of consumption [125,126,127,128]. Particularly, the hippocampus and the cerebellum are key regions of the predictive coding system. NAS manifests as poor feeding, gastrointestinal disorders, abnormal sleeping patterns, jitteriness, tremors and seizures [129]. Studies with small samples of infants have shown FAS-related changes in the brain using EEG and MRI showing epileptic-type activity and changes in brain volume, specifically in the basal ganglia [130,131] which is also an important brain structure in the predictive coding system. Newborns are also exposed to other kinds of insults such as hydrocephalus, i.e., where there is a build-up of cerebrospinal fluid, and cerebral palsy (CP), a movement disorder that also affects co-ordination, swallowing, speaking and is accompanied by muscle stiffness weakness and tremors, etc.
Changes in the brain structure because of any of the above reasons, including pre-natal stress, can cause developmental delays due to the reduced ability of sensory-motor integration, which is the key step to cognitive development [132]. Furthermore, research suggests that more than half of the babies who are born pre-term (<=30 weeks) suffer from sensory processing disorders (SPD), which is a reliable precursor to developing developmental delays [133]. This is backed up by another systematic review of articles looking into SPD in children from birth—three born pre-term—showing sensory over-reactivity (82% of findings positive) in these children [134]. SPD is characterised by hyper-responsiveness, hypo-responsiveness or craving to sensory stimuli occurring in 5% of children in the general population and in 40–80% of the population with developmental disorders [135]. Furthermore, the severity of SPD in children predicts the severity of SPD in adulthood and whether or not these subjects will have high emotional dysregulation [136], which is suggested to be an important factor in the development and maintenance of chronic pain [137]. A detailed study using different sensory stimuli showed that children with SPD perceive more pain that lasts longer [138]. Although not as extensively researched in tinnitus, we know that tinnitus is sometimes accompanied by hyperacusis—or increased sensitivity to auditory stimuli—which is a subset of SPD.
6.2. Environmental Factors
The environment we are exposed to is constantly changing throughout our lifetime. From a predictive coding perspective, this changing environment plays a major role in shaping us into who we are, influences our belief systems and is paramount in forming associations. A meta-analysis showed a moderate risk of developing chronic pain in people with low and medium socioeconomic statuses [139]. This could be because one’s socioeconomic status determines the kind of obstetric care mothers receive during pregnancy, the nutritional value of the food infants receive during gestation, etc., which are important in determining the health of the new-born and are, in turn, key in the development of the sensory-motor system and cognition. An earlier study determined that infants with a lower birth weight and gestational age were more affected by birth-related complications than their peers with a normal birth weight and normal gestational age [140]. Furthermore, there is sufficient research stating the relationship between pre-term birth and SPD [133,134].
The next major factor is the health of the family environment. Several studies have shown the relationship between chronic pain and a history of physical and sexual abuse [141,142] and emotional neglect [143]. A meta-analysis examining the relationship between sexual abuse and a lifetime diagnosis of somatic disorders shows a significant risk of developing chronic pain (OR = 2.20; 95% CI, 1.54–3.15, I2 = 82%), particularly chronic pelvic pain (OR, 2.73; 95% CI, 1.73–4.30, I2 = 40%) [144]. Not only adverse childhood trauma, such as physical and sexual abuse, but also dysfunctional family dynamics can affect the neurodevelopment of a child. Although children coming from divorced parents are less likely to have gone through physical abuse, they are more likely to be diagnosed with ADHD [145]. Furthermore, there remains a strong association between early inpatient psychiatric hospitalisation and the development of mental illness later in their lives [145]. Children with a traumatic childhood background have also shown to have an increased association with dysfunctional sensory processing [146] which, as stated, is a risk factor for developing chronic pain and often observed with tinnitus.
In addition to the socioeconomic status and family dynamics, the work environment, exposure to chemicals, toxins, noise, pollution, etc., can also have a cumulative effect on the sensory system, leading to dysfunctions in the predictive coding system and to disorders like tinnitus. The characteristics of hearing loss in people exposed to continuous noise (an uninterrupted sound level that varies around less than 5 dB during the period of observation) may vary from those exposed to intermittent noise (continuous noise that persists for more than 1 s and is interrupted for more than 1 s) or impulse noise (a change of sound pressure of 40 dB or more within 0.5 s within a duration of less than 1 s) [147]. Noise-induced hearing loss is one of the major co-morbidities of tinnitus, putting construction and factory workers, musicians, soldiers and others who are exposed to either intermittent noise or impulsive noise at a higher risk of developing tinnitus. Furthermore, occupational or environmental exposure to organic solvents such as toluene, styrene, xylene and ethyl benzene can significantly impact auditory perception [148,149]. Exposure to BTEX (Benzene, Toluene, Ethylbenzene and Xylene), a cocktail of highly soluble and volatile organic compounds naturally occurring in crude oil and petroleum products, is also a major risk factor for hearing loss, as ototoxic levels of BTEX can be present in both indoor and outdoor environments [150,151,152]. Emissions from motor vehicles, petrol stations and refineries are major sources of BTEX in the outdoor environment. BTEX is estimated to constitute up to 60% of non-methane volatile organic compounds in the urban atmosphere. In the indoor environment, BTEX is found in consumer goods, such as paints and lacquers, thinners, rubber products, adhesives, inks, cosmetics and pharmaceutical products. The degree of hearing loss associated with exposure to these compounds varies with the organic solvent type and exposure level. Studies also show that patients who have an inherent sensitivity to noise and chemicals are at a higher risk of developing stress and tinnitus [153]. Per the predictive coding model, changes in the weighting of the bottom–up input are hypothesised to be one of the primary reasons that change the perceptual inference [9].
Some of the more recent events that have had a significant impact on health and economy are the COVID-19 pandemic and ongoing wars in different parts of the world. A recent study showed that 40% of a multi-country sample with tinnitus experienced exacerbated tinnitus, especially in those who were self-isolating, experiencing loneliness or sleeping poorly [154]. Furthermore, a meta-analysis of the impact of COVID-19 on cognitive health 12 weeks post-COVID showed an elevation of pro-inflammatory markers and considerable functional impairment [155]. A systematic review showed a potential relationship between pro-inflammatory markers and the development of chronic lower back pain [156].
Exposure to war is a risk factor for the development of chronic phantom perceptions owing to the exposure to loud noise and traumatic injury to the brain and other parts of the body. This relationship has been established from previous studies of war veterans [157,158]. Studies show changes in the hippocampus, anterior cingulate cortex and ventromedial prefrontal cortex which are all key regions of the predictive coding system [159]. A systematic review of the literature looking at the effect of war on children’s health reports a direct and indirect effect on their health [160]. Direct effects include a range of physical injuries affecting all bodily organs and indirect effects include a higher burden of infectious, communicable and non-communicable diseases reducing the disability-adjusted life years (DALYs) in children exposed to war. All these factors alter the signalling to the brain, likely affecting the development of the predictive coding system and resulting in maladaptive coping strategies.
6.3. Genetic Risk Factors
The transmission of neurological disorders through the transfer of genes is another major risk factor influencing the development of the predictive coding system and its damage later in life. From twin studies in different disorders, we infer that developmental disorders and sensory disorders such as tinnitus may be passed genetically [161,162,163]. The more interesting associations of genetic factors that may affect the health of the predictive coding system, exposing certain people more susceptible to aberrant predictive coding, are the polymorphisms of certain genes that control the signalling of specific neurotransmitter receptors.
The most prominent polymorphisms for chronic tinnitus and pain are GABA-ergic and dopaminergic receptor-related genes. Particularly, fibromyalgia is associated with the GABRB3, a GABA receptor subunit. One of the disorder-wide polymorphisms observed is that of the COMT (Val158Met) in tinnitus [164] and pain. COMT (Val158Met) is a gene that regulates the dopamine metabolism whose dysregulation is observed in all these disorders. Changes in the dopamine metabolism also affects the amplitude of the P300 component depending on the task, as explained above, potentially explaining the neural correlate of the genotype. Furthermore a genome-wide association study showed that tinnitus is related to the single-nucleotide polymorphism of GMP6A which is involved in the development of nuclear membranes [165]. Furthermore, both tinnitus and pain showed a shared genetic risk with other psychiatric conditions [165,166]. An array of studies also show mutations in genes affecting the cholinergic system [167] and noradrenergic system [168] in order to play a role in the generation of different neuropathologies; however, there is still a lot of variability in the results between different genetic studies. Furthermore, it is still not clear as to how these genetic risk factors affect the predictive coding system of the brain and, in turn, the development of chronic conditions.
A summary of these different risk factors is presented in Figure 3.
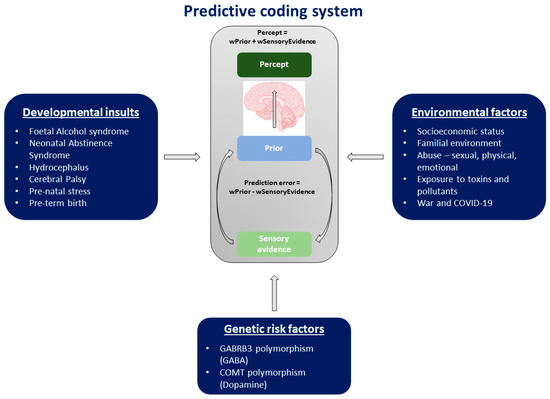
Figure 3.
Summary of the different risk factors that can predispose a person to aberrant predictive coding. These include developmental insults and environmental and genetic risk factors.
7. Limitations
The current manuscript is a perspective phrasing chronic perceptual disorders from the lens of predictive coding using a life-course approach. One of the limitations of the manuscript is not covering all the literature in this topic since this is built as a narrative review/perspective and not a systematic review. We also recognise that the predisposing factors for maladaptive changes in the predictive coding system are not limited to the ones presented here. This manuscript is built to encourage re-thinking about perceptual disorders from a predictive coding perspective and inform future research to not only probe this perspective from an experimental and systems neuroscience angle, but also consider the factors presented here from epidemiological studies and community interventions. Hence, there may be other factors which may be revealed from further prospective studies that may not be included in this manuscript and factors included in this manuscript that may not play as much of an important role as hypothesised here.
8. Future Directions
The current manuscript provides a basis to conduct further research in this topic of taking a life-course approach to understanding changes in the predictive coding system. From the current perspective, it is clear that there is a lot more comprehensive research performed in the field of chronic pain than tinnitus in all stages—acute, transition and chronic. Acute tinnitus is not fully understood partly because people do not see a specialist in the acute phase very quickly. Increasing awareness about tinnitus and understanding the role of acute tinnitus in terms of neural and biomarkers in the development of chronic tinnitus through longitudinal studies are the needs of the hour. The field also calls for the development of human models for tinnitus and pain to better understand the objective markers for both disorders. It is also clear that a comprehensive biopsychosocial model for tinnitus is required. This calls for epidemiological studies looking at different developmental and environmental factors for tinnitus to expand tinnitus from being an auditory problem, similar to what is being done in chronic pain. This will not only open up avenues for pharmacological and neuromodulatory interventions and the investigation of preventative factors and pre-clinical biomarkers, but also community-based, culturally appropriate interventions that can complement traditional medicine.
9. Conclusions
The predictive coding system is a constantly changing entity from gestation to death. In this study, we particularly studied the changes in the predictive coding system through the lens of chronic perceptual disorders. Several factors such as developmental insults and environmental and genetic factors affect different aspects of the predictive coding system influencing its development, the way associations are formed and how it processes sensory information. These factors help us identify specific groups of people who may be more vulnerable to the aberration of the predictive coding system. Future research that seeks to integrate these different factors into their questionnaires will be able to investigate biomarkers that can help us in the early detection of perceptual disorders across the life-course, thereby preventing the incidence and/or aiding in decreasing the severity of the illness which will make a significant contribution to the development of preclinical biomarkers in the predictive coding system.
Author Contributions
Both authors wrote this review. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
No applicable.
Data Availability Statement
No data are included in the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Knill, D.C.; Pouget, A. The Bayesian brain: The role of uncertainty in neural coding and computation. Trends Neurosci. 2004, 27, 712–719. [Google Scholar] [CrossRef]
- Friston, K.; FitzGerald, T.; Rigoli, F.; Schwartenbeck, P.; Doherty, J.O.; Pezzulo, G. Active inference and learning. Neurosci. Biobehav. Rev. 2016, 68, 862–879. [Google Scholar] [CrossRef]
- Powers, A.R.; Mathys, C.; Corlett, P.R. Pavlovian conditioning–induced hallucinations result from overweighting of perceptual priors. Science 2017, 357, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Bhamoo, N.; Riquelme, J.S.; Long, S.; Norena, A.; Vanneste, S. Investigating functional changes in the brain to intermittently induced auditory illusions and its relevance to chronic tinnitus. Hum. Brain Mapp. 2020, 41, 1819–1832. [Google Scholar] [CrossRef]
- Ryan, A.F.; Kujawa, S.G.; Hammill, T.; Le Prell, C.; Kil, J. Temporary and Permanent Noise-induced Threshold Shifts: A Review of Basic and Clinical Observations. Otol. Neurotol. 2016, 37, e271–e275. [Google Scholar] [CrossRef] [PubMed]
- Amaya, F.; Izumi, Y.; Matsuda, M.; Sasaki, M. Tissue injury and related mediators of pain exacerbation. Curr. Neuropharmacol. 2013, 11, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Vanneste, S. Adaptive and maladaptive neural compensatory consequences of sensory deprivation—From a phantom percept perspective. Prog. Neurobiol. 2017, 153, 1–17. [Google Scholar] [CrossRef]
- Corlett, P.R.; Horga, G.; Fletcher, P.C.; Alderson-Day, B.; Schmack, K.; Powers, A.R., III. Hallucinations and strong priors. Trends Cogn. Sci. 2019, 23, 114–127. [Google Scholar] [CrossRef]
- Sedley, W.; Friston, K.J.; Gander, P.E.; Kumar, S.; Griffiths, T.D. An Integrative Tinnitus Model Based on Sensory Precision. Trends Neurosci. 2016, 39, 799–812. [Google Scholar] [CrossRef]
- Rauschecker, J.P.; Leaver, A.M.; Mühlau, M. Tuning out the noise: Limbic-auditory interactions in tinnitus. Neuron 2010, 66, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Rauschecker, J.P.; May, E.S.; Maudoux, A.; Ploner, M. Frontostriatal Gating of Tinnitus and Chronic Pain. Trends Cogn. Sci. 2015, 19, 567–578. [Google Scholar] [CrossRef] [PubMed]
- De Ridder, D.; Vanneste, S. Chapter 15—The Bayesian brain in imbalance: Medial, lateral and descending pathways in tinnitus and pain: A perspective. In Progress in Brain Research; Langguth, B., Kleinjung, T., De Ridder, D., Schlee, W., Vanneste, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 262, pp. 309–334. [Google Scholar]
- Vanneste, S.; Alsalman, O.; De Ridder, D. Top-down and Bottom-up Regulated Auditory Phantom Perception. J. Neurosci. 2019, 39, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Luckey, A.; Weisz, N.; Vanneste, S. Predisposition to domain-wide maladaptive changes in predictive coding in auditory phantom perception. NeuroImage 2021, 248, 118813. [Google Scholar] [CrossRef] [PubMed]
- Rubin, J.J. Psychosomatic pain: New insights and management strategies. South Med. J. 2005, 98, 1099–1111. [Google Scholar] [CrossRef]
- Jarach, C.M.; Lugo, A.; Scala, M.; van den Brandt, P.A.; Cederroth, C.R.; Odone, A.; Garavello, W.; Schlee, W.; Langguth, B.; Gallus, S. Global Prevalence and Incidence of Tinnitus: A Systematic Review and Meta-analysis. JAMA Neurol. 2022, 79, 888–900. [Google Scholar] [CrossRef]
- Dahlhamer, J.; Lucas, J.; Zelaya, C.; Nahin, R.; Mackey, S.; DeBar, L.; Kerns, R.; Von Korff, M.; Porter, L.; Helmick, C. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. Morb. Mortal. Wkly. Rep. 2018, 67, 1001. [Google Scholar] [CrossRef]
- Goldstein, E.; Ho, C.-X.; Hanna, R.; Elinger, C.; Yaremchuk, K.L.; Seidman, M.D.; Jesse, M.T. Cost of Care for Subjective Tinnitus in Relation to Patient Satisfaction. Otolaryngol. Head Neck Surg. 2015, 152, 518–523. [Google Scholar] [CrossRef]
- Steglitz, J.; Buscemi, J.; Ferguson, M.J. The future of pain research, education, and treatment: A summary of the IOM report “Relieving pain in America: A blueprint for transforming prevention, care, education, and research”. Transl. Behav. Med. 2012, 2, 6–8. [Google Scholar] [CrossRef]
- Nagai, Y. Predictive learning: Its key role in early cognitive development. Philos. Trans. R. Soc. B 2019, 374, 20180030. [Google Scholar] [CrossRef]
- Näätänen, R.; Paavilainen, P.; Rinne, T.; Alho, K. The mismatch negativity (MMN) in basic research of central auditory processing: A review. Clin. Neurophysiol. 2007, 118, 2544–2590. [Google Scholar] [CrossRef]
- Wacongne, C.; Labyt, E.; van Wassenhove, V.; Bekinschtein, T.; Naccache, L.; Dehaene, S. Evidence for a hierarchy of predictions and prediction errors in human cortex. Proc. Natl. Acad. Sci. USA 2011, 108, 20754–20759. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Hotson, L.; Trainor, L.J. Mismatch responses to pitch changes in early infancy. J. Cogn. Neurosci. 2007, 19, 878–892. [Google Scholar] [CrossRef]
- He, C.; Trainor, L.J. Finding the pitch of the missing fundamental in infants. J. Neurosci. 2009, 29, 7718–8822. [Google Scholar] [CrossRef] [PubMed]
- Trainor, L.J.; Samuel, S.S.; Desjardins, R.N.; Sonnadara, R.R. Measuring temporal resolution in infants using mismatch negativity. Neuroreport 2001, 12, 2443–2448. [Google Scholar] [CrossRef] [PubMed]
- Trainor, L.; McFadden, M.; Hodgson, L.; Darragh, L.; Barlow, J.; Matsos, L.; Sonnadara, R. Changes in auditory cortex and the development of mismatch negativity between 2 and 6 months of age. Int. J. Psychophysiol. 2003, 51, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Tew, S.; Fujioka, T.; He, C.; Trainor, L. Neural representation of transposed melody in infants at 6 months of age. Ann. N. Y. Acad. Sci. 2009, 1169, 287–290. [Google Scholar] [CrossRef]
- Trainor, L.J.; Lee, K.; Bosnyak, D.J. Cortical plasticity in 4-month-old infants: Specific effects of experience with musical timbres. Brain Topogr. 2011, 24, 192–203. [Google Scholar] [CrossRef]
- Wacongne, C.; Changeux, J.-P.; Dehaene, S. A neuronal model of predictive coding accounting for the mismatch negativity. J. Neurosci. 2012, 32, 3665–3678. [Google Scholar] [CrossRef]
- Chennu, S.; Noreika, V.; Gueorguiev, D.; Blenkmann, A.; Kochen, S.; Ibáñez, A.; Owen, A.M.; Bekinschtein, T.A. Expectation and attention in hierarchical auditory prediction. J. Neurosci. 2013, 33, 11194–11205. [Google Scholar] [CrossRef]
- Emberson, L.L.; Richards, J.E.; Aslin, R.N. Top-down modulation in the infant brain: Learning-induced expectations rapidly affect the sensory cortex at 6 months. Proc. Natl. Acad. Sci. USA 2015, 112, 9585–9590. [Google Scholar] [CrossRef]
- Kouider, S.; Long, B.; Le Stanc, L.; Charron, S.; Fievet, A.-C.; Barbosa, L.S.; Gelskov, S.V. Neural dynamics of prediction and surprise in infants. Nat. Commun. 2015, 6, 8537. [Google Scholar] [CrossRef] [PubMed]
- De Haan, M. Infant EEG and Event-Related Potentials; Psychology Press: London, UK, 2013. [Google Scholar]
- Elsner, B.; Jeschonek, S.; Pauen, S. Event-related potentials for 7-month-olds’ processing of animals and furniture items. Dev. Cogn. Neurosci. 2013, 3, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Marinović, V.; Hoehl, S.; Pauen, S. Neural correlates of human–animal distinction: An ERP-study on early categorical differentiation with 4-and 7-month-old infants and adults. Neuropsychologia 2014, 60, 60–76. [Google Scholar] [CrossRef] [PubMed]
- Polich, J. Updating P300: An integrative theory of P3a and P3b. Clin. Neurophysiol. 2007, 118, 2128–2148. [Google Scholar] [CrossRef] [PubMed]
- Harms, L.; Parras, G.G.; Michie, P.T.; Malmierca, M.S. The Role of Glutamate Neurotransmission in Mismatch Negativity (MMN), A Measure of Auditory Synaptic Plasticity and Change-detection. Neuroscience 2021, 456, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Carbajal, G.V.; Malmierca, M.S. The neuronal basis of predictive coding along the auditory pathway: From the subcortical roots to cortical deviance detection. Trends Hear. 2018, 22, 2331216518784822. [Google Scholar] [CrossRef] [PubMed]
- Frodl-Bauch, T.; Bottlender, R.; Hegerl, U. Neurochemical substrates and neuroanatomical generators of the event-related P300. Neuropsychobiology 1999, 40, 86–94. [Google Scholar] [CrossRef]
- Albrecht, M.A.; Martin-Iverson, M.T.; Price, G.; Lee, J.; Iyyalol, R. Dexamphetamine-induced reduction of P3a and P3b in healthy participants. J. Psychopharmacol. 2011, 25, 1623–1631. [Google Scholar] [CrossRef]
- Vanneste, S.; Mohan, A.; Yoo, H.B.; Huang, Y.; Luckey, A.M.; McLeod, S.L.; Tabet, M.N.; Souza, R.R.; McIntyre, C.K.; Chapman, S. The peripheral effect of direct current stimulation on brain circuits involving memory. Sci. Adv. 2020, 6, eaax9538. [Google Scholar] [CrossRef]
- Sales, A.C.; Friston, K.J.; Jones, M.W.; Pickering, A.E.; Moran, R.J. Locus Coeruleus tracking of prediction errors optimises cognitive flexibility: An Active Inference model. PLoS Comput. Biol. 2019, 15, e1006267. [Google Scholar] [CrossRef]
- Murphy, P.R.; Robertson, I.H.; Balsters, J.H.; O’connell, R.G. Pupillometry and P3 index the locus coeruleus–noradrenergic arousal function in humans. Psychophysiology 2011, 48, 1532–1543. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, C.M.; Balsam, P.D.; Weinstein, J.J.; Rosengard, R.J.; Slifstein, M.; Daw, N.D.; Abi-Dargham, A.; Horga, G. A perceptual inference mechanism for hallucinations linked to striatal dopamine. Curr. Biol. 2018, 28, 503–514.e504. [Google Scholar] [CrossRef]
- Iglesias, S.; Mathys, C.; Brodersen, K.H.; Kasper, L.; Piccirelli, M.; den Ouden, H.E.; Stephan, K.E. Hierarchical prediction errors in midbrain and basal forebrain during sensory learning. Neuron 2013, 80, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, S.; Kasper, L.; Harrison, S.J.; Manka, R.; Mathys, C.; Stephan, K.E. Cholinergic and dopaminergic effects on prediction error and uncertainty responses during sensory associative learning. NeuroImage 2020, 226, 117590. [Google Scholar] [CrossRef] [PubMed]
- Moran, R.J.; Campo, P.; Symmonds, M.; Stephan, K.E.; Dolan, R.J.; Friston, K.J. Free energy, precision and learning: The role of cholinergic neuromodulation. J. Neurosci. 2013, 33, 8227–8236. [Google Scholar] [CrossRef]
- Conn, P.M. Sourcebook of Models for Biomedical Research; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Martucci, K.T.; Mackey, S.C. Imaging Pain. Anesth. Clin. 2016, 34, 255–269. [Google Scholar] [CrossRef] [PubMed]
- De Ridder, D.; Vanneste, S.; Smith, M.; Adhia, D. Pain and the Triple Network Model. Front. Neurol. 2022, 13, 757241. [Google Scholar] [CrossRef] [PubMed]
- Leaver, A.M.; Renier, L.; Chevillet, M.A.; Morgan, S.; Kim, H.J.; Rauschecker, J.P. Dysregulation of limbic and auditory networks in tinnitus. Neuron 2011, 69, 33–43. [Google Scholar] [CrossRef]
- Sedley, W.; Alter, K.; Gander, P.E.; Berger, J.; Griffiths, T.D. Exposing Pathological Sensory Predictions in Tinnitus Using Auditory Intensity Deviant Evoked Responses. J. Neurosci. 2019, 39, 10096–10103. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Y.; Sun, Y.; Wang, J.Y. A Brain Signature to Differentiate Acute and Chronic Pain in Rats. Front. Comput. Neurosci. 2016, 10, 41. [Google Scholar] [CrossRef]
- Legrain, V.; Guérit, J.-M.; Bruyer, R.; Plaghki, L. Electrophysiological correlates of attentional orientation in humans to strong intensity deviant nociceptive stimuli, inside and outside the focus of spatial attention. Neurosci. Lett. 2003, 339, 107–110. [Google Scholar] [CrossRef]
- Leske, S.; Tse, A.; Oosterhof, N.N.; Hartmann, T.; Müller, N.; Keil, J.; Weisz, N. The strength of alpha and beta oscillations parametrically scale with the strength of an illusory auditory percept. NeuroImage 2014, 88, 69–78. [Google Scholar] [CrossRef]
- Norena, A.; Micheyl, C.; Chery-Croze, S. An auditory negative after-image as a human model of tinnitus. Hear. Res. 2000, 149, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Knipper, M.; Zimmermann, U.; Müller, M. Molecular aspects of tinnitus. Hear. Res. 2010, 266, 60–69. [Google Scholar] [CrossRef] [PubMed]
- De Ridder, D.; Schlee, W.; Vanneste, S.; Londero, A.; Weisz, N.; Kleinjung, T.; Shekhawat, G.S.; Elgoyhen, A.B.; Song, J.J.; Andersson, G.; et al. Tinnitus and tinnitus disorder: Theoretical and operational definitions (an international multidisciplinary proposal). Prog. Brain Res. 2021, 260, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef]
- Baguley, D.; McFerran, D.; Hall, D. Tinnitus. Lancet 2013, 382, 1600–1607. [Google Scholar] [CrossRef]
- Cohen, S.P.; Vase, L.; Hooten, W.M. Chronic pain: An update on burden, best practices, and new advances. Lancet 2021, 397, 2082–2097. [Google Scholar] [CrossRef]
- Hearing Loss Association of America. Hearing Loss Facts and Statistics; Hearing Loss Association of America: Bethesda, MD, USA, 2019. [Google Scholar]
- Treede, R.D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. A classification of chronic pain for ICD-11. Pain 2015, 156, 1003–1007. [Google Scholar] [CrossRef]
- Volcheck, M.M.; Graham, S.M.; Fleming, K.C.; Mohabbat, A.B.; Luedtke, C.A. Central sensitization, chronic pain, and other symptoms: Better understanding, better management. Cleve. Clin. J. Med. 2023, 90, 245–254. [Google Scholar] [CrossRef] [PubMed]
- De Meulemeester, K.; Meeus, M.; De Pauw, R.; Cagnie, B.; Keppler, H.; Lenoir, D. Suffering from chronic tinnitus, chronic neck pain, or both: Does it impact the presence of signs and symptoms of central sensitization? PLoS ONE 2023, 18, e0290116. [Google Scholar] [CrossRef] [PubMed]
- Noreña, A.J. An integrative model of tinnitus based on a central gain controlling neural sensitivity. Neurosci. Biobehav. Rev. 2011, 35, 1089–1109. [Google Scholar] [CrossRef] [PubMed]
- Harte, S.E.; Harris, R.E.; Clauw, D.J. The neurobiology of central sensitization. J. Appl. Biobehav. Res. 2018, 23, e12137. [Google Scholar] [CrossRef]
- Zeng, F.G. Tinnitus and hyperacusis: Central noise, gain and variance. Curr. Opin. Physiol. 2020, 18, 123–129. [Google Scholar] [CrossRef]
- Latremoliere, A.; Woolf, C.J. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef]
- Leong, S.L.; Tchen, S.; Robertson, I.H.; Alsalman, O.; To, W.T.; Vanneste, S. The potential interruptive effect of tinnitus-related distress on attention. Sci. Rep. 2020, 10, 11911. [Google Scholar] [CrossRef]
- Attridge, N.; Keogh, E.; Eccleston, C. The effect of pain on task switching: Pain reduces accuracy and increases reaction times across multiple switching paradigms. Pain 2016, 157, 2179–2193. [Google Scholar] [CrossRef]
- Moore, D.J.; Meints, S.M.; Lazaridou, A.; Johnson, D.; Franceschelli, O.; Cornelius, M.; Schreiber, K.; Edwards, R.R. The Effect of Induced and Chronic Pain on Attention. J. Pain 2019, 20, 1353–1361. [Google Scholar] [CrossRef]
- Amaro-Díaz, L.; Montoro, C.I.; Fischer-Jbali, L.R.; Galvez-Sánchez, C.M. Chronic Pain and Emotional Stroop: A Systematic Review. J. Clin. Med. 2022, 11, 3259. [Google Scholar] [CrossRef]
- Bhatt, J.M.; Bhattacharyya, N.; Lin, H.W. Relationships between tinnitus and the prevalence of anxiety and depression. Laryngoscope 2017, 127, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Mullins, P.M.; Yong, R.J.; Bhattacharyya, N. Associations between chronic pain, anxiety, and depression among adults in the United States. Pain Pract. 2023, 23, 589–594. [Google Scholar] [CrossRef] [PubMed]
- McKenna, L.; Baguley, D.; McFerran, D. Living with Tinnitus and Hyperacusis: New Edition; Sheldon Press: London, UK, 2021. [Google Scholar]
- Tang, N.K. Insomnia Co-Occurring with Chronic Pain: Clinical Features, Interaction, Assessments and Possible Interventions. Rev. Pain 2008, 2, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Alster, J.; Shemesh, Z.; Ornan, M.; Attias, J. Sleep disturbance associated with chronic tinnitus. Biol. Psychiatry 1993, 34, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Milinski, L.; Nodal, F.R.; Vyazovskiy, V.V.; Bajo, V.M. Tinnitus: At a crossroad between phantom perception and sleep. Brain Commun. 2022, 4, fcac089. [Google Scholar] [CrossRef] [PubMed]
- Mathias, J.L.; Cant, M.L.; Burke, A.L.J. Sleep disturbances and sleep disorders in adults living with chronic pain: A meta-analysis. Sleep Med. 2018, 52, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Vanneste, S.; De Ridder, D. The auditory and non-auditory brain areas involved in tinnitus. An emergent property of multiple parallel overlapping subnetworks. Front. Syst. Neurosci. 2012, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Chang, M.C. Chronic Pain: Structural and Functional Changes in Brain Structures and Associated Negative Affective States. Int. J. Mol. Sci. 2019, 20, 3130. [Google Scholar] [CrossRef]
- Hullfish, J.; Sedley, W.; Vanneste, S. Prediction and perception: Insights for (and from) tinnitus. Neurosci. Biobehav. Rev. 2019, 102, 1–12. [Google Scholar] [CrossRef]
- Chen, Z.S. Hierarchical predictive coding in distributed pain circuits. Front. Neural Circuits 2023, 17, 1073537. [Google Scholar] [CrossRef]
- Song, Y.; Yao, M.; Kemprecos, H.; Byrne, A.; Xiao, Z.; Zhang, Q.; Singh, A.; Wang, J.; Chen, Z.S. Predictive coding models for pain perception. J. Comput. Neurosci. 2021, 49, 107–127. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.A.; Akrofi, K.; Carpenter-Thompson, J.R.; Husain, F.T. Default Mode, Dorsal Attention and Auditory Resting State Networks Exhibit Differential Functional Connectivity in Tinnitus and Hearing Loss. PLoS ONE 2013, 8, e76488. [Google Scholar] [CrossRef] [PubMed]
- Shahsavarani, S.; Schmidt, S.A.; Khan, R.A.; Tai, Y.; Husain, F.T. Salience, emotion, and attention: The neural networks underlying tinnitus distress revealed using music and rest. Brain Res. 2021, 1755, 147277. [Google Scholar] [CrossRef] [PubMed]
- Vanneste, S.; Plazier, M.; der Loo, E.v.; de Heyning, P.V.; Congedo, M.; De Ridder, D. The neural correlates of tinnitus-related distress. NeuroImage 2010, 52, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Davidson, C.; De Ridder, D.; Vanneste, S. Effective connectivity analysis of inter- and intramodular hubs in phantom sound perception–identifying the core distress network. Brain Imaging Behav. 2020, 14, 289–307. [Google Scholar] [CrossRef] [PubMed]
- De Ridder, D.; Vanneste, S.; Song, J.J.; Adhia, D. Tinnitus and the Triple Network Model: A Perspective. Clin. Exp. Otorhinolaryngol. 2022, 15, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Dick, B.D.; Connolly, J.F.; McGrath, P.J.; Finley, G.A.; Stroink, G.; Houlihan, M.E.; Clark, A.J. The disruptive effect of chronic pain on mismatch negativity. Clin. Neurophysiol. 2003, 114, 1497–1506. [Google Scholar] [CrossRef]
- Fan, L.; Sun, Y.-B.; Sun, Z.-K.; Wang, N.; Luo, F.; Yu, F.; Wang, J.-Y. Modulation of auditory sensory memory by chronic clinical pain and acute experimental pain: A mismatch negativity study. Sci. Rep. 2018, 8, 15673. [Google Scholar] [CrossRef]
- Yao, S.; Liu, X.; Yang, W.; Wang, X. Preattentive processing abnormalities in chronic pain: Neurophysiological evidence from mismatch negativity. Pain Med. 2011, 12, 773–781. [Google Scholar] [CrossRef]
- Choi, W.; Lim, M.; Kim, J.S.; Kim, D.J.; Chung, C.K. Impaired pre-attentive auditory processing in fibromyalgia: A mismatch negativity (MMN) study. Clin. Neurophysiol. 2015, 126, 1310–1318. [Google Scholar] [CrossRef]
- Guo, Y.; Tian, Q.; Xu, S.; Han, M.; Sun, Y.; Hong, Y.; Hou, X.; Liu, X. The impact of attack frequency and duration on neurocognitive processing in migraine sufferers: Evidence from event-related potentials using a modified oddball paradigm. BMC Neurol. 2019, 19, 73. [Google Scholar] [CrossRef] [PubMed]
- Tomasevic-Todorovic, S.; Boskovic, K.; Filipovic, D.; Milekic, B.; Grajic, M.; Hanna, F. Auditory Event-Related P300 Potentials in Rheumatoid Arthritis Patients. Neurophysiology 2015, 47, 138–143. [Google Scholar] [CrossRef]
- Niso, G.; Tjepkema-Cloostermans, M.C.; Lenders, M.W.P.M.; de Vos, C.C. Modulation of the Somatosensory Evoked Potential by Attention and Spinal Cord Stimulation. Front. Neurol. 2021, 12, 4310. [Google Scholar] [CrossRef] [PubMed]
- Broadbent, P.; Liossi, C.; Schoth, D.E. Attentional bias to somatosensory stimuli in chronic pain patients: A systematic review and meta-analysis. Pain 2021, 162, 332–352. [Google Scholar] [CrossRef] [PubMed]
- Sendesen, E.; Erbil, N.; Türkyılmaz, M.D. The mismatch negativity responses of individuals with tinnitus with normal extended high-frequency hearing-is it possible to use mismatch negativity in the evaluation of tinnitus? Eur. Arch. Otorhinolaryngol. 2022, 279, 3425–3434. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Lu, X.; Sun, S. Application of auditory mismatch negativity in tinnitus patients based on high-resolution electroencephalogram signals. Transl. Neurosci. 2022, 13, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Mohebbi, M.; Daneshi, A.; Asadpour, A.; Mohsen, S.; Farhadi, M.; Mahmoudian, S. The potential role of auditory prediction error in decompensated tinnitus: An auditory mismatch negativity study. Brain Behav. 2019, 9, e01242. [Google Scholar] [CrossRef] [PubMed]
- Weisz, N.; Voss, S.; Berg, P.; Elbert, T. Abnormal auditory mismatch response in tinnitus sufferers with high-frequency hearing loss is associated with subjective distress level. BMC Neurosci. 2004, 5, 8. [Google Scholar] [CrossRef][Green Version]
- Cardon, E.; Joossen, I.; Vermeersch, H.; Jacquemin, L.; Mertens, G.; Vanderveken, O.M.; Topsakal, V.; Van de Heyning, P.; Van Rompaey, V.; Gilles, A. Systematic review and meta-analysis of late auditory evoked potentials as a candidate biomarker in the assessment of tinnitus. PLoS ONE 2020, 15, e0243785. [Google Scholar] [CrossRef]
- Møller, A.R. Tinnitus and pain. Prog. Brain Res. 2007, 166, 47–53. [Google Scholar] [CrossRef]
- Isler, B.; von Burg, N.; Kleinjung, T.; Meyer, M.; Stämpfli, P.; Zölch, N.; Neff, P. Lower glutamate and GABA levels in auditory cortex of tinnitus patients: A 2D-JPRESS MR spectroscopy study. Sci. Rep. 2022, 12, 4068. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lei, Y.; Tian, Y.; Xu, S.; Shen, X.; Wu, H.; Bao, S.; Wang, F. The etiological contribution of GABAergic plasticity to the pathogenesis of neuropathic pain. Mol. Pain 2019, 15, 1744806919847366. [Google Scholar] [CrossRef] [PubMed]
- Stolzberg, D.; Salvi, R.J.; Allman, B.L. Salicylate toxicity model of tinnitus. Front. Syst. Neurosci. 2012, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Goljanian Tabrizi, A.; Safavi Naini, A.; Baradaran, N. Short-Term Effect of Gabapentin on Subjective Tinnitus in Acoustic Trauma Patients. Iran. J. Otorhinolaryngol. 2017, 29, 95–100. [Google Scholar] [PubMed]
- Peek, A.L.; Rebbeck, T.; Puts, N.A.J.; Watson, J.; Aguila, M.-E.R.; Leaver, A.M. Brain GABA and glutamate levels across pain conditions: A systematic literature review and meta-analysis of 1H-MRS studies using the MRS-Q quality assessment tool. NeuroImage 2020, 210, 116532. [Google Scholar] [CrossRef]
- Barr, M.S.; Farzan, F.; Davis, K.D.; Fitzgerald, P.B.; Daskalakis, Z.J. Measuring GABAergic Inhibitory Activity with TMS-EEG and Its Potential Clinical Application for Chronic Pain. J. Neuroimmune Pharmacol. 2013, 8, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Gonzalez, M.A.; Esteban-Ortega, F. Tinnitus dopaminergic pathway. Ear noises treatment by dopamine modulation. Med. Hypotheses 2005, 65, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, S.; Lu, X.; Tao, F. Role of Descending Dopaminergic Pathways in Pain Modulation. Curr. Neuropharmacol. 2019, 17, 1176–1182. [Google Scholar] [CrossRef]
- Hruschak, V.; Cochran, G. Psychosocial predictors in the transition from acute to chronic pain: A systematic review. Psychol. Health Med. 2018, 23, 1151–1167. [Google Scholar] [CrossRef]
- Wallhäusser-Franke, E.; D’Amelio, R.; Glauner, A.; Delb, W.; Servais, J.J.; Hörmann, K.; Repik, I. Transition from Acute to Chronic Tinnitus: Predictors for the Development of Chronic Distressing Tinnitus. Front. Neurol. 2017, 8, 605. [Google Scholar] [CrossRef]
- Vielsmeier, V.; Santiago Stiel, R.; Kwok, P.; Langguth, B.; Schecklmann, M. From Acute to Chronic Tinnitus: Pilot Data on Predictors and Progression. Front. Neurol. 2020, 11, 997. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ji, L.; Wang, L.; Yin, Z.; Cen, J.; Guo, Y. Clinical characteristics and psychoacoustic analysis of acute and chronic subjective tinnitus. Laryngoscope Investig. Otolaryngol. 2023, 8, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Lan, L.; Li, J.; Chen, Y.; Chen, W.; Li, W.; Zhao, F.; Chen, G.; Liu, J.; Chen, Y.; Li, Y.; et al. Alterations of brain activity and functional connectivity in transition from acute to chronic tinnitus. Hum. Brain Mapp. 2021, 42, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Baliki, M.N.; Petre, B.; Torbey, S.; Herrmann, K.M.; Huang, L.; Schnitzer, T.J.; Fields, H.L.; Apkarian, A.V. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat. Neurosci. 2012, 15, 1117–1119. [Google Scholar] [CrossRef] [PubMed]
- Mutso, A.A.; Petre, B.; Huang, L.; Baliki, M.N.; Torbey, S.; Herrmann, K.M.; Schnitzer, T.J.; Apkarian, A.V. Reorganization of hippocampal functional connectivity with transition to chronic back pain. J. Neurophysiol. 2014, 111, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Apkarian, A.V.; Baliki, M.N.; Farmer, M.A. Predicting transition to chronic pain. Curr. Opin. Neurol. 2013, 26, 360. [Google Scholar] [CrossRef]
- Glare, P.; Overton, S.; Aubrey, K. Transition from acute to chronic pain: Where cells, systems and society meet. Pain Manag. 2020, 10, 421–436. [Google Scholar] [CrossRef] [PubMed]
- Mifflin, K.A.; Kerr, B.J. The transition from acute to chronic pain: Understanding how different biological systems interact. Can. J. Anesth. 2014, 61, 112–122. [Google Scholar] [CrossRef]
- Murawski, N.J.; Moore, E.M.; Thomas, J.D.; Riley, E.P. Advances in diagnosis and treatment of fetal alcohol spectrum disorders: From animal models to human studies. Alcohol Res. Curr. Rev. 2015, 37, 97. [Google Scholar]
- O’Leary-Moore, S.K.; Parnell, S.E.; Lipinski, R.J.; Sulik, K.K. Magnetic resonance-based imaging in animal models of fetal alcohol spectrum disorder. Neuropsychol. Rev. 2011, 21, 167–185. [Google Scholar] [CrossRef]
- Godin, E.A.; O’Leary-Moore, S.K.; Khan, A.A.; Parnell, S.E.; Ament, J.J.; Dehart, D.B.; Johnson, B.W.; Allan Johnson, G.; Styner, M.A.; Sulik, K.K. Magnetic resonance microscopy defines ethanol-induced brain abnormalities in prenatal mice: Effects of acute insult on gestational day 7. Alcohol. Clin. Exp. Res. 2010, 34, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Parnell, S.E.; Holloway, H.T.; O’Leary-Moore, S.K.; Dehart, D.B.; Paniaqua, B.; Oguz, I.; Budin, F.; Styner, M.A.; Johnson, G.A.; Sulik, K.K. Magnetic resonance microscopy-based analyses of the neuroanatomical effects of gestational day 9 ethanol exposure in mice. Neurotoxicol. Teratol. 2013, 39, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.M.; Migliorini, R.; Infante, M.A.; Riley, E.P. Fetal alcohol spectrum disorders: Recent neuroimaging findings. Curr. Dev. Disord. Rep. 2014, 1, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Hudak, M.L.; Tan, R.C. The Committee on Drugs and the Committee on Fetus and Newborn. Neonatal Drug Withdrawal (vol 129, pg e540, 2012). Pediatrics 2014, 133, 937–938. [Google Scholar]
- Palla, M.R.; Khan, G.; Haghighat, Z.M.; Bada, H. EEG findings in infants with neonatal abstinence syndrome presenting with clinical seizures. Front. Pediatr. 2019, 7, 111. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Rubic, M.; Seah, J.; Rae, C.; Wright, I.M.; Kaltenbach, K.; Feller, J.; Abdel-Latif, M.E.; Chu, C.; Oei, J.L. Do maternal opioids reduce neonatal regional brain volumes? A pilot study. J. Perinatol. 2014, 34, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.L.; Moore, C.F.; Gajewski, L.L.; Larson, J.A.; Roberts, A.D.; Converse, A.K.; DeJesus, O.T. Sensory processing disorder in a primate model: Evidence from a longitudinal study of prenatal alcohol and prenatal stress effects. Child Dev. 2008, 79, 100–113. [Google Scholar] [CrossRef]
- Ryckman, J.; Hilton, C.; Rogers, C.; Pineda, R. Sensory processing disorder in preterm infants during early childhood and relationships to early neurobehavior. Early Hum. Dev. 2017, 113, 18–22. [Google Scholar] [CrossRef]
- Mitchell, A.W.; Moore, E.M.; Roberts, E.J.; Hachtel, K.W.; Brown, M.S. Sensory processing disorder in children ages birth–3 years born prematurely: A systematic review. Am. J. Occup. Ther. 2015, 69, 6901220030. [Google Scholar] [CrossRef]
- Ahn, R.R.; Miller, L.J.; Milberger, S.; McIntosh, D.N. Prevalence of parents’ perceptions of sensory processing disorders among kindergarten children. Am. J. Occup. Ther. 2004, 58, 287–293. [Google Scholar] [CrossRef]
- McMahon, K.; Anand, D.; Morris-Jones, M.; Rosenthal, M.Z. A path from childhood sensory processing disorder to anxiety disorders: The mediating role of emotion dysregulation and adult sensory processing disorder symptoms. Front. Integr. Neurosci. 2019, 13, 22. [Google Scholar] [CrossRef]
- Koechlin, H.; Coakley, R.; Schechter, N.; Werner, C.; Kossowsky, J. The role of emotion regulation in chronic pain: A systematic literature review. J. Psychosom. Res. 2018, 107, 38–45. [Google Scholar] [CrossRef]
- Bar-Shalita, T.; Vatine, J.-J.; Seltzer, Z.e.; Parush, S. Psychophysical correlates in children with sensory modulation disorder (SMD). Physiol. Behav. 2009, 98, 631–639. [Google Scholar] [CrossRef]
- Prego-Domínguez, J.; Khazaeipour, Z.; Mallah, N.; Takkouche, B. Socioeconomic status and occurrence of chronic pain: A meta-analysis. Rheumatology 2021, 60, 1091–1105. [Google Scholar] [CrossRef]
- Gill, S.V.; May-Benson, T.A.; Teasdale, A.; Munsell, E.G. Birth and developmental correlates of birth weight in a sample of children with potential sensory processing disorder. BMC Pediatr. 2013, 13, 29. [Google Scholar] [CrossRef]
- Bailey, B.E.; Freedenfeld, R.N.; Kiser, R.S.; Gatchel, R.J. Lifetime physical and sexual abuse in chronic pain patients: Psychosocial correlates and treatment outcomes. Disabil. Rehabil. 2003, 25, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Linton, S.J. A population-based study of the relationship between sexual abuse and back pain: Establishing a link. Pain 1997, 73, 47–53. [Google Scholar] [CrossRef]
- Davis, D.A.; Luecken, L.J.; Zautra, A.J. Are Reports of Childhood Abuse Related to the Experience of Chronic Pain in Adulthood?: A Meta-analytic Review of the Literature. Clin. J. Pain 2005, 21, 398–405. [Google Scholar] [CrossRef]
- Paras, M.L.; Murad, M.H.; Chen, L.P.; Goranson, E.N.; Sattler, A.L.; Colbenson, K.M.; Elamin, M.B.; Seime, R.J.; Prokop, L.J.; Zirakzadeh, A. Sexual abuse and lifetime diagnosis of somatic disorders: A systematic review and meta-analysis. JAMA 2009, 302, 550–561. [Google Scholar] [CrossRef]
- Behere, A.P.; Basnet, P.; Campbell, P. Effects of family structure on mental health of children: A preliminary study. Indian J. Psychol. Med. 2017, 39, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Howard, A.R.H.; Lynch, A.K.; Call, C.D.; Cross, D.R. Sensory processing in children with a history of maltreatment: An occupational therapy perspective. Vulnerable Child. Youth Stud. 2020, 15, 60–67. [Google Scholar] [CrossRef]
- Rosati, R.; Jamesdaniel, S. Environmental exposures and hearing loss. Int. J. Environ. Res. Public Health 2020, 17, 4879. [Google Scholar] [CrossRef]
- Morata, T.C.; Dunn, D.E.; Sieber, W.K. Occupational exposure to noise and ototoxic organic solvents. Arch. Environ. Health Int. J. 1994, 49, 359–365. [Google Scholar] [CrossRef]
- Sliwinska-Kowalska, M.; Zamyslowska-Szmytke, E.; Szymczak, W.; Kotylo, P.; Fiszer, M.; Wesolowski, W.; Pawlaczyk-Luszczynska, M. Ototoxic effects of occupational exposure to styrene and co-exposure to styrene and noise. J. Occup. Environ. Med. 2003, 45, 15–24. [Google Scholar] [CrossRef]
- Staudt, A.M.; Whitworth, K.W.; Chien, L.-C.; Whitehead, L.W.; de Porras, D.G.R. Association of organic solvents and occupational noise on hearing loss and tinnitus among adults in the US, 1999–2004. Int. Arch. Occup. Environ. Health 2019, 92, 403–413. [Google Scholar] [CrossRef]
- Roggia, S.M.; de França, A.G.; Morata, T.C.; Krieg, E.; Earl, B.R. Auditory system dysfunction in Brazilian gasoline station workers. Int. J. Audiol. 2019, 58, 484–496. [Google Scholar] [CrossRef]
- Bolden, A.L.; Kwiatkowski, C.F.; Colborn, T. New look at BTEX: Are ambient levels a problem? Environ. Sci. Technol. 2015, 49, 5261–5276. [Google Scholar] [CrossRef]
- Danioth, L.; Brotschi, G.; Croy, I.; Friedrich, H.; Caversaccio, M.-D.; Negoias, S. Multisensory environmental sensitivity in patients with chronic tinnitus. J. Psychosom. Res. 2020, 135, 110155. [Google Scholar] [CrossRef] [PubMed]
- Beukes, E.W.; Baguley, D.M.; Jacquemin, L.; Lourenco, M.P.; Allen, P.M.; Onozuka, J.; Stockdale, D.; Kaldo, V.; Andersson, G.; Manchaiah, V. Changes in tinnitus experiences during the COVID-19 pandemic. Front. Public Health 2020, 8, 681. [Google Scholar] [CrossRef] [PubMed]
- Ceban, F.; Ling, S.; Lui, L.M.W.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav. Immun. 2022, 101, 93–135. [Google Scholar] [CrossRef] [PubMed]
- Morris, P.; Ali, K.; Merritt, M.; Pelletier, J.; Macedo, L.G. A systematic review of the role of inflammatory biomarkers in acute, subacute and chronic non-specific low back pain. BMC Musculoskelet. Disord. 2020, 21, 142. [Google Scholar] [CrossRef]
- Eisen, S.A.; Kang, H.K.; Murphy, F.M.; Blanchard, M.S.; Reda, D.J.; Henderson, W.G.; Toomey, R.; Jackson, L.W.; Alpern, R.; Parks, B.J. Gulf War veterans’ health: Medical evaluation of a US cohort. Ann. Intern. Med. 2005, 142, 881–890. [Google Scholar] [CrossRef]
- Henry, J.A.; Griest, S.E.; Blankenship, C.; Thielman, E.J.; Theodoroff, S.M.; Hammill, T.; Carlson, K.F. Impact of Tinnitus on Military Service Members. Mil. Med. 2019, 184, 604–614. [Google Scholar] [CrossRef]
- Kühn, S.; Butler, O.; Willmund, G.; Wesemann, U.; Zimmermann, P.; Gallinat, J. The brain at war: Effects of stress on brain structure in soldiers deployed to a war zone. Transl. Psychiatry 2021, 11, 247. [Google Scholar] [CrossRef]
- Kadir, A.; Shenoda, S.; Goldhagen, J. Effects of armed conflict on child health and development: A systematic review. PLoS ONE 2019, 14, e0210071. [Google Scholar] [CrossRef]
- Constantino, J.N.; Todd, R.D. Autistic traits in the general population: A twin study. Arch. Gen. Psychiatry 2003, 60, 524–530. [Google Scholar] [CrossRef]
- Maas, I.L.; Brüggemann, P.; Requena, T.; Bulla, J.; Edvall, N.K.; vB Hjelmborg, J.; Szczepek, A.J.; Canlon, B.; Mazurek, B.; Lopez-Escamez, J.A. Genetic susceptibility to bilateral tinnitus in a Swedish twin cohort. Genet. Med. 2017, 19, 1007–1012. [Google Scholar] [CrossRef]
- Gejman, P.V.; Sanders, A.R.; Duan, J. The role of genetics in the etiology of schizophrenia. Psychiatr. Clin. 2010, 33, 35–66. [Google Scholar] [CrossRef]
- Vanneste, S.; Alsalman, O.; De Ridder, D. COMT and the neurogenetic architecture of hearing loss induced tinnitus. Hear. Res. 2018, 365, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, I.S.; Wilson, N.; Dias, R.; Torkamani, A. A genome-wide association study of tinnitus reveals shared genetic links to neuropsychiatric disorders. Sci. Rep. 2022, 12, 22511. [Google Scholar] [CrossRef] [PubMed]
- Johnston, K.J.A.; Adams, M.J.; Nicholl, B.I.; Ward, J.; Strawbridge, R.J.; Ferguson, A.; McIntosh, A.M.; Bailey, M.E.S.; Smith, D.J. Genome-wide association study of multisite chronic pain in UK Biobank. PLoS Genet. 2019, 15, e1008164. [Google Scholar] [CrossRef]
- Schaaf, C.P. Nicotinic acetylcholine receptors in human genetic disease. Genet. Med. 2014, 16, 649–656. [Google Scholar] [CrossRef]
- Nagatsu, T. The catecholamine system in health and disease -Relation to tyrosine 3-monooxygenase and other catecholamine-synthesizing enzymes. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2007, 82, 388–415. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).