Investigating Aphasia Recovery: Demographic and Clinical Factors
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
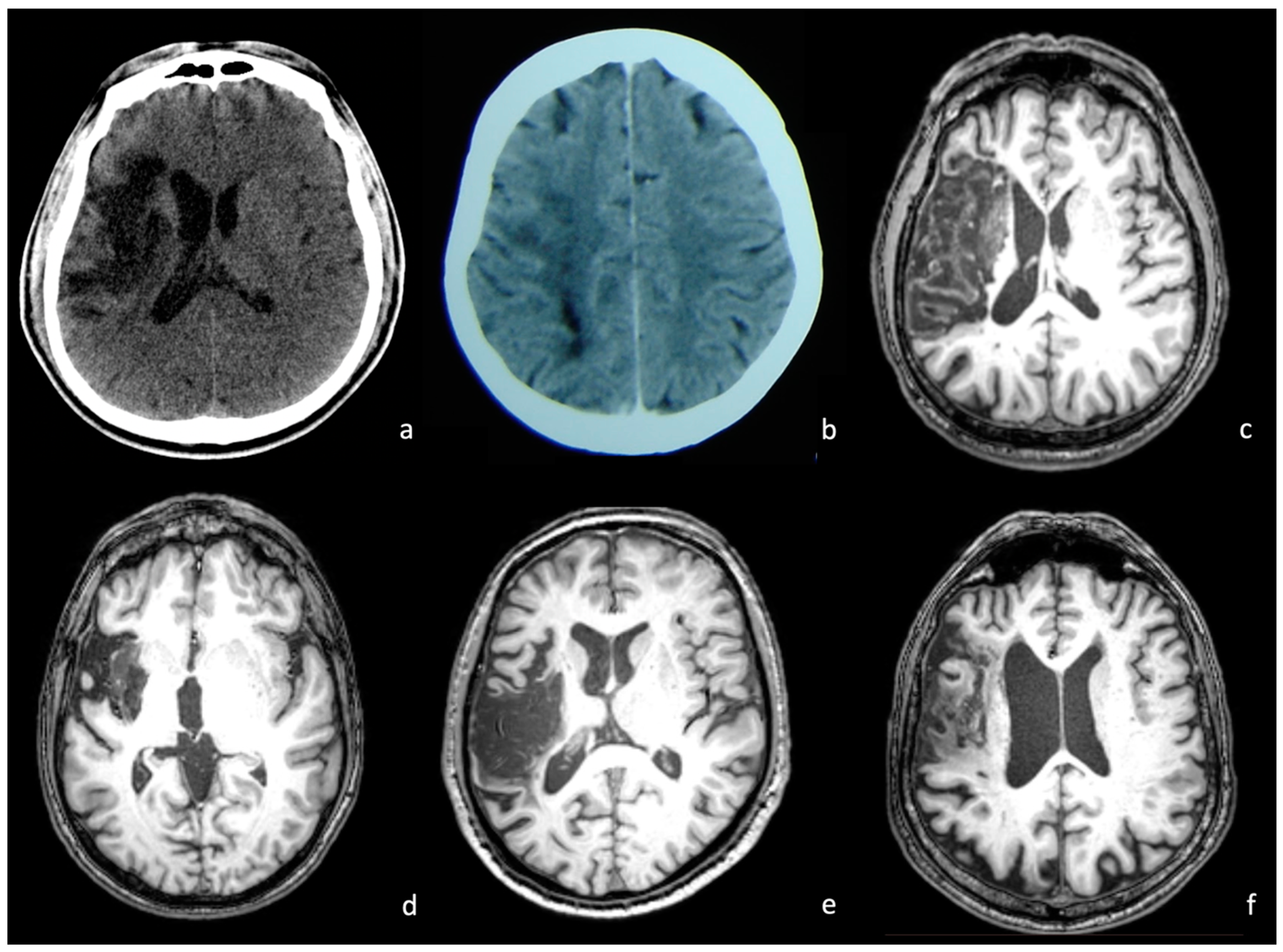
2.2. Neuroimaging Data
2.3. Procedures
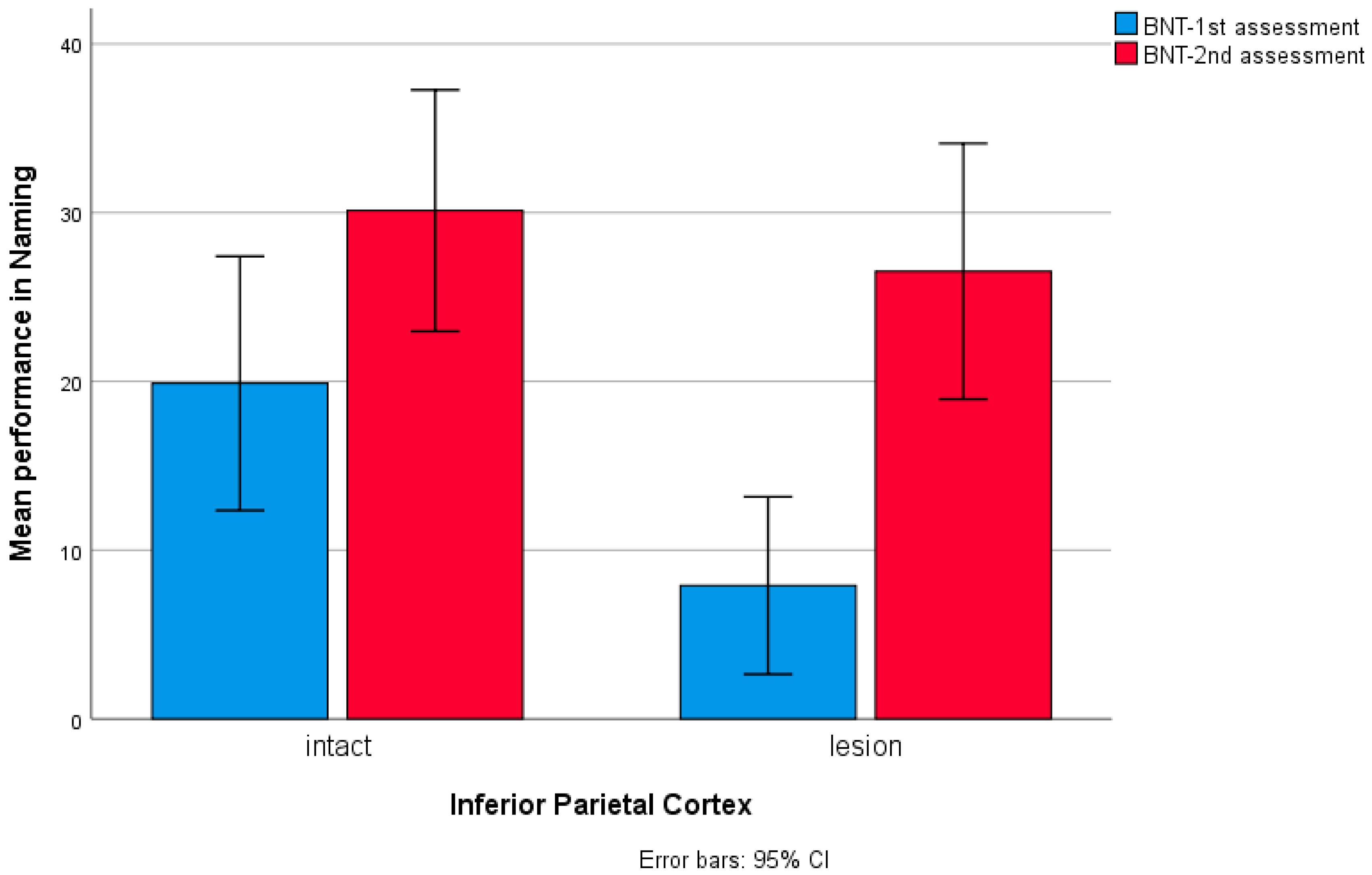
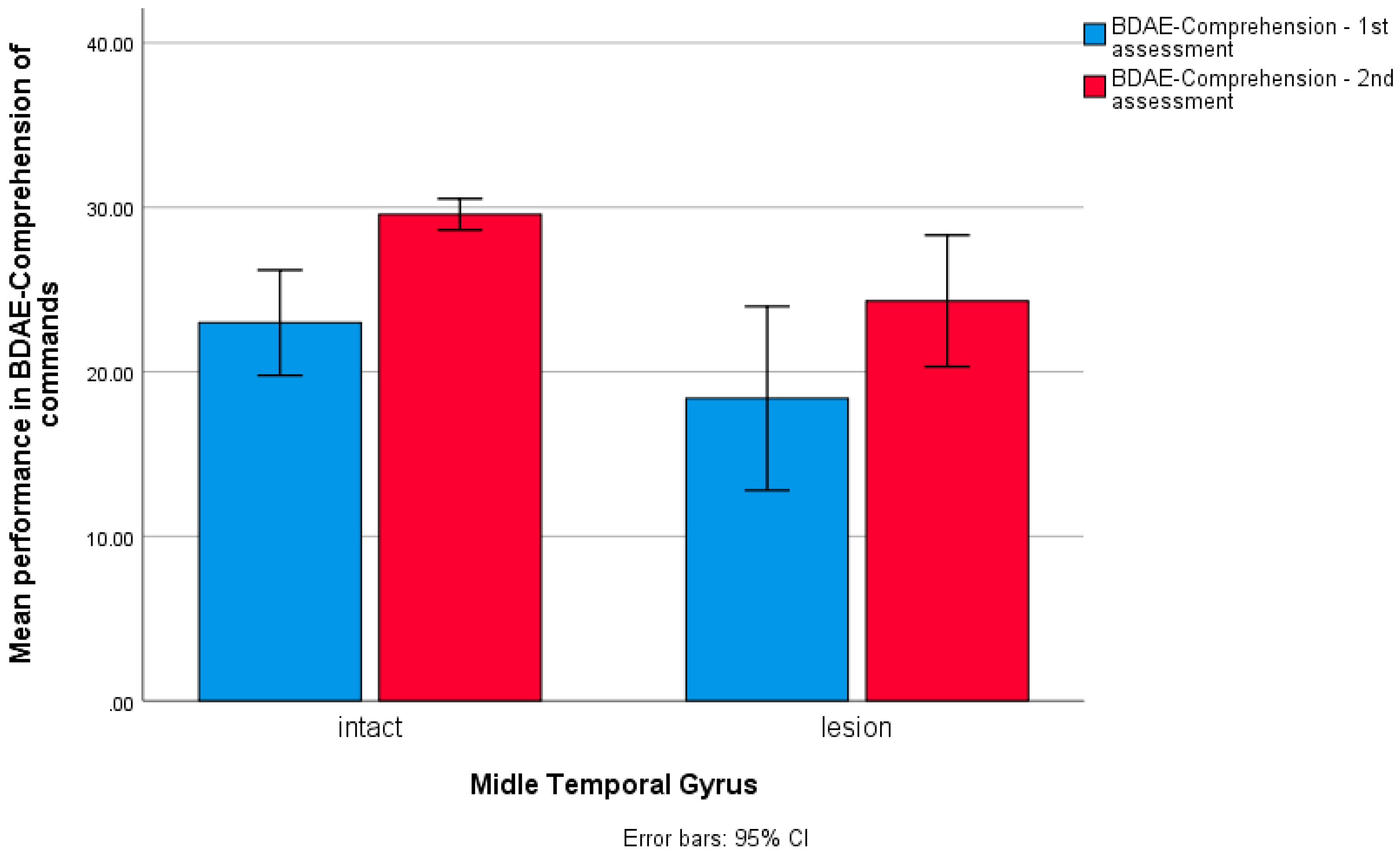
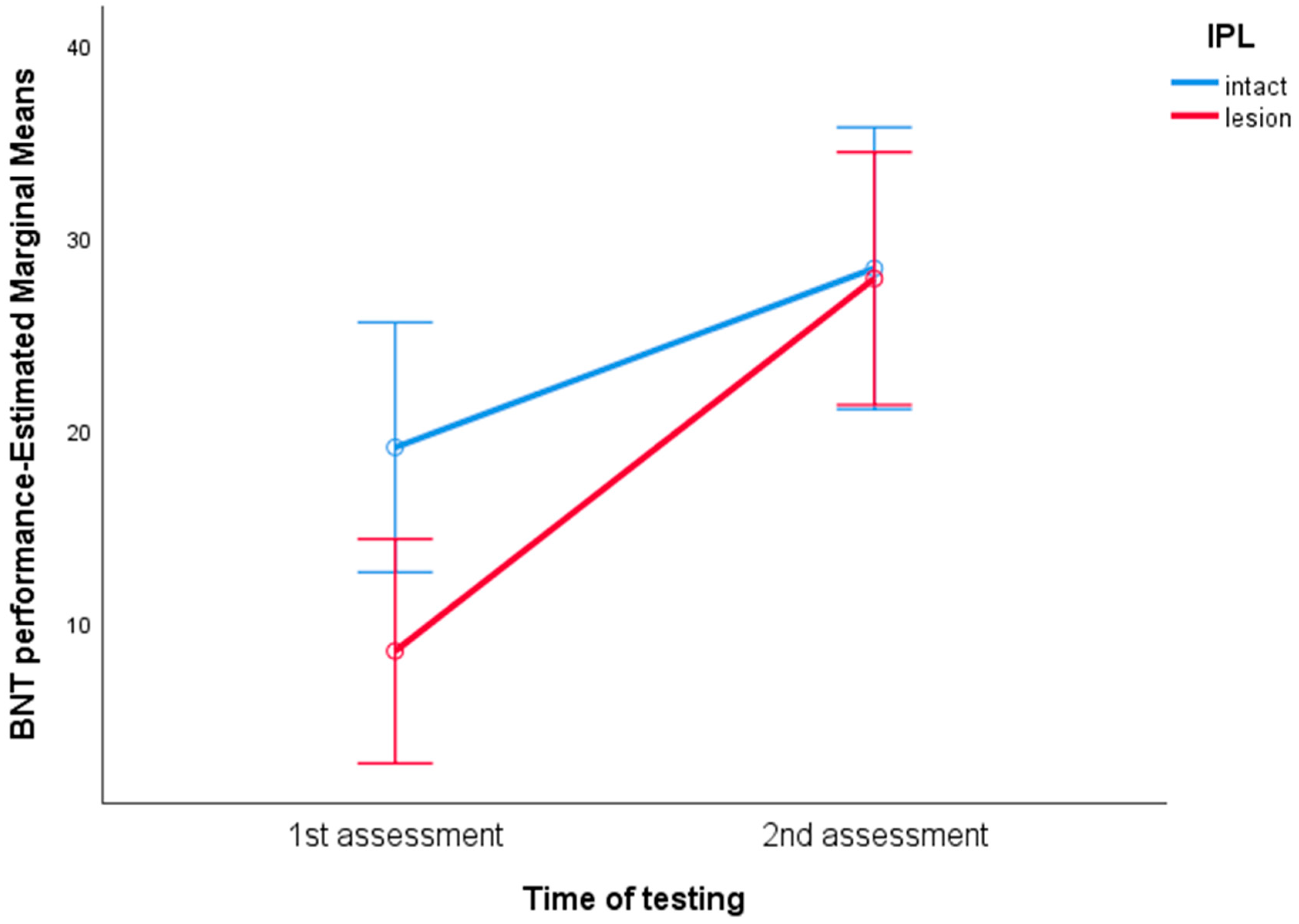
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Papanicolaou, A.C.; Moore, B.D.; Deutsch, G.; Levin, H.S.; Eisenberg, H.M. Evidence for Right-Hemisphere Involvement in Recovery From Aphasia. Arch. Neurol. 1988, 45, 1025–1029. [Google Scholar] [CrossRef] [PubMed]
- Weiller, C.; Isensee, C.; Rijntjes, M.; Huber, W.; Müller, S.; Bier, D.; Dutschka, K.; Woods, R.P.; Noth, J.; Diener, H.C. Recovery from Wernicke’s Aphasia: A Positron Emission Tomographic Study. Ann. Neurol. 1995, 37, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Basso, A. Efficacy of Aphasia Therapy. Aphasia Ther. 2003, 74–104. [Google Scholar] [CrossRef]
- de Riesthal, M.; Wertz, R. Prognosis for Aphasia: Relationship between Selected Biographical and Behavioural Variables and Outcome and Improvement. Aphasiology 2004, 18, 899–915. [Google Scholar] [CrossRef]
- Kasselimis, D.; Potagas, C. Language Disorders, Treatment and Remediation of. In International Encyclopedia of the Social & Behavioral Sciences; James, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 13, pp. 329–336. [Google Scholar]
- Hillis, A.E.; Beh, Y.Y.; Sebastian, R.; Breining, B.; Tippett, D.C.; Wright, A.; Saxena, S.; Rorden, C.; Bonilha, L.; Basilakos, A.; et al. Predicting Recovery in Acute Poststroke Aphasia. Ann. Neurol. 2018, 83, 612–622. [Google Scholar] [CrossRef] [PubMed]
- McClung, J.S.; Rothi, L.J.G.; Nadeau, S.E. Ambient Experience in Restitutive Treatment of Aphasia. Front. Hum. Neurosci. 2010, 4, 183. [Google Scholar] [CrossRef]
- Basso, A. Prognostic Factors in Aphasia. Aphasiology 1992, 6, 337–348. [Google Scholar] [CrossRef]
- Laska, A.C.; Hellblom, A.; Murray, V.; Kahan, T.; Von Arbin, M. Aphasia in Acute Stroke and Relation to Outcome. J. Intern. Med. 2001, 249, 413–422. [Google Scholar] [CrossRef]
- Lazar, R.M.; Antoniello, D. Variability in Recovery from Aphasia. Curr. Neurol. Neurosci. Rep. 2008, 8, 497–502. [Google Scholar] [CrossRef]
- Raymer, A.M.; Beeson, P.; Holland, A.; Kendall, D.; Maher, L.M.; Martin, N.; Murray, L.; Rose, M.; Thompson, C.K.; Turkstra, L.; et al. Translational Research in Aphasia: From Neuroscience to Neurorehabilitation. J. Speech Lang. Hear. Res. 2008, 51, S259–S275. [Google Scholar] [CrossRef]
- Kasselimis, D.S.; Papageorgiou, G.; Angelopoulou, G.; Tsolakopoulos, D.; Potagas, C. Translational Neuroscience of Aphasia and Adult Language Rehabilitation. Contempor. Clin. Neurosci. 2020, 5–20. [Google Scholar] [CrossRef]
- Papageorgiou, G.; Kasselimis, D.; Laskaris, N.; Potagas, C. Unraveling the Thread of Aphasia Rehabilitation: A Translational Cognitive Perspective. Biomedicines 2023, 11, 2856. [Google Scholar] [CrossRef] [PubMed]
- Marsh, E.B.; Hillis, A.E. Recovery from Aphasia Following Brain Injury: The Role of Reorganization. Prog. Brain Res. 2006, 157, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Kiran, S.; Thompson, C.K. Neuroplasticity of Language Networks in Aphasia: Advances, Updates, and Future Challenges. Front. Neurol. 2019, 10, 295. [Google Scholar] [CrossRef] [PubMed]
- Lazar, R.M.; Minzer, B.; Antoniello, D.; Festa, J.R.; Krakauer, J.W.; Marshall, R.S. Improvement in Aphasia Scores After Stroke is Well Predicted by Initial Severity. Stroke 2010, 41, 1485–1488. [Google Scholar] [CrossRef]
- El Hachioui, H.; Lingsma, H.F.; Sandt-Koenderman, M.E.; Dippel, D.W.J.; Koudstaal, P.J.; Visch-Brink, E.G. Recovery of Aphasia after Stroke: A 1-Year Follow-up Study. J. Neurol. 2012, 260, 166–171. [Google Scholar] [CrossRef]
- Pedersen, P.M.; Vinter, K.; Olsen, T.S. Aphasia after Stroke: Type, Severity and Prognosis. Cerebrovasc. Dis. 2003, 17, 35–43. [Google Scholar] [CrossRef]
- Kertesz, A.; Mccabe, P. Recovery Patterns and Prognosis in Aphasia. Brain 1977, 100, 1–18. [Google Scholar] [CrossRef]
- Watila, M.M.; Balarabe, S.A. Factors Predicting Post-Stroke Aphasia Recovery. J. Neurol. Sci. 2015, 352, 12–18. [Google Scholar] [CrossRef]
- Inatomi, Y.; Yonehara, T.; Omiya, S.; Hashimoto, Y.; Hirano, T.; Uchino, M. Aphasia during the Acute Phase in Ischemic Stroke. Cerebrovasc. Dis. 2008, 25, 316–323. [Google Scholar] [CrossRef]
- Godefroy, O.; Dubois, C.; Debachy, B.; Leclerc, M.; Kreisler, A.; Sakatani, K.; Xie, Y.; Lichty, W.; Li, S.; Zuo, H.; et al. Vascular Aphasias. Stroke 2002, 33, 702–705. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lendrem, W.; Lincoln, N.B. Spontaneous Recovery of Language in Patients with Aphasia between 4 and 34 Weeks after Stroke. J. Neurol. Neurosurg. Psychiatry 1985, 48, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, P.M.; Jørgensen, H.S.; Nakayama, H.; Raaschou, H.O.; Olsen, T.S. Aphasia in Acute Stroke: Incidence, Determinants, and Recovery. Ann. Neurol. 1995, 38, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Connor, L.T.; Obler, L.K.; Tocco, M.; Fitzpatrick, P.M.; Albert, M.L. Effect of Socioeconomic Status on Aphasia Severity and Recovery. Brain Lang. 2001, 78, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Lazar, R.M.; Speizer, A.E.; Festa, J.R.; Krakauer, J.W.; Marshall, R.S. Variability in Language Recovery after First-Time Stroke. J. Neurol. Neurosurg. Psychiatry 2008, 79, 530–534. [Google Scholar] [CrossRef]
- Seniów, J.; Litwin, M.; Leśniak, M. The Relationship between Non-Linguistic Cognitive Deficits and Language Recovery in Patients with Aphasia. J. Neurol. Sci. 2009, 283, 91–94. [Google Scholar] [CrossRef]
- Mazzoni, M.; Vista, M.; Pardossi, L.; Avila, L.; Bianchi, F.; Moretti, P. Spontaneous Evolution of Aphasia after Ischaemic Stroke. Aphasiology 1992, 6, 387–396. [Google Scholar] [CrossRef]
- Goldenberg, G.; Spatt, J. Influence of Size and Site of Cerebral Lesions on Spontaneous Recovery of Aphasia and on Success of Language Therapy. Brain Lang. 1994, 47, 684–698. [Google Scholar] [CrossRef]
- Maas, M.B.; Lev, M.H.; Ay, H.; Singhal, A.B.; Greer, D.M.; Smith, W.S.; Harris, G.J.; Halpern, E.F.; Koroshetz, W.J.; Furie, K.L. The Prognosis for Aphasia in Stroke. J. Stroke Cerebrovasc. Dis. 2010, 21, 350–357. [Google Scholar] [CrossRef]
- Naeser, M.A.; Palumbo, C.L.; Helm-Estabrooks, N.; Stiassny-Eder, D.; Albert, M.L. Severe Nonfluency in Aphasia. Brain 1989, 112, 1–38. [Google Scholar] [CrossRef]
- Naeser, M.A.; Palumbo, C.L.; Prete, M.N.; Fitzpatrick, P.M.; Mimura, M.; Samaraweera, R.; Albert, M.L. Visible Changes in Lesion Borders on CT Scan after Five Years Poststroke, and Long-Term Recovery in Aphasia. Brain Lang. 1998, 62, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Henseler, I.; Regenbrecht, F.; Obrig, H. Lesion Correlates of Patholinguistic Profiles in Chronic Aphasia: Comparisons of Syndrome-, Modality- and Symptom-Level Assessment. Brain 2014, 137, 918–930. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, R.E.; Lux, W.E.; Dromerick, A.W. Global Aphasia without Hemiparesis: Language Profiles and Lesion Distribution. J. Neurol. Neurosurg. Psychiatry 1999, 66, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Demeurisse, G.; Capon, A. Language Recovery in Aphasic Stroke Patients: Clinical, CT and CBF Studies. Aphasiology 1987, 1, 301–315. [Google Scholar] [CrossRef]
- Heiss, W.-D.; Thiel, A.; Kessler, J.; Herholz, K. Disturbance and Recovery of Language Function: Correlates in PET Activation Studies. NeuroImage 2003, 20, S42–S49. [Google Scholar] [CrossRef]
- Kang, E.K.; Sohn, H.M.; Han, M.-K.; Kim, W.; Han, T.R.; Paik, N.-J. Severity of Post-Stroke Aphasia According to Aphasia Type and Lesion Location in Koreans. J. Korean Med. Sci. 2010, 25, 123–127. [Google Scholar] [CrossRef]
- Pashek, G.V.; Holland, A.L. Evolution of Aphasia in the First Year Post-Onset. Cortex 1988, 24, 411–423. [Google Scholar] [CrossRef]
- Kasselimis, D.S.; Simos, P.G.; Peppas, C.; Evdokimidis, I.; Potagas, C. The Unbridged Gap between Clinical Diagnosis and Contemporary Research on Aphasia: A Short Discussion on the Validity and Clinical Utility of Taxonomic Categories. Brain Lang. 2017, 164, 63–67. [Google Scholar] [CrossRef]
- Tremblay, P.; Dick, A.S. Broca and Wernicke are Dead, or Moving Past the Classic Model of Language Neurobiology. Brain Lang. 2016, 162, 60–71. [Google Scholar] [CrossRef]
- García, A.O.; Brambati, S.M.; Brisebois, A.; Désilets-Barnabé, M.; Houzé, B.; Bedetti, C.; Rochon, E.; Leonard, C.; Desautels, A.; Marcotte, K. Predicting Early Post-Stroke Aphasia Outcome from Initial Aphasia Severity. Front. Neurol. 2020, 11, 120. [Google Scholar] [CrossRef]
- Selnes, O.A.; Knopman, D.S.; Niccum, N.; Rubens, A.B.; Larson, D. Computed Tomographic Scan Correlates of Auditory Comprehension Deficits in Aphasia: A Prospective Recovery Study. Ann. Neurol. 1983, 13, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Kertesz, A.; Lau, W.; Polk, M. The Structural Determinants of Recovery in Wernicke′s Aphasia. Brain Lang. 1993, 44, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Selnes, O.A.; Niccum, N.; Knopman, D.S.; Rubens, A.B. Recovery of Single Word Comprehension: CT-Scan Correlates. Brain Lang. 1984, 21, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Smania, N.; Gandolfi, M.; Aglioti, S.M.; Girardi, P.; Fiaschi, A.; Girardi, F. How Long Is the Recovery of Global Aphasia? Twenty-Five Years of Follow-up in a Patient with Left Hemisphere Stroke. Neurorehabilit. Neural Repair 2010, 24, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Kiran, S. What Is the Nature of Poststroke Language Recovery and Reorganization? ISRN Neurol. 2012, 2012, 786872. [Google Scholar] [CrossRef] [PubMed]
- Fridriksson, J.; Richardson, J.D.; Fillmore, P.; Cai, B. Left Hemisphere Plasticity and Aphasia Recovery. NeuroImage 2012, 60, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Ochfeld, E.; Newhart, M.; Molitoris, J.; Leigh, R.; Cloutman, L.; Davis, C.; Crinion, J.; Hillis, A.E. Ischemia in Broca Area Is Associated with Broca Aphasia More Reliably in Acute than in Chronic Stroke. Stroke 2010, 41, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Standring, S. Gray’s Anatomy: The Anatomical Basis of Clinical Practice; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Schuenke, M.; Schulte, E.; Schumacher, U.; Ross, L.M.; Lamperti, E.D.; Taub, E. Thieme Atlas of Anatomy: Head and Neuroanatomy; Thieme: Stuttgart, Germany, 2007. [Google Scholar]
- Ross, L.M. Thieme Atlas of Anatomy; George Thieme Verlag: Stuttgart, Germany, 2006. [Google Scholar]
- Kiernan, J.A.; Rajakumar, N.; Barr, M.L. Barr’s the Human Nervous System: An Anatomical Viewpoint; Wolters Kluwer Health Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2014. [Google Scholar]
- Efthymiopoulou, E.; Kasselimis, D.S.; Ghika, A.; Kyrozis, A.; Peppas, C.; Evdokimidis, I.; Petrides, M.; Potagas, C. The Effect of Cortical and Subcortical Lesions on Spontaneous Expression of Memory-Encoded and Emotionally Infused Information: Evidence for a Role of the Ventral Stream. Neuropsychologia 2017, 101, 115–120. [Google Scholar] [CrossRef]
- Kasselimis, D.S.; Simos, P.G.; Economou, A.; Peppas, C.; Evdokimidis, I.; Potagas, C. Are Memory Deficits Dependent on the Presence of Aphasia in Left Brain Damaged Patients? Neuropsychologia 2013, 51, 1773–1776. [Google Scholar] [CrossRef]
- Goodglass, H.; Kaplan, E. The Assessment of Aphasia and Related Disorders; Lea & Febiger: Philadelphia, PA, USA, 1972. [Google Scholar]
- Tsapkini, K.; Vlahou, C.H.; Potagas, C. Adaptation and Validation of Standardized Aphasia Tests in Different Languages: Lessons from the Boston Diagnostic Aphasia Examination–Short Form in Greek. Behav. Neurol. 2010, 22, 111–119. [Google Scholar] [CrossRef]
- Kaplan, E.; Goodglass, H.; Weintraub, S. Boston Naming Test. PsycTESTS Dataset 1983. Available online: https://psycnet.apa.org/doiLanding?doi=10.1037%2Ft27208-000 (accessed on 1 January 2016).
- Simos, P.G.; Kasselimis, D.; Mouzaki, A. Age, Gender, and Education Effects on Vocabulary Measures in Greek. Aphasiology 2010, 25, 475–491. [Google Scholar] [CrossRef]
- Simos, P.G.; Kasselimis, D.; Mouzaki, A. Effects of Demographic Variables and Health Status on Brief Vocabulary Measures in Greek. Aphasiology 2010, 25, 492–504. [Google Scholar] [CrossRef]
- Stockbridge, M.D.; Bunker, L.D.; Hillis, A.E. Reversing the Ruin: Rehabilitation, Recovery, and Restoration After Stroke. Curr. Neurol. Neurosci. Rep. 2022, 22, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Shaywitz, B.A.; Shaywltz, S.E.; Pugh, K.R.; Constable, R.T.; Skudlarski, P.; Fulbright, R.K.; Bronen, R.A.; Fletcher, J.M.; Shankweiler, D.P.; Katz, L.; et al. Sex Differences in the Functional Organization of the Brain for Language. Nature 1995, 373, 607–609. [Google Scholar] [CrossRef] [PubMed]
- Hirnstein, M.; Hugdahl, K.; Hausmann, M. Cognitive Sex Differences And Hemispheric Asymmetry: A Critical Review of 40 Years of Research. Asymmetries Body Brain Cognit. 2018, 24, 204–252. [Google Scholar] [CrossRef]
- Holland, A.L.; Greenhouse, J.B.; Fromm, D.; Swindell, C.S. Predictors of Language Restitution Following Stroke. J. Speech Lang. Hear. Res. 1989, 32, 232–238. [Google Scholar] [CrossRef]
- Etchell, A.; Adhikari, A.; Weinberg, L.S.; Choo, A.L.; Garnett, E.O.; Chow, H.M.; Chang, S.-E. A Systematic Literature Review of Sex Differences In Childhood Language And Brain Development. Neuropsychologia 2018, 114, 19–31. [Google Scholar] [CrossRef]
- Angelopoulou, G.; Meier, E.L.; Kasselimis, D.; Pan, Y.; Tsolakopoulos, D.; Velonakis, G.; Karavasilis, E.; Kelekis, N.L.; Goutsos, D.; Potagas, C.; et al. Investigating Gray and White Matter Structural Substrates of Sex Differences in the Narrative Abilities of Healthy Adults. Front. Neurosci. 2020, 13, 1424. [Google Scholar] [CrossRef]
- Wallentin, M. Putative Sex Differences in Verbal Abilities and Language Cortex: A Critical Review. Brain Lang. 2009, 108, 175–183. [Google Scholar] [CrossRef]
- Cahana-Amitay, D.; Albert, M.L. Redefining Recovery from Aphasia; Oxford University Press: New York, NY, USA, 2015; pp. 255–270. [Google Scholar] [CrossRef]
- Ferro, J.M.; Madureira, S. Aphasia Type, Age and Cerebral Infarct Localisation. J. Neurol. 1997, 244, 505–509. [Google Scholar] [CrossRef]
- De Renzi, E.; Faglioni, P.; Ferrari, P. The Influence of Sex and Age on the Incidence and Type of Aphasia. Cortex 1980, 16, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Payabvash, S.; Kamalian, S.; Fung, S.; Wang, Y.; Passanese, J.; Souza, L.; Kemmling, A.; Harris, G.; Halpern, E.; González, R.; et al. Predicting Language Improvement in Acute Stroke Patients Presenting with Aphasia: A Multivariate Logistic Model Using Location-Weighted Atlas-Based Analysis of Admission CT Perfusion Scans. Am. J. Neuroradiol. 2010, 31, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.M.; Entrup, J.L.; Schneck, S.M.; Onuscheck, C.F.; Levy, D.F.; Rahman, M.; Willey, E.; Casilio, M.; Yen, M.; Brito, A.C.; et al. Recovery from Aphasia in the First Year After Stroke. Brain 2022, 146, 1021–1039. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.d.C.V.; Grimsley-Moore, T.; Sakka, E.; Thrippleton, M.J.; Chappell, F.M.; Armitage, P.A.; Makin, S.; Wardlaw, J.M. Lacunar Stroke Lesion Extent and Location and White Matter Hyperintensities Evolution 1 Year Post-lacunar Stroke. Front. Neurol. 2021, 12, 640498. [Google Scholar] [CrossRef] [PubMed]
- Saur, D.; Kreher, B.W.; Schnell, S.; Kümmerer, D.; Kellmeyer, P.; Vry, M.-S.; Umarova, R.; Musso, M.; Glauche, V.; Abel, S.; et al. Ventral and Dorsal Pathways for Language. Proc. Natl. Acad. Sci. USA 2008, 105, 18035–18040. [Google Scholar] [CrossRef] [PubMed]
- Spezzano, L.C.; Radanovic, M. Naming Abilities: Differentiation between Objects and Verbs in Aphasia. Dement. Neuropsychol. 2010, 4, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Damasio, H.; Grabowski, T.J.; Tranel, D.; Hichwa, R.D.; Damasio, A.R. A Neural Basis For Lexical Retrieval. Nature 1996, 380, 499–505. [Google Scholar] [CrossRef]
- Piai, V.; Eikelboom, D. Brain Areas Critical for Picture Naming: A Systematic Review and meta-Analysis of Lesion-Symptom Mapping Studies. Neurobiol. Lang. 2023, 4, 280–296. [Google Scholar] [CrossRef]
- Gleichgerrcht, E.; Fridriksson, J.; Bonilha, L. Neuroanatomical Foundations of naming Impairments Across Different Neurologic Conditions. Neurology 2015, 85, 284–292. [Google Scholar] [CrossRef]
- Hickok, G.; Poeppel, D. Dorsal and Ventral Streams: A Framework for Understanding Aspects of the Functional Anatomy of Language. Cognition 2004, 92, 67–99. [Google Scholar] [CrossRef]
- Kourtidou, E.; Kasselimis, D.; Angelopoulou, G.; Karavasilis, E.; Velonakis, G.; Kelekis, N.; Zalonis, I.; Evdokimidis, I.; Potagas, C.; Petrides, M. The Role of the Right Hemisphere White Matter Tracts in Chronic Aphasic Patients After Damage of the Language Tracts in the Left Hemisphere. Front. Hum. Neurosci. 2021, 15, 635750. [Google Scholar] [CrossRef] [PubMed]
- Kargar, Y.; Jalilian, M. Anatomo-Functional Profile of White Matter Tracts in Relevance to Language: A systematic review. J. Neurolinguist. 2024, 69, 101175. [Google Scholar] [CrossRef]
- Kourtidou, E.; Kasselimis, D.; Angelopoulou, G.; Karavasilis, E.; Velonakis, G.; Kelekis, N.; Zalonis, I.; Evdokimidis, I.; Potagas, C.; Petrides, M. Specific Disruption of the Ventral Anterior Temporo-Frontal Network Reveals Key Implications for Language Comprehension and Cognition. Commun. Biol. 2022, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Busby, N.; Wilmskoetter, J.; Gleichgerrcht, E.; Rorden, C.; Roth, R.; Newman-Norlund, R.; Hillis, A.E.; Keller, S.S.; de Bezenac, C.; Kristinsson, S.; et al. Advanced Brain Age and Chronic Poststroke Aphasia Severity. Neurology 2023, 100, e1166–e1176. [Google Scholar] [CrossRef] [PubMed]
- Kolb, B.; Muhammad, A. Harnessing the Power of Neuroplasticity for Intervention. Front. Hum. Neurosci. 2014, 8, 377. [Google Scholar] [CrossRef] [PubMed]
- Jarso, S.; Li, M.; Faria, A.; Davis, C.; Leigh, R.; Sebastian, R.; Tsapkini, K.; Mori, S.; Hillis, A.E. Distinct Mechanisms and Timing of Language Recovery after Stroke. Cogn. Neuropsychol. 2013, 30, 454–475. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.; Basilakos, A.; Yourganov, G.; Cai, B.; Bonilha, L.; Rorden, C.; Fridriksson, J. Progression of Aphasia Severity in the Chronic Stages of Stroke. Am. J. Speech-Lang. Pathol. 2019, 28, 639–649. [Google Scholar] [CrossRef]
- Price, C.J. A review and synthesis of the first 20years of PET and fMRI Studies of Heard Speech, Spoken Language and Reading. NeuroImage 2012, 62, 816–847. [Google Scholar] [CrossRef]
- Murtha, S.; Chertkow, H.; Beauregard, M.; Evans, A. The Neural Substrate of Picture Naming. J. Cogn. Neurosci. 1999, 11, 399–423. [Google Scholar] [CrossRef]
- Salmelin, R.; Hari, R.; Lounasmaa, O.V.; Sams, M. Dynamics of Brain Activation during Picture Naming. Nature 1994, 368, 463–465. [Google Scholar] [CrossRef]
- Hillis, A.E.; Tuffiash, E.; Wityk, R.J.; Barker, P.B. Regions of Neural Dysfunction Associated with Impaired Naming of Actions and Objects in Acute Stroke. Cogn. Neuropsychol. 2002, 19, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Carrera, E.; Tononi, G. Diaschisis: Past, Present, Future. Brain 2014, 137, 2408–2422. [Google Scholar] [CrossRef] [PubMed]
- Catani, M.; Ffytche, D.H. The Rises and Falls of Disconnection Syndromes. Brain 2005, 128, 2224–2239. [Google Scholar] [CrossRef] [PubMed]
- DeLeon, J.; Gottesman, R.F.; Kleinman, J.T.; Newhart, M.; Davis, C.; Heidler-Gary, J.; Lee, A.; Hillis, A.E. Neural Regions Essential for Distinct Cognitive Processes Underlying Picture Naming. Brain 2007, 130, 1408–1422. [Google Scholar] [CrossRef] [PubMed]
- Døli, H.; Helland, W.A.; Helland, T.; Næss, H.; Hofstad, H.; Specht, K. Associations between Stroke Severity, Aphasia Severity, Lesion Location, and Lesion Size in Acute Stroke, and Aphasia Severity One Year Post Stroke. Aphasiology 2021, 37, 307–329. [Google Scholar] [CrossRef]
- Baldo, J.V.; Arévalo, A.; Patterson, J.P.; Dronkers, N.F. Grey and White Matter Correlates of Picture Naming: Evidence from a Voxel-Based Lesion Analysis of the Boston Naming Test. Cortex 2013, 49, 658–667. [Google Scholar] [CrossRef]
- Okada, T.; Tanaka, S.; Nakai, T.; Nishizawa, S.; Inui, T.; Sadato, N.; Yonekura, Y.; Konishi, J. Naming of Animals and Tools: A Functional Magnetic Resonance Imaging Study of Categorical Differences in the Human Brain Areas Commonly Used for Naming Visually Presented Objects. Neurosci. Lett. 2000, 296, 33–36. [Google Scholar] [CrossRef]
- Fatterpekar, G.M.; Naidich, T.P.; Delman, B.N.; Aguinaldo, J.G.; Gultekin, S.H.; Sherwood, C.C.; Hof, P.R.; Drayer, B.P.; Fayad, Z.A. Cytoarchitecture of the Human Cerebral Cortex: MR Microscopy of Excised Specimens at 9.4 Tesla. Am. J. Neuroradiol. 2002, 23, 1313–1321. [Google Scholar]
- Caspers, S.; Eickhoff, S.B.; Geyer, S.; Scheperjans, F.; Mohlberg, H.; Zilles, K.; Amunts, K. The Human Inferior Parietal Lobule in Stereotaxic Space. Brain Struct. Funct. 2008, 212, 481–495. [Google Scholar] [CrossRef]
- Obler, L.K.; Rykhlevskaia, E.; Schnyer, D.; Clark-Cotton, M.R.; Spiro III, A.; Hyun, J.; Kim, D.-S.; Goral, M.; Albert, M.L. Bilateral Brain Regions Associated with Naming in Older Adults. Brain Lang. 2010, 113, 113–123. [Google Scholar] [CrossRef]
- Geranmayeh, F.; Brownsett, S.L.E.; Wise, R.J.S. Task-Induced Brain Activity in Aphasic Stroke Patients: What Is Driving Recovery? Brain 2014, 137, 2632–2648. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, K. The Organism: A Holistic Approach to Biology Derived from Pathological Data in Man; American Book Company: New York, NY, USA, 1939. [Google Scholar]
- Willmes, K.; Poeck, K. To What Extent Can Aphasic Syndromes be Localized? Brain 1993, 116, 1527–1540. [Google Scholar] [CrossRef] [PubMed]


| Mean | (SD) | Min–Max | |
|---|---|---|---|
| Age in acute phase (years) | 56.45 | 14.6 | 23–84 |
| Education (years in formal schooling) | 11.31 | 3.6 | 6–17 |
| TPO (in acute phase in days) | 17.83 | 18.6 | 1–84 |
| TPO (in chronic phase in days) | 308.8 | 219.7 | 42–1154 |
| Sex | 28 Males 14 Females |
| Mean | (SD) | Min–Max | |
|---|---|---|---|
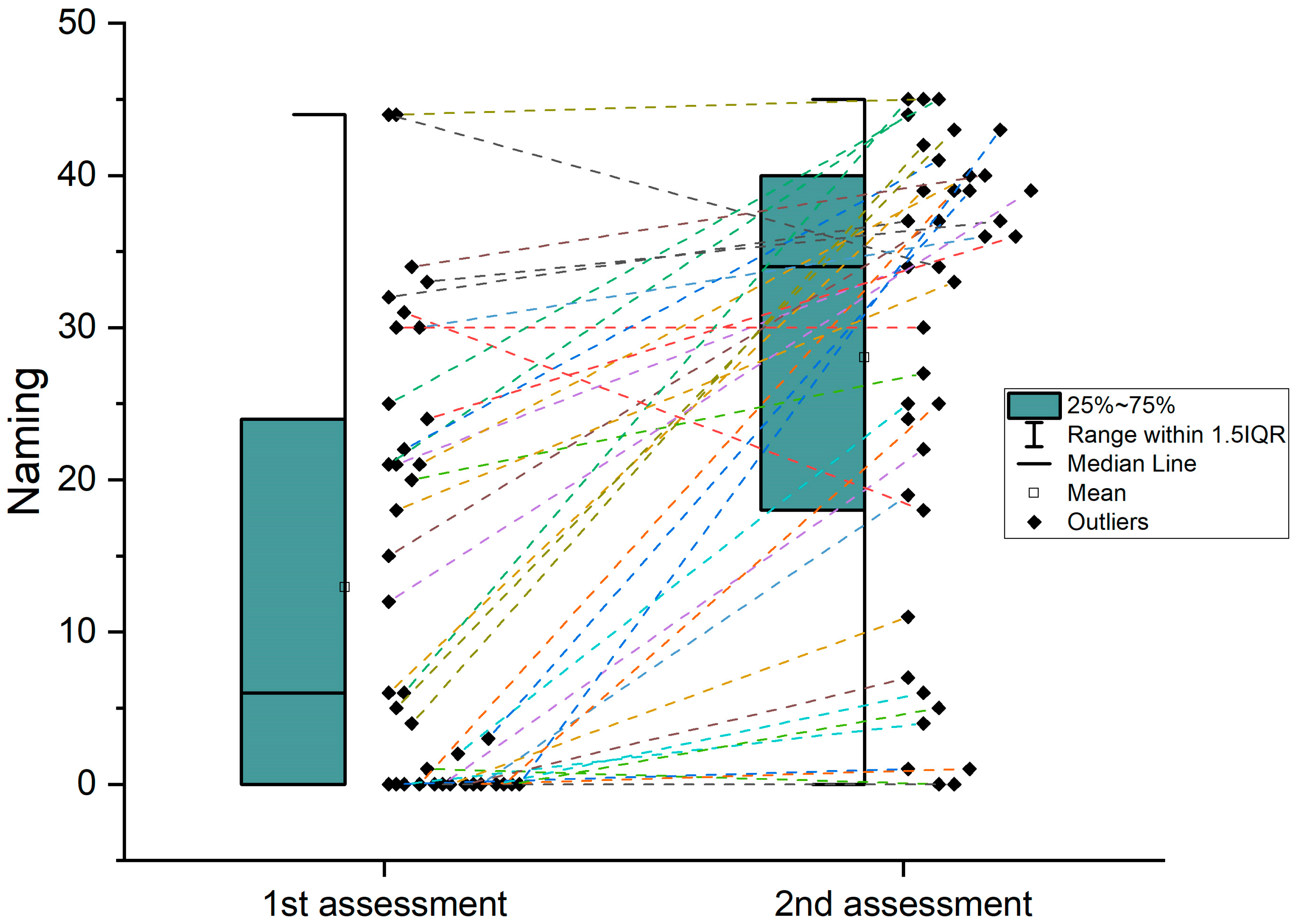
| Boston Naming Test (acute) | 12.92 | 14.2 | 0–44 |
| Boston Naming Test (chronic) | 28.03 | 15.2 | 0–45 |
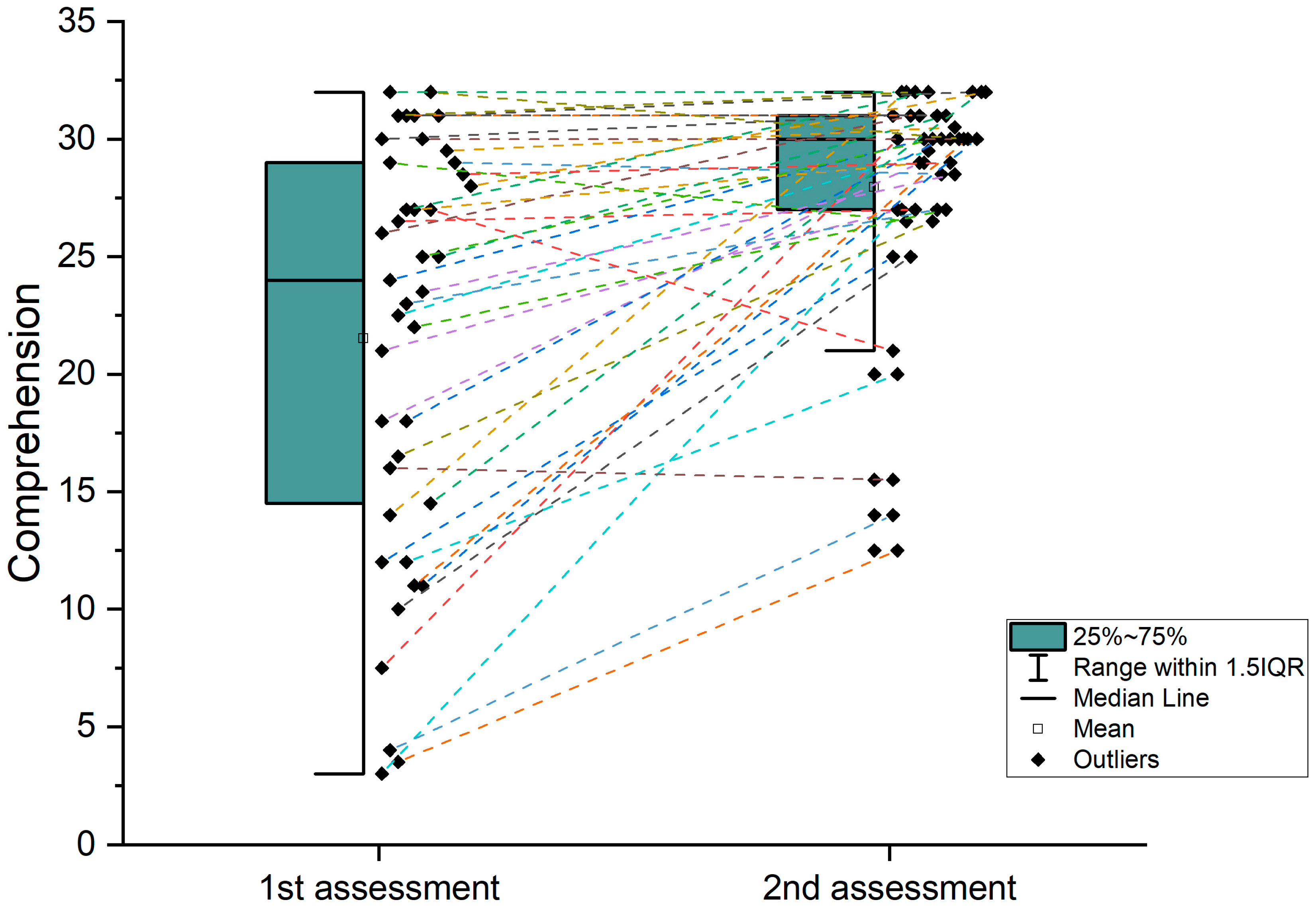
| Comprehension BDAE (acute) | 21.52 | 8.7 | 3–32 |
| Comprehension BDAE (chronic) | 27.95 | 4.8 | 12.5–32 |
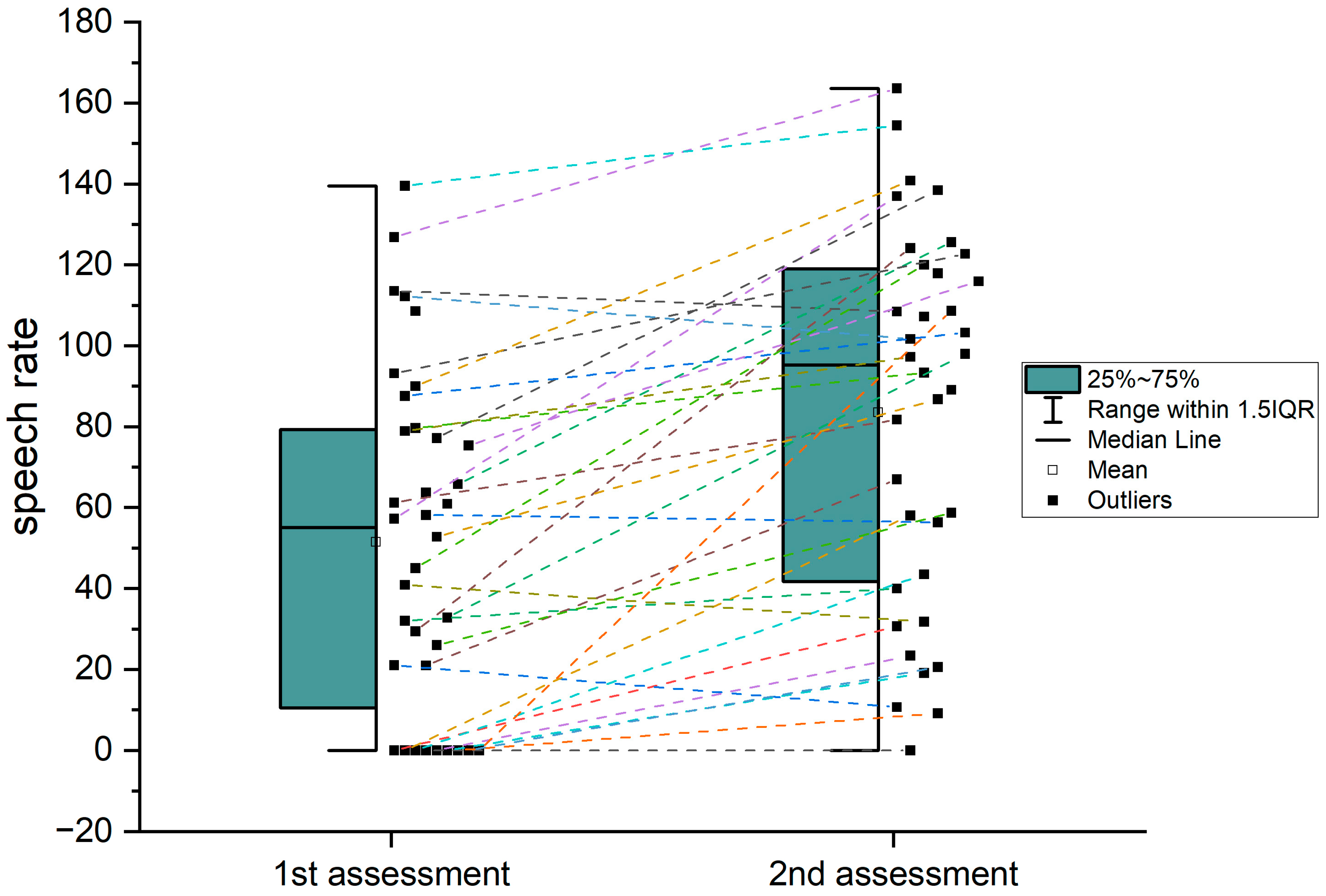
| Speech rate (acute) Speech rate (chronic) | 51.41 83.48 | 41.4 41.7 | 0–139.53 0–163.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papageorgiou, G.; Kasselimis, D.; Angelopoulou, G.; Laskaris, N.; Tsolakopoulos, D.; Velonakis, G.; Tountopoulou, A.; Vassilopoulou, S.; Potagas, C. Investigating Aphasia Recovery: Demographic and Clinical Factors. Brain Sci. 2024, 14, 7. https://doi.org/10.3390/brainsci14010007
Papageorgiou G, Kasselimis D, Angelopoulou G, Laskaris N, Tsolakopoulos D, Velonakis G, Tountopoulou A, Vassilopoulou S, Potagas C. Investigating Aphasia Recovery: Demographic and Clinical Factors. Brain Sciences. 2024; 14(1):7. https://doi.org/10.3390/brainsci14010007
Chicago/Turabian StylePapageorgiou, Georgios, Dimitrios Kasselimis, Georgia Angelopoulou, Nikolaos Laskaris, Dimitrios Tsolakopoulos, Georgios Velonakis, Argyro Tountopoulou, Sophia Vassilopoulou, and Constantin Potagas. 2024. "Investigating Aphasia Recovery: Demographic and Clinical Factors" Brain Sciences 14, no. 1: 7. https://doi.org/10.3390/brainsci14010007
APA StylePapageorgiou, G., Kasselimis, D., Angelopoulou, G., Laskaris, N., Tsolakopoulos, D., Velonakis, G., Tountopoulou, A., Vassilopoulou, S., & Potagas, C. (2024). Investigating Aphasia Recovery: Demographic and Clinical Factors. Brain Sciences, 14(1), 7. https://doi.org/10.3390/brainsci14010007







