Enhancing Speech Rehabilitation in a Young Adult with Trisomy 21: Integrating Transcranial Direct Current Stimulation (tDCS) with Rapid Syllable Transition Training for Apraxia of Speech
Abstract
1. Introduction
2. Methods
2.1. Participant
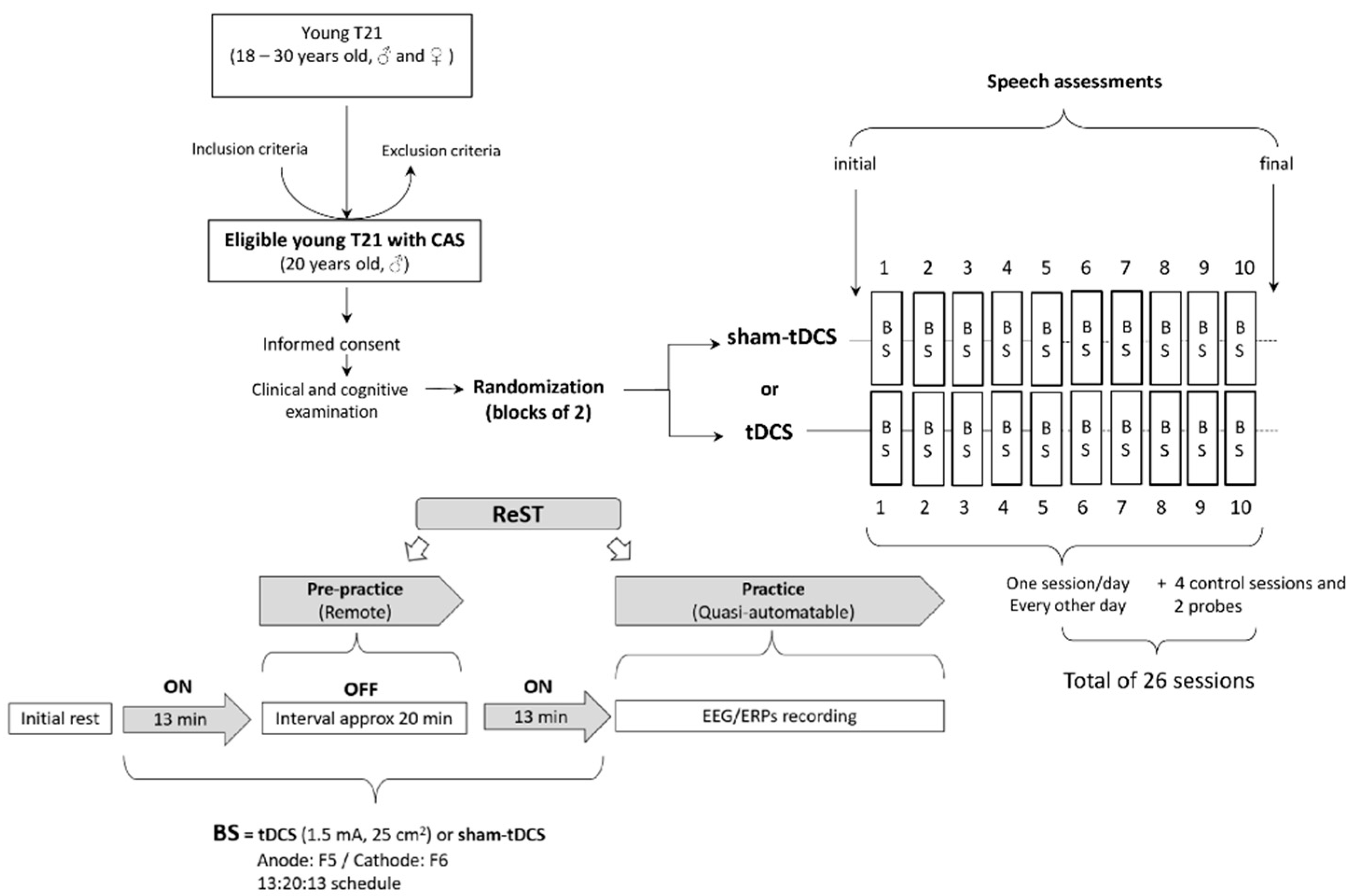
2.2. N-of-1 Study Design
2.3. Experimental Procedures
2.3.1. Non-Invasive Brain Stimulation
2.3.2. Speech Intervention
2.3.3. Speech Assessments
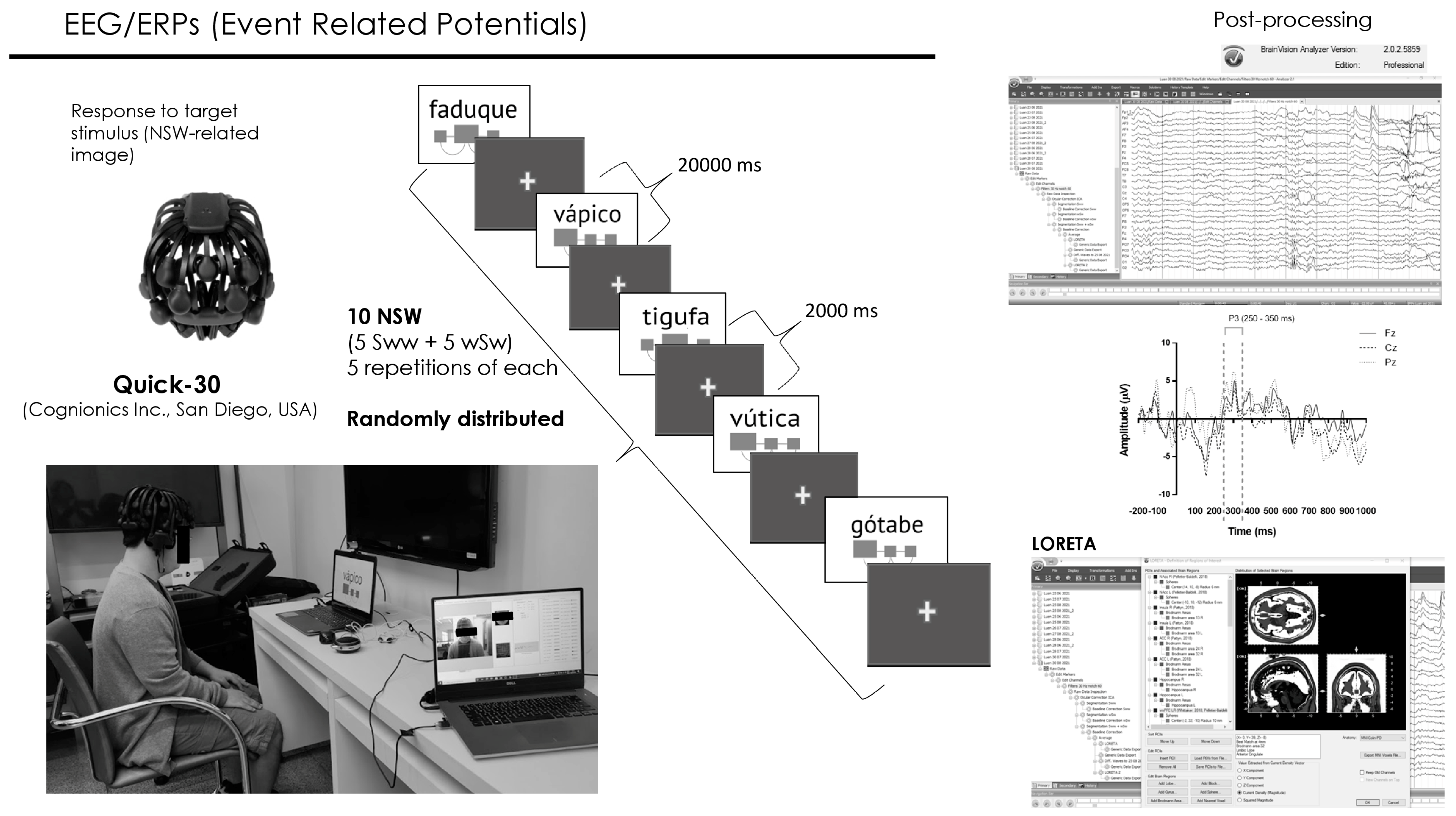
2.3.4. Event-Related Potentials
2.3.5. Statistical Analysis
3. Results
3.1. Participant
3.2. ReST Performance
3.2.1. Speech Sound Production
3.2.2. Probe: Pre- and Post-Analysis
3.2.3. Consistency of NSWs Utterances
3.3. Speech Assessments
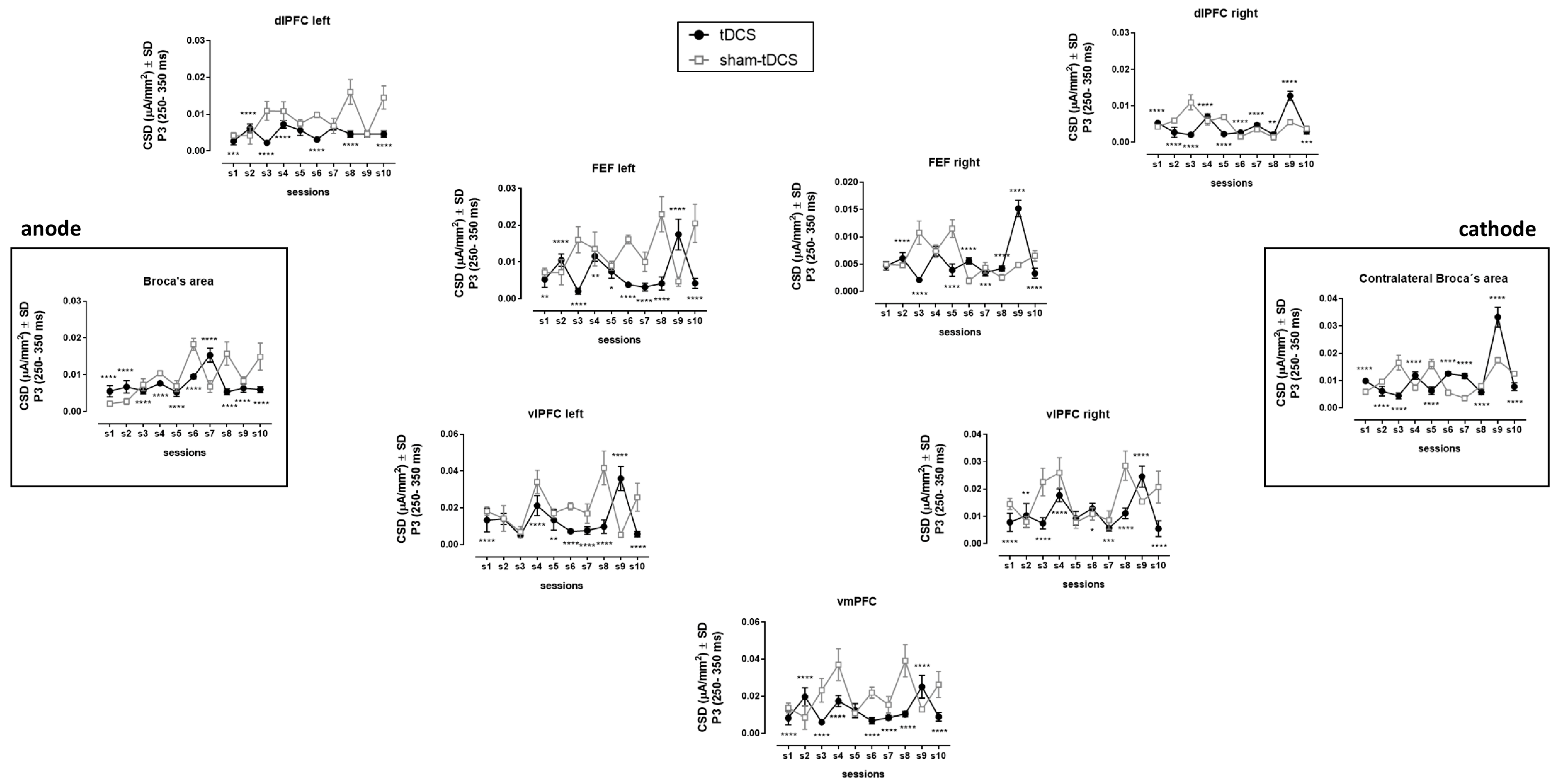
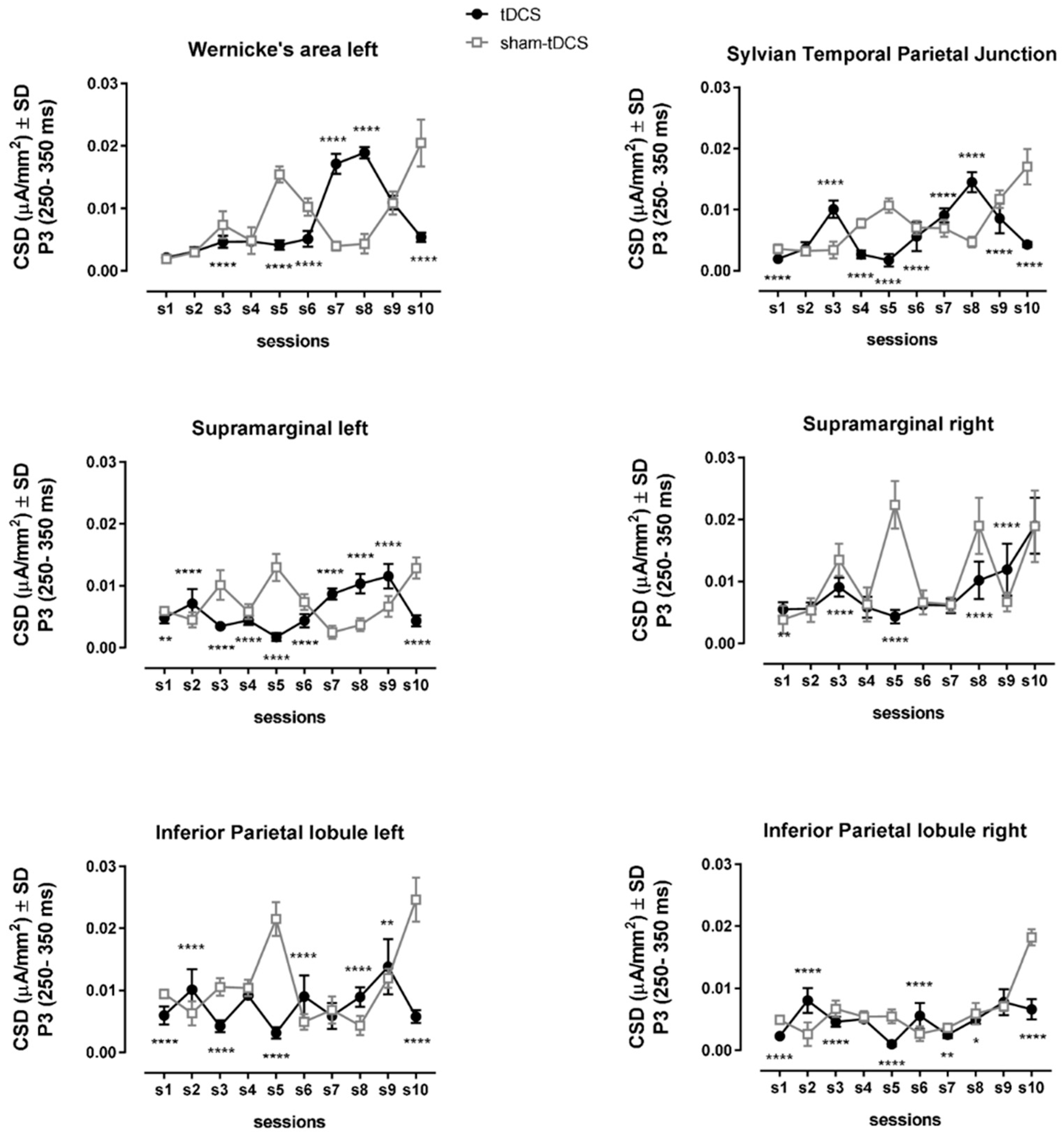
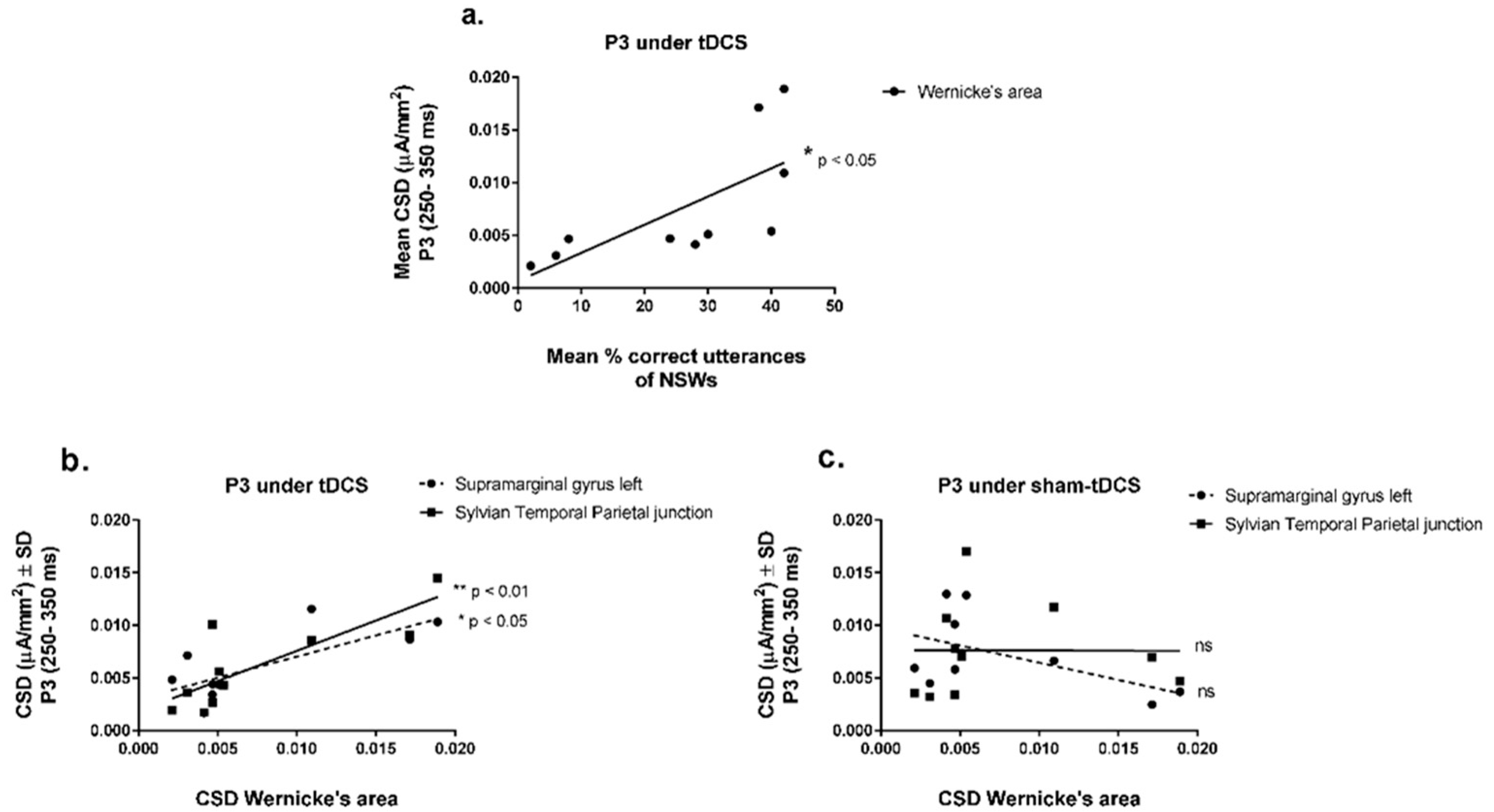
3.4. EEG/ERPs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumin, L. Speech intelligibility and childhood verbal apraxia in children with Down syndrome. Down Syndr. Res. Pract. 2006, 10, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.M.; Abbeduto, L.; Camarata, S.M.; Shriberg, L.D. Estimates of the prevalence of speech and motor speech disorders in adolescents with Down syndrome. Clin. Linguist. Phon. 2019, 33, 772–789. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.M.; Abbeduto, L.; Camarata, S.M.; Shriberg, L.D. Speech and motor speech disorders and intelligibility in adolescents with Down syndrome. Clin. Linguist. Phon. 2019, 33, 790–814. [Google Scholar] [CrossRef] [PubMed]
- ASHA. Evidence-Based Practice in Communication Disorders: An Introduction [Technical Report]. Available online: https://www.asha.org/policy/tr2004-00001/ (accessed on 4 February 2021).
- Cassar, C.; McCabe, P.; Cumming, S. “I still have issues with pronunciation of words”: A mixed methods investigation of the psychosocial and speech effects of Childhood Apraxia of Speech in adults. Int. J. Speech Lang. Pathol. 2022, 25, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.A.; Freebairn, L.A.; Hansen, A.J.; Iyengar, S.K.; Taylor, H.G. School-age follow-up of children with childhood apraxia of speech. Lang. Speech Hear. Serv. Sch. 2004, 35, 122–140. [Google Scholar] [CrossRef]
- Morgan, A.T.; Murray, E.; Liegeois, F.J. Interventions for childhood apraxia of speech. Cochrane Database Syst. Rev. 2018, 5, CD006278. [Google Scholar] [CrossRef] [PubMed]
- Fregni, F.; El-Hagrassy, M.M.; Pacheco-Barrios, K.; Carvalho, S.; Leite, J.; Simis, M.; Brunelin, J.; Nakamura-Palacios, E.M.; Marangolo, P.; Venkatasubramanian, G.; et al. Evidence-Based Guidelines and Secondary Meta-Analysis for the Use of Transcranial Direct Current Stimulation in Neurological and Psychiatric Disorders. Int. J. Neuropsychopharmacol. 2021, 24, 256–313. [Google Scholar] [CrossRef]
- Zettin, M.; Bondesan, C.; Nada, G.; Varini, M.; Dimitri, D. Transcranial Direct-Current Stimulation and Behavioral Training, a Promising Tool for a Tailor-Made Post-stroke Aphasia Rehabilitation: A Review. Front. Hum. Neurosci. 2021, 15, 742136. [Google Scholar] [CrossRef]
- Marangolo, P.; Fiori, V.; Cipollari, S.; Campana, S.; Razzano, C.; Di Paola, M.; Koch, G.; Caltagirone, C. Bihemispheric stimulation over left and right inferior frontal region enhances recovery from apraxia of speech in chronic aphasia. Eur. J. Neurosci. 2013, 38, 3370–3377. [Google Scholar] [CrossRef]
- Marangolo, P.; Marinelli, C.V.; Bonifazi, S.; Fiori, V.; Ceravolo, M.G.; Provinciali, L.; Tomaiuolo, F. Electrical stimulation over the left inferior frontal gyrus (IFG) determines long-term effects in the recovery of speech apraxia in three chronic aphasics. Behav. Brain Res. 2011, 225, 498–504. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Schauenburg, A.; Lang, N.; Liebetanz, D.; Exner, C.; Paulus, W.; Tergau, F. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J. Cogn. Neurosci. 2003, 15, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Themistocleous, C.; Webster, K.; Tsapkini, K. Effects of tDCS on Sound Duration in Patients with Apraxia of Speech in Primary Progressive Aphasia. Brain Sci. 2021, 11, 335. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, A.; Calhoun, H.; Rimikis, S.; Lowe, M.S.; Wellner, R.; Edwards, D.J. Using tDCS to facilitate motor learning in speech production: The role of timing. Cortex 2019, 111, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.L.; Combs, A.B.; Acosta, D. Experimental models for the investigation of toxicological mechanisms. In Comprehensive Toxicology, 2nd ed.; McQueen, C., Ed.; Elsevier Ltd.: Oxford, UK, 2010; pp. 203–224. [Google Scholar]
- Kadis, D.S.; Goshulak, D.; Namasivayam, A.; Pukonen, M.; Kroll, R.; De Nil, L.F.; Pang, E.W.; Lerch, J.P. Cortical thickness in children receiving intensive therapy for idiopathic apraxia of speech. Brain Topogr. 2014, 27, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Namasivayam, A.K.; Yan, T.; Bali, R.; Hayden, D.; van Lieshout, P. Cross-Modal Somatosensory Repetition Priming and Speech Processing. J. Integr. Neurosci. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Yu, V.Y.; Kadis, D.S.; Goshulak, D.; Namasivayam, A.K.; Pukonen, M.; Kroll, R.M.; De Nil, L.F.; Pang, E.W. Impact of Motor Speech Intervention on Neural Activity in Children with Speech Sound Disorders: Use of Magnetoencephalography. J. Behav. Brain Sci. 2018, 8, 415–429. [Google Scholar] [CrossRef][Green Version]
- Antal, A.; Luber, B.; Brem, A.K.; Bikson, M.; Brunoni, A.R.; Cohen Kadosh, R.; Dubljevic, V.; Fecteau, S.; Ferreri, F.; Floel, A.; et al. Non-invasive brain stimulation and neuroenhancement. Clin. Neurophysiol. Pract. 2022, 7, 146–165. [Google Scholar] [CrossRef] [PubMed]
- Terranova, C.; Rizzo, V.; Cacciola, A.; Chillemi, G.; Calamuneri, A.; Milardi, D.; Quartarone, A. Is There a Future for Non-invasive Brain Stimulation as a Therapeutic Tool? Front. Neurol. 2018, 9, 1146. [Google Scholar] [CrossRef]
- Luck, S.J. An Introduction to the Event-Related Potential Technique; MIT Press: Cambridge, MA, USA, 2005; p. xii. 374p. [Google Scholar]
- Polich, J. Updating P300: An integrative theory of P3a and P3b. Clin. Neurophysiol. 2007, 118, 2128–2148. [Google Scholar] [CrossRef]
- Romero, R.; Polich, J. P3(00) habituation from auditory and visual stimuli. Physiol. Behav. 1996, 59, 517–522. [Google Scholar] [CrossRef]
- International Classification of Diseases, Eleventh Revision (ICD-11), World Health Organization (WHO) 2019/2021. Available online: https://icd.who.int/browse11 (accessed on 4 February 2021).
- Edgington, E.S. Randomized single-subject experiments and statistical tests. J. Couns. Psychol. 1987, 34, 437–442. [Google Scholar] [CrossRef]
- Perdices, M.; Tate, R.L. Single-subject designs as a tool for evidence-based clinical practice: Are they unrecognised and undervalued? Neuropsychol. Rehabil. 2009, 19, 904–927. [Google Scholar] [CrossRef] [PubMed]
- Rvachew, S. Application of single subject randomization designs to communicative disorders research. Hum. Commun. Can. 1988, 12, 7–13. [Google Scholar]
- Rvachew, S.; Matthews, T. Demonstrating treatment efficacy using the single subject randomization design: A tutorial and demonstration. J. Commun. Disord. 2017, 67, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ferron, J.; Ware, W. Using randomization tests with responsive single-case designs. Behav. Res. Ther. 1994, 32, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Margolis, A.; Giuliano, C. Making the switch: From case studies to N-of-1 trials. Epilepsy Behav. Rep. 2019, 12, 100336. [Google Scholar] [CrossRef] [PubMed]
- Samuel, J.P.; Wootton, S.H.; Tyson, J.E. N-of-1 trials: The epitome of personalized medicine? J. Clin. Transl. Sci. 2023, 7, e161. [Google Scholar] [CrossRef]
- Klauss, J.; Penido Pinheiro, L.C.; Silva Merlo, B.L.; Correia Santos Gde, A.; Fregni, F.; Nitsche, M.A.; Miyuki Nakamura-Palacios, E. A randomized controlled trial of targeted prefrontal cortex modulation with tDCS in patients with alcohol dependence. Int. J. Neuropsychopharmacol. 2014, 17, 1793–1803. [Google Scholar] [CrossRef]
- Monte-Silva, K.; Kuo, M.F.; Hessenthaler, S.; Fresnoza, S.; Liebetanz, D.; Paulus, W.; Nitsche, M.A. Induction of late LTP-like plasticity in the human motor cortex by repeated non-invasive brain stimulation. Brain Stimul. 2013, 6, 424–432. [Google Scholar] [CrossRef]
- Murray, E.; McCabe, P.; Ballard, K.J. A Randomized Controlled Trial for Children With Childhood Apraxia of Speech Comparing Rapid Syllable Transition Treatment and the Nuffield Dyspraxia Programme-Third Edition. J. Speech Lang. Hear. Res. 2015, 58, 669–686. [Google Scholar] [CrossRef]
- Bahar, N.; Namasivayam, A.K.; van Lieshout, P. Telehealth intervention and childhood apraxia of speech: A scoping review. Speech Lang. Hear. 2021, 25, 450–462. [Google Scholar] [CrossRef]
- Thomas, D.C.; McCabe, P.; Ballard, K.J.; Lincoln, M. Telehealth delivery of Rapid Syllable Transitions (ReST) treatment for childhood apraxia of speech. Int. J. Lang. Commun. Disord. 2016, 51, 654–671. [Google Scholar] [CrossRef] [PubMed]
- Namasivayam, A.K.; Huynh, A.; Bali, R.; Granata, F.; Law, V.; Rampersaud, D.; Hard, J.; Ward, R.; Helms-Park, R.; van Lieshout, P.; et al. Development and Validation of a Probe Word List to Assess Speech Motor Skills in Children. Am. J. Speech Lang. Pathol. 2021, 30, 622–648. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C.R.F.; Béfi-Lopes, D.M.; Fernandes, F.D.M.; Wertzner, W.H. ABFW: Teste de Linguagem Infantil nas áreas de Fonologia, Vocabulário, Fluência e Pragmática; Pró-Fono: Carapicuíba, Brazil, 2004. [Google Scholar]
- Shriberg, L.D.; Kwiatkowski, J. Phonological disorders III: A procedure for assessing severity of involvement. J. Speech Hear. Disord. 1982, 47, 256–270. [Google Scholar] [CrossRef] [PubMed]
- Pagliarin, K.C.; Ortiz, K.Z.; Barreto Sdos, S.; Pimenta Parente, M.A.; Nespoulous, J.L.; Joanette, Y.; Fonseca, R.P. Montreal-Toulouse Language Assessment Battery: Evidence of criterion validity from patients with aphasia. J. Neurol. Sci. 2015, 357, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Pagliarin, K.C.; Ortiz, K.Z.; Parente, M.A.; Arteche, A.; Joanette, Y.; Nespoulous, J.L.; Fonseca, R.P. Montreal-Toulouse language assessment battery for aphasia: Validity and reliability evidence. NeuroRehabilitation 2014, 34, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Oddson, B.; Thomas-Stonell, N.; Robertson, B.; Rosenbaum, P. Validity of a streamlined version of the Focus on the Outcomes of Communication Under Six: Process and outcome. Child. Care Health Dev. 2019, 45, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Anderer, P.; Saletu, B.; Pascual-Marqui, R.D. Effect of the 5-HT(1A) partial agonist buspirone on regional brain electrical activity in man: A functional neuroimaging study using low-resolution electromagnetic tomography (LORETA). Psychiatry Res. 2000, 100, 81–96. [Google Scholar] [CrossRef]
- Pascual-Marqui, R.D.; Esslen, M.; Kochi, K.; Lehmann, D. Functional imaging with low-resolution brain electromagnetic tomography (LORETA): A review. Methods Find. Exp. Clin. Pharmacol. 2002, 24 (Suppl. C), 91–95. [Google Scholar]
- Pascual-Marqui, R.D.; Lehmann, D.; Koenig, T.; Kochi, K.; Merlo, M.C.; Hell, D.; Koukkou, M. Low resolution brain electromagnetic tomography (LORETA) functional imaging in acute, neuroleptic-naive, first-episode, productive schizophrenia. Psychiatry Res. 1999, 90, 169–179. [Google Scholar] [CrossRef]
- Worrell, G.A.; Lagerlund, T.D.; Sharbrough, F.W.; Brinkmann, B.H.; Busacker, N.E.; Cicora, K.M.; O’Brien, T.J. Localization of the epileptic focus by low-resolution electromagnetic tomography in patients with a lesion demonstrated by MRI. Brain Topogr. 2000, 12, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Androulakis, X.M.; Krebs, K.A.; Jenkins, C.; Maleki, N.; Finkel, A.G.; Rorden, C.; Newman, R. Central Executive and Default Mode Network Intranet work Functional Connectivity Patterns in Chronic Migraine. J. Neurol. Disord. 2018, 6, 393. [Google Scholar] [CrossRef] [PubMed]
- Pelletier-Baldelli, A.; Orr, J.M.; Bernard, J.A.; Mittal, V.A. Social reward processing: A biomarker for predicting psychosis risk? Schizophr. Res. 2020, 226, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, J.R.; Foley, S.F.; Ackling, E.; Murphy, K.; Caseras, X. The Functional Connectivity Between the Nucleus Accumbens and the Ventromedial Prefrontal Cortex as an Endophenotype for Bipolar Disorder. Biol. Psychiatry 2018, 84, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Namasivayam, A.K.; Pukonen, M.; Goshulak, D.; Hard, J.; Rudzicz, F.; Rietveld, T.; Maassen, B.; Kroll, R.; van Lieshout, P. Treatment intensity and childhood apraxia of speech. Int. J. Lang. Commun. Disord. 2015, 50, 529–546. [Google Scholar] [CrossRef] [PubMed]
- Wertzner, H.F.; Papp, A.C.; Galea, D.E. Picture naming and imitation tests as tools for the diagnosis of phonological disorder. Pró-Fono Rev. Atualização Científica 2006, 18, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Barrozo, T.F.; Pagan-Neves, L.O.; Pinheiro da Silva, J.; Wertzner, H.F. Sensitivity and specificity of the Percentage of Consonants Correct-Revised in the identification of speech sound disorder. Codas 2017, 29, e20160038. [Google Scholar] [CrossRef]
- Preschool Speech and Language Outcome Measurement Guide. 2015. Available online: https://www.toronto.ca/wp-content/uploads/2018/04/958f-Preschool-Speech-and-Lanaguage-Outcome-Measurement-Guide-2018.pdf (accessed on 4 February 2021).
- Binder, J.R. The Wernicke area: Modern evidence and a reinterpretation. Neurology 2015, 85, 2170–2175. [Google Scholar] [CrossRef]
- Binder, J.R. Current Controversies on Wernicke’s Area and its Role in Language. Curr. Neurol. Neurosci. Rep. 2017, 17, 58. [Google Scholar] [CrossRef]
- Tremblay, P.; Dick, A.S. Broca and Wernicke are dead, or moving past the classic model of language neurobiology. Brain Lang. 2016, 162, 60–71. [Google Scholar] [CrossRef]
- Pillay, S.B.; Stengel, B.C.; Humphries, C.; Book, D.S.; Binder, J.R. Cerebral localization of impaired phonological retrieval during rhyme judgment. Ann. Neurol. 2014, 76, 738–746. [Google Scholar] [CrossRef]
- Buchsbaum, B.R.; Baldo, J.; Okada, K.; Berman, K.F.; Dronkers, N.; D’Esposito, M.; Hickok, G. Conduction aphasia, sensory-motor integration, and phonological short-term memory—An aggregate analysis of lesion and fMRI data. Brain Lang. 2011, 119, 119–128. [Google Scholar] [CrossRef]
- Ferpozzi, V.; Fornia, L.; Montagna, M.; Siodambro, C.; Castellano, A.; Borroni, P.; Riva, M.; Rossi, M.; Pessina, F.; Bello, L.; et al. Broca’s Area as a Pre-articulatory Phonetic Encoder: Gating the Motor Program. Front. Hum. Neurosci. 2018, 12, 64. [Google Scholar] [CrossRef]
- Ficek, B.N.; Wang, Z.; Zhao, Y.; Webster, K.T.; Desmond, J.E.; Hillis, A.E.; Frangakis, C.; Vasconcellos Faria, A.; Caffo, B.; Tsapkini, K. The effect of tDCS on functional connectivity in primary progressive aphasia. Neuroimage Clin. 2018, 19, 703–715. [Google Scholar] [CrossRef]

| Brain Area | Factor | DF | F | MSE | p Value | ωp2 | Bonferroni’s Multiple Comparisons Test |
|---|---|---|---|---|---|---|---|
| Broca (left IFG) | Between condition | (1,49) | 244.7 | 4.1 × 10−6 | <0.0001 | 0.048 | <0.0001 all comparisons |
| Within sessions | (9,441) | 359.0 | 2.8 × 10−6 | <0.0001 | 0.430 | ||
| Interaction | (9,441) | 415.9 | 2.3 × 10−6 | <0.0001 | 0.407 | ||
| Contralateral (right IFG) | Between condition | (1,49) | 49.1 | 2.4 × 10−6 | <0.0001 | 0.026 | <0.0001 all comparisons |
| Within sessions | (9,441) | 1642.0 | 1.7 × 10−6 | <0.0001 | 0.576 | ||
| Interaction | (9,441) | 716.4 | 2.6 × 10−6 | <0.0001 | 0.374 | ||
| dlPFC left | Between condition | (1,49) | 5646.0 | 7.7 × 10−7 | <0.0001 | 0.268 | <0.001, 0.0001, except sessions 7 and 9 |
| Within sessions | (9,441) | 175.8 | 2.8 × 10−6 | <0.0001 | 0.266 | ||
| Interaction | (9,441) | 173.3 | 3.1 × 10−6 | <0.0001 | 0.300 | ||
| dlPFC right | Between condition | (1,49) | 146.8 | 4.2 × 10−7 | <0.0001 | 0.067 | <0.01, 0.001, 0.0001 all comparisons |
| Within sessions | (9,441) | 789.6 | 6.1 × 10−7 | <0.0001 | 0.470 | ||
| Interaction | (9,441) | 555.8 | 8.4 × 10−7 | <0.0001 | 0.450 | ||
| FEF left | Between condition | (1,49) | 4266.0 | 1.9 × 10−6 | <0.0001 | 0.195 | <0.05, 0.01, 0.0001 all comparisons |
| Within sessions | (9,441) | 79.6 | 7.9 × 10−6 | <0.0001 | 0.133 | ||
| Interaction | (9,441) | 324.7 | 7.4 × 10−6 | <0.0001 | 0.505 | ||
| FEF right | Between condition | (1,49) | 67.3 | 5.1 × 10−7 | <0.0001 | 0.029 | <0.001, 0.0001, except sessions 1 and 4 |
| Within sessions | (9,441) | 598.9 | 7.3 × 10−7 | <0.0001 | 0.343 | ||
| Interaction | (9,441) | 629.2 | 1.1 × 10−6 | <0.0001 | 0.572 | ||
| vlPFC left | Between condition | (1,49) | 1071.0 | 1.1 × 10−5 | <0.0001 | 0.087 | <0.05, 0.01, 0.0001, except sessions 2 and 3 |
| Within sessions | (9,441) | 163.8 | 2.5 × 10−5 | <0.0001 | 0.283 | ||
| Interaction | (9,441) | 274.0 | 2.4 × 10−5 | <0.0001 | 0.456 | ||
| vlPFC right | Between condition | (1,49) | 1745.0 | 3.7 × 10−6 | <0.0001 | 0.107 | p < 0.05, 0.01, 0.001, 0.0001, except session 5 |
| Within sessions | (9,441) | 194.5 | 1.4 × 10−5 | <0.0001 | 0.408 | ||
| Interaction | (9,441) | 192.5 | 1.0 × 10−5 | <0.0001 | 0.301 | ||
| vmPFC | Between condition | (1,49) | 1810.0 | 1.0 × 10−5 | <0.0001 | 0.167 | <0.0001, except session 5 |
| Within sessions | (9,441) | 154.5 | 1.0 × 10−5 | <0.0001 | 0.257 | ||
| Interaction | (9,441) | 179.8 | 2.5 × 10−5 | <0.0001 | 0.382 | ||
| Wernicke | Between condition | (1,49) | 29.9 | 3.1 × 10−6 | <0.0001 | 0.003 | <0.0001, except sessions 1, 2, 4 and 9 |
| Within sessions | (9,441) | 843.2 | 1.7 × 10−6 | <0.0001 | 0.379 | ||
| Interaction | (9,441) | 1033.0 | 1.0 × 10−6 | <0.0001 | 0.558 | ||
| Sylvian Temporal Parietal junction | Between condition | (1,49) | 273.7 | 1.8 × 10−6 | <0.0001 | 0.026 | <0.0001, except session 2 |
| Within sessions | (9,441) | 377.1 | 1.9 × 10−6 | <0.0001 | 0.343 | ||
| Interaction | (9,441) | 514.8 | 2.2 × 10−6 | <0.0001 | 0.529 | ||
| Supramarginal left | Between condition | (1,49) | 228.8 | 1.5 × 10−6 | <0.0001 | 0.027 | <0.01, 0.0001 all comparisons |
| Within sessions | (9,441) | 116.6 | 1.6 × 10−6 | <0.0001 | 0.130 | ||
| Interaction | (9,441) | 398.9 | 2.5 × 10−6 | <0.0001 | 0.672 | ||
| Supramarginal right | Between condition | (1,49) | 128.2 | 1.2 × 10−5 | <0.0001 | 0.040 | <0.0001, except sessions 1, 2, 4, 6, 7 and 10 |
| Within sessions | (9,441) | 473.6 | 4.8 × 10−6 | <0.0001 | 0.520 | ||
| Interaction | (9,441) | 101.3 | 1.1 × 10−5 | <0.0001 | 0.246 | ||
| Inferior Parietal Lobule left | Between condition | (1,49) | 1164.0 | 2.6 × 10−6 | <0.0001 | 0.094 | <0.01, <0.0001, except sessions 4 and 7 |
| Within sessions | (9,441) | 236.7 | 4.0 × 10−6 | <0.0001 | 0.257 | ||
| Interaction | (9,441) | 311.3 | 6.1 × 10−6 | <0.0001 | 0.512 | ||
| Inferior Parietal Lobule right | Between condition | (1,49) | 277.0 | 1.8 × 10−6 | <0.0001 | 0.037 | <0.05, 0.01, 0.0001, except sessions 4 and 9 |
| Within sessions | (9,441) | 751.9 | 1.0 × 10−6 | <0.0001 | 0.503 | ||
| Interaction | (9,441) | 221.9 | 2.3 × 10−6 | <0.0001 | 0.343 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura-Palacios, E.M.; Falçoni Júnior, A.T.; Tanese, G.L.; Vogeley, A.C.E.; Namasivayam, A.K. Enhancing Speech Rehabilitation in a Young Adult with Trisomy 21: Integrating Transcranial Direct Current Stimulation (tDCS) with Rapid Syllable Transition Training for Apraxia of Speech. Brain Sci. 2024, 14, 58. https://doi.org/10.3390/brainsci14010058
Nakamura-Palacios EM, Falçoni Júnior AT, Tanese GL, Vogeley ACE, Namasivayam AK. Enhancing Speech Rehabilitation in a Young Adult with Trisomy 21: Integrating Transcranial Direct Current Stimulation (tDCS) with Rapid Syllable Transition Training for Apraxia of Speech. Brain Sciences. 2024; 14(1):58. https://doi.org/10.3390/brainsci14010058
Chicago/Turabian StyleNakamura-Palacios, Ester Miyuki, Aldren Thomazini Falçoni Júnior, Gabriela Lolli Tanese, Ana Carla Estellita Vogeley, and Aravind Kumar Namasivayam. 2024. "Enhancing Speech Rehabilitation in a Young Adult with Trisomy 21: Integrating Transcranial Direct Current Stimulation (tDCS) with Rapid Syllable Transition Training for Apraxia of Speech" Brain Sciences 14, no. 1: 58. https://doi.org/10.3390/brainsci14010058
APA StyleNakamura-Palacios, E. M., Falçoni Júnior, A. T., Tanese, G. L., Vogeley, A. C. E., & Namasivayam, A. K. (2024). Enhancing Speech Rehabilitation in a Young Adult with Trisomy 21: Integrating Transcranial Direct Current Stimulation (tDCS) with Rapid Syllable Transition Training for Apraxia of Speech. Brain Sciences, 14(1), 58. https://doi.org/10.3390/brainsci14010058






