The Use of Intraoperative Microvascular Doppler in Vascular Neurosurgery: Rationale and Results—A Systematic Review
Abstract
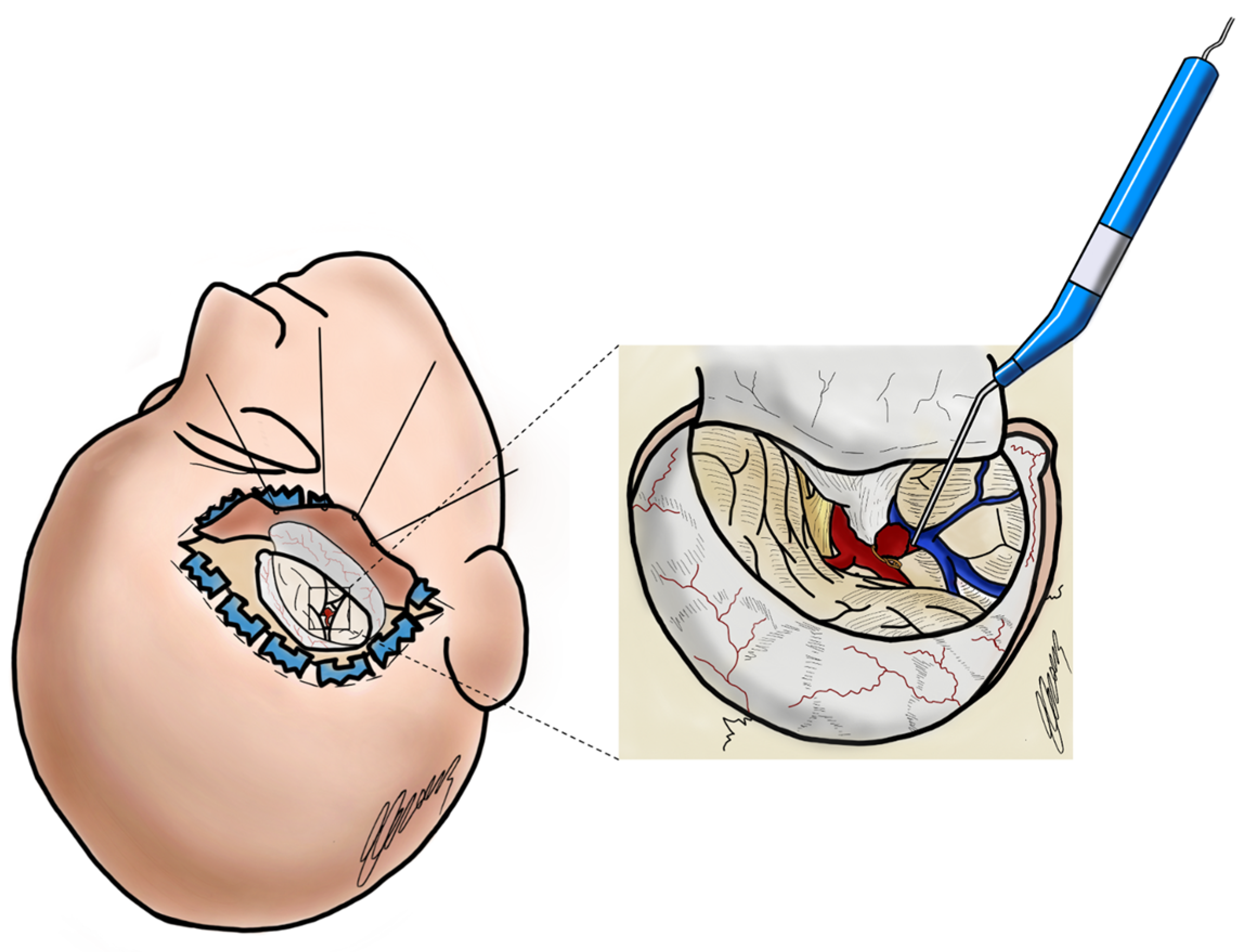
1. Introduction
2. Materials and Methods
3. Results
| Author, Year of Publication | N Patient/ Lesions | Study Design | iMDS Characteristics | Other Intraoperative Methods | Vessel Characteristics | iMDS Employment | Outcomes |
|---|---|---|---|---|---|---|---|
| Xing XZ et al., 2019 [9] | 7 | R | Transcranial Doppler ultra-sonography machines (Viasys Healthcare, USA). Probe diameter: 1 mm, Frequency: 20 MHz | None | 2 lumbar and 5 thoracic spinal arteriovenous fistulas. | Flow measurement before and after dural opening, during temporary clip occlusion when considered relevant, after final obliteration, on venous drainage | Final flow measurements showed arterial spectrum flow disappeared in all cases. |
| Della Puppa A et al., 2018 [10] | 85/96 | R | (Charbel Microflowprobe; Transonic System, Ithaca, NY, USA) | IONM, ICGA-VA | 46 ruptured, 50 unruptured aneurysms. Proximal group (ICA, A1, M1) (45); Distal group (pericallosal, MCA bifurcation), (51) | Flow measurement before and after clipping to exclude a flow drop and verify flow preservation. | Clip repositioning performed 24 times according to blood flow impairment (24/42; 57.1%). Decreased flow recorded in 24 patients. |
| Della Puppa A et al., 2016 [12] | 12 | R | (Charbel Microflow probe; Transonic Systems, Inc., Ithaca, NY, USA). | ICGA-VA | 10 thoracic and 2 lumbar spinal arteriovenous fistulas. | Identification of fistula point, assessment of final fistula point exclusion, flow measurement before and after temporary/permanent occlusion of the fistula. | Flow drop in all procedures, confirming complete fistula occlusion. Flowmetry presents a sensitivity similar to ICG-VA and helps to clarify ICG-VA. |
| Della Puppa A et al., 2016 [11] | 27 | R | Charbel Micro Flowprobe, Transonic Systems, Inc., Ithaca, NY, USA). | ICGA-VA, temporary artery clipping test | 21 ruptured, 6 unruptured brain AVMs | Flow assessment before resection, after ICG-VA, during dissection on the same vessels or on different vessels and after surgical resection | iMDS was helpful in making ICG-VA data clearer before resection in 22% of cases. No AVM remnants reported at postoperative DSA when flowmetry was performed. iMDS was more reliable than ICG-VA in detecting residual nidus missed at resection of deep or subcortical/interhemispheric AVMs. |
| Li Z et al., 2016 [13] | 158 | R | DIGI-LITE Doppler blood flow analyzer. Probe diameter: 1 mm; Frequency: 16 MHz; M-mode depth bounds of 0.8~13.6 mm for doppler monitoring | SSEP, ICGA-VA | ICA (36.7%); ACoA (31%); MCA (16.5%); OA (13.3%); ICA bifourcation (2.5%) aneurysms | Monitoring BF spectrum and velocity of proximal and distal fragments of parent artery | Perforating vessel occlusion in 10 patients with MCA a. >10% increase in flow velocity of parent arteries in 12 patients with ACoA a. and 8 patients with OA a. |
| Pereira BJ et al., 2015 [6] | 50 | R | Mizuho Surgical Doppler (Mizuho Corporation, Tokyo, Japan). Frequency: 20 MHz | None | MCA (21), ACA (9), PCoA (9), ICA (9) | Cerebral flow assessment. Data compared with a control group without iMDS. | Information provided by iMDS influenced clip repositioning in 11 patients (22% of cases). Ischemic infarct rate (4% in the group with iMDS vs 18% in the group without iMDS). |
| Burkhardt T et al., 2015 [7] | 32 | R | DWL SmartDop®, Compumedics, Singen, Germany. Probe diameter: 1 mm, Frequency: 16 MHz | None | brain AVM | Systolic- and diastolic-flow velocity main feeders and drainage vessels of AVM priorly verified by preoperative DSA | Normalized venous flow patterns without arterial flow turbulences at the end of the surgical procedure in all 32 cases |
| Malinova V et al., 2015 [3] | 39 | R | DWL SmartDop®, Compumedics, Singen, Germany. Probe diameter: 1 mm, Frequency: 20 MHz. Probe coupled with a neuronavigational pointer | None | 29 unruptured, 10 ruptured a. MCA (23); ACoA (7); ICA (6); PCoA (2); ACA (1) aneurysms | BVF measurement before and after aneurysm clipping | Mean deviation of the BFV before and after clipping was only 2.12 cm/s. NO BFV drop, no need of repositioning of clipping. A postoperative hemiparesis occurred due to occlusion of a perforating vessel not monitored by iMDS. |
| Della Puppa A et al., 2014 [11] | 26/34 | R | Charbel Micro Flowprobe, Transonic Systems, Inc., Ithaca, NY, USA). | ICG-VA | MCA (21), ACA (8), ICA (5); 25 unruptured, 9 ruptured. | BFV pre and postoperative assessment. A drop >25% in postclipping blood flow indicates a clipping repositioned. | No flow reduction recorded after clip reposition. Flow reduction >25% in 8 out of 48 cases (16%). |
| Hui PJ et al., 2013 [4] | 92/101 | R | Doppler Blood Flow Monitoring System (Companion III, Germany). Probe diameter: 1.5mm, Frequency: 20 MHz. Insonation angle: 30–60° | None | 82 aneurysms ligated with a single clip; 19 paraclinoid, ophthalmic segment, large or giant aneurysms with two or three clips. | BFV before and after the clip application. Data compared with a control group without iMDS | iMDS identified compromised blood flow due to inaccurate clip placement in 11 of the 101 (10.9%) aneurysms. Stenosis of the parent artery in 19 cases (18.8%), increased of BFV in parent artery in 16 of these cases (15.8%). No residual aneurysm or vessels stenosis in iMDS group. |
| Cui H et al., 2011 [8] | 79/85 | R | Companion III (SciMed, Bristol, UK). Probe diameter: 1mm, Frequency: 20 MHz. Insonation angle: 30–60° | None | 50 aneurysms <10 mm; 24 between 10 and 20 mm, 11 aneurysms >20 mm | BFV before and after the clip application | Incomplete occlusion in 9/85 a. (10.6%). Vessel stenosis induced by the clip in 10 of 79 cases (12.7%). |
| Gruber A et al., 2011 [5] | 104/123 | R | DWL SmartDop®, Compumedics, Singen, Germany. Probe diameter: 1mm, Frequency: 20 MHz | ICGA-VA, DSA, endoscopy | AcoA (31), ACA (11), ICA (15), MCA (63), posterior circulation (3) aneurysms | Assessment of the parent arteries before and clipping | Absent parent artery flow documented in 4 out of 15 cases. |
4. Discussion
4.1. Use of Intraoperative Microdoppler Sonography in Aneurysm Surgery
4.2. Use of Intraoperative Microdoppler Sonography in Arterio-Venous Malformations Surgery
4.3. Use of Intraoperative Microdoppler Sonography in Dural Arterio-Venous Fistula Surgery
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kandregula, S.; Savardekar, A.R.; Terrell, D.; Adeeb, N.; Whipple, S.; Beyl, R.; Birk, H.S.; Newman, W.C.; Kosty, J.; Cuellar, H.; et al. Microsurgical clipping and endovascular management of unruptured anterior circulation aneurysms: How age, frailty, and comorbidity indexes influence outcomes. J. Neurosurg. 2023, 138, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Malinova, V.; von Eckardstein, K.; Rohde, V.; Mielke, D. Neuronavigated microvascular Doppler sonography for intraoperative monitoring of blood flow velocity changes during aneurysm surgery—A feasible monitoring technique. Clin. Neurol. Neurosurg. 2015, 137, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Hui, P.-J.; Yan, Y.-H.; Zhang, S.-M.; Wang, Z.; Yu, Z.-Q.; Zhou, Y.-X.; Li, X.-D.; Cui, G.; Zhou, D.; Hui, G.-Z.; et al. Intraoperative microvascular Doppler monitoring in intracranial aneurysm surgery. Chin. Med. J. 2013, 126, 2424–2429. [Google Scholar] [PubMed]
- Gruber, A.; Dorfer, C.; Standhardt, H.; Bavinzski, G.; Knosp, E. Prospective comparison of intraoperative vascular monitoring technologies during cerebral aneurysm surgery. Neurosurgery 2011, 68, 657–673, discussion 673. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.J.A.; Holanda, V.M.; Giudicissi-Filho, M.; Borba, L.A.B.; de Holanda, C.V.M.; de Oliveira, J.G. Assessment of Cerebral Blood Flow with Micro-Doppler Vascular Reduces the Risk of Ischemic Stroke During the Clipping of Intracranial Aneurysms. World Neurosurg. 2015, 84, 1747–1751. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, T.; Siasios, G.; Schmidt, N.O.; Reitz, M.; Regelsberger, J.; Westphal, M. Intraoperative Micro-Doppler in Cerebral Arteriovenous Malformations. J. Neurol. Surg. Part Cent. Eur. Neurosurg. 2015, 76, 451–455. [Google Scholar] [CrossRef]
- Cui, H.; Wang, Y.; Yin, Y.; Wan, J.; Fei, Z.; Gao, W.; Jiang, J. Role of intraoperative microvascular Doppler in the microsurgical management of intracranial aneurysms. J. Clin. Ultrasound 2011, 39, 27–31. [Google Scholar] [CrossRef]
- Xing, X.Z.; Guan, B.L.; Jie, L.X. Directional multistage intraoperative microvascular Doppler in the hemilaminectomy surgical obliteration of spinal dural arteriovenous fistular. Clin. Neurol. Neurosurg. 2019, 176, 61–66. [Google Scholar] [CrossRef]
- Della Puppa, A.; Rossetto, M.; Volpin, F.; Rustemi, O.; Grego, A.; Gerardi, A.; Ortolan, R.; Causin, F.; Munari, M.; Scienza, R. Microsurgical Clipping of Intracranial Aneurysms Assisted by Neurophysiological Monitoring, Microvascular Flow Probe, and ICG-VA: Outcomes and Intraoperative Data on a Multimodal Strategy. World Neurosurg. 2018, 113, e336–e344. [Google Scholar] [CrossRef]
- Della Puppa, A.; Volpin, F.; Gioffre, G.; Rustemi, O.; Troncon, I.; Scienza, R. Microsurgical clipping of intracranial aneurysms assisted by green indocyanine videoangiography (ICGV) and ultrasonic perivascular microflow probe measurement. Clin. Neurol. Neurosurg. 2016, 116, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Della Puppa, A.; Rustemi, O.; Scienza, R. Intraoperative Flow Measurement by Microflow Probe During Spinal Dural Arteriovenous Fistula Surgery. World Neurosurg. 2016, 89, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, G.; Huang, G.; Wang, Z.; Tan, H.; Liu, J.; Li, A. Intraoperative Combined Use of Somatosensory Evoked Potential, Microvascular Doppler Sonography, and Indocyanine Green Angiography in Clipping of Intracranial Aneurysm. Med. Sci. Monit. 2016, 22, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Della Puppa, A.; Scienza, R. Multimodal Flow-Assisted Resection of Brain AVMs. Acta Neurochir. Suppl. 2016, 123, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Imbault, M.; Chauvet, D.; Gennisson, J.L.; Capelle, L.; Tanter, M. Intraoperative Functional Ultrasound Imaging of Human Brain Activity. Sci. Rep. 2017, 7, 7304. [Google Scholar] [CrossRef] [PubMed]
- Gilsbach, J.M.; Hassler, W.E. Intraoperative Doppler and real time sonography in neurosurgery. Neurosurg. Rev. 1984, 7, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Kato, K. Measurement of Cerebral Blood Flow by Ultrasonic Doppler Technique. Jpn. Circ. J. 1965, 29, 375–382. [Google Scholar] [CrossRef]
- Aaslid, R.; Markwalder, T.M.; Nornes, H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J. Neurosurg. 1982, 57, 769–774. [Google Scholar] [CrossRef]
- Nornes, H.; Grip, A.; Wikeby, P. Intraoperative evaluation of cerebral hemodynamics using directional Doppler technique. Part 2: Saccular aneurysms. J. Neurosurg. 1979, 50, 570–577. [Google Scholar] [CrossRef]
- Gilsbach, J.M.; Harders, A. Early aneurysm operation and vasospasm. Intracranial Doppler findings. Neurochirurgia 1985, 28 (Suppl. S1), 100–102. [Google Scholar] [CrossRef]
- Charbel, F.T.; Hoffman, W.E.; Misra, M.; Hannigan, K.; Ausman, J.I. Role of a perivascular ultrasonic micro-flow probe in aneurysm surgery. Neurol. Med. Chir. 1998, 38, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Amin-Hanjani, S.; Charbel, F.T. Flow-assisted surgical technique in cerebrovascular surgery. Surg Neurol. 2007, 68 (Suppl. S1), S4–S11. [Google Scholar] [CrossRef] [PubMed]
- Akdemir, H.; Oktem, I.S.; Tucer, B.; Menkü, A.; Başaslan, K.; Günaldi, O. Intraoperative microvascular Doppler sonography in aneurysm surgery. Minim. Invasive Neurosurg. 2006, 49, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Andrychowski, J.; Czernicki, Z.; Bogucki, J.; Mierzejewski, M. [The usefulness of intraoperative high-frequency doppler flowmetry in surgeries for intracranial aneurysms]. Neurol. Neurochir. Pol. 1998, 32, 331–339. [Google Scholar] [PubMed]
- Bailes, J.E.; Tantuwaya, L.S.; Fukushima, T.; Schurman, G.W.; Davis, D. Intraoperative microvascular Doppler sonography in aneurysm surgery. Neurosurgery 1997, 40, 965–970, discussion 970–972. [Google Scholar] [CrossRef] [PubMed]
- Critchley, G.R.; O’Neill, K.S.; Bell, B.A. Cerebral blood flow and tissue oxygenation monitoring during aneurysm surgery. Neurol. Res. 1998, 20 (Suppl. S1), S44–S47. [Google Scholar] [CrossRef]
- Eliava, S.S.; Shekhtman, O.D.; Zolotukhin, S.P.; Sazonov, I.A.; Kheĭreddin, A.S. [Intraoperative contact Doppler ultrasonography in the surgery of cerebral aneurysm]. Zh. Vopr. Neirokhir. Im. N N Burdenko. 2006, 2, 42–47, discussion 47–48. [Google Scholar]
- Gilsbach, J.M. [Microvascular intraoperative Doppler sonography]. Ultraschall. Med. Stuttg. Ger. 1980 1984, 5, 246–254. [Google Scholar] [CrossRef]
- Marchese, E.; Della Pepa, G.M.; La Rocca, G.; Albanese, A.; Ius, T.; Simboli, G.A.; Sabatino, G. Application of indocyanine green video angiography in vascular neurosurgery. J. Neurosurg. Sci. 2019, 63, 656–660. [Google Scholar] [CrossRef]
- Scerrati, A.; Trovalusci, F.; Albanese, A.; Ponticelli, G.S.; Tagliaferri, V.; Sturiale, C.L.; Cavallo, M.A.; Marchese, E. A workflow to generate physical 3D models of cerebral aneurysms applying open source freeware for CAD modeling and 3D printing. Interdiscip. Neurosurg. 2019, 17, 1–6. [Google Scholar] [CrossRef]
- Di Bonaventura, R.; Sturiale, C.L.; Latour, K.; Mazzucchi, E.; Marchese, E.; Albanese, A. Comparison Between Minipterional Craniotomy Associated With Focused Sylvian Fissure Opening and Standard Pterional Approach With Extended Sylvian Fissure Dissection for Treatment of Unruptured Middle Cerebral Artery Aneurysms. World Neurosurg. 2021, 146, e1293–e1300. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, E.G.; Deshmukh, P.; Nakaji, P.; Crusius, M.U.; Crawford, N.; Spetzler, R.F.; Preul, M.C. The minipterional craniotomy: Technical description and anatomic assessment. Neurosurgery 2007, 61 (Suppl. 2), 256–264, discussion 264–265. [Google Scholar] [CrossRef] [PubMed]
- Elsharkawy, A.; Niemelä, M.; Lehečka, M.; Lehto, H.; Jahromi, B.R.; Goehre, F.; Kivisaari, R.; Hernesniemi, J. Focused opening of the sylvian fissure for microsurgical management of MCA aneurysms. Acta Neurochir. 2014, 156, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Sturiale, C.L.; La Rocca, G.; Puca, A.; Fernandez, E.; Visocchi, M.; Marchese, E.; Sabatino, G.; Albanese, A. Minipterional Craniotomy for Treatment of Unruptured Middle Cerebral Artery Aneurysms. A Single-Center Comparative Analysis with Standard Pterional Approach as Regard to Safety and Efficacy of Aneurysm Clipping and the Advantages of Reconstruction. Acta Neurochir. Suppl. 2017, 124, 93–100. [Google Scholar] [CrossRef]
- La Rocca, G.; Della Pepa, G.M.; Sturiale, C.L.; Sabatino, G.; Auricchio, A.M.; Puca, A.; Olivi, A.; Marchese, E.; Albanese, A. Lateral Supraorbital Versus Pterional Approach: Analysis of Surgical, Functional, and Patient-Oriented Outcomes. World Neurosurg. 2018, 119, e192–e199. [Google Scholar] [CrossRef]
- Marchese, E.; Albanese, A.; Denaro, L.; Vignati, A.; Fernandez, E.; Maira, G. Intraoperative microvascular Doppler in intracranial aneurysm surgery. Surg. Neurol. 2005, 63, 336–342, discussion 342. [Google Scholar] [CrossRef]
- Laborde, G.; Gilsbach, J.; Harders, A. The microvascular Doppler--an intraoperative tool for the treatment of large and giant aneurysms. Acta Neurochir. Suppl. 1988, 42, 75–80. [Google Scholar] [CrossRef]
- Firsching, R.; Synowitz, H.J.; Hanebeck, J. Practicability of intraoperative microvascular Doppler sonography in aneurysm surgery. Minim. Invasive Neurosurg. 2000, 43, 144–148. [Google Scholar] [CrossRef]
- Stendel, R.; Pietilä, T.; Al Hassan, A.A.; Schilling, A.; Brock, M. Intraoperative microvascular Doppler ultrasonography in cerebral aneurysm surgery. J. Neurol. Neurosurg. Psychiatry 2000, 68, 29–35. [Google Scholar] [CrossRef]
- Woydt, M.; Greiner, K.; Perez, J.; Krone, A.; Roosen, K. Intraoperative color duplex sonography of basal arteries during aneurysm surgery. J. Neuroimaging 1997, 7, 203–207. [Google Scholar] [CrossRef]
- Kapsalaki, E.Z.; Lee, G.P.; Robinson, J.S.; Grigorian, A.A.; Fountas, K.N. The role of intraoperative micro-Doppler ultrasound in verifying proper clip placement in intracranial aneurysm surgery. J. Clin. Neurosci. 2008, 15, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Friedman, W.A.; Chadwick, G.M.; Verhoeven, F.J.; Mahla, M.; Day, A.L. Monitoring of somatosensory evoked potentials during surgery for middle cerebral artery aneurysms. Neurosurgery 1991, 29, 83–88. [Google Scholar] [CrossRef]
- Skrap, B.; Di Bonaventura, R.; Di Domenico, M.; Sturiale, C.L.; Auricchio, A.M.; Maugeri, R.; Giammalva, G.R.; Iacopino, D.G.; Olivi, A.; Marchese, E.; et al. Has intraoperative neuromonitoring changed the surgery for unruptured middle cerebral artery aneurysms? A retrospective comparative study. Neurosurg. Rev. 2023, 46, 191. [Google Scholar] [CrossRef] [PubMed]
- Shibata, Y.; Fujita, S.; Kawaguchi, T.; Hosoda, K.; Komatsu, H.; Tamaki, N. Use of microvascular Doppler sonography in aneurysm surgery on the anterior choroidal artery. Neurol. Med. Chir. 2000, 40, 30–35, discussion 35–37. [Google Scholar] [CrossRef] [PubMed]
- Ondra, S.L.; Troupp, H.; George, E.D.; Schwab, K. The natural history of symptomatic arteriovenous malformations of the brain: A 24-year follow-up assessment. J. Neurosurg. 1990, 73, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Della Pepa, G.M.; Di Bonaventura, R.; Latour, K.; Sturiale, C.L.; Marchese, E.; Puca, A.; Sabatino, G.; Albanese, A. Combined Use of Color Doppler Ultrasound and Contrast-Enhanced Ultrasound in the Intraoperative Armamentarium for Arteriovenous Malformation Surgery. World Neurosurg. 2021, 147, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Fiorenza, V.; Brunasso, L.; Ascanio, F.; Maugeri, R.; Odierna Contino, A.; Iacopino, D.G.; Tringali, G. Glomus Cervical Arteriovenous Malformation Presenting with Intracranial Subarachnoid Hemorrhage: Clinical Case and Pial Resection Surgical Technique Description. World Neurosurg. 2023, 179, 1–4. [Google Scholar] [CrossRef]
- Hacein-Bey, L.; Konstas, A.A.; Pile-Spellman, J. Natural history, current concepts, classification, factors impacting endovascular therapy, and pathophysiology of cerebral and spinal dural arteriovenous fistulas. Clin. Neurol. Neurosurg. 2014, 121, 64–75. [Google Scholar] [CrossRef]
- Della Pepa, G.M.; Parente, P.; D’Argento, F.; Pedicelli, A.; Sturiale, C.L.; Sabatino, G.; Albanese, A.; Puca, A.; Fernandez, E.; Olivi, A.; et al. Angio-Architectural Features of High-Grade Intracranial Dural Arteriovenous Fistulas: Correlation With Aggressive Clinical Presentation and Hemorrhagic Risk. Neurosurgery 2017, 81, 315. [Google Scholar] [CrossRef]
- Signorelli, F.; Della Pepa, G.M.; Sabatino, G.; Marchese, E.; Maira, G.; Puca, A.; Albanese, A. Diagnosis and management of dural arteriovenous fistulas: A 10 years single-center experience. Clin. Neurol. Neurosurg. 2015, 128, 123–129. [Google Scholar] [CrossRef]
- Endo, T.; Endo, H.; Sato, K.; Matsumoto, Y.; Tominaga, T. Surgical and Endovascular Treatment for Spinal Arteriovenous Malformations. Neurol. Med. Chir. 2016, 56, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.; Stapleton, C.J.; Agarwalla, P.K.; Torok, C.M.; Shin, J.H.; Coumans, J.-V.; Borges, L.F.; Ogilvy, C.S.; Rabinov, J.D.; Patel, A.B. 157 Open and Endovascular Treatment of Spinal Dural Arteriovenous Fistulae: A 10-Year Experience. Neurosurgery 2016, 63, 163. [Google Scholar] [CrossRef]
- Della Pepa, G.M.; Sabatino, G.; Sturiale, C.L.; Marchese, E.; Puca, A.; Olivi, A.; Albanese, A. Integration of Real-Time Intraoperative Contrast-Enhanced Ultrasound and Color Doppler Ultrasound in the Surgical Treatment of Spinal Cord Dural Arteriovenous Fistulas. World Neurosurg. 2018, 112, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Colby, G.P.; Coon, A.L.; Sciubba, D.M.; Bydon, A.; Gailloud, P.E.; Tamargo, R.J. Intraoperative indocyanine green angiography for obliteration of a spinal dural arteriovenous fistula: Case report. J. Neurosurg. Spine 2009, 11, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Amin-Hanjani, S.; Meglio, G.; Gatto, R.; Bauer, A.; Charbel, F.T. The utility of intraoperative blood flow measurement during aneurysm surgery using an ultrasonic perivascular flow probe. Neurosurgery 2006, 58 (Suppl. 2), ONS-305–ONS-312, discussion ONS-312. [Google Scholar] [CrossRef]
- Giller, C.A.; Meyer, Y.J.; Batjer, H.H. Hemodynamic assessment of the spinal cord arteriovenous malformation with intraoperative microvascular Doppler ultrasound: Case report. Neurosurgery 1989, 25, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Hassler, W.; Thron, A. Flow velocity and pressure measurements in spinal dural arteriovenous fistulas. Neurosurg. Rev. 1994, 17, 29–36. [Google Scholar] [CrossRef]
- Kataoka, H.; Miyamoto, S.; Nagata, I.; Ueno, Y.; Hashimoto, N. Intraoperative microdoppler monitoring for spinal dural arteriovenous fistulae. Surg. Neurol. 1999, 52, 466–472. [Google Scholar] [CrossRef]
- Randel, S.; Gooding, G.A.; Dillon, W.P. Sonography of intraoperative spinal arteriovenous malformations. J. Ultrasound Med. 1987, 6, 539–544. [Google Scholar] [CrossRef][Green Version]
- Iacopino, D.G.; Giusa, M.; Conti, A.; Cardali, S.; Tomasello, F. Intraoperative microvascular Doppler monitoring of blood flow within a spinal dural arteriovenous fistula: A precious surgical tool. Case report. Neurosurg. Focus 2001, 10, ECP1. [Google Scholar] [CrossRef]
- Iacopino, D.G.; Conti, A.; Giusa, M.; Cardali, S.; Tomasello, F. Assistance of intraoperative microvascular Doppler in the surgical obliteration of spinal dural arteriovenous fistula: Cases description and technical considerations. Acta Neurochir. 2003, 145, 133–137, discussion 137. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gulino, V.; Brunasso, L.; Avallone, C.; Campisi, B.M.; Bonosi, L.; Costanzo, R.; Cammarata, E.; Sturiale, C.L.; Cordova, A.; Iacopino, D.G.; et al. The Use of Intraoperative Microvascular Doppler in Vascular Neurosurgery: Rationale and Results—A Systematic Review. Brain Sci. 2024, 14, 56. https://doi.org/10.3390/brainsci14010056
Gulino V, Brunasso L, Avallone C, Campisi BM, Bonosi L, Costanzo R, Cammarata E, Sturiale CL, Cordova A, Iacopino DG, et al. The Use of Intraoperative Microvascular Doppler in Vascular Neurosurgery: Rationale and Results—A Systematic Review. Brain Sciences. 2024; 14(1):56. https://doi.org/10.3390/brainsci14010056
Chicago/Turabian StyleGulino, Vincenzo, Lara Brunasso, Chiara Avallone, Benedetta Maria Campisi, Lapo Bonosi, Roberta Costanzo, Emanuele Cammarata, Carmelo Lucio Sturiale, Adriana Cordova, Domenico Gerardo Iacopino, and et al. 2024. "The Use of Intraoperative Microvascular Doppler in Vascular Neurosurgery: Rationale and Results—A Systematic Review" Brain Sciences 14, no. 1: 56. https://doi.org/10.3390/brainsci14010056
APA StyleGulino, V., Brunasso, L., Avallone, C., Campisi, B. M., Bonosi, L., Costanzo, R., Cammarata, E., Sturiale, C. L., Cordova, A., Iacopino, D. G., & Maugeri, R. (2024). The Use of Intraoperative Microvascular Doppler in Vascular Neurosurgery: Rationale and Results—A Systematic Review. Brain Sciences, 14(1), 56. https://doi.org/10.3390/brainsci14010056






