Abstract
Cancer survivors experience cancer-related cognitive impairment (CRCI) secondary to treatment. Chemotherapy and radiation therapy independently contribute to cognitive dysfunction; however, the underlying mechanisms leading to dysfunction remain unclear. We characterized brain gene expression changes in a mouse model of CRCI to identify the mechanistic underpinnings. Eleven-to-twelve-week-old SKH1 mice were treated with doxorubicin (DOX), hindlimb radiation (RT), concurrent hindlimb radiation and doxorubicin (DOX-RT), or no treatment (control). Sixteen days following treatment, gene expression was measured from murine brains using the NanoString nCounter® glial profiling panel. Gene expression was normalized and compared between groups. No two groups shared the same expression pattern, and only Gnb1 and Srpr were upregulated in multiple treatment groups. Brains from DOX-treated mice had upregulated Atf2, Atp5b, Gnb1, Rad23b, and Srpr and downregulated Sirt5 expression compared to control brains. Brains from RT-treated mice demonstrated increased Abcg2 and Fgf2 and decreased C1qa and C1qb expression compared to control brains. Brains from DOX-RT-treated mice had upregulated Adar, E2f3, Erlec1, Gnb1, Srpr, Vim, and Pdgfra expression and downregulated Rock2 and Inpp5f expression compared to control brains. The gene expression changes demonstrated here highlight roles for neuronal transmission and oxidative stress in the pathogenesis of doxorubicin-related CRCI and inflammation in RT-related CRCI.
1. Introduction
Improvements in cancer detection and treatment have increased the number of cancer survivors with long-term survival [1,2,3,4]. Although the lifespan of the average cancer patient has increased, the adverse effects of cancer treatment can be detrimental to long-term quality of life. Cancer-related cognitive impairment (CRCI) affects patients with and without central nervous system cancer and significantly contributes to survivorship challenges. CRCI is a neurocognitive syndrome estimated to affect up to 75% of cancer patients, continuing for 5–10 years following treatment [1,2,5]. CRCI is clinically defined by learning and memory deficits and impaired concentration, processing speed, and executive function [1,5,6]. Cancer survivors’ quality of life is detrimentally impacted by CRCI, and it negatively affects their ability to return to work, perform daily tasks, and maintain their personal relationships [7].
CRCI is particularly well documented in breast cancer survivors, as treatment improvements have resulted in a 90% five-year survival rate [8]. Breast cancer patients are frequently prescribed chemotherapy and radiation therapy, both of which have been linked to CRCI [1,2,9,10]. Imaging studies in female breast cancer patients treated with chemotherapy have revealed decreases in grey matter volume, reductions in white matter microstructure, neuroinflammation, altered cerebral blood flow, and changes in brain connectivity [4,9,10,11,12,13]. Several proposed mechanisms leading to neurocognitive symptoms after chemotherapy include disruption of the blood–brain barrier, neuronal apoptosis, decreased neurogenesis, oxidative stress, myelin degeneration, DNA damage, genetic predispositions, altered brain blood flow, and cytokine dysregulation [1,2,3].
Radiation therapy is a standard-of-care treatment for multiple cancers, and over 50% of all newly diagnosed cancer patients will receive radiation therapy during their course of treatment [2]. The direct effects of radiation therapy on the brain include cognitive impairment, white matter necrosis, vascular changes, demyelination, and neuroinflammation [14,15]. Much less is known regarding the ability of radiation to induce cognitive deficits when anatomic sites distant to the brain are irradiated. Previous reports have documented persistent impairment in memory and executive function following non-brain-directed radiation therapy [2,13].
The 2021 GLOBOCAN cancer burden survey estimated that 19.3 million new cancer cases occurred in 2020 and that this number will grow to 28.4 million in 2040 [16]. This increase in cancer incidence will result in increased usage of therapeutic chemotherapy and radiation. It is estimated by the year 2040, there will be 15 million cancer patients requiring chemotherapy, and by the year 2030, there will be 4.17 million 5-year cancer survivors who received radiation therapy [17,18]. Given this increase in chemotherapy- and radiation-treated cancer patients and improved cancer outcomes, the characterization of the role of cancer treatment in cancer patient survivorship issues, including CRCI, is an urgent need.
The exact mechanisms causing chemotherapy- and/or radiotherapy-induced CRCI are not understood, which hinders solutions to mitigate its occurrence. In prior work, we demonstrated that the studied mice developed multifocal brain gliosis and hippocampal memory deficits when treated with doxorubicin, hindlimb radiation, or the concurrent administration of doxorubicin and radiation [1]. To shed mechanistic insight on the link between non-brain-directed radiation, brain gliosis, and cognitive impairment, we performed gene expression profiling in the brains of mice treated with doxorubicin, non-brain-directed radiation, or concurrent doxorubicin and radiation using the NanoString nCounter® platform (NanoString Technologies, Seattle, WA, USA).
2. Materials and Methods
2.1. Experimental Animals
Eleven-to-twelve-week-old female SKH1 mice were purchased from Charles River Laboratories (Wilmington, MA, USA). As female breast cancer survivors are disproportionately affected by CRCI, only female mice were used in this study [19]. To isolate treatment-associated changes from tumor changes, non-tumor-bearing mice were used in this study [1,5,9,13]. Mice were assigned to one of four groups according to body weight: (1) mice treated with doxorubicin only (DOX), (2) mice treated with non-brain-directed radiation treatment (RT), (3) mice treated with both (DOX-RT), and (4) untreated mice (control). Mice were housed in static cages, with four female mice from the same treatment group per cage. This study was performed with approval by and in accordance with the University of Minnesota Institutional Animal Care and Use Committee (UMN-IACUC).
2.2. Animal Treatment
The therapeutic methodology was identical to our previous study [1]. Briefly, doxorubicin HCL (Hikma Pharmaceuticals USA Inc., Berkeley Heights, NJ, USA) was administered intraperitoneally at 5 mg/kg. We chose to administer a single dose of 5 mg/kg (equivalent to 18 mg/m2), which is lower than the claimed lethal dose in mice of 7–10 mg/kg IP and comparable to doxorubicin dosages (10–20 mg/m2) administrated with radiation therapy in human cancer patients [20,21,22,23]. The radiation protocol performed on the RT and DOX-RT mice consisted of 20 Gy applied to the skin of the right hindlimb with 6 MeV electrons (Varian 2100 iX; Varian Medical Systems, Inc., Palo Alto, CA, USA) using a 1 cm tissue-equivalent bolus and a 2 × 2 cm2 electron cutout [1]. The dose of radiation was quantified using radiochromic film dosimetry (GAFchromicTM EBT2, Ashland AdvancedMaterials, Bridgewater, NJ, USA) to confirm the prescribed dose was given to the right hindlimb and that the dose was undetectable at the level of the skin over the skull [24]. The dose of radiation administered was comparable to the dose recommended to be given over 7 days in early-stage breast cancer patients [25]. Control mice were anesthetized with xylazine (4 mg/kg) and ketamine (90 mg/kg) and treated with intraperitoneal saline. Cognitive deficits were analyzed through standardized behavioral testing, including the open field test, novel location recognition test, novel object recognition test, and spontaneous alternation y-maze, prior to collection of brain tissue [1].
2.3. Tissue Preparation
Sixteen days post treatment, mice were euthanized by carbon dioxide followed by exsanguination in accordance the UMN-IACUC Criteria for Carbon Dioxide Euthanasia Guidelines. This timepoint was selected to evaluate acute-term effects of cancer therapy translational to almost 2 years post cancer treatment in adult humans [26]. Mouse brains were collected, immersion-fixed in 10% neutral buffered formalin, and transferred into 70% ethanol. Samples were sectioned and subjected to routine tissue processing before paraffin embedding.
2.4. NanoString Gene Expression Profiling
Brain tissues from six mice from each treatment group were processed for gene expression profiling. The brains of mice were split into two paraffin blocks. A total of twenty 10 µm coronal sections of the brains were collected for RNA extraction. The first coronal sections from two paraffin blocks of each brain included the caudal cortex (coronal sections near −3.38 to −4.08 Bregma), cerebellum (coronal sections near −6.355 to −7.255 Bregma), hippocampus (coronal sections near −2.78 to −3.455 Bregma), medulla (coronal sections near −6.355 to −7.255 Bregma), midbrain (coronal sections near −3.38 to −4.08 Bregma), rostral cortex (coronal sections near −0.08 to −1.455 Bregma), and striatum (coronal sections near −0.08 to −1.455 Bregma) [1,27]. RNA was extracted using the PureLink FFPE total RNA isolation kit (Invitrogen, Carlsbad, CA, USA) according to manufacturer’s instructions. A total of 100 nanograms of RNA from each sample was used to evaluate gene expression by the nCounter® glial cell profiling panel from NanoString (NanoString Technologies, Seattle, WA, USA).
2.5. Data Analysis
The nSolver™ Analysis Software version 4.0 (NanoString Technologies, Seattle, WA, USA) quality control parameters were used to assess quality of imaging, binding density, positive control linearity, and positive control limit of detection. All samples passed the quality controls assessed [28,29]. The nSolver™ Analysis Software Advanced Analysis Module (version 2.0.134) was used to analyze gene expression data including gene normalization as well as differential expression volcano plots with p-values from linear regression [30]. The Advanced Analysis Module was used to normalize raw gene data for each sample to the geometric mean of the endogenous housekeeping genes using the geNorm algorithm [28,29]. The normalized gene expression data were analyzed using Prism 10.1.2 (GraphPad Software, San Diego, CA, USA). One-way analysis of variance (ANOVA) with the Tukey post hoc test was used to evaluate differences between groups.
A pathway enrichment analysis was performed using Enrichr on the 17 genes differentially expressed in the brains of mice treated with cancer therapy compared to control mice [31,32,33]. The databases included were BioPlanet 2019, WikiPathway 2023 Human, KEGG 2021 Human, and Elsevier Pathway Collection. From each database, the top 10 significant p-values enrichment results were reported.
3. Results
Cancer Treatment Is Associated with Unintended Molecular Changes in the Normal Brain
Glial cell activation is associated with worse cognitive impairment in breast cancer patients after cancer treatment [4]. We previously found widespread glial cell activation and cognitive impairment following cytotoxic therapy in this mouse model [1]. To better understand molecular signals associated with chemotherapy, radiation, or concurrent treatment, we used the nCounter® glial cell profiling panel to characterize gene expression patterns in the brains of normal control mice and DOX-, RT-, and DOX-RT-treated mice. Unsupervised hierarchical clustering of the normalized gene expression data for all mice (Figure 1A) and the heat map of gene pathway cluster scores (Figure 1B) demonstrated unique changes to all treated groups.
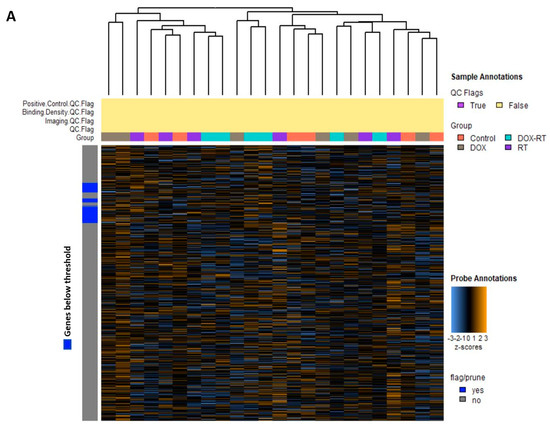
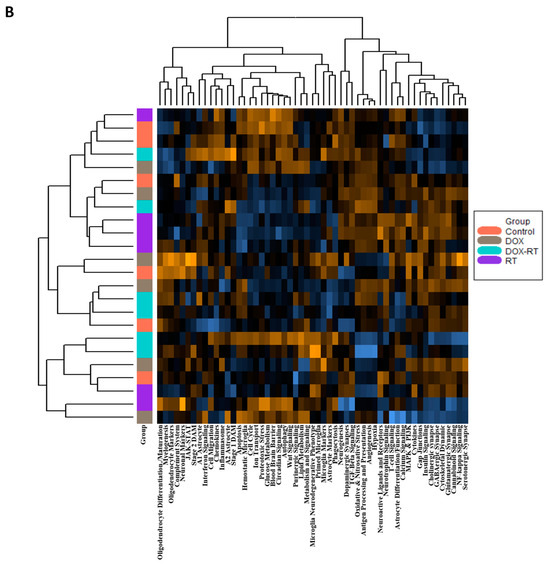
Figure 1.
Overview of gene changes in the brain of mice treated with cancer treatment. (A) Heat map of normalized data, with orange indicating high expression and blue indicating low expression. (B) Heatmap showing gene pathway clustering of the groups. Control, control mice; DOX, doxorubicin-treated mice; RT, hindlimb-radiation-treated mice; DOX-RT, doxorubicin- and hindlimb-radiation-treated mice. n = 6 mice per group.
Brain tissue from DOX-treated mice demonstrated significant (p-value < 0.01) upregulation in Gnb1, Atf2, Srpr, Atp5b, Rad23b, and Mtmr4 and downregulation in Sirt5 compared to control in a log linear regression model (Figure 2A). Upon ANOVA and post hoc analysis, DOX-treated brains had significantly upregulated Atf2 (p = 0.0130, Figure 3A), Atp5b (p = 0.0245, Figure 3B), Gnb1 (p = 0.0105, Figure 3C), Rad23b (p = 0.0298, Figure 3D) and Srpr (p = 0.0137, Figure 3F) gene expression compared to control brains. Only Sirt5 (p = 0.0341, Figure 3E) expression was downregulated compared to the control.
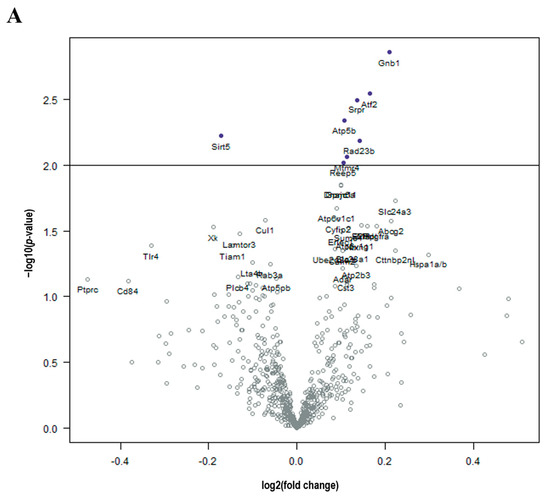
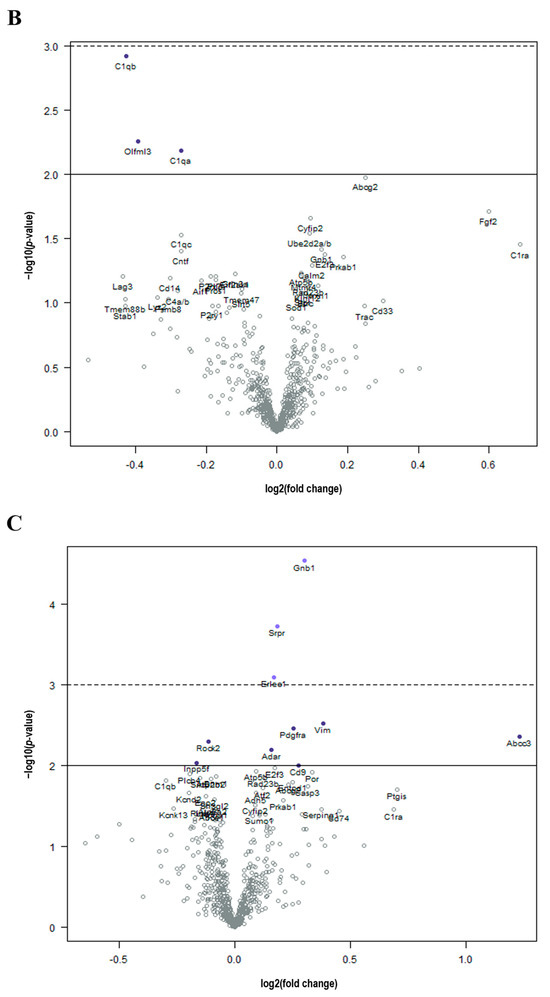
Figure 2.
Cancer treatment is associated with molecular changes in the brain. (A) Volcano plot shows all differentially expressed genes above background between brains from DOX and control mice, with genes of high statistical significance on top and high fold change on either side using a log linear regression. (B) Volcano plot shows all differential expressed genes above background between brains from RT and control mice, with genes of high statistical significance on top and high fold change on either side using a log linear regression. (C) Volcano plot shows all differential expressed genes above background between brains from DOX-RT and control mice, with genes of high statistical significance on top and high fold change on either side using a log linear regression. Solid line p-value < 0.01; dotted line p-value < 0.001. Control, control mice; DOX, doxorubicin-treated mice; RT, hindlimb-radiation-treated mice; DOX-RT, doxorubicin- and hindlimb-radiation-treated mice. n = 6 mice per group.
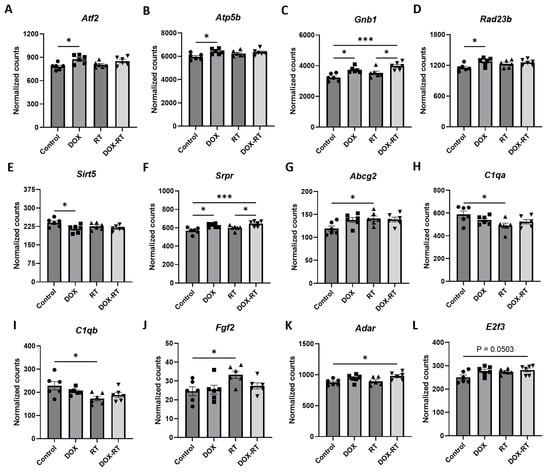
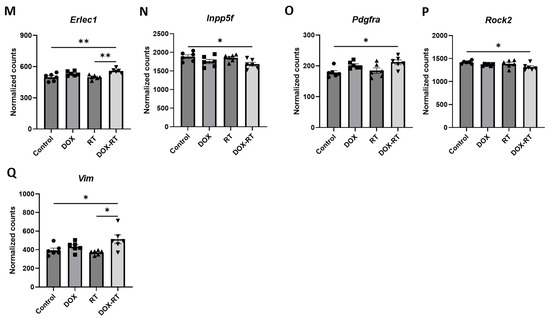
Figure 3.
Cancer treatment causes significant gene changes in the brain of mice. Mice treated with DOX had significant upregulation of genes in their brain, including Atf2 (A), Atp5b (B), Gnb1 (C), Rad23b (D), and Srpr (F), compared to control mice. Mice treated with DOX-RT had significant downregulation of the Sirt5 (E) gene in their brain compared to control mice. Comparison showed brains from mice treated with RT had significant upregulation of Abcg2 (G) and Fgf2 (J) genes and downregulation of C1qa (H) and C1qb (I) compared to control mice. Mice treated with DOX-RT had significant upregulation of Adar (K), E2f3 (L), Erlec1 (M), Gnb1 (C), Pdgfra (O), Srpr (F), and Vim (Q) gene expression and downregulation of Inpp5f (N) and Rock2 (P) gene expression compared to control mice. Data represent the mean and SEM evaluated by ANOVA and post hoc analysis. DOX, doxorubicin-treated group; RT, hindlimb-radiation-treated group; DOX-RT, doxorubicin- and hindlimb-radiation-treated group. n = 6 mice per group. * p-value < 0.05; ** p-value < 0.01; *** p-value < 0.001.
Compared to control mice, brains from mice treated with RT demonstrated significant (p-value < 0.01) decreased expression of C1qb, Olfml3, and C1qa in a log linear regression model (Figure 2B). Upon ANOVA and post hoc analysis, the upregulated genes from RT-treated brains as compared to the control included Abcg2 (p = 0.0495, Figure 3G) and Fgf2 (p = 0.0350, Figure 3J). The downregulated genes as compared to control were C1qa (p = 0.0270, Figure 3H) and C1qb (p = 0.0190, Figure 3I).
As compared to control mice, mice treated with DOX-RT demonstrated significant (p-value < 0.01) increased expression of Gnb1, Srpr, Erlec1, Vim, Pdgfra, Abcc3, Adar, and CD9 and decreased expression of Rock2 and Inpp5f in a log linear regression model (Figure 2C). Upon ANOVA and post hoc analysis, upregulated Adar (p = 0.0278, Figure 3K), E2f3 (p = 0.0503, Figure 3L), Erlec1 (p = 0.0034, Figure 3M), Gnb1 (p = 0.0002, Figure 3C), Pdgfra (p = 0.0147, Figure 3O), Srpr (p = 0.0008, Figure 3F), and Vim (p = 0.0377, Figure 3Q) expression was measured compared to control brains. Only two genes were downregulated (Figure 3), including Inpp5f (p = 0.0442, Figure 3N) and Rock2 (p = 0.0252, Figure 3P).
When comparing treatment groups, brains from DOX-treated mice did not differ in gene expression from RT- or DOX-RT-treated mice. RT-treated mice had significant gene expression changes in their brains compared to DOX-RT-treated mice (Table 1). RT-treated mice had increased expression of Atp2b2 compared to DOX-RT treated mice and decreased Cd74, Erlec1 (Figure 3M), Gnb1 (Figure 3C), Lyz2, Olfml3, Srpr (Figure 3F), and Vim (Figure 3Q) expression compared to DOX-RT-treated mice (Table 1).

Table 1.
Gene differences between treatment groups. p-values represent results from group comparisons in a one-way ANOVA with a post hoc Tukey test. Mean = normalized mRNA counts.
A pathway enrichment analysis was performed on the 17 differentially expressed genes in the DOX-, RT-, and DOX-RT-treated mice compared to the control (Table 2). The 40 pathway enrichments included 13 associated with cancer and 5 associated with infectious agents. Other functions included in the enrichment analysis were complement cascade activation, cytoskeleton regulation, angiogenesis, and oxidative stress (Table 2).

Table 2.
Pathway enrichment for the 17 genes differentially expressed in the brains of mice after cancer treatment.
4. Discussion
In this study, we sought to identify gene expression changes in the brains of a previously published mouse model of CRCI to better understand the mechanistic links between cognitive dysfunction and distinctive cancer therapies, namely focal radiation treatment and systemic doxorubicin. The main overall findings of this study highlight roles for neuronal transmission and oxidative stress in the pathogenesis of doxorubicin-related CRCI and inflammation in RT-related CRCI. This study builds upon our previous work demonstrating that these mice show extensive brain gliosis and hippocampal-dependent memory deficits after treatment with either hindlimb radiation, doxorubicin, or concurrent treatment [1]. The results highlight the importance of documenting distinct, treatment-specific mechanisms to define potential mitigators for CRCI, as our data demonstrated differentially expressed gene changes in the brains of SKH1 mice after treatment with DOX, RT, or DOX-RT compared to control mice. In addition, we observed significant gene expression changes between the RT-treated mice and DOX-RT-treated mice. Surprisingly, neither RT- nor DOX-treated mice shared similar gene expression patterns with the DOX-RT-treated mice. The results of this study suggest an interaction effect on the brain in mice treated with DOX-RT. An explanation for this finding is that radiation and doxorubicin have unique mechanisms of action leading to distinct systemic effects. Doxorubicin induces DNA breaks and interrupts DNA replication via impeding the action of topoisomerases in cancer cells [34]. Doxorubicin also causes multifaceted toxic effects leading to damage in the heart, brain, liver, and kidney [34]. On the other hand, radiation therapy causes DNA breaks directly and indirectly by creating free radicals from water molecules leading to cell death [35]. Radiation therapy toxicity is seen not only at the site of irradiation in the skin but at distant sites via cytokine signaling [36]. The majority of gene changes observed were in connections to cancer gene pathways, behavior, cell stress, astrocyte activity, and neuronal transmission.
In the DOX-RT treated mice, we identified increased expression of genes associated with cognitive impairment, namely Adar, Gnb1, and Vim. Adar encodes for a protein called ADAR1, and increased expression of ADAR1 has been associated with stress-induced cognitive impairment in mice [37]. Gnb1 expression engages in G-protein-coupled receptors by acting as a molecular switch in the signal transduction [38]. The increased expression of brain Gnb1, which we also observed in the DOX-only-treated mice, has been linked to anxiety and depressive behavior [39]. Doxorubicin has been shown to cause anxiety in humans and anxiety-like behavior in mice and rats [40,41,42,43]. In humans, Gnb1 expression has been shown to be increased in neurological diseases including Alzheimer’s disease [38]. Lastly, Vim encodes for vimentin, which is a marker of reactive astrocytes in reactive gliosis, and its expression is increased in neurodegenerative diseases [44,45]. We previously showed reactive gliosis in multiple regions of the brain in these mice treated with DOX and DOX-RT [1].
In addition to documenting gene expression abnormalities that corroborate our previous observations of cognitive derangements in cancer treatment mice, we also identified changes in gene expression that suggest oxidative stress is a key contributor to DOX- and DOX-RT-related CRCI [1]. Specifically, increased expression of E2f3, Erlec1, Srpr, and Pdgfra in DOX-RT-treated mice implicates apoptosis, cell/endoplasmic reticulum stress, and alterations in the blood–brain barrier as pathways altered in brain tissue, although doxorubicin and hindlimb irradiation did not directly impact the brain. The E2f3 gene encodes the e2f transcription factor 3 that is involved in DNA-damage-induced apoptosis and neurogenesis including neuronal precursor proliferation and neuronal migration [46,47]. Other researchers have previously reported doxorubicin treatment to cause increased E2f3 mRNA and protein expression in both human and mouse cell lines [46,48]. The Erlec1 gene encodes for endoplasmic reticulum lectin 1 and is involved in cell stress response, including endoplasmic reticulum stress, and is frequently overexpressed in human cancers [49,50,51]. Another gene associated with endoplasmic reticulum stress that was significantly increased in both the DOX and DOX-RT mice was Srpr [52]. In rats treated with doxorubicin, endoplasmic reticulum stress is induced in the hippocampus [43]. Increased expression of Erlec1 and Srpr in the brains of the DOX-RT mice and DOX mice could indicate endoplasmic reticulum stress within the brains of the mice [51]. Pdgfra is an oncogene that encodes for the platelet-derived growth factor receptor alpha subunit [53]. Pdgfra is expressed in the hippocampus of mice, and its increased expression is linked to blood–brain barrier integrity and learning and memory [53,54]. Doxorubicin is known to cause oxidative stress within the brain that can lead to blood–brain barrier disruption [55].
We also identified the decreased expression of two genes, namely Inpp5f and Rock2, following DOX-RT treatment, which supports that abscopal effects may underlie RT-related contributions to CRCI. The Inpp5f gene encodes for inositol polyphosphate phosphatase F, which inhibits the PI3K/AKT signaling pathway [56]. Downregulation of the Inpp5f gene has been shown to be involved in neuropathic pain and cognitive impairment in rats [56]. The Rock2 gene is highly expressed in the brain, especially within neurons. It encodes for the rho-associated coiled-coil kinase (ROCK) isoform 2 [57]. ROCK2 inhibition has been shown to reduce DNA damage repair proteins [58]. As previous studies have shown that radiation treatment can cause abscopal DNA damage, there is a possibility that the treatment could impact the repair of DNA damage in neurons affected by radiation treatment [59].
In RT-treated mice, the downregulation of C1qa and C1qb and upregulation of Abcg2 and Fgf2 suggests microglial and astrocyte reactivity may contribute to RT-related CRCI. C1qa and C1qb genes are two of three genes (C1qa, C1qb, and C1qc) that encode C1q, a protein primarily originating from microglia within the brain [60,61]. Notably, C1qc expression was decreased in the RT-treated mice compared to the control, but this did not reach statistical significance. Our findings of decreased C1qa and C1qb expression at 16 days post a RT are similar to those reported in study evaluating the impact of direct brain irradiation in mice. In this study, irradiation of the brain induced a transient increase in C1q expression in both astrocytes and microglia that lasted hours after treatment but dropped below control levels weeks post radiation exposure [60]. Abcg2 encodes for ATP-binding cassette sub-family G member 2 (ABCG2), which is a transporter protein expressed in brain endothelium in the blood–brain barrier, neurons, astrocytes, microglia, and pericytes [62,63]. ABCG2 is upregulated in neurologic diseases such as Alzheimer’s disease and amyotrophic lateral sclerosis [62,64]. Fgf2 encodes for the fibroblast growth factor 2 (FGF2) and is expressed in reactive astrocytes and neurons [65]. FGF2 has been previously shown to be upregulated after radiation treatment and is associated with radiation-induced fibrosis following cellular injury, which could contribute to cognitive dysfunction [65,66]. We showed previously that RT-treated SKH1 mice have an increased number of reactive astrocytes within multiple regions of the brain, which could explain the increased expression of Fgf2 [1].
The DOX-only-treated mice showed significant increases in the expression of Atf2, Atp5b, and Rad23b and significant decreases in expression of Sirt5 compared to control mice. Atf2 encodes for activating transcription factor 2 (ATF2), which plays a role in normal cellular development, cellular survival, and the cellular response to stress and DNA damage [67]. Although our identification of increased Atf2 expression aligns with similar findings from human patients with Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease, it contrasts with a previous study demonstrating that mice treated with doxorubicin-based multiagent protocols had significant downregulation of brain Atf2 [8,67]. Although the precise cause for these discordant results is unknown, they may reflect differences in chemotherapy drug treatment regimes, mouse strain, and/or the timepoint at which expression in mouse brains was measured [8]. The Atp5b gene encodes the ATP synthase subunit beta enzyme, which is part of catalytic portion of complex V in the mitochondrial electron transport chain [68]. Our identification of increased expression of brain Atp5b is consistent with previous work demonstrating increased Atp5b and suppressed complex V activity in the hearts of doxorubicin-treated mice [68,69]. Rad23b is a DNA repair gene, and doxorubicin systemically damages DNA in dividing cells and can cause DNA damage to neurons [70,71,72]. This could explain why Rad23b was increased in the brains from DOX-treated mice. The gene Sirt5 encodes for sirtuin 5, which resides in the mitochondria and promotes glycolysis but also has antioxidant capacity [73]. This finding corroborates a prior murine study in which doxorubicin decreased expression of Sirt5, which may promote doxorubicin-induced oxidative stress [74]. Doxorubicin has been shown to cause indirect oxidative stress in the nervous system, and oxidative stress can cause neuronal degeneration and cognitive impairment [72,75].
This is the first study to examine whole-brain gene expression in mice treated with concurrent doxorubicin and non-brain-directed radiation therapy or non-brain-directed radiation therapy alone. We previously demonstrated that these mice developed cognitive deficits and glial pathology following each of these treatment regimens [1]. Our data reflect gene changes associated with brain injury and cognitive dysfunction following a single post-treatment timepoint and provide novel mechanistic insights.
There are limitations to this work that can be addressed in future studies. Gene expression changes were evaluated using NanoString, which may be less sensitive to small changes in gene expression compared to RT-qPCR [76]. The effect of hormones was not evaluated in this study that used female mice only, given the predilection of women with CRCI following treatment for breast cancer. Differences in gene expression between male and female mice may shed light on unique molecular pathways for CRCI. In this study, we evaluated gene expression changes in the whole brain post treatment. There are known cognitive domains associated with CRCI that are brain-region-specific [1,19]. Additional focus is needed to study treatment-related injury to specific regions of the brain related to memory and learning. Our study investigated molecular changes after cancer treatment in a non-tumor-bearing mouse model to separate treatment-associated changes from tumor-associated changes since cancer itself causes cognitive deficits [5,9,13]. Future studies will need to evaluate the effects of cancer and anti-cancer treatments on molecular changes in the brain. Finally, all of the mice in this study underwent behavioral testing one day before the mice were euthanized by an AVMA-approved method. It is possible that both behavioral tests and euthanasia itself can alter gene expression in the brain [77,78,79,80]. Additional studies would be needed to evaluate the effect of behavioral testing and humane euthanasia methods on brain gene expression. Nonetheless, we feel that the changes in gene expression described here are important since the treatment effect was identified using similarly euthanized control mice that underwent the same behavioral testing as the treatment groups. Our study evaluated gene expression changes in mice at one timepoint translational to cancer patients years after treatment [26]. Further work will be needed to determine additional peracute and chronic changes, as our work and that of others supports a spectrum of changes that develop within hours to days and persist for weeks in rodents [1,2,21,81].
5. Conclusions
This study demonstrates that in adult female SKH1 mice, doxorubicin, hindlimb radiation, or concurrent doxorubicin and hindlimb radiation substantially altered gene expression patterns distinct to each treatment group. Mechanisms leading to DOX- and RT-related CRCI are unique, and the gene expression alterations identified here shed light on mitigation strategies. This study supports additional work needed to evaluate protein and gene expression changes within anatomic regions of the brain related to memory and learning following commonly used cancer treatments.
Author Contributions
Conceptualization, K.D.-D., J.L., C.F. and D.S.; methodology, K.D.-D., J.L., C.F. and D.S.; software, K.D.-D., J.L., C.F. and D.S.; validation, K.D.-D., J.L., C.F. and D.S.; formal analysis, K.D.-D., J.L., C.F. and D.S.; investigation, K.D.-D., J.L., C.F. and D.S.; resources, K.D.-D., J.L., C.F. and D.S.; data curation, K.D.-D., J.L., C.F. and D.S.; writing—original draft preparation, K.D.-D., J.L., C.F. and D.S.; writing—review and editing, K.D.-D., J.L., C.F. and D.S.; visualization, K.D.-D., J.L. and D.S.; supervision, K.D.-D., J.L., C.F. and D.S.; project administration, K.D.-D., J.L., C.F. and D.S.; funding acquisition, K.D.-D., J.L. and D.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the NIH Office of The Director T32OD010993 and the 2020 College of Veterinary Medicine Resident and Graduate Student Research Grants provided by the University of Minnesota College of Veterinary Medicine Research Office (https://vetmed.umn.edu/research/research-office), accessed on 23 December 2023. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health.
Institutional Review Board Statement
The study was conducted in accordance with and approved by University of Minnesota Institutional Animal Care and Use Committee (UMN-IACUC) (protocol code 2005-38146A and 8/2020, approval date: 20 August 2020).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are openly available in (GEO repository) at (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE235567), accessed on 21 December 2023, reference number (GSE235567).
Acknowledgments
We thank Amy Morgan and Jessica Coffey from the University of Minnesota Veterinary Medical Center’s Radiation Oncology Service for their assistance in radiation treatment of the mice. We thank the University of Minnesota Genomics Core for their assistance in processing our samples for the NanoString nCounter® glial cell profiling panel. We thank Erin Lind, the Director of University of Minnesota Mouse Behavior Core, for her expertise in rodent behavior testing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Demos-Davies, K.; Lawrence, J.; Rogich, A.; Lind, E.; Seelig, D. Cancer treatment induces neuroinflammation and behavioral deficits in mice. Front. Behav. Neurosci. 2022, 16, 1067298. [Google Scholar] [CrossRef] [PubMed]
- Feiock, C.; Yagi, M.; Maidman, A.; Rendahl, A.; Hui, S.; Seelig, D. Central Nervous System Injury—A Newly Observed Bystander Effect of Radiation. PLoS ONE 2016, 11, e0163233. [Google Scholar] [CrossRef] [PubMed]
- Kerstens, C.; Wildiers, H.P.M.W.; Schroyen, G.; Almela, M.; Mark, R.E.; Lambrecht, M.; Deprez, S.; Sleurs, C. A Systematic Review on the Potential Acceleration of Neurocognitive Aging in Older Cancer Survivors. Cancers 2023, 15, 1215. [Google Scholar] [CrossRef] [PubMed]
- Schroyen, G.; Blommaert, J.; van Weehaeghe, D.; Sleurs, C.; Vandenbulcke, M.; Dedoncker, N.; Hatse, S.; Goris, A.; Koole, M.; Smeets, A.; et al. Neuroinflammation and Its Association with Cognition, Neuronal Markers and Peripheral Inflammation after Chemotherapy for Breast Cancer. Cancers 2021, 13, 4198. [Google Scholar] [CrossRef] [PubMed]
- Janelsins, M.C.; Kohli, S.; Mohile, S.G.; Usuki, K.; Ahles, T.A.; Morrow, G.R. An update on cancer- and chemotherapy-related cognitive dysfunction: Current status. Semin. Oncol. 2011, 38, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Seigers, R.; Loos, M.; Van Tellingen, O.; Boogerd, W.; Smit, A.B.; Schagen, S.B. Cognitive impact of cytotoxic agents in mice. Psychopharmacology 2015, 232, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Oppegaard, K.R.; Armstrong, T.S.; Anguera, J.A.; Kober, K.M.; Kelly, D.L.; Laister, R.C.; Saligan, L.N.; Ayala, A.P.; Kuruvilla, J.; Alm, M.W.; et al. Blood-based biomarkers of cancer-related cognitive impairment in non-central nervous system cancer: A scoping review. Crit. Rev. Oncol. Hematol. 2022, 180, 103822. [Google Scholar] [CrossRef]
- Brown, T.; McElroy, T.; Simmons, P.; Walters, H.; Ntagwabira, F.; Wang, J.; Byrum, S.D.; Allen, A.R. Cognitive impairment resulting from treatment with docetaxel, doxorubicin, and cyclophosphamide. Brain Res. 2021, 1760, 147397. [Google Scholar] [CrossRef]
- Lange, M.; Joly, F.; Vardy, J.; Ahles, T.; Dubois, M.; Tron, L.; Winocur, G.; De Ruiter, M.; Castel, H. Cancer-related cognitive impairment: An update on state of the art, detection, and management strategies in cancer survivors. Ann. Oncol. 2019, 30, 1925–1940. [Google Scholar] [CrossRef]
- McDonald, B.C.; Conroy, S.K.; Ahles, T.A.; West, J.D.; Saykin, A.J. Gray matter reduction associated with systemic chemotherapy for breast cancer: A prospective MRI study. Breast Cancer Res. Treat. 2010, 123, 819–828. [Google Scholar] [CrossRef]
- Fleming, B.; Edison, P.; Kenny, L. Cognitive impairment after cancer treatment: Mechanisms, clinical characterization, and management. BMJ 2023, 380, e071726. [Google Scholar] [CrossRef] [PubMed]
- Matsos, A.; Johnston, I.N. Chemotherapy-induced cognitive impairments: A systematic review of the animal literature. Neurosci. Biobehav. Rev. 2019, 102, 382–399. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.C.; Pyter, L.M. Neuroimmunology of Behavioral Comorbidities Associated with Cancer and Cancer Treatments. Front. Immunol. 2018, 9, 1195. [Google Scholar] [CrossRef] [PubMed]
- Cuccurullo, V.; Di Stasio, G.D.; Cascini, G.L.; Gatta, G.; Bianco, C. The Molecular Effects of Ionizing Radiations on Brain Cells: Radiation Necrosis vs. Tumor Recurrence. Diagnostics 2019, 9, 127. [Google Scholar] [CrossRef] [PubMed]
- Lumniczky, K.; Szatmári, T.; Sáfrány, G. Ionizing Radiation-Induced Immune and Inflammatory Reactions in the Brain. Front. Immunol. 2017, 8, 517. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F.; Bsc, M.F.B.; Me, J.F.; Soerjomataram, M.I.; et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Bryant, A.K.; Banegas, M.P.; Martinez, M.E.; Mell, L.K.; Murphy, J.D. Trends in Radiation Therapy among Cancer Survivors in the United States, 2000–2030. Cancer Epidemiol. Biomark. Prev. 2017, 26, 963–970. [Google Scholar] [CrossRef]
- Wilson, B.E.; Jacob, S.; Yap, M.L.; Ferlay, J.; Bray, F.; Barton, M.B. Estimates of global chemotherapy demands and corresponding physician workforce requirements for 2018 and 2040: A population-based study. Lancet Oncol. 2019, 20, 769–780. [Google Scholar] [CrossRef]
- Wefel, J.S.; Kesler, S.R.; Noll, K.R.; Schagen, S.B. Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA Cancer J. Clin. 2015, 65, 123–138. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Johansen, P.B. Doxorubicin pharmacokinetics after intravenous and intraperitoneal administration in the nude mouse. Cancer Chemother. Pharmacol. 1981, 5, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Pisters, P.W.; Patel, S.R.; Prieto, V.G.; Thall, P.F.; Lewis, V.O.; Feig, B.W.; Hunt, K.K.; Yasko, A.W.; Lin, P.P.; Jacobson, M.G.; et al. Phase I trial of preoperative doxorubicin-based concurrent chemoradiation and surgical resection for localized extremity and body wall soft tissue sarcomas. J. Clin. Oncol. 2004, 22, 3375–3380. [Google Scholar] [CrossRef] [PubMed]
- Romesser, P.B.; Sherman, E.J.; Whiting, K.; Ho, M.L.; Shaha, A.R.; Sabra, M.M.; Riaz, N.; Waldenberg, T.E.; Sabol, C.R.; Ganly, I.; et al. Intensity-modulated radiation therapy and doxorubicin in thyroid cancer: A prospective phase 2 trial. Cancer 2021, 127, 4161–4170. [Google Scholar] [CrossRef] [PubMed]
- Baghani, H.R.; Aghamiri, S.M.R.; Mahdavi, S.R.; Robatjazi, M.; Zadeh, A.R.; Akbari, M.E.; Mirzaei, H.R.; Nafissi, N.; Samsami, M. Dosimetric evaluation of Gafchromic EBT2 film for breast intraoperative electron radiotherapy verification. Phys. Med. 2015, 31, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Aglan, O. StatPearls. Radiation Therapy for Early-Stage Breast Cancer; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef]
- Dong, H.-W. Allen Reference Atlas: A Digital Color Brain Atlas of the C57Black/6J Male Mouse; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2008. [Google Scholar]
- Vider, J.; Croaker, A.; Cox, A.J.; Raymond, E.; Rogers, R.; Adamson, S.; Doyle, M.; O’brien, B.; Cripps, A.W.; West, N.P. Comparison of skin biopsy sample processing and storage methods on high dimensional immune gene expression using the Nanostring nCounter system. Diagn. Pathol. 2020, 15, 57. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Nelson, M.; Basu, M.; Srinivasan, P.; Lazarski, C.; Zhang, P.; Zheng, P.; Sandler, A.D. MYC oncogene is associated with suppression of tumor immunity and targeting Myc induces tumor cell immunogenicity for therapeutic whole cell vaccination. J. Immunother. Cancer 2021, 9, e001388. [Google Scholar] [CrossRef]
- Ma, C.; Hunt, J.B.; Kovalenko, A.; Liang, H.; Selenica, M.-L.B.; Orr, M.B.; Zhang, B.; Gensel, J.C.; Feola, D.J.; Gordon, M.N.; et al. Myeloid Arginase 1 Insufficiency Exacerbates Amyloid-β Associated Neurodegenerative Pathways and Glial Signatures in a Mouse Model of Alzheimer’s Disease: A Targeted Transcriptome Analysis. Front. Immunol. 2021, 12, 628156. [Google Scholar] [CrossRef]
- Chen, E.Y.; Tan, C.M.; Kou, Y.; Duan, Q.; Wang, Z.; Meirelles, G.V.; Clark, N.R.; Ma’Ayan, A. Enrichr: Interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinform. 2013, 14, 128. [Google Scholar] [CrossRef]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef]
- Xie, Z.; Bailey, A.; Kuleshov, M.V.; Clarke, D.J.B.; Evangelista, J.E.; Jenkins, S.L.; Lachmann, A.; Wojciechowicz, M.L.; Kropiwnicki, E.; Jagodnik, K.M.; et al. Gene Set Knowledge Discovery with Enrichr. Curr. Protoc. 2021, 1, e90. [Google Scholar] [CrossRef] [PubMed]
- Kciuk, M.; Gielecińska, A.; Mujwar, S.; Kołat, D.; Kałuzińska-Kołat, Ż.; Celik, I.; Kontek, R. Doxorubicin-An Agent with Multiple Mechanisms of Anticancer Activity. Cells 2023, 12, 659. [Google Scholar] [CrossRef] [PubMed]
- Baskar, R.; Dai, J.; Wenlong, N.; Yeo, R.; Yeoh, K.W. Biological response of cancer cells to radiation treatment. Front. Mol. Biosci. 2014, 1, 24. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jenrow, K.A.; Brown, S.L. Mechanisms of radiation-induced normal tissue toxicity and implications for future clinical trials. Radiat. Oncol. J. 2014, 32, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; An, D.; Xu, H.; Cheng, X.; Wang, S.; Yu, W.; Yu, D.; Zhao, D.; Sun, Y.; Deng, W.; et al. Effects of social isolation and re-socialization on cognition and ADAR1 (p110) expression in mice. PeerJ 2016, 4, e2306. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Yang, J.; Yu, W. Promoter Hypomethylation of TGFBR3 as a Risk Factor of Alzheimer’s Disease: An Integrated Epigenomic-Transcriptomic Analysis. Front. Cell Dev. Biol. 2021, 9, 825729. [Google Scholar] [CrossRef]
- Benton, C.S.; Miller, B.H.; Skwerer, S.; Suzuki, O.; Schultz, L.E.; Cameron, M.D.; Marron, J.S.; Pletcher, M.T.; Wiltshire, T. Evaluating genetic markers and neurobiochemical analytes for fluoxetine response using a panel of mouse inbred strains. Psychopharmacology 2012, 221, 297–315. [Google Scholar] [CrossRef]
- Anwar, M.J.; Pillai, K.K.; Khanam, R.; Akhtar, M.; Vohora, D. Effect of alprazolam on anxiety and cardiomyopathy induced by doxorubicin in mice. Fundam. Clin. Pharmacol. 2012, 26, 356–362. [Google Scholar] [CrossRef]
- Aziriova, S.; Bednarova, K.R.; Krajcirovicova, K.; Hrenak, J.; Rajkovicova, R.; Arendasova, K.; Kamodyova, N.; Celec, P.; Zorad, S.; Adamcova, M.; et al. Doxorubicin-induced behavioral disturbances in rats: Protective effect of melatonin and captopril. Pharmacol. Biochem. Behav. 2014, 124, 284–289. [Google Scholar] [CrossRef]
- Cavalier, A.N.; Clayton, Z.S.; Hutton, D.A.; Wahl, D.; Lark, D.S.; Reisz, J.A.; Melov, S.; Campisi, J.; Seals, D.R.; LaRocca, T.J. Accelerated aging of the brain transcriptome by the common chemotherapeutic doxorubicin. Exp. Gerontol. 2021, 152, 111451. [Google Scholar] [CrossRef]
- Liao, D.; Xiang, D.; Dang, R.; Xu, P.; Wang, J.; Han, W.; Fu, Y.; Yao, D.; Cao, L.; Jiang, P. Neuroprotective Effects of dl-3-n-Butylphthalide against Doxorubicin-Induced Neuroinflammation, Oxidative Stress, Endoplasmic Reticulum Stress, and Behavioral Changes. Oxid. Med. Cell Longev. 2018, 2018, 9125601. [Google Scholar] [CrossRef] [PubMed]
- Hol, E.M.; Pekny, M. Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr. Opin. Cell Biol. 2015, 32, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Castro, C.; Noori, A.; Magdamo, C.G.; Li, Z.; Marks, J.D.; Frosch, M.P.; Das, S.; Hyman, B.T.; Serrano-Pozo, A. Cyclic multiplex fluorescent immunohistochemistry and machine learning reveal distinct states of astrocytes and microglia in normal aging and Alzheimer’s disease. J. Neuroinflamm. 2022, 19, 30. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.A.; Goluszko, E.; Chen, H.-Z.; Leone, G.; Post, S.; Lozano, G.; Chen, Z.; Chauchereau, A. E2F3 is a mediator of DNA damage-induced apoptosis. Mol. Cell Biol. 2010, 30, 524–536. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McClellan, K.A.; Ruzhynsky, V.A.; Douda, D.N.; Vanderluit, J.L.; Ferguson, K.L.; Chen, D.; Bremner, R.; Park, D.S.; Leone, G.; Slack, R.S. Unique requirement for Rb/E2F3 in neuronal migration: Evidence for cell cycle-independent functions. Mol. Cell Biol. 2007, 27, 4825–4843. [Google Scholar] [CrossRef] [PubMed]
- Jamialahmadi, K.; Zahedipour, F.; Karimi, G. The role of microRNAs on doxorubicin drug resistance in breast cancer. J. Pharm. Pharmacol. 2021, 73, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Ji, H.; Lu, M.; Li, Z.; Qiao, X.; Sun, B.; Zhang, W.; Xue, D. Proteomic analysis of apoptotic and oncotic pancreatic acinar AR42J cells treated with caerulein. Mol. Cell Biochem. 2013, 382, 1–17. [Google Scholar] [CrossRef]
- Stankiewicz, E.; Mao, X.; Mangham, D.C.; Xu, L.; Yeste-Velasco, M.; Fisher, G.; North, B.; Chaplin, T.; Young, B.; Wang, Y.; et al. Identification of FBXL4 as a Metastasis Associated Gene in Prostate Cancer. Sci. Rep. 2017, 7, 5124. [Google Scholar] [CrossRef]
- Yanagisawa, K.; Konishi, H.; Arima, C.; Tomida, S.; Takeuchi, T.; Shimada, Y.; Yatabe, Y.; Mitsudomi, T.; Osada, H.; Takahashi, T. Novel metastasis-related gene CIM functions in the regulation of multiple cellular stress-response pathways. Cancer Res. 2010, 70, 9949–9958. [Google Scholar] [CrossRef]
- Ryu, E.J.; Angelastro, J.M.; Greene, L.A. Analysis of gene expression changes in a cellular model of Parkinson disease. Neurobiol. Dis. 2005, 18, 54–74. [Google Scholar] [CrossRef]
- Sil, S.; Periyasamy, P.; Thangaraj, A.; Chivero, E.T.; Buch, S. PDGF/PDGFR axis in the neural systems. Mol. Aspects Med. 2018, 62, 63–74. [Google Scholar] [CrossRef]
- Järvelä, I. Genomics studies on musical aptitude, music perception, and practice. Ann. N. Y. Acad. Sci. 2018, 1423, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Tabaczar, S.; Czepas, J.; Koceva-Chyla, A.; Kilanczyk, E.; Piasecka-Zelga, J.; Gwozdzinski, K. The effect of the nitroxide pirolin on oxidative stress induced by doxorubicin and taxanes in the rat brain. J. Physiol. Pharmacol. 2017, 68, 295–308. [Google Scholar] [PubMed]
- Yuan, L.; Liu, C.; Wan, Y.; Yan, H.; Li, T. Effect of HDAC2/Inpp5f on neuropathic pain and cognitive function through regulating PI3K/Akt/GSK-3β signal pathway in rats with neuropathic pain. Exp. Ther. Med. 2019, 18, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.J.; Herskowitz, J.H. Perspectives on ROCK2 as a Therapeutic Target for Alzheimer’s Disease. Front. Cell Neurosci. 2021, 15, 636017. [Google Scholar] [CrossRef] [PubMed]
- Pranatharthi, A.; Thomas, P.; Udayashankar, A.H.; Bhavani, C.; Suresh, S.B.; Krishna, S.; Thatte, J.; Srikantia, N.; Ross, C.R.; Srivastava, S. RhoC regulates radioresistance via crosstalk of ROCK2 with the DNA repair machinery in cervical cancer. J. Exp. Clin. Cancer Res. 2019, 38, 392. [Google Scholar] [CrossRef] [PubMed]
- Siva, S.; Lobachevsky, P.N.; MacManus, M.P.; Kron, T.; Möller, A.; Lobb, R.J.; Ventura, J.; Best, N.; Smith, J.; Ball, D.; et al. Radiotherapy for Non-Small Cell Lung Cancer Induces DNA Damage Response in Both Irradiated and Out-of-field Normal Tissues. Clin. Cancer Res. 2016, 22, 4817–4826. [Google Scholar] [CrossRef]
- Markarian, M.; Krattli, R.P.; Baddour, J.D.; Alikhani, L.; Giedzinski, E.; Usmani, M.T.; Agrawal, A.; Baulch, J.E.; Tenner, A.J.; Acharya, M.M. Glia-Selective Deletion of Complement. Cancer Res. 2021, 81, 1732–1744. [Google Scholar] [CrossRef]
- Son, M. Understanding the contextual functions of C1q and LAIR-1 and their applications. Exp. Mol. Med. 2022, 54, 567–572. [Google Scholar] [CrossRef]
- Katzeff, J.S.; Kim, W.S. ATP-binding cassette transporters and neurodegenerative diseases. Essays Biochem. 2021, 65, 1013–1024. [Google Scholar]
- Zeng, Y.; Callaghan, D.; Xiong, H.; Yang, Z.; Huang, P.; Zhang, W. Abcg2 deficiency augments oxidative stress and cognitive deficits in Tg-SwDI transgenic mice. J. Neurochem. 2012, 122, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Callaghan, D.; Jones, A.; Bai, J.; Rasquinha, I.; Smith, C.; Pei, K.; Walker, D.; Lue, L.F.; Stanimirovic, D.; et al. ABCG2 is upregulated in Alzheimer’s brain with cerebral amyloid angiopathy and may act as a gatekeeper at the blood-brain barrier for Abeta(1-40) peptides. J. Neurosci. 2009, 29, 5463–5475. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Chung, Y.G.; Kim, C.Y.; Kim, H.K.; Lee, H.K. Upregulation of VEGF and FGF2 in normal rat brain after experimental intraoperative radiation therapy. J. Korean Med. Sci. 2004, 19, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Houchen, C.W.; George, R.J.; Sturmoski, M.A.; Cohn, S.M. FGF-2 enhances intestinal stem cell survival and its expression is induced after radiation injury. Am. J. Physiol. 1999, 276, G249–G258. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.; Ronai, Z.A. ATF2—At the crossroad of nuclear and cytosolic functions. J. Cell Sci. 2012, 125 Pt 12, 2815–2824. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Miriyala, S.; Miao, L.; Mitov, M.; Schnell, D.; Dhar, S.; Cai, J.; Klein, J.; Sultana, R.; Butterfield, D.; et al. Redox proteomic identification of HNE-bound mitochondrial proteins in cardiac tissues reveals a systemic effect on energy metabolism after doxorubicin treatment. Free Radic. Biol. Med. 2014, 72, 55–65. [Google Scholar] [CrossRef]
- Vijay, V.; Moland, C.L.; Han, T.; Fuscoe, J.C.; Lee, T.; Herman, E.H.; Jenkins, G.R.; Lewis, S.M.; Cummings, C.A.; Gao, Y.; et al. Early transcriptional changes in cardiac mitochondria during chronic doxorubicin exposure and mitigation by dexrazoxane in mice. Toxicol. Appl. Pharmacol. 2016, 295, 68–84. [Google Scholar] [CrossRef]
- You, X.; Guo, W.; Wang, L.; Hou, Y.; Zhang, H.; Pan, Y.; Han, R.; Huang, M.; Liao, L.; Chen, Y. Subcellular distribution of RAD23B controls XPC degradation and DNA damage repair in response to chemotherapy drugs. Cell Signal 2017, 36, 108–116. [Google Scholar] [CrossRef]
- Ma, L.Y.; Liu, J.M.; Du, G.L.; Dang, X.B. Irisin attenuates lipopolysaccharide-induced acute lung injury by downregulating inflammatory cytokine expression through miR-199a-mediated Rad23b overexpression. Exp. Cell Res. 2021, 404, 112593. [Google Scholar] [CrossRef]
- Du, J.; Zhang, A.; Li, J.; Liu, X.; Wu, S.; Wang, B.; Wang, Y.; Jia, H. Doxorubicin-Induced Cognitive Impairment: The Mechanistic Insights. Front. Oncol. 2021, 11, 673340. [Google Scholar] [CrossRef]
- He, L.; Liu, F.; Li, J. Mitochondrial Sirtuins and Doxorubicin-induced Cardiotoxicity. Cardiovasc. Toxicol. 2021, 21, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.; Ma, L.; Li, Y.; Yang, J.; Yang, Q.; Yao, W.; Li, S. CoQ10 Improves Myocardial Damage in Doxorubicin-Induced Heart Failure in C57BL/6 Mice. Front. Biosci. (Landmark Ed.) 2022, 27, 244. [Google Scholar] [CrossRef]
- Eide, S.; Feng, Z.P. Doxorubicin chemotherapy-induced “chemo-brain”: Meta-analysis. Eur. J. Pharmacol. 2020, 881, 173078. [Google Scholar] [CrossRef] [PubMed]
- Bergbower, E.A.S.; Pierson, R.N.; Azimzadeh, A.M. Multi-gene technical assessment of qPCR and NanoString n-Counter analysis platforms in cynomolgus monkey cardiac allograft recipients. Cell Immunol. 2020, 347, 104019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Good, D.J. Comparison of hypothalamic mRNA levels in mice euthanized by CO₂ inhalation and focused-beam microwave irradiation. Lab Anim. 2011, 40, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Staib-Lasarzik, I.; Kriege, O.; Timaru-Kast, R.; Pieter, D.; Werner, C.; Engelhard, K.; Thal, S.C. Anesthesia for euthanasia influences mRNA expression in healthy mice and after traumatic brain injury. J. Neurotrauma 2014, 31, 1664–1671. [Google Scholar] [CrossRef]
- Scott, H.; Rogers, M.F.; Scott, H.L.; Campbell, C.; Warburton, E.C.; Uney, J.B. Recognition memory-induced gene expression in the perirhinal cortex: A transcriptomic analysis. Behav. Brain Res. 2017, 328, 1–12. [Google Scholar] [CrossRef]
- Mendez, M.; Arias, N.; Uceda, S.; Arias, J.L. c-Fos expression correlates with performance on novel object and novel place recognition tests. Brain Res. Bull. 2015, 117, 16–23. [Google Scholar] [CrossRef]
- Tangpong, J.; Cole, M.P.; Sultana, R.; Estus, S.; Vore, M.; Clair, W.S.; Ratanachaiyavong, S.; Clair, D.K.S.; Butterfield, D.A. Adriamycin-mediated nitration of manganese superoxide dismutase in the central nervous system: Insight into the mechanism of chemobrain. J. Neurochem. 2007, 100, 191–201. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).