Data Mining of Microarray Datasets in Translational Neuroscience
Abstract
1. Introduction
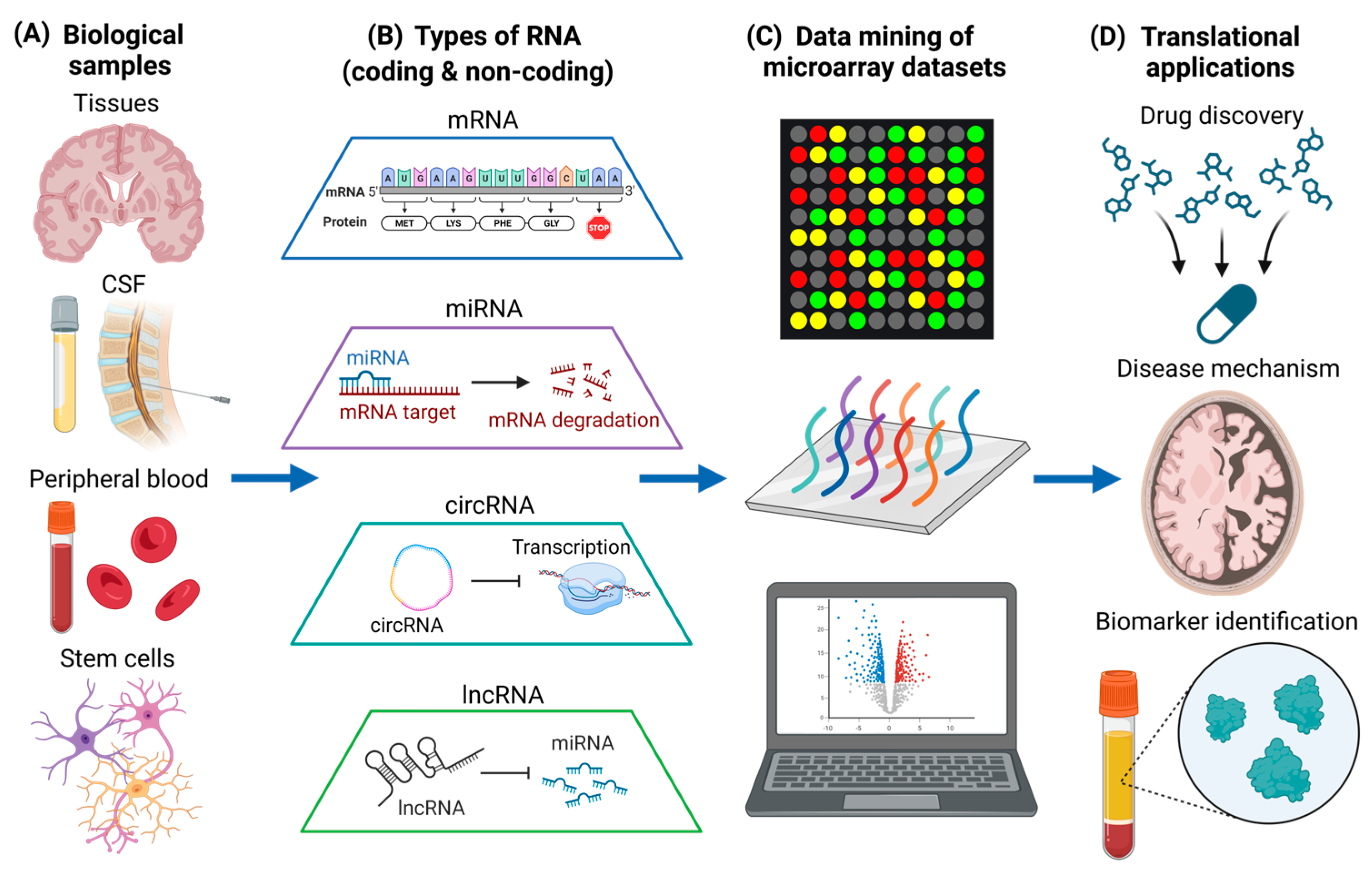
2. Biological Samples for Microarray Analysis
2.1. Brain Tissues
2.2. CSF and Peripheral Blood
2.3. Human Stem Cells
3. RNA Based Microarray Gene Expression Analysis
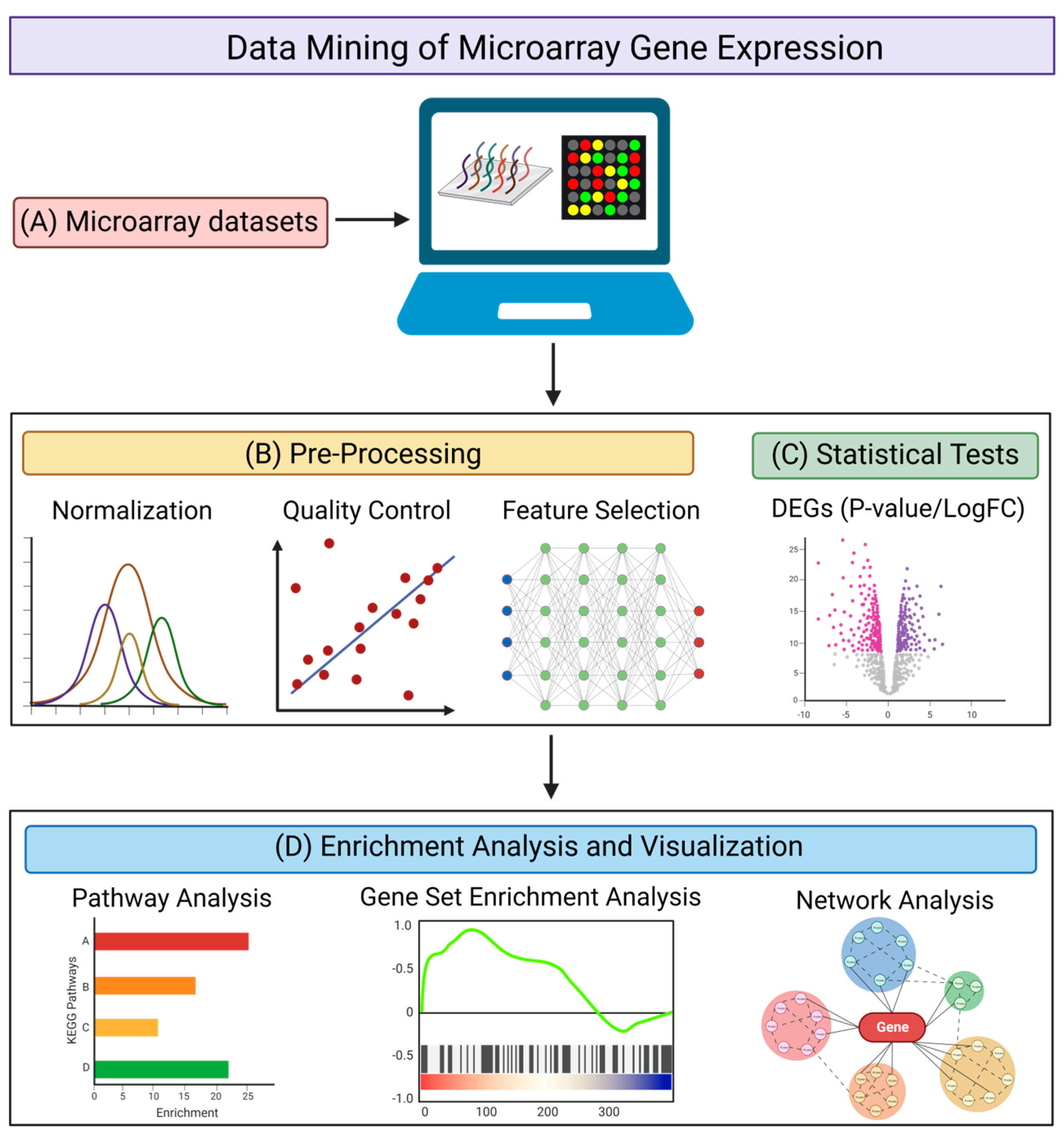
3.1. Pipeline for the Data Mining of Microarray Datasets
3.2. Microarray Analysis of Coding RNA (mRNA)
3.3. Microarray Analysis of Non-Coding RNA (miRNA, circRNA, and lncRNA)
3.4. Bridging Gaps between Microarray and RNA-Seq Analysis
3.5. Experimental Validation to Advance Therapeutic Development and Biomarker Identification
4. Summary and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Negi, A.; Shukla, A.; Jaiswar, A.; Shrinet, J.; Jasrotia, R.S. Chapter 6-Applications and Challenges of Microarray and RNA-Sequencing; Singh, D.B., Pathak, R.K.B.T.-B., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 91–103. ISBN 978-0-323-89775-4. [Google Scholar]
- Costa, C.; Giménez-Capitán, A.; Karachaliou, N.; Rosell, R. Comprehensive Molecular Screening: From the RT-PCR to the RNA-Seq. Transl. lung cancer Res. 2013, 2, 87–91. [Google Scholar] [PubMed]
- Kukurba, K.R.; Montgomery, S.B. RNA Sequencing and Analysis. Cold Spring Harb. Protoc. 2015, 2015, 951–969. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.R.; Neff, N.F.; Kalisky, T.; Dalerba, P.; Treutlein, B.; Rothenberg, M.E.; Mburu, F.M.; Mantalas, G.L.; Sim, S.; Clarke, M.F.; et al. Quantitative Assessment of Single-Cell RNA-Sequencing Methods. Nat. Methods 2014, 11, 41–46. [Google Scholar] [CrossRef]
- Mantione, K.J.; Kream, R.M.; Kuzelova, H.; Ptacek, R.; Raboch, J.; Samuel, J.M.; Stefano, G.B. Comparing Bioinformatic Gene Expression Profiling Methods: Microarray and RNA-Seq. Med. Sci. Monit. Basic Res. 2014, 20, 138–142. [Google Scholar]
- Cui, X.; Churchill, G.A. Statistical Tests for Differential Expression in CDNA Microarray Experiments. Genome Biol. 2003, 4, 210. [Google Scholar] [CrossRef] [PubMed]
- Kogenaru, S.; Yan, Q.; Guo, Y.; Wang, N. RNA-Seq and Microarray Complement Each Other in Transcriptome Profiling. BMC Genomics 2012, 13, 629. [Google Scholar] [CrossRef] [PubMed]
- Oshlack, A.; Wakefield, M.J. Transcript Length Bias in RNA-Seq Data Confounds Systems Biology. Biol. Direct 2009, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Tarca, A.L.; Romero, R.; Draghici, S. Analysis of Microarray Experiments of Gene Expression Profiling. Am. J. Obstet. Gynecol. 2006, 195, 373–388. [Google Scholar] [CrossRef] [PubMed]
- Harbola, A.; Negi, D.; Manchanda, M.; Kesharwani, R.K. Chapter 27-Bioinformatics and Biological Data Mining; Singh, D.B., Pathak, R.K.B.T.-B., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 457–471. ISBN 978-0-323-89775-4. [Google Scholar]
- Wu, W.-T.; Li, Y.-J.; Feng, A.-Z.; Li, L.; Huang, T.; Xu, A.-D.; Lyu, J. Data Mining in Clinical Big Data: The Frequently Used Databases, Steps, and Methodological Models. Mil. Med. Res. 2021, 8, 44. [Google Scholar] [CrossRef]
- Hadar, A.; Gurwitz, D. Peripheral Transcriptomic Biomarkers for Early Detection of Sporadic Alzheimer Disease? Dialogues Clin. Neurosci. 2018, 20, 293–300. [Google Scholar] [CrossRef]
- Lake, J.; Storm, C.S.; Makarious, M.B.; Bandres-Ciga, S. Genetic and Transcriptomic Biomarkers in Neurodegenerative Diseases: Current Situation and the Road Ahead. Cells 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Dou, L.; Li, X.; Zhang, Y. Review of Bioinformatics in Azheimer’s Disease Research. Comput. Biol. Med. 2022, 143, 105269. [Google Scholar] [CrossRef] [PubMed]
- Paananen, J. Bioinformatics in the Identification of Novel Targets and Pathways in Neurodegenerative Diseases. Curr. Genet. Med. Rep. 2017, 5, 15–21. [Google Scholar] [CrossRef]
- Koh, E.J.; Kim, S.H.; Hwang, S.Y. Sample Management: A Primary Critical Starting Point for Successful Omics Studies. Mol. Cell. Toxicol. 2022, 18, 141–148. [Google Scholar] [CrossRef]
- Clement, C.; Hill, J.M.; Dua, P.; Culicchia, F.; Lukiw, W.J. Analysis of RNA from Alzheimer’s Disease Post-Mortem Brain Tissues. Mol. Neurobiol. 2016, 53, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Stan, A.D.; Ghose, S.; Gao, X.M.; Roberts, R.C.; Lewis-Amezcua, K.; Hatanpaa, K.J.; Tamminga, C.A. Human Postmortem Tissue: What Quality Markers Matter? Brain Res. 2006, 1123, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sturm, G.; List, M.; Zhang, J.D. Tissue Heterogeneity Is Prevalent in Gene Expression Studies. NAR Genomics Bioinforma. 2021, 3, lqab077. [Google Scholar] [CrossRef]
- Wu, M.; Fang, K.; Wang, W.; Lin, W.; Guo, L.; Wang, J. Identification of Key Genes and Pathways for Alzheimer’s Disease via Combined Analysis of Genome-Wide Expression Profiling in the Hippocampus. Biophys. Reports 2019, 5, 98–109. [Google Scholar] [CrossRef]
- Young, A.L.; Marinescu, R.V.; Oxtoby, N.P.; Bocchetta, M.; Yong, K.; Firth, N.C.; Cash, D.M.; Thomas, D.L.; Dick, K.M.; Cardoso, J.; et al. Uncovering the Heterogeneity and Temporal Complexity of Neurodegenerative Diseases with Subtype and Stage Inference. Nat. Commun. 2018, 9, 4273. [Google Scholar] [CrossRef]
- Yin, X.; Wu, Q.; Hao, Z.; Chen, L. Identification of Novel Prognostic Targets in Glioblastoma Using Bioinformatics Analysis. Biomed. Eng. Online 2022, 21, 26. [Google Scholar] [CrossRef]
- Yang, L.; Zeng, W.; Sun, H.; Huang, F.; Yang, C.; Cai, X.; Lu, Y.; Zeng, J.; Yang, K. Bioinformatical Analysis of Gene Expression Omnibus Database Associates TAF7/CCNB1, TAF7/CCNA2, and GTF2E2/CDC20 Pathways with Glioblastoma Development and Prognosis. World Neurosurg. 2020, 138, e492–e514. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Langfelder, P.; Fuller, T.F.; Oldham, M.C.; Luo, R.; van den Berg, L.H.; Ophoff, R.A.; Horvath, S. Is Human Blood a Good Surrogate for Brain Tissue in Transcriptional Studies? BMC Genomics 2010, 11, 589. [Google Scholar] [CrossRef] [PubMed]
- McEwen, A.E.; Leary, S.E.S.; Lockwood, C.M. Beyond the Blood: CSF-Derived CfDNA for Diagnosis and Characterization of CNS Tumors. Front. Cell Dev. Biol. 2020, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Robey, T.T.; Panegyres, P.K. Cerebrospinal Fluid Biomarkers in Neurodegenerative Disorders. Future Neurol. 2019, 14, FNL6. [Google Scholar] [CrossRef]
- Niemantsverdriet, E.; Valckx, S.; Bjerke, M.; Engelborghs, S. Alzheimer’s Disease CSF Biomarkers: Clinical Indications and Rational Use. Acta Neurol. Belg. 2017, 117, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Katayama, T.; Sawada, J.; Takahashi, K.; Yahara, O. Cerebrospinal Fluid Biomarkers in Parkinson’s Disease: A Critical Overview of the Literature and Meta-Analyses. Brain Sci. 2020, 10, 466. [Google Scholar] [CrossRef] [PubMed]
- Parnetti, L.; Gaetani, L.; Eusebi, P.; Paciotti, S.; Hansson, O.; El-Agnaf, O.; Mollenhauer, B.; Blennow, K.; Calabresi, P. CSF and Blood Biomarkers for Parkinson’s Disease. Lancet Neurol. 2019, 18, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hamade, M.; Wu, Q.; Wang, Q.; Axtell, R.; Giri, S.; Mao-Draayer, Y. Current and Future Biomarkers in Multiple Sclerosis. Int. J. Mol. Sci. 2022, 23, 5877. [Google Scholar] [CrossRef]
- Deisenhammer, F.; Zetterberg, H.; Fitzner, B.; Zettl, U.K. The Cerebrospinal Fluid in Multiple Sclerosis. Front. Immunol. 2019, 10, 726. [Google Scholar] [CrossRef]
- Mankhong, S.; Kim, S.; Lee, S.; Kwak, H.B.; Park, D.H.; Joa, K.L.; Kang, J.H. Development of Alzheimer’s Disease Biomarkers: From CSF-to Blood-Based Biomarkers. Biomedicines 2022, 10, 850. [Google Scholar] [CrossRef]
- D’Ambrosio, A.; Pontecorvo, S.; Colasanti, T.; Zamboni, S.; Francia, A.; Margutti, P. Peripheral Blood Biomarkers in Multiple Sclerosis. Autoimmun. Rev. 2015, 14, 1097–1110. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Li, J.; Zhang, R. Current Research Status of Blood Biomarkers in Alzheimer’s Disease: Diagnosis and Prognosis. Ageing Res. Rev. 2021, 72, 101492. [Google Scholar] [CrossRef]
- Park, S.A.; Jang, Y.J.; Kim, M.K.; Lee, S.M.; Moon, S.Y. Promising Blood Biomarkers for Clinical Use in Alzheimer’s Disease: A Focused Update. J. Clin. Neurol. 2022, 18, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Obrocki, P.; Khatun, A.; Ness, D.; Senkevich, K.; Hanrieder, J.; Capraro, F.; Mattsson, N.; Andreasson, U.; Portelius, E.; Ashton, N.J.; et al. Perspectives in Fluid Biomarkers in Neurodegeneration from the 2019 Biomarkers in Neurodegenerative Diseases Course—A Joint PhD Student Course at University College London and University of Gothenburg. Alzheimers. Res. Ther. 2020, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Roser, A.E.; Gomes, L.C.; Schünemann, J.; Maass, F.; Lingor, P. Circulating MiRNAs as Diagnostic Biomarkers for Parkinson’s Disease. Front. Neurosci. 2018, 12, 625. [Google Scholar] [CrossRef] [PubMed]
- Swarbrick, S.; Wragg, N.; Ghosh, S.; Stolzing, A. Systematic Review of MiRNA as Biomarkers in Alzheimer’s Disease. Mol. Neurobiol. 2019, 56, 6156–6167. [Google Scholar] [CrossRef] [PubMed]
- Søndergaard, H.B.; Hesse, D.; Krakauer, M.; Sørensen, P.S.; Sellebjerg, F. Differential MicroRNA Expression in Blood in Multiple Sclerosis. Mult. Scler. J. 2013, 19, 1849–1857. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, Y.; Han, J. Wang Peripheral Blood MicroRNAs: A Novel Tool for Diagnosing Disease? Intractable Rare Dis. Res. 2012, 1, 98–102. [Google Scholar]
- Zou, K.; Abdullah, M.; Michikawa, M. Current Biomarkers for Alzheimer’s Disease: From CSF to Blood. J. Pers. Med. 2020, 10, 85. [Google Scholar] [CrossRef]
- Janeiro, M.H.; Ardanaz, C.G.; Sola-Sevilla, N.; Dong, J.; Cortés-Erice, M.; Solas, M.; Puerta, E.; Ramírez, M.J. Biomarkers in Alzheimer’s Disease. Adv. Lab. Med. / Av. en Med. Lab. 2021, 2, 27–37. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem Cells: Past, Present, and Future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.W.; Liou, Y.J.; Lu, S.W.; Tseng, L.M.; Kao, C.L.; Chen, S.J.; Chiou, S.H.; Chang, C.J. Stem Cell-Based Neuroprotective and Neurorestorative Strategies. Int. J. Mol. Sci. 2010, 11, 2039–2055. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.M.; Pham, P.T.; Bach, T.Q.; Ngo, A.T.L.; Nguyen, Q.T.; Phan, T.T.K.; Nguyen, G.H.; Le, P.T.T.; Hoang, V.T.; Forsyth, N.R.; et al. Stem Cell-Based Therapy for Human Diseases. Signal Transduct. Target. Ther. 2022, 7, 272. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Koo, B.-K.; Knoblich, J.A. Human Organoids: Model Systems for Human Biology and Medicine. Nat. Rev. Mol. Cell Biol. 2020, 21, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.H.; O’Connor, M.D. Stems Cells, Big Data and Compendium-Based Analyses for Identifying Cell Types, Signalling Pathways and Gene Regulatory Networks. Biophys. Rev. 2019, 11, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Müller, G.A.; Tarasov, K.V.; Gundry, R.L.; Boheler, K.R. Human ESC/IPSC-Based “omics” and Bioinformatics for Translational Research. Drug Discov. Today Dis. Model. 2012, 9, e161–e170. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Novak, G.; Kyriakis, D.; Grzyb, K.; Bernini, M.; Rodius, S.; Dittmar, G.; Finkbeiner, S.; Skupin, A. Single-Cell Transcriptomics of Human IPSC Differentiation Dynamics Reveal a Core Molecular Network of Parkinson’s Disease. Commun. Biol. 2022, 5, 49. [Google Scholar] [CrossRef]
- Billing, A.M.; Dib, S.S.; Bhagwat, A.M.; da Silva, I.T.; Drummond, R.D.; Hayat, S.; Al-Mismar, R.; Ben-Hamidane, H.; Goswami, N.; Engholm-Keller, K.; et al. A Systems-Level Characterization of the Differentiation of Human Embryonic Stem Cells into Mesenchymal Stem Cells. Mol. Cell. Proteomics 2019, 18, 1950–1966. [Google Scholar] [CrossRef]
- Yagi, T.; Ito, D.; Okada, Y.; Akamatsu, W.; Nihei, Y.; Yoshizaki, T.; Yamanaka, S.; Okano, H.; Suzuki, N. Modeling Familial Alzheimer’s Disease with Induced Pluripotent Stem Cells. Hum. Mol. Genet. 2011, 20, 4530–4539. [Google Scholar] [CrossRef]
- Pandey, S.; Jirásko, M.; Lochman, J.; Chvátal, A.; Chottova Dvorakova, M.; Kučera, R. IPSCs in Neurodegenerative Disorders: A Unique Platform for Clinical Research and Personalized Medicine. J. Pers. Med. 2022, 12, 1485. [Google Scholar] [CrossRef]
- Kang, W.; Jin, T.; Zhang, T.; Ma, S.; Yan, H.; Liu, Z.; Ji, Z.; Cai, Y.; Wang, S.; Song, M.; et al. Regeneration Roadmap: Database Resources for Regenerative Biology. Nucleic Acids Res. 2022, 50, D1085–D1090. [Google Scholar] [PubMed]
- Hyvärinen, E.; Savolainen, M.; Mikkonen, J.J.W.; Kullaa, A.M. Salivary Metabolomics for Diagnosis and Monitoring Diseases: Challenges and Possibilities. Metabolites 2021, 11, 587. [Google Scholar] [CrossRef] [PubMed]
- Minale, G.; Saesong, T.; Temkitthawon, P.; Waranuch, N.; Nuengchamnong, N.; Chootip, K.; Kamkaew, N.; Kongbangkerd, T.; Engsuwan, J.; Ingkaninan, K. Characterization of Metabolites in Plasma, Urine and Feces of Healthy Participants after Taking Brahmi Essence for Twelve Weeks Using Lc-Esi-Qtof-Ms Metabolomic Approach. Molecules 2021, 26, 2944. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Koh, I.S.; Rho, M. Deciphering the Human Microbiome Using Next-Generation Sequencing Data and Bioinformatics Approaches. Methods 2015, 79, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Hong, X.; Li, S.; Zhang, Y.; Zhao, Q.; Du, W.; Wang, Y.; Ni, J. Urine-Based Biomarkers for Alzheimer’s Disease Identified Through Coupling Computational and Experimental Methods. J. Alzheimers. Dis. 2018, 65, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Gomez, J.L.; Chen, A.; Diaz, M.P.; Zirn, N.; Gupta, A.; Britto, C.; Sauler, M.; Yan, X.; Stewart, E.; Santerian, K.; et al. A Network of Sputum Micrornas Is Associated with Neutrophilic Airway Inflammation in Asthma. Am. J. Respir. Crit. Care Med. 2020, 202, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Liao, J.; Qian, J.; Chen, W.; Fan, X. MetaGeneBank: A Standardized Database to Study Deep Sequenced Metagenomic Data from Human Fecal Specimen. BMC Microbiol. 2021, 21, 263. [Google Scholar] [CrossRef]
- Jangi, S.; Gandhi, R.; Cox, L.M.; Li, N.; Von Glehn, F.; Yan, R.; Patel, B.; Mazzola, M.A.; Liu, S.; Glanz, B.L.; et al. Alterations of the Human Gut Microbiome in Multiple Sclerosis. Nat. Commun. 2016, 7, 12015. [Google Scholar] [CrossRef]
- Wang, Y.; Kasper, L.H. The Role of Microbiome in Central Nervous System Disorders. Brain. Behav. Immun. 2014, 38, 1–12. [Google Scholar] [CrossRef]
- Lipponen, A.; Natunen, T.; Hujo, M.; Ciszek, R.; Hämäläinen, E.; Tohka, J.; Hiltunen, M.; Paananen, J.; Poulsen, D.; Kansanen, E.; et al. In Vitro and In Vivo Pipeline for Validation of Disease-Modifying Effects of Systems Biology-Derived Network Treatments for Traumatic Brain Injury—Lessons Learned. Int. J. Mol. Sci. 2019, 20, 5395. [Google Scholar] [CrossRef]
- Collaborators, G.B.D. 2017 U.S.N.D. Burden of Neurological Disorders Across the US From 1990-2017: A Global Burden of Disease Study. JAMA Neurol. 2021, 78, 165–176. [Google Scholar]
- Gan, L.; Cookson, M.R.; Petrucelli, L.; La Spada, A.R. Converging Pathways in Neurodegeneration, from Genetics to Mechanisms. Nat. Neurosci. 2018, 21, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Cree, B.A.C.; Arnold, D.L.; Chataway, J.; Chitnis, T.; Fox, R.J.; Ramajo, A.P.; Murphy, N.; Lassmann, H. Secondary Progressive Multiple Sclerosis. Neurology 2021, 97, 378 LP–388. [Google Scholar] [CrossRef]
- Thakur, S.; Dhapola, R.; Sarma, P.; Medhi, B.; Reddy, D.H. Neuroinflammation in Alzheimer’s Disease: Current Progress in Molecular Signaling and Therapeutics. Inflammation 2023, 46, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Dong-Chen, X.; Yong, C.; Yang, X.; Chen-Yu, S.; Li-Hua, P. Signaling Pathways in Parkinson’s Disease: Molecular Mechanisms and Therapeutic Interventions. Signal Transduct. Target. Ther. 2023, 8, 73. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.M., 3rd; Cookson, M.R.; Van Den Bosch, L.; Zetterberg, H.; Holtzman, D.M.; Dewachter, I. Hallmarks of Neurodegenerative Diseases. Cell 2023, 186, 693–714. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.H.; Sachs, J.N. The Role of Wild-Type Tau in Alzheimer’s Disease and Related Tauopathies. J. life Sci. 2020, 2, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.H. Heterogeneous Tau Oligomers as Molecular Targets for for Alzheimer’s Disease and Related Tauopathies. Biophysica 2022, 2, 440–451. [Google Scholar] [CrossRef]
- Lo, C.H. Recent Advances in Cellular Biosensor Technology to Investigate Tau Oligomerization. Bioeng. Transl. Med. 2021, 6, e10231. [Google Scholar] [CrossRef]
- Lo, C.H.; Lim, C.K.W.; Ding, Z.; Wickramasinghe, S.P.; Braun, A.R.; Ashe, K.H.; Rhoades, E.; Thomas, D.D.; Sachs, J.N. Targeting the Ensemble of Heterogeneous Tau Oligomers in Cells: A Novel Small Molecule Screening Platform for Tauopathies. Alzheimer’s Dement. 2019, 15, 1489–1502. [Google Scholar] [CrossRef]
- McAlary, L.; Plotkin, S.S.; Cashman, N.R. Emerging Developments in Targeting Proteotoxicity in Neurodegenerative Diseases. CNS Drugs 2019, 33, 883–904. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Dekens, D.W.; De Deyn, P.P.; Naudé, P.J.W.; Eisel, U.L.M. Targeting of Tumor Necrosis Factor Alpha Receptors as a Therapeutic Strategy for Neurodegenerative Disorders. Antibodies 2015, 4, 369–408. [Google Scholar] [CrossRef]
- Ghosh, R.; Tabrizi, S.J. Gene Suppression Approaches to Neurodegeneration. Alzheimer’s Res. Ther. 2017, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- Sealfon, S.C.; Chu, T.T. RNA and DNA Microarrays. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; Volume 671, pp. 3–34. [Google Scholar]
- Kodama, Y.; Mashima, J.; Kosuge, T.; Ogasawara, O. DDBJ Update: The Genomic Expression Archive (GEA) for Functional Genomics Data. Nucleic Acids Res. 2019, 47, D69–D73. [Google Scholar] [CrossRef] [PubMed]
- Bono, H. All of Gene Expression (AOE): An Integrated Index for Public Gene Expression Databases. PLoS ONE 2020, 15, e0227076. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for Functional Genomics Data Sets-Update. Nucleic Acids Res. 2013, 41, D991–D995. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Betanzos, A.; Bolón-Canedo, V.; Morán-Fernández, L.; Sánchez-Maroño, N. A Review of Microarray Datasets: Where to Find Them and Specific Characteristics. Methods Mol. Biol. 2019, 1986, 65–85. [Google Scholar]
- Park, T.; Yi, S.-G.; Kang, S.-H.; Lee, S.; Lee, Y.-S.; Simon, R. Evaluation of Normalization Methods for Microarray Data. BMC Bioinformatics 2003, 4, 33. [Google Scholar] [CrossRef]
- Zhou, Q.; Su, X.; Jing, G.; Chen, S.; Ning, K. RNA-QC-Chain: Comprehensive and Fast Quality Control for RNA-Seq Data. BMC Genomics 2018, 19, 144. [Google Scholar] [CrossRef]
- Li, Z.; Xie, W.; Liu, T. Efficient Feature Selection and Classification for Microarray Data. PLoS ONE 2018, 13, e0202167. [Google Scholar] [CrossRef]
- Townes, F.W.; Hicks, S.C.; Aryee, M.J.; Irizarry, R.A. Feature Selection and Dimension Reduction for Single-Cell RNA-Seq Based on a Multinomial Model. Genome Biol. 2019, 20, 295. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma Powers Differential Expression Analyses for RNA-Sequencing and Microarray Studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Dennis, G.; Sherman, B.T.; Hosack, D.A.; Yang, J.; Gao, W.; Lane, H.C.; Lempicki, R.A. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003, 4, R60. [Google Scholar] [CrossRef]
- Krämer, A.; Green, J.; Pollard, J.J.; Tugendreich, S. Causal Analysis Approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene Set Enrichment Analysis: A Knowledge-Based Approach for Interpreting Genome-Wide Expression Profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Liu, J.; Cao, K.; Zhang, J.; Wang, J. Centrality-Based Pathway Enrichment: A Systematic Approach for Finding Significant Pathways Dominated by Key Genes. BMC Syst. Biol. 2012, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- SPIA: Signaling Pathway Impact Analysis (SPIA) Using Combined Evidence of Pathway Over-Representation and Unusual Signaling Perturbations. Available online: https://rdrr.io/bioc/SPIA/ (accessed on 21 July 2023).
- Pathan, M.; Keerthikumar, S.; Ang, C.-S.; Gangoda, L.; Quek, C.Y.J.; Williamson, N.A.; Mouradov, D.; Sieber, O.M.; Simpson, R.J.; Salim, A.; et al. FunRich: An Open Access Standalone Functional Enrichment and Interaction Network Analysis Tool. Proteomics 2015, 15, 2597–2601. [Google Scholar] [CrossRef]
- Liu, P.; Ewald, J.; Pang, Z.; Legrand, E.; Jeon, Y.S.; Sangiovanni, J.; Hacariz, O.; Zhou, G.; Head, J.A.; Basu, N.; et al. ExpressAnalyst: A Unified Platform for RNA-Sequencing Analysis in Non-Model Species. Nat. Commun. 2023, 14, 2995. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING V11: Protein-Protein Association Networks with Increased Coverage, Supporting Functional Discovery in Genome-Wide Experimental Datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Stark, C.; Breitkreutz, B.-J.; Reguly, T.; Boucher, L.; Breitkreutz, A.; Tyers, M. BioGRID: A General Repository for Interaction Datasets. Nucleic Acids Res. 2006, 34, D535-9. [Google Scholar] [CrossRef]
- Zhou, G.; Soufan, O.; Ewald, J.; Hancock, R.E.W.; Basu, N.; Xia, J. NetworkAnalyst 3.0: A Visual Analytics Platform for Comprehensive Gene Expression Profiling and Meta-Analysis. Nucleic Acids Res. 2019, 47, W234–W241. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Zhang, D.; Hua, P.; Yang, S. The Glial-Specific Hypermethylated 3′ Untranslated Region of Histone Deacetylase 1 May Modulates Several Signal Pathways in Alzheimer’s Disease. Life Sci. 2021, 265, 118760. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.W.; Yu, W.W.; Yang, Y.; Lü, Y. Exploring the Key Genes and Identification of Potential Diagnosis Biomarkers in Alzheimer’s Disease Using Bioinformatics Analysis. Front. Aging Neurosci. 2021, 13, 602781. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Liu, M.; Du, K.; Zhong, X.; Gong, S.; Jiao, L.; Wei, M. Differential Expression of MRNAs in the Brain Tissues of Patients with Alzheimer’s Disease Based on GEO Expression Profile and Its Clinical Significance. Biomed Res. Int. 2019, 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, J.; Weng, L. Identification and Validation of Aging-Related Genes in Alzheimer’s Disease. Front. Neurosci. 2022, 16, 905722. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Zhang, Y.; Zheng, C.; Yang, S.; Chen, X.; Wang, H.H.; Gao, S. A 3-Gene-Based Diagnostic Signature in Alzheimer’s Disease. Eur. Neurol. 2022, 85, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Li, B.; Jia, Z.; Guo, L. Sirtuin 3 MRNA Expression Is Downregulated in the Brain Tissues of Alzheimer’s Disease Patients: A Bioinformatic and Data Mining Approach. Med. Sci. Monit. 2020, 26, e923547. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Zhao, Y.; Wang, J.; Zhou, Q.; Xu, L.; Kang, D.; Liu, A.L.; Du, G.H. The Bioinformatic Analysis of the Dysregulated Genes and MicroRNAs in Entorhinal Cortex, Hippocampus, and Blood for Alzheimer’s Disease. Biomed Res. Int. 2017, 2017, 1–16. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Long, J.; Pan, G.; He, T.; Anichtchik, O.; Belshaw, R.; Albani, D.; Edison, P.; Green, E.K.; et al. Systematic Analysis and Biomarker Study for Alzheimer’s Disease. Sci. Rep. 2018, 8, 17394. [Google Scholar] [CrossRef]
- Qin, H.; Hu, C.; Zhao, X.; Tian, M.; Zhu, B. Usefulness of Candidate MRNAs and MiRNAs as Biomarkers for Mild Cognitive Impairment and Alzheimer’s Disease. Int. J. Neurosci. 2021, 133, 89–102. [Google Scholar] [CrossRef]
- Liu, W.; Lin, H.; He, X.; Chen, L.L.; Dai, Y.; Jia, W.; Xue, X.; Tao, J.; Chen, L.L. Neurogranin as a Cognitive Biomarker in Cerebrospinal Fluid and Blood Exosomes for Alzheimer’s Disease and Mild Cognitive Impairment. Transl. Psychiatry 2020, 10, 125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Gaiteri, C.; Bodea, L.G.; Wang, Z.; McElwee, J.; Podtelezhnikov, A.A.; Zhang, C.; Xie, T.; Tran, L.; Dobrin, R.; et al. Integrated Systems Approach Identifies Genetic Nodes and Networks in Late-Onset Alzheimer’s Disease. Cell 2013, 153, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Zhang, K.; Zhang, Y.; Guo, Y.; Li, A.; Xiao, S.; Liu, Q.; Shen, L.; Ni, J. Identification of Blood Biomarkers for Alzheimer’s Disease through Computational Prediction and Experimental Validation. Front. Neurol. 2019, 9, 1158. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Chang, W.; Zhao, Y. Diagnosis and Drug Prediction of Parkinson’s Disease Based on Immune-Related Genes. J. Mol. Neurosci. 2022, 72, 1809–1819. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Liu, X.; Chen, J. Microarray Analysis of the Molecular Mechanism Involved in Parkinson’s Disease. Parkinsons. Dis. 2018, 2018, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.; Xie, Q.; Jia, J.; Liu, X.; Sun, J.; Deng, Y.; Yi, L. Integrated Analysis and Identification of Novel Biomarkers in Parkinson’s Disease. Front. Aging Neurosci. 2018, 10, 178. [Google Scholar] [CrossRef] [PubMed]
- Shehadeh, L.A.; Yu, K.; Wang, L.; Guevara, A.; Singer, C.; Vance, J.; Papapetropoulos, S. SRRM2, a Potential Blood Biomarker Revealing High Alternative Splicing in Parkinson’s Disease. PLoS ONE 2010, 5, e9104. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Jia, A.; Cui, Y.; Feng, J. Cerebrospinal Fluid Cells Immune Landscape in Multiple Sclerosis. J. Transl. Med. 2021, 19, 125. [Google Scholar] [CrossRef]
- Hagan, N.; Kane, J.L.; Grover, D.; Woodworth, L.; Madore, C.; Saleh, J.; Sancho, J.; Liu, J.; Li, Y.; Proto, J.; et al. CSF1R Signaling Is a Regulator of Pathogenesis in Progressive MS. Cell Death Dis. 2020, 11, 904. [Google Scholar] [CrossRef]
- Olcum, M.; Tastan, B.; Kiser, C.; Genc, S.; Genc, K. Microglial NLRP3 Inflammasome Activation in Multiple Sclerosis. Adv. Protein Chem. Struct. Biol. 2020, 119, 247–308. [Google Scholar]
- Van Wageningen, T.A.; Gerrits, E.; Brouwer, N.; Breve, J.J.P.; Geurts, J.J.G.; Eggen, B.J.L.; Erik Boddeke, H.W.G.M.; Van Dam, A.M. Distinct Gene Expression in Demyelinated White and Grey Matter Areas of Patients with Multiple Sclerosis. Brain Commun. 2022, 4, fcac005. [Google Scholar] [CrossRef] [PubMed]
- Qiu, D.; Zhang, D.; Yu, Z.; Jiang, Y.; Zhu, D. Bioinformatics Approach Reveals the Critical Role of the NOD-like Receptor Signaling Pathway in COVID-19-Associated Multiple Sclerosis Syndrome. J. Neural Transm. 2022, 129, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Moni, M.A.; Rana, H.K.; Islam, M.B.; Ahmed, M.B.; Xu, H.; Hasan, M.A.M.; Lei, Y.; Quinn, J.M.W. A Computational Approach to Identify Blood Cell-Expressed Parkinson’s Disease Biomarkers That Are Coordinately Expressed in Brain Tissue. Comput. Biol. Med. 2019, 113, 103385. [Google Scholar] [CrossRef] [PubMed]
- Chiu, I.M.; Morimoto, E.T.A.; Goodarzi, H.; Liao, J.T.; O’Keeffe, S.; Phatnani, H.P.; Muratet, M.; Carroll, M.C.; Levy, S.; Tavazoie, S.; et al. A Neurodegeneration-Specific Gene-Expression Signature of Acutely Isolated Microglia from an Amyotrophic Lateral Sclerosis Mouse Model. Cell Rep. 2013, 4, 385–401. [Google Scholar] [CrossRef]
- Premkumar, T.; Sajitha Lulu, S. Molecular Crosstalk between COVID-19 and Alzheimer’s Disease Using Microarray and RNA-Seq Datasets: A System Biology Approach. Front. Med. 2023, 10, 1151046. [Google Scholar] [CrossRef] [PubMed]
- Irmady, K.; Hale, C.R.; Qadri, R.; Fak, J.; Simelane, S.; Carroll, T.; Przedborski, S.; Darnell, R.B. Blood Transcriptomic Signatures Associated with Molecular Changes in the Brain and Clinical Outcomes in Parkinson’s Disease. Nat. Commun. 2023, 14, 3956. [Google Scholar] [CrossRef] [PubMed]
- Salta, E.; De Strooper, B. Noncoding RNAs in Neurodegeneration. Nat. Rev. Neurosci. 2017, 18, 627–640. [Google Scholar] [CrossRef]
- Salta, E.; De Strooper, B. Non-Coding RNAs with Essential Roles in Neurodegenerative Disorders. Lancet Neurol. 2012, 11, 189–200. [Google Scholar] [CrossRef]
- Latowska, J.; Grabowska, A.; Zarębska, Ż.; Kuczyński, K.; Kuczyńska, B.; Rolle, K. Non-Coding RNAs in Brain Tumors, the Contribution of LncRNAs, CircRNAs, and SnoRNAs to Cancer Development—Their Diagnostic and Therapeutic Potential. Int. J. Mol. Sci. 2020, 21, 7001. [Google Scholar] [CrossRef]
- Wang, J.; Yan, S.; Yang, J.; Lu, H.; Xu, D.; Wang, Z. Non-Coding RNAs in Rheumatoid Arthritis: From Bench to Bedside. Front. Immunol. 2020, 10, 3129. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, J.; Zhang, Z.; Wang, X.; Hu, S.; Yu, J. Ribogenomics: The Science and Knowledge of RNA. Genomics, Proteomics Bioinforma. 2014, 12, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Condrat, C.E.; Thompson, D.C.; Barbu, M.G.; Bugnar, O.L.; Boboc, A.; Cretoiu, D.; Suciu, N.; Cretoiu, S.M.; Voinea, S.C. MiRNAs as Biomarkers in Disease: Latest Findings Regarding Their Role in Diagnosis and Prognosis. Cells 2020, 9, 276. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, S.; Zoltowska, K.M.; Laskowska-Kaszub, K.; Wojda, U. MicroRNA Diagnostic Panel for Alzheimer’s Disease and Epigenetic Trade-off between Neurodegeneration and Cancer. Ageing Res. Rev. 2019, 49, 125–143. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Pan, W.; Qian, L. Identification of the MiRNA–MRNA Regulatory Network in Multiple Sclerosis. Neurol. Res. 2017, 39, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Ehya, F.; Tehrani, H.A.; Garshasbi, M.; Nabavi, S.M. Identification of MiR-24 and MiR-137 as Novel Candidate Multiple Sclerosis MiRNA Biomarkers Using Multi-Staged Data Analysis Protocol. Mol. Biol. Res. Commun. 2017, 6, 127–140. [Google Scholar] [PubMed]
- Faruqui, N.A.; Prium, D.H.; Afrin Mowna, S.; Rahaman, T.I.; Dutta, A.R.; Farjana Akter, M. Identification of Common Molecular Signatures Shared between Alzheimer’s and Parkinson’s Diseases and Therapeutic Agents Exploration: An Integrated Genomics Approach. bioRxiv 2021. bioRxiv:2020.12.31.424962. [Google Scholar]
- Lu, L.; Dai, W.Z.; Zhu, X.C.; Ma, T. Analysis of Serum MiRNAs in Alzheimer’s Disease. Am. J. Alzheimers. Dis. Other Demen. 2021, 36, 153331752110217. [Google Scholar] [CrossRef] [PubMed]
- Candido, S.; Lupo, G.; Pennisi, M.; Basile, M.S.; Anfuso, C.D.; Petralia, M.C.; Gattuso, G.; Vivarelli, S.; Spandidos, D.A.; Libra, M.; et al. The Analysis of MiRNA Expression Profiling Datasets Reveals Inverse MicroRNA Patterns in Glioblastoma and Alzheimer’s Disease. Oncol. Rep. 2019, 42, 911–922. [Google Scholar] [CrossRef]
- Li, H.; Zou, L.; Shi, J.; Han, X. Bioinformatics Analysis of Differentially Expressed Genes and Identification of an MiRNA–MRNA Network Associated with Entorhinal Cortex and Hippocampus in Alzheimer’s Disease. Hereditas 2021, 158, 25. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Y.; Ren, R.; Dammer, E.B.; Xie, X.; Chen, S.; Huang, Q.; Huang, W.; Zhang, R.; Chen, H.; et al. MicroRNA-425 Loss Mediates Amyloid Plaque Microenvironment Heterogeneity and Promotes Neurodegenerative Pathologies. Aging Cell 2021, 20, e13454. [Google Scholar] [CrossRef]
- Sabaie, H.; Talebi, M.; Gharesouarn, J.; Asadi, M.R.; Jalaiei, A.; Arsang-Jang, S.; Hussen, B.M.; Taheri, M.; Jalili Khoshnoud, R.; Rezazadeh, M. Identification and Analysis of BCAS4/Hsa-MiR-185-5p/SHISA7 Competing Endogenous RNA Axis in Late-Onset Alzheimer’s Disease Using Bioinformatic and Experimental Approaches. Front. Aging Neurosci. 2022, 14, 812169. [Google Scholar] [CrossRef] [PubMed]
- Brito, L.M.; Ribeiro-dos-Santos, Â.; Vidal, A.F.; de Araújo, G.S. Differential Expression and MiRNA–Gene Interactions in Early and Late Mild Cognitive Impairment. Biology 2020, 9, 251. [Google Scholar] [CrossRef] [PubMed]
- Soreq, L.; Salomonis, N.; Bronstein, M.; Greenberg, D.; Israel, Z.; Bergman, H.; Soreq, H. Small RNA Sequencing-Microarray Analyses in Parkinson Leukocytes Reveal Deep Brain Stimulation-Induced Splicing Changes That Classify Brain Region Transcriptomes. Front. Mol. Neurosci. 2013, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Scimone, C.; Donato, L.; Alafaci, C.; Granata, F.; Rinaldi, C.; Longo, M.; D’Angelo, R.; Sidoti, A. High-Throughput Sequencing to Detect Novel Likely Gene-Disrupting Variants in Pathogenesis of Sporadic Brain Arteriovenous Malformations. Front. Genet. 2020, 11, 146. [Google Scholar] [CrossRef] [PubMed]
- Donato, L.; Scimone, C.; Alibrandi, S.; Scalinci, S.Z.; Rinaldi, C.; D’Angelo, R.; Sidoti, A. Epitranscriptome Analysis of Oxidative Stressed Retinal Epithelial Cells Depicted a Possible RNA Editing Landscape of Retinal Degeneration. Antioxidants 2022, 11, 1967. [Google Scholar] [CrossRef] [PubMed]
- Donato, L.; Alibrandi, S.; Scimone, C.; Rinaldi, C.; Dascola, A.; Calamuneri, A.; D’Angelo, R.; Sidoti, A. The Impact of Modifier Genes on Cone-Rod Dystrophy Heterogeneity: An Explorative Familial Pilot Study and a Hypothesis on Neurotransmission Impairment. PLoS ONE 2022, 17, e0278857. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, C.; Sun, H.; Wang, J.; Liang, Y.; Wang, Y.; Wong, G. The Bioinformatics Toolbox for CircRNA Discovery and Analysis. Brief. Bioinform. 2021, 22, 1706–1728. [Google Scholar] [CrossRef] [PubMed]
- Cochran, K.R.; Veeraraghavan, K.; Kundu, G.; Mazan-Mamczarz, K.; Coletta, C.; Thambisetty, M.; Gorospe, M.; De, S. Systematic Identification of Circrnas in Alzheimer’s Disease. Genes 2021, 12, 1258. [Google Scholar] [CrossRef]
- Junn, E.; Lee, K.-W.; Jeong, B.S.; Chan, T.W.; Im, J.-Y.; Mouradian, M.M. Repression of α-Synuclein Expression and Toxicity by MicroRNA-7. Proc. Natl. Acad. Sci. USA 2009, 106, 13052–13057. [Google Scholar] [CrossRef]
- Dolinar, A.; Koritnik, B.; Glavač, D.; Ravnik-Glavač, M. Circular RNAs as Potential Blood Biomarkers in Amyotrophic Lateral Sclerosis. Mol. Neurobiol. 2019, 56, 8052–8062. [Google Scholar] [CrossRef]
- Li, K.; Wang, Z. LncRNA NEAT1: Key Player in Neurodegenerative Diseases. Ageing Res. Rev. 2023, 86, 101878. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Zhu, W.; Sun, M.; Shi, L. Bioinformatics Analysis of Long Non-Coding RNA and Related Diseases: An Overview. Front. Genet. 2021, 12, 813873. [Google Scholar] [CrossRef] [PubMed]
- Chi, L.M.; Wang, L.P.; Jiao, D. Identification of Differentially Expressed Genes and Long Noncoding RNAs Associated with Parkinson’s Disease. Parkinsons. Dis. 2019, 2019, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, L.; Zheng, C.; Xu, S.; Gao, Y.; Wang, J. Co-Expression Network Analysis Revealing the Potential Regulatory Roles of LncRNAs in Alzheimer’s Disease. Interdiscip. Sci. Comput. Life Sci. 2019, 11, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Xue, W.; Tao, L.; Zhu, F. Identification of Key Long Non-Coding RNAs in the Pathology of Alzheimer’s Disease and Their Functions Based on Genome-Wide Associations Study, Microarray, and RNA-Seq Data. J. Alzheimers. Dis. 2019, 68, 339–355. [Google Scholar] [CrossRef] [PubMed]
- van der Kloet, F.M.; Buurmans, J.; Jonker, M.J.; Smilde, A.K.; Westerhuis, J.A. Increased Comparability between RNA-Seq and Microarray Data by Utilization of Gene Sets. PLOS Comput. Biol. 2020, 16, e1008295. [Google Scholar] [CrossRef]
- Jain, M.; Abu-Shumays, R.; Olsen, H.E.; Akeson, M. Advances in Nanopore Direct RNA Sequencing. Nat. Methods 2022, 19, 1160–1164. [Google Scholar] [CrossRef]
- Sena, J.A.; Galotto, G.; Devitt, N.P.; Connick, M.C.; Jacobi, J.L.; Umale, P.E.; Vidali, L.; Bell, C.J. Unique Molecular Identifiers Reveal a Novel Sequencing Artefact with Implications for RNA-Seq Based Gene Expression Analysis. Sci. Rep. 2018, 8, 13121. [Google Scholar] [CrossRef]
- Hwang, B.; Lee, J.H.; Bang, D. Single-Cell RNA Sequencing Technologies and Bioinformatics Pipelines. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef]
- Slovin, S.; Carissimo, A.; Panariello, F.; Grimaldi, A.; Bouché, V.; Gambardella, G.; Cacchiarelli, D. Single-Cell RNA Sequencing Analysis: A Step-by-Step Overview. Methods Mol Biol. 2021, 2284, 343–365. [Google Scholar]
- Svensson, V.; da Veiga Beltrame, E.; Pachter, L. A Curated Database Reveals Trends in Single-Cell Transcriptomics. Database 2020, 2020, baaa073. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.-X.; Lim, S.B. Single-Cell RNA Sequencing in Parkinson’s Disease. Biomed. 2021, 9, 368. [Google Scholar] [CrossRef] [PubMed]
- Kamme, F.; Salunga, R.; Yu, J.; Tran, D.-T.; Zhu, J.; Luo, L.; Bittner, A.; Guo, H.-Q.; Miller, N.; Wan, J.; et al. Single-Cell Microarray Analysis in Hippocampus CA1: Demonstration and Validation of Cellular Heterogeneity. J. Neurosci. Off. J. Soc. Neurosci. 2003, 23, 3607–3615. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.S.; Van Vleet, T.R.; Ciurlionis, R.; Buck, W.R.; Mittelstadt, S.W.; Blomme, E.A.G.; Liguori, M.J. Comparison of RNA-Seq and Microarray Gene Expression Platforms for the Toxicogenomic Evaluation of Liver from Short-Term Rat Toxicity Studies. Front. Genet. 2019, 9, 636. [Google Scholar] [CrossRef] [PubMed]
- GEO2R. Available online: https://www.ncbi.nlm.nih.gov/geo/info/geo2r.html (accessed on 21 July 2023).
- Amaral, M.L.; Erikson, G.A.; Shokhirev, M.N. BART: Bioinformatics Array Research Tool. BMC Bioinformatics 2018, 19, 296. [Google Scholar] [CrossRef] [PubMed]
- Perampalam, P.; Dick, F.A. BEAVR: A Browser-Based Tool for the Exploration and Visualization of RNA-Seq Data. BMC Bioinformatics 2020, 21, 221. [Google Scholar] [CrossRef] [PubMed]
- Teichman, G.; Cohen, D.; Ganon, O.; Dunsky, N.; Shani, S.; Gingold, H.; Rechavi, O. RNAlysis: Analyze Your RNA Sequencing Data without Writing a Single Line of Code. BMC Biol. 2023, 21, 74. [Google Scholar] [CrossRef] [PubMed]
- La Ferlita, A.; Alaimo, S.; Di Bella, S.; Martorana, E.; Laliotis, G.I.; Bertoni, F.; Cascione, L.; Tsichlis, P.N.; Ferro, A.; Bosotti, R.; et al. RNAdetector: A Free User-Friendly Stand-Alone and Cloud-Based System for RNA-Seq Data Analysis. BMC Bioinformatics 2021, 22, 298. [Google Scholar] [CrossRef]
- Li, R.; Hu, K.; Liu, H.; Green, M.R.; Zhu, L.J. OneStopRNAseq: A Web Application for Comprehensive and Efficient Analyses of RNA-Seq Data. Genes 2020, 11, 1165. [Google Scholar] [CrossRef]
- Jiménez-Jacinto, V.; Sanchez-Flores, A.; Vega-Alvarado, L. Integrative Differential Expression Analysis for Multiple EXperiments (IDEAMEX): A Web Server Tool for Integrated RNA-Seq Data Analysis. Front. Genet. 2019, 10, 279. [Google Scholar] [CrossRef]
- Malhotra, A.; Das, S.; Rai, S.N. Analysis of Single-Cell RNA-Sequencing Data: A Step-by-Step Guide. BioMedInformatics 2022, 2, 43–61. [Google Scholar] [CrossRef]
- He, J.; Lin, L.; Chen, J. Practical Bioinformatics Pipelines for Single-Cell RNA-Seq Data Analysis. Biophys. Rep. 2022, 8, 158–169. [Google Scholar]
- Bertolini, A.; Prummer, M.; Tuncel, M.A.; Menzel, U.; Rosano-González, M.L.; Kuipers, J.; Stekhoven, D.J.; consortium, T.P.; Beerenwinkel, N.; Singer, F. ScAmpi—A Versatile Pipeline for Single-Cell RNA-Seq Analysis from Basics to Clinics. PLOS Comput. Biol. 2022, 18, e1010097. [Google Scholar] [CrossRef] [PubMed]
- Gardeux, V.; David, F.P.A.; Shajkofci, A.; Schwalie, P.C.; Deplancke, B. ASAP: A Web-Based Platform for the Analysis and Interactive Visualization of Single-Cell RNA-Seq Data. Bioinformatics 2017, 33, 3123–3125. [Google Scholar] [CrossRef] [PubMed]
- Mohanraj, S.; Díaz-Mejía, J.J.; Pham, M.D.; Elrick, H.; Husić, M.; Rashid, S.; Luo, P.; Bal, P.; Lu, K.; Patel, S.; et al. CReSCENT: CanceR Single Cell ExpressioN Toolkit. Nucleic Acids Res. 2020, 48, W372–W379. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.A.; Menon, V.; Goldy, J.; Kaykas, A.; Lee, C.-K.; Smith, K.A.; Shen, E.H.; Phillips, J.W.; Lein, E.S.; Hawrylycz, M.J. Improving Reliability and Absolute Quantification of Human Brain Microarray Data by Filtering and Scaling Probes Using RNA-Seq. BMC Genomics 2014, 15, 154. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Pedrosa, E.; Shah, A.; Hrabovsky, A.; Maqbool, S.; Zheng, D.; Lachman, H.M. RNA-Seq of Human Neurons Derived from IPS Cells Reveals Candidate Long Non-Coding RNAs Involved in Neurogenesis and Neuropsychiatric Disorders. PLoS ONE 2011, 6, e23356. [Google Scholar] [CrossRef]
- Abedi, M.; Fatehi, R.; Moradzadeh, K.; Gheisari, Y. Big Data to Knowledge: Common Pitfalls in Transcriptomics Data Analysis and Representation. RNA Biol. 2019, 16, 1531–1533. [Google Scholar] [CrossRef]
- Li, Y.; Ge, X.; Peng, F.; Li, W.; Li, J.J. Exaggerated False Positives by Popular Differential Expression Methods When Analyzing Human Population Samples. Genome Biol. 2022, 23, 79. [Google Scholar] [CrossRef]
- Eilertsen, I.A.; Moosavi, S.H.; Strømme, J.M.; Nesbakken, A.; Johannessen, B.; Lothe, R.A.; Sveen, A. Technical Differences between Sequencing and Microarray Platforms Impact Transcriptomic Subtyping of Colorectal Cancer. Cancer Lett. 2020, 469, 246–255. [Google Scholar] [CrossRef]
- Tang, K.; Ji, X.; Zhou, M.; Deng, Z.; Huang, Y.; Zheng, G.; Cao, Z. Rank-in: Enabling Integrative Analysis across Microarray and RNA-Seq for Cancer. Nucleic Acids Res. 2021, 49, e99. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.X.; Son, E.W.; Yao, R. IDEP: An Integrated Web Application for Differential Expression and Pathway Analysis of RNA-Seq Data. BMC Bioinformatics 2018, 19, 534. [Google Scholar] [CrossRef] [PubMed]
- Can, T. Introduction to Bioinformatics. Methods Mol. Biol. 2014, 1107, 51–71. [Google Scholar] [PubMed]
- Taub, M.A.; Corrada Bravo, H.; Irizarry, R.A. Overcoming Bias and Systematic Errors in next Generation Sequencing Data. Genome Med. 2010, 2, 87. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, L.M.; O’Connor, B.A.; Lim, S.B.; Zeng, J.; Lo, C.H. Integrative Multi-Omics and Systems Bioinformatics in Translational Neuroscience: A Data Mining Perspective. J. Pharm. Anal. 2023, 13, 836–850. [Google Scholar] [CrossRef]
- Subramanian, I.; Verma, S.; Kumar, S.; Jere, A.; Anamika, K. Multi-Omics Data Integration, Interpretation, and Its Application. Bioinform. Biol. Insights 2020, 14, 1177932219899051. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.H.; Zeng, J. Defective Lysosomal Acidification: A New Prognostic Marker and Therapeutic Target for Neurodegenerative Diseases. Transl. Neurodegener. 2023, 12, 29. [Google Scholar] [CrossRef] [PubMed]
- Quick, J.D.; Silva, C.; Wong, J.H.; Lim, K.L.; Reynolds, R.; Barron, A.M.; Zeng, J.; Lo, C.H. Lysosomal acidification dysfunction in microglia: An emerging pathogenic mechanism of neuroinflammation and neurodegeneration. J. Neuroinflammation 2023, 20, 185. [Google Scholar] [CrossRef]
- Pitt, D.; Lo, C.H.; Gauthier, S.A.; Hickman, R.A.; Longbrake, E.; Airas, L.M.; Mao-Draayer, Y.; Riley, C.; De Jager, P.L.; Wesley, S.; et al. Toward Precision Phenotyping of Multiple Sclerosis. Neurol.-Neuroimmunol. Neuroinflammation 2022, 9, e200025. [Google Scholar] [CrossRef]
- Lo, C.H.; Skarica, M.; Mansoor, M.; Bhandarkar, S.; Toro, S.; Pitt, D. Astrocyte Heterogeneity in Multiple Sclerosis: Current Understanding and Technical Challenges. Front. Cell. Neurosci. 2021, 15, 726479. [Google Scholar] [CrossRef]
- Rossi, S.L.; Subramanian, P.; Bovenkamp, D.E. The Future Is Precision Medicine-Guided Diagnoses, Preventions and Treatments for Neurodegenerative Diseases. Front. Aging Neurosci. 2023, 15, 1128619. [Google Scholar] [CrossRef]
- Hampel, H.; Gao, P.; Cummings, J.; Toschi, N.; Thompson, P.M.; Hu, Y.; Cho, M.; Vergallo, A. The Foundation and Architecture of Precision Medicine in Neurology and Psychiatry. Trends Neurosci. 2023, 46, 176–198. [Google Scholar] [CrossRef]
| AD | Brain tissue |  HDAC1, WWTR1, ITGB1, PDGFRB, PLOD1, MAP4K4, NFKBIA, TYROBP, GSN, TIMP1 HDAC1, WWTR1, ITGB1, PDGFRB, PLOD1, MAP4K4, NFKBIA, TYROBP, GSN, TIMP1 |
 SIRT3, RAB7A, BDNF, VLDLR, APLP2 SIRT3, RAB7A, BDNF, VLDLR, APLP2 | ||
| Blood |  VCAM1, TYK2, TCIRG1, PPP3CB, SNCB, SACS, GSN, TIMP1 VCAM1, TYK2, TCIRG1, PPP3CB, SNCB, SACS, GSN, TIMP1 | |
 CTSD, RPL11, SNCA, FKBP1B, BDNF, VLDLR, APLP2 CTSD, RPL11, SNCA, FKBP1B, BDNF, VLDLR, APLP2 | ||
| PD | Brain tissue | 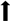 SRRM2 SRRM2 |
 MAPK8, CDC42, NDUFS1, COX4I1, SDHC MAPK8, CDC42, NDUFS1, COX4I1, SDHC | ||
| Blood | 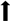 LILRB3, CSF3R, SRRM2 LILRB3, CSF3R, SRRM2 | |
 ICAM1 ICAM1 | ||
| MS | CSF |  NLRP3, LILRB2, C1QB, CD86, C1QA, CSF1R, IL1B, TLR2 NLRP3, LILRB2, C1QB, CD86, C1QA, CSF1R, IL1B, TLR2 |
| Data Mining Tools/Programs | Datasets Analyzed | References |
|---|---|---|
| GEO2R | Microarray data | [159] |
| BART | Microarray data | [160] |
| BEAVR | RNA-seq data | [161] |
| RNAlysis | RNA-seq data | [162] |
| RNAdetector | RNA-seq data | [163] |
| OneStopRNAseq | RNA-seq data | [164] |
| IDEAMEX | RNA-seq data | [165] |
| ScAmpi | ScRNA-seq data | [168] |
| ASAP | Sc/snRNA-seq data | [169] |
| CReSCENT | Sc/snRNA-seq data | [170] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Connor, L.M.; O’Connor, B.A.; Zeng, J.; Lo, C.H. Data Mining of Microarray Datasets in Translational Neuroscience. Brain Sci. 2023, 13, 1318. https://doi.org/10.3390/brainsci13091318
O’Connor LM, O’Connor BA, Zeng J, Lo CH. Data Mining of Microarray Datasets in Translational Neuroscience. Brain Sciences. 2023; 13(9):1318. https://doi.org/10.3390/brainsci13091318
Chicago/Turabian StyleO’Connor, Lance M., Blake A. O’Connor, Jialiu Zeng, and Chih Hung Lo. 2023. "Data Mining of Microarray Datasets in Translational Neuroscience" Brain Sciences 13, no. 9: 1318. https://doi.org/10.3390/brainsci13091318
APA StyleO’Connor, L. M., O’Connor, B. A., Zeng, J., & Lo, C. H. (2023). Data Mining of Microarray Datasets in Translational Neuroscience. Brain Sciences, 13(9), 1318. https://doi.org/10.3390/brainsci13091318





