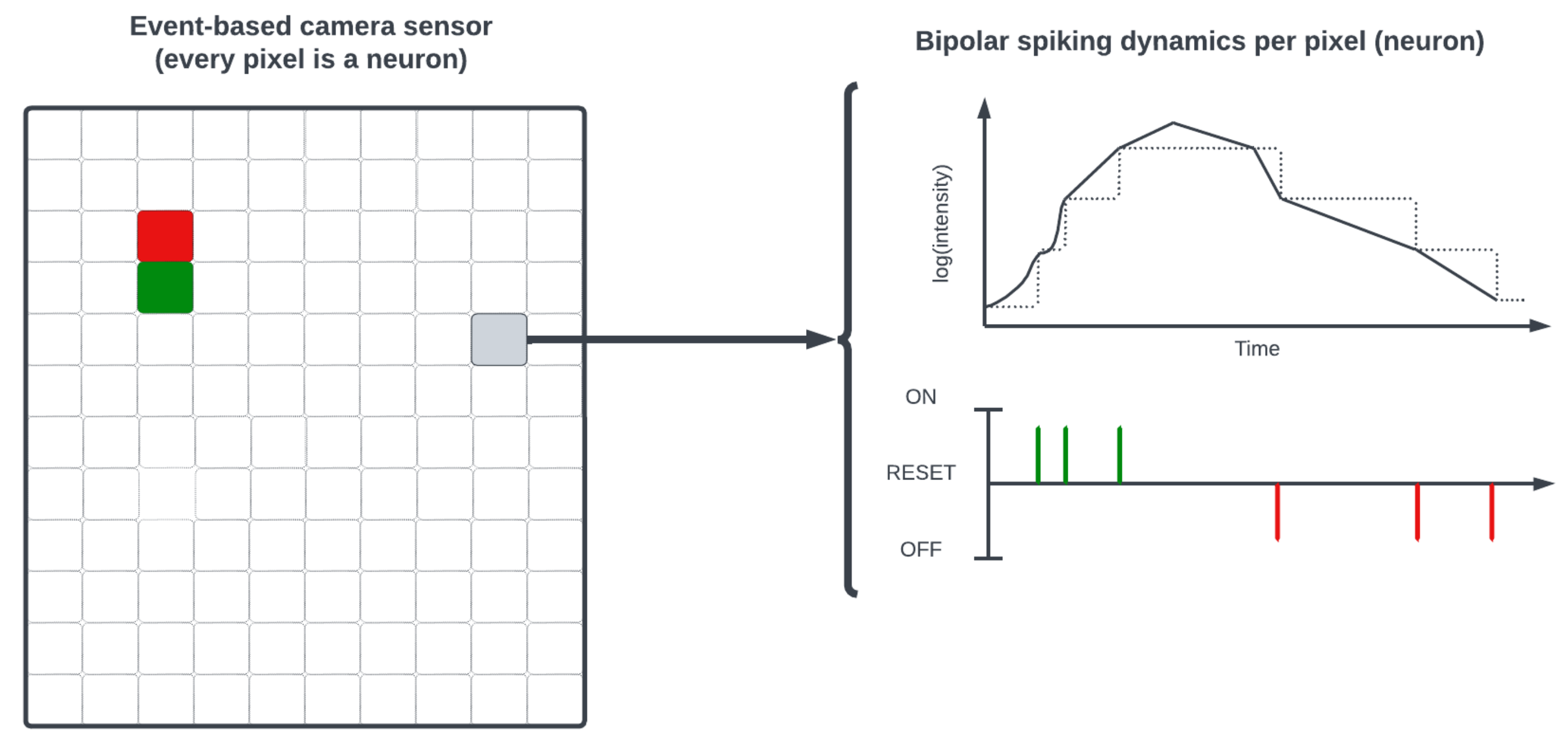
1. Introduction
Understanding how living organisms function within their surrounding environment reveals the key properties essential to designing and operating next-generation intelligent systems. We refer to a system as intelligent if it has the capability of fast adapting to the changes in its components, environment, or mission requirements. A central actor enabling organisms’ complex behavioral interactions with the environment is indeed their efficient information processing through neuronal circuitries. Although biological neurons transfer information in the form of trains of impulses or spikes, Artificial Neural Networks (ANNs) that are mostly employed in the current Artificial Intelligence (AI) practices do not necessarily admit a binary behavior. In addition to the obvious scientific curiosity of studying intelligent living organisms, the interest in the biologically plausible neuronal models, i.e., Spiking Neural Networks (SNNs) that can capture a model of organisms’ nervous systems, may be simply justified by their unparalleled energy/computational efficiency.
Inspired by the spike-based activity of biological neuronal circuitries, several neuromorphic chips, such as Intel’s Loihi chip [
1], have been emerging. These pieces of hardware process information through distributed spiking activities across analog neurons, which, to some extent, trivially assume biological plausibility; hence, they can inherently leverage neuroscientific advancements in understanding neuronal dynamics to improve our computational solutions for, e.g., robotics and AI applications. Due to their unique processing characteristics, neuromorphic chips fundamentally differ from traditional von Neumann computing machines, where a large energy/time cost is incurred whenever data must be moved from memory to processor and vice versa. On the other hand, coordinating parallel and asynchronous activities of a massive SNN on a neuromorphic chip to perform an algorithm, such as a real-time AI task, is non-trivial and requires new developments of software, compilers, and simulations of dynamical systems. The main advantages of such hardware are (i) computing architectures organized closely to known biological counterparts may offer energy efficiency, (ii) well-known theories in cognitive science can be leveraged to define high-level cognition for artificial systems, and (iii) robotic systems equipped with neuromorphic technologies can establish an empirical testbench to contribute to the research on embodied cognition and to explainability of SNNs. Particularly, this arranged marriage between biological plausibility and computational efficiency perceived from studying embodied human cognition has greatly influenced the field of AI.
In any interdisciplinary research, where neural networks are used to construct entirely artificial systems or to simulate living intelligent systems, defining cognition from different disciplines’ points of view becomes a “wicked problem” [
2]. For an engineer, it may be enough to include artificial neural systems capable of state-of-the-art task performance that are designed based on biological principles and are not necessarily faithful to in silico representations of neural circuitry in vivo. On the other hand, a neuroscientist may only be interested in seeking biologically-equivalent representations that are not necessarily computationally efficient. Both describe what they investigate as “cognition”, and both are indeed partially correct (we distinguish between artificial and biological cognition here) until a strong link is drawn from the in vivo observations to in silico realizations. A clear distinction can be observed when considering neuromorphic technologies, where better energy efficiency overshadows the inherent biological plausibility of what can be deemed cognition.
Connecting the dots from in vivo to in silico neural systems requires not only an account of physical substrates dynamics supporting cognition, as observed in nature, but also their replication in viable neural computing architectures to reproduce such observations. This perspective provides a platform to test purely embodied versus purely functionalist computational hypotheses of mind: Are certain classes of biologically embodied computations (e.g., sensorimotor processing for perception and locomotion) inherent features of the physical substrates? Or, is the symbolic information processing universal to the substrates? Embodied analog-hardware cognition suggests that certain features of the mind are contingent upon the physical substrates (sensing organs and actuators) in which it is embodied. That is, mental processes are the exclusive province of the brain and they are naturally dependent on and constrained by the physical characteristics of the neural system through which their operations are implemented (the so-called neural dependency). Although symbolic computationalist theories of mind may also have roots in neural activities, they hold the belief that the mind is the consequence of what neural information processing does, i.e., its functionality, not the specific hardware [
3]. Without being bogged down in the endless philosophical debate around the classic mind-body problem, we can pragmatically conclude that a dialectical synthesis of these tensions suggests that a limited class of substrates is capable of performing a limited class of computations and that these classes are constrained by the biological entity and task at hand [
4]. Consistent with this pragmatic approach, the interdisciplinary link between neuroscience and AI may in fact be determining these reciprocal constraints. At this critical junction, robotic experimentation of embodied cognition developed predicated on SNNs becomes the necessary test bench [
5]. Notably, many outstanding questions of the field then collapse into the following: (i) Do biological neuronal models that better reproduce observed empirical data better capture cognition (i.e., intelligent behaviors), when implemented on robotic agents?, and (ii) How do the embodiment of neural networks affect the network dynamics and topology, when interacting with the robot’s physical environment?
This article examines some recent multidisciplinary developments in the use of SNNs for both natural and engineering sciences. We summarize and critically review new developments in three separate areas: (i) vision and image processing using Dynamic Vision Sensors (DVS), (ii) SNN-based analysis of the Electroencephalography (EEG), and (iii) robust spiking control systems applicable in robotic Guidance, Navigation, and Control (GNC). By connecting the dots from perception to signal processing for brain data to GNC systems for robotics, we highlight where SNNs may be used to further research goals across disciplines and how they may be constituent to a theory of embodied cognition. A survey of control based on learning-inspired spiking neural networks can be found in [
6] where different learning rules are identified and classified for robotic applications. More recently, [
7] exclusively reviews the application of neuromorphic computing for socially interactive robotics and [
8] focuses on the implementation of neuromorphic hardware in robotics. However, comprehensive research on closing the cognition loop in neurorobotics is still missing in the literature, which is our main agenda in the current paper.
The paper is structured as follows:
Section 2 provides a deep description of SNN modeling, distinguishing between descriptive models and functional models.
Section 3 discusses the bottlenecks in the computational simulation and emulation of SNNs.
Section 4 discusses the embodiment of SNN into cognitive robotic systems and reviews multiple perspectives that SNNs can play a crucial role in developing an intelligent cognitive robot. It also explores the consequences of the technological advancements to future brain-inspired computational cognition research in relation to simulating adaptable and reconfigurable neurorobotic systems.
Section 5 summarizes the trends in the literature and the limitations of the existing technologies. Some concluding remarks are included in
Section 6.
2. SNNs and Neuromorphic Modeling
In this section, we recall the basic components of SNN modeling and discuss their neuromorphic implementation [
9,
10]. For a more detailed review of SNNs and their applications, we refer the reader to [
11]. Note that the category of bio-inspired computing is an unsettled one, and it is unclear how such systems can be designed to integrate into existing computing architectures and solutions [
12]. Consider the neuron as the basic computational element of a SNN [
13].
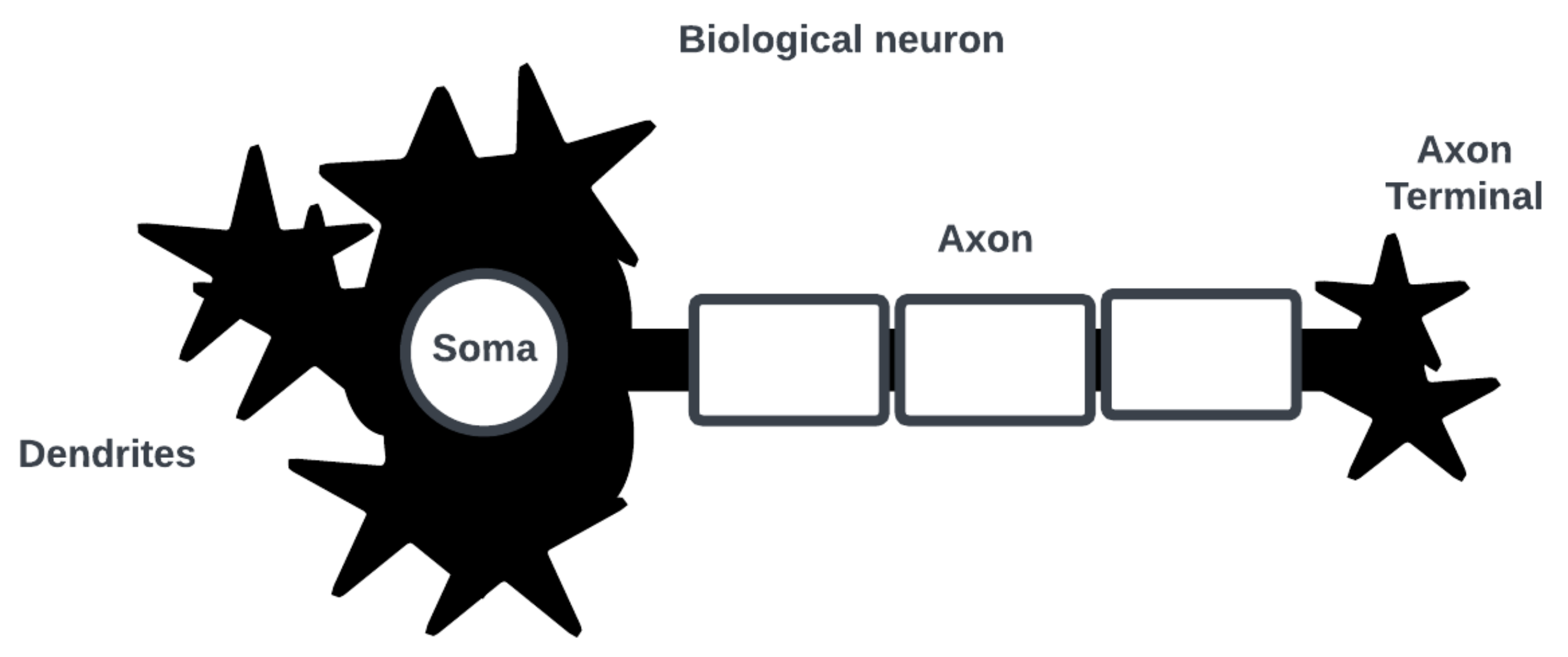
Figure 1 shows a simplified biological neuron consisting of dendrites where post-synaptic interactions with other neurons occur, a soma where an action potential (i.e., a spike in cell membrane voltage potential) is generated, and an axon with a terminal where an action potential is triggered for pre-synaptic interactions.
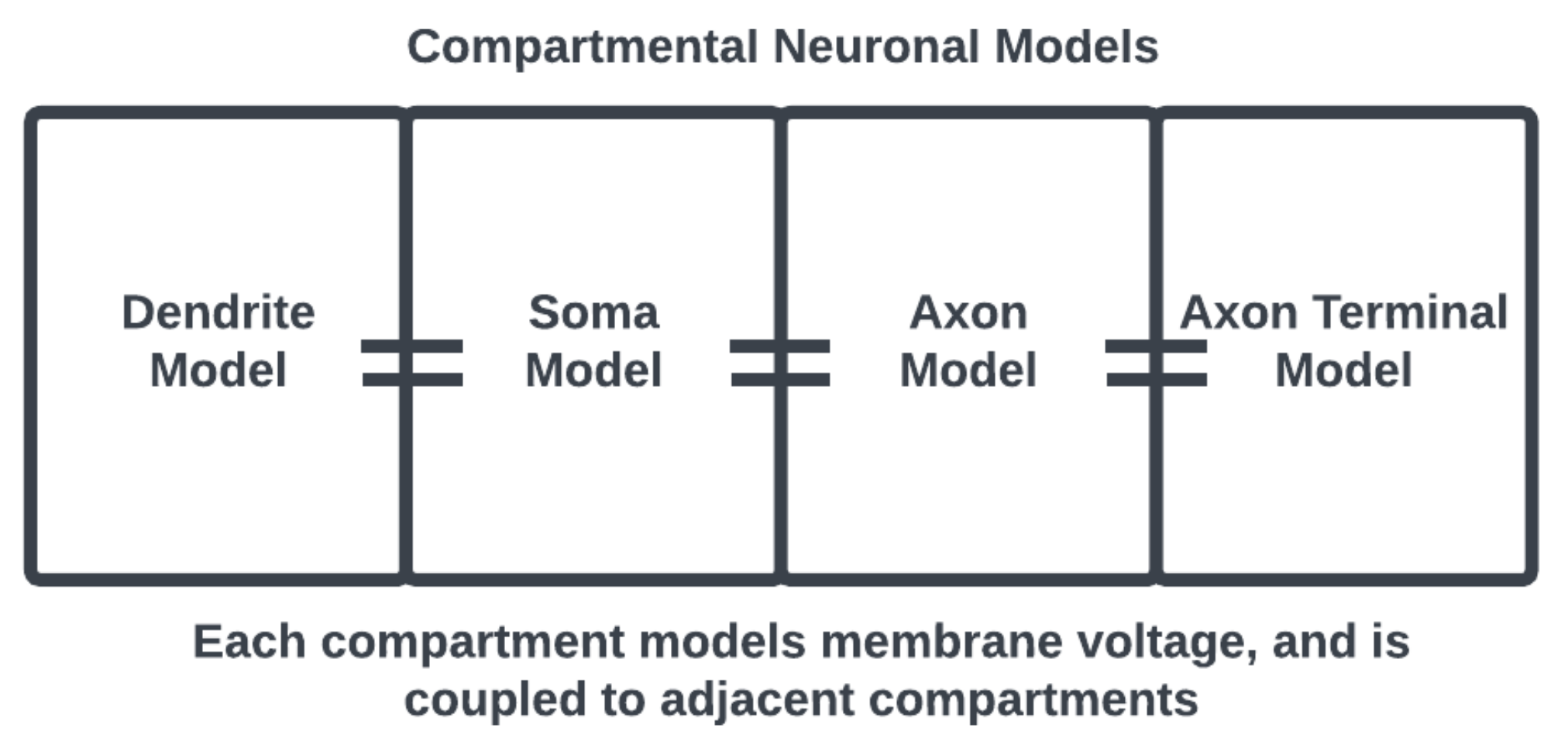
One way to model a spiking neuron is using compartmental models [
14] that consider each of these morphological components separately, with their own sets of dynamical equations coupled together to represent the functional anatomy of a neuronal cell [
15]. For example, as in
Figure 2, the equations describing the membrane potential associated with the dendrites are only coupled to the soma but the equations associated with the soma are coupled to the axon as well. Thus, interactions at the subcellular level are modeled locally to represent cell anatomy.
The advantage of this model is the structural details and subcellular local effects that may be included; however, the model of a neural network becomes too complex and difficult to compute. In this sense, compartmental models may be viewed as biologically plausible but computationally unfeasible for many applications beyond investigations at the cellular level. The work of [
16] implemented a multi-compartmental model on the SpiNNaker neuromorphic hardware to incorporate dendritic processing into the model of computation.
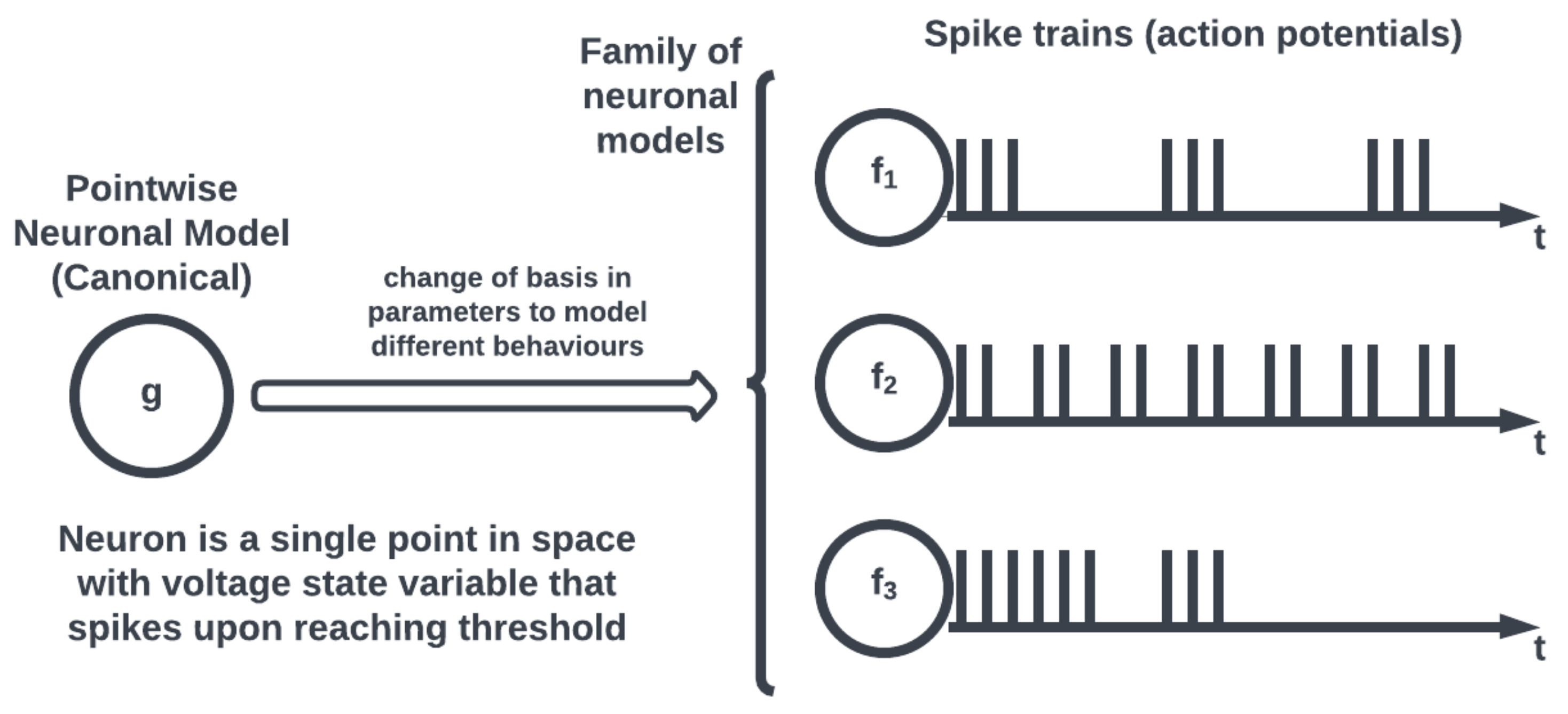
On the other hand, most neuromorphic hardware implements a simplified form of neuronal models, known as a pointwise model (see
Figure 3), where a neuron is conceptualized as a single point. Pointwise models abstract the intracellular dynamics into a state voltage variable that records a spike when the voltage exceeds a threshold to effectively capture the action potential behavior of the neuron without having to model its structure. Therefore, unlike compartmental neuronal models that capture the spatiotemporal neuronal dynamics allowing for electrochemical process simulations, pointwise models offer computationally efficient simulations for large-scale networks neglecting spatial complexity.
The difference between compartmental and pointwise neuronal models is just one design decision when simulating neural systems, and it can be argued that the latter lacks biological plausibility. A now near-canonical example of neuronal modeling is the Hodgkin–Huxley model describing the dynamics of action potentials in neurons [
17]. In the case of Hodgkin–Huxley, a continuous-time system of differential equations forming a mathematical template for neuronal modeling was derived by selecting squid as a biological specimen and analyzing its nervous system. Despite its descriptive completeness and prescriptive accuracy, this formalization is still an abstraction suffering from the representation problem of the map-territory relation [
18]. Furthermore, the required computational machinery to implement the simulation of a single neuron based on the Hodgkin–Huxley model outweighs its biological completeness [
19]. Generally, it is undeniable that the computational feasibility of any model inherently comes at the cost of some inevitable reduction in biological veridicality. Following Hodgkin–Huxley’s seminal work, much development has been made in capturing the dynamics of neuronal cells. The work of Izhikevich [
20] showed that many observed spiking patterns could be reproduced using a small set of differential equations and that these equations were also simpler to compute while making a tradeoff in physically meaningful quantities. The eponymously named model consists of a pair of first-order differential equations on the space of the membrane potential and recovery variable of a neuron along with a simple spiking thresholding logic. Related works named these equations the canonical model approach, where the variability of spiking behaviors could be accounted for using a change of basis in the variables of the dynamical equations [
21]. Accordingly, a neuronal model can be re-written in the form of the canonical system which allows for a consolidation of neuronal activity analysis under one dynamical system. The advantage of this approach is that an empirically observed spiking behavior may be modeled by a complicated and biologically detailed model or a simplified and reduced form, depending on needs and resources. The canonical approach also allows for the investigation of different kinds of spiking behavior (bursting, phasic) generated by the same neuronal dynamics under different parameter regimes, which is a step towards the standardization of neuronal models matching observed empirical data. Under this approach, dynamical systems theory bears upon our neurophysiological understanding via simulating the (biological) spiking behavior of different types of neuronal cells employing the appropriate canonical model with specific parameterizations [
22]. The problem of modeling neurons’ spiking behavior then transforms into selecting the differential equation and estimating its parameters. It is from this formalized neuronal dynamics position that the GNC of neurorobotic systems is revisited in a later section. A discussion and summary table of biological plausibility and computational efficiency for different neuronal models, including Hodgkin–Huxley, Izhikevich, and integrate-and-fire, are documented in [
19].
Table A1 in
Appendix A discusses different criteria of interest regarding biological plausibility and their relation to neuromorphic hardware.
Canonical models are advantageous in studying the behavior of a single spiking neuron because they standardize the mechanisms of action potentials using the mathematical theory of dynamical systems. To investigate a network of neurons we must also model the neurochemical interaction and neural plasticity at the synaptic cleft of connected neurons, introducing certain challenges especially when concerning biological plausibility [
17]. Together, populations of homogenous or heterogenous neurons may be connected to form a SNN and simulate its time evolution of neural activity. The resulting neural network fundamentally differs from an ANN where the input/output information and its process are not embedded in a temporal dimension. It is still an open problem how the information is encoded in a spike train, whether the frequency of spikes in a time window encodes information (rate-based) or the time between the spikes (spike-based). Brette suggests that rate-based encoding is a useful but limited ad-hoc heuristic for understanding neural activities and that spike-based encoding is more supported both empirically and theoretically [
23]. For example, there are studies supporting interspike interval (a spike-based method) information encoding in the primary visual cortex [
24].
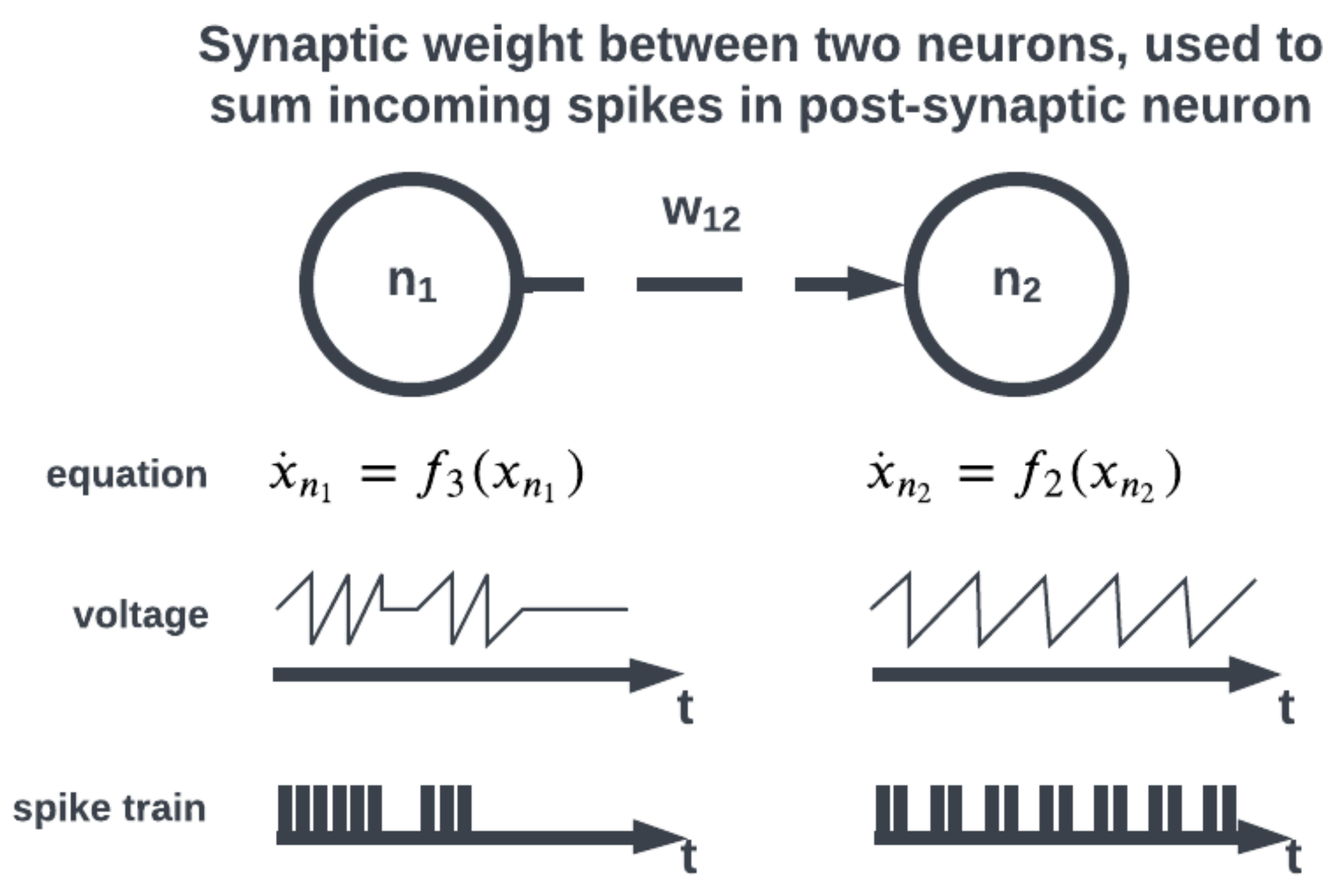
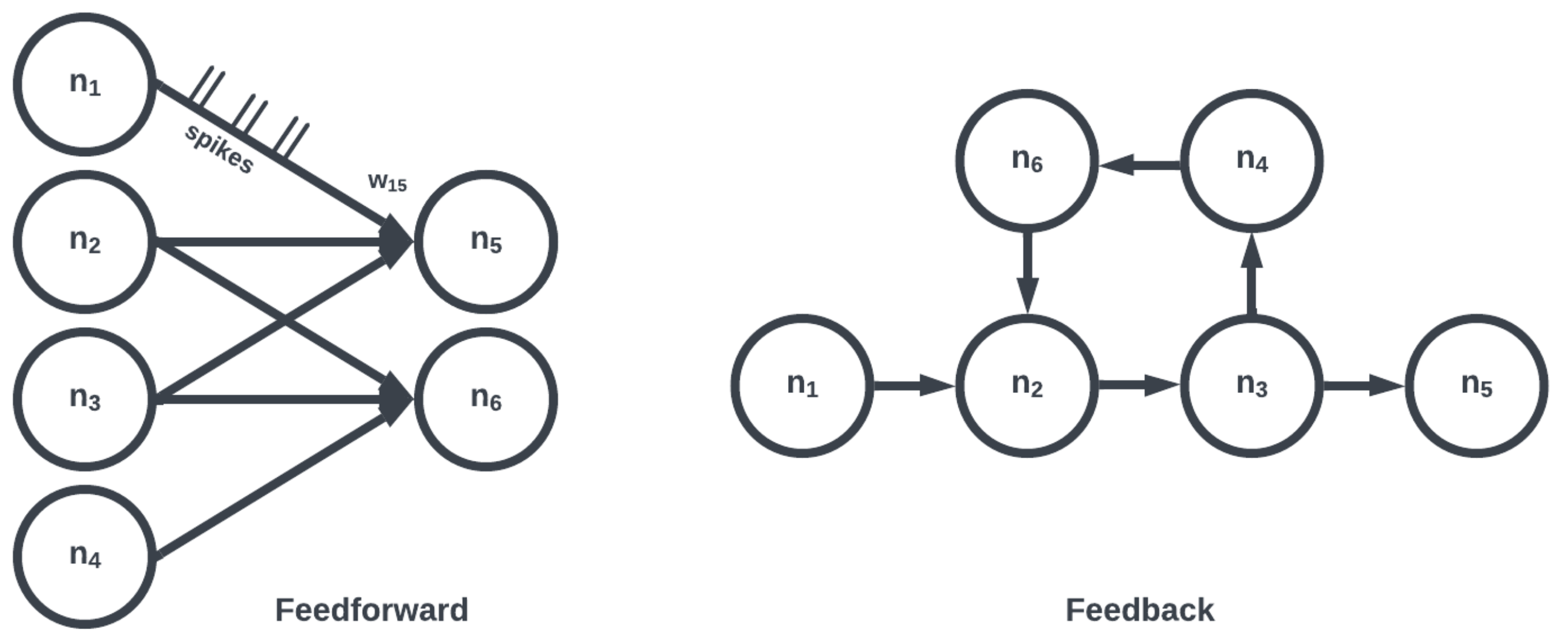
The topology of a SNN prescribes how the neuronal activities are connected to each other by weights representing synaptic modulation between neurons (see
Figure 4). The work of [
25] showed that there are highly non-random small patterns of connectivity in cortical networks, an idea expanded upon more broadly for the brain by [
26]. These non-random patterns between neurons are called motifs [
27], an example of which is a typical convolutional (non-recurrent, feedforward) organization (
Figure 5). Organizing sets of motifs, we may construct layers or populations of coordinated neural activities (
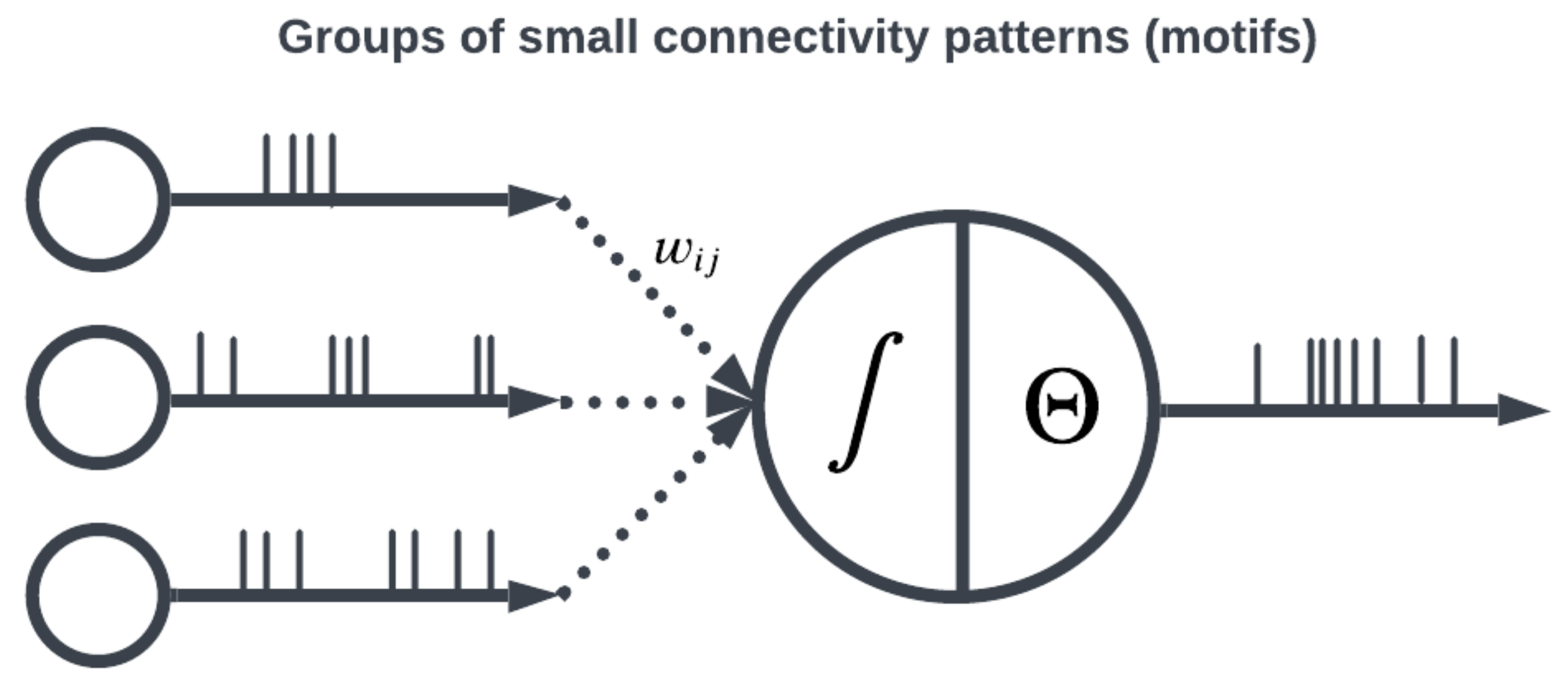
Figure 6). The connectivity pattern and structure of the network influence information flow, signal processing, and the emergence of network dynamics. The choice of topology impacts how neurons communicate and interact with each other, affecting the propagation of spiking activity, synchronization, and formation of functional circuits. Different topologies, such as feedforward, recurrent, or hierarchical structures, offer unique computational capabilities. For example, feedforward networks excel at information propagation and feature extraction, while recurrent networks enable memory and feedback mechanisms. The topology of a SNN is a fundamental design parameter that shapes its computational properties and determines its ability to perform specific tasks, making it a key consideration in designing and understanding neuronal computation.
Large organizations of coordinated neural populations may be trained to perform AI tasks or approximate functions [
28] (see
Figure 7). In addition to the SNN topology, the adaptation rule that specifies the weights between interconnected neurons plays a crucial role in determining the computation performed by the network. Here, Hebb’s postulates, what are aphoristically understood as “cells that fire together wire together” [
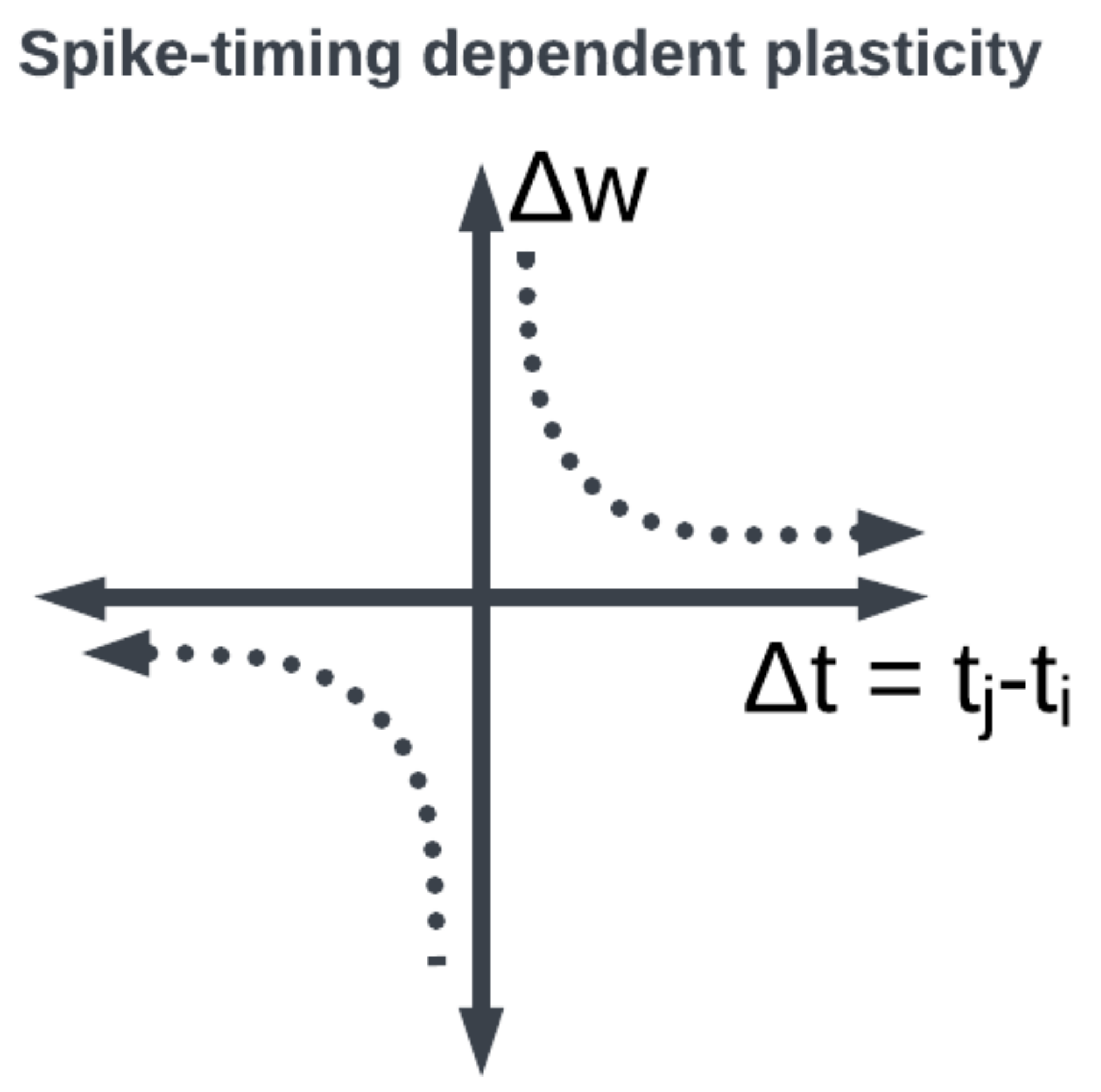
29], remains the dominant neuroscientific insight for learning. A seminal work in modeling synaptic plasticity, this is a well-known unsupervised learning technique called Spike-Timing-Dependent Plasticity (STDP) [
30]. The STDP method works based on the selection of a learning window (see
Figure 8) describing the asymmetric modification of the synaptic conductance between two neurons: when the presynaptic neuron fires before (after) the postsynaptic neuron, the conductance is strengthened (attenuated). The smaller the time interval between spikes, the greater the magnitude of modification in either direction. By applying a learning algorithm such as STDP to a population of spiking neurons, the learned (conductance) connection weights between neurons capture the topology of the network. A historical account of the developments from Hebb’s synaptic plasticity postulate to the STDP learning rule and its modern variations can be found in [
31]. A survey of methods for learning via SNNs in [
32] reveals that most learning models are STDP-based [
33].
Alternative methods exist to learn synaptic weights, and their development is an active area of research for applications where biological fidelity may not be a priority. One such method is to convert ANNs to SNNs by mapping their weights [
34,
35,
36]. Another approach is to use surrogate-gradient descent [
37] such that traditional deep learning methods may be used by choosing an appropriate substitute for the spiking neurons’ Heaviside activation function. The substitute is typically a function that approximates the Heaviside function but has smooth gradients, such as the tangent hyperbolic function.
3. Neuromorphic Computing: Hardware vs. Software
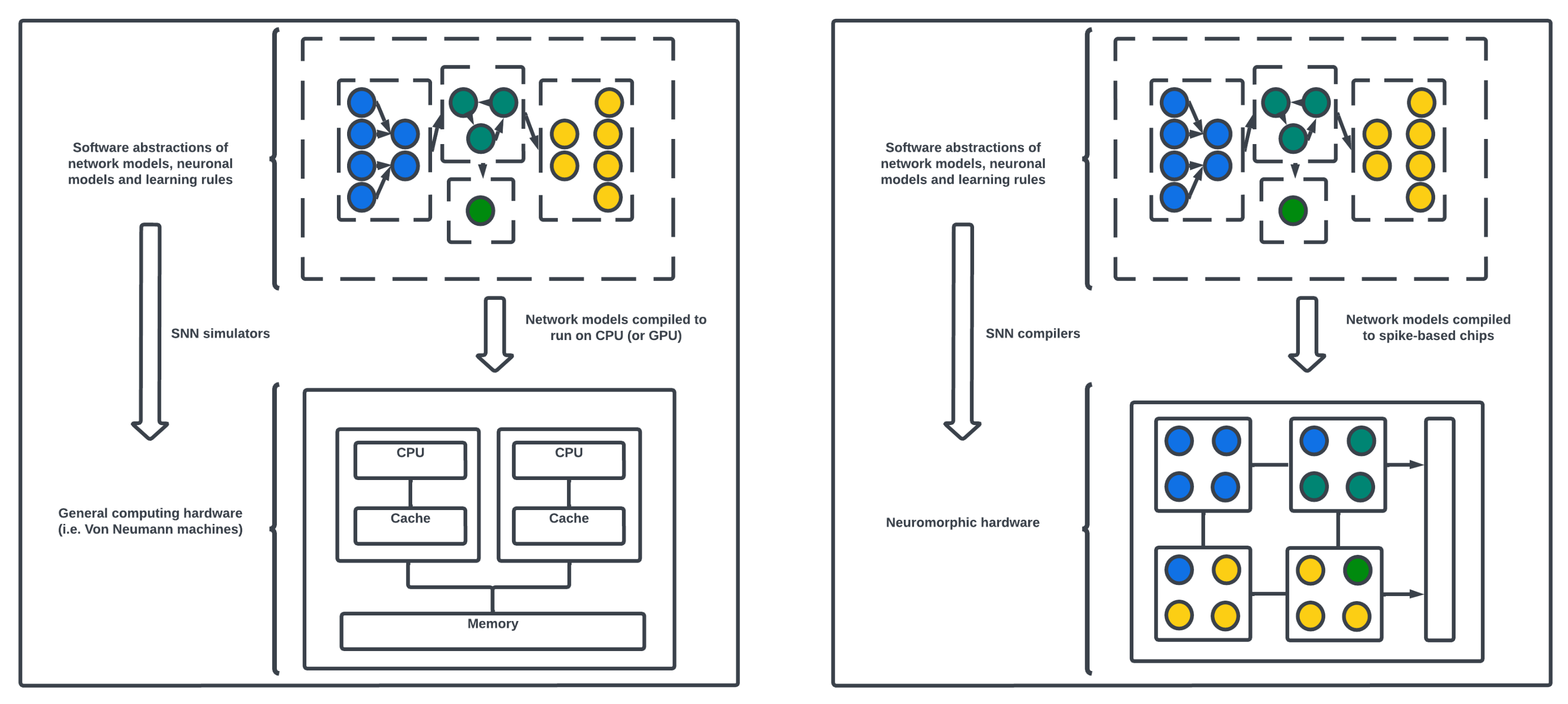
SNN models may be simulated on von Neumann computing machines as well as compiled onto neuromorphic chips.
Figure 9 describes the subtle difference between the two types of computing architectures and distinguishes where power gains can be made by utilizing the asynchronous event-based chips. Von Neumann computing, the traditional computing architecture, is based on the separation of processing and memory units, with sequential instruction execution and a focus on fast and precise arithmetic operations. In contrast, neuromorphic computing draws inspiration from the structure and function of biological neural networks. Neuromorphic systems typically feature specialized hardware that integrates processing and memory, enabling parallel and distributed computation with low power consumption. While von Neumann architectures excel at general-purpose computation and algorithmic tasks, neuromorphic computing emphasizes efficient and event-driven processing, enabling the simulation of large-scale neural networks and the implementation of brain-inspired algorithms. Neuromorphic computing offers advantages in areas such as real-time sensory processing, pattern recognition, and cognitive tasks that require high parallelism, low-power operation, and adaptive learning capabilities. Processors such as Central Processing Units (CPUs) and Graphics Processing Units (GPUs) are examples of von Neumann architectures. Often, GPUs (in contrast to CPUs) are used to accelerate the training and simulation of SNNs up to orders of magnitude by introducing parallel computing [
38,
39,
40]. However, in practice, a heterogeneous system architecture consisting of some CPU/GPU and neuromorphic chips would be preferred [
41,
42].
Another option for efficiently implementing SNNs is simulating them on Field-Programmable Gate Arrays (FPGAs), often used when the application is low-power and not large-scale [
43] or when real-time processing is a requirement [
44]. FPGAs are reconfigurable integrated circuits that allow for custom hardware design and implementation through digital logic circuits, making them suitable for a variety of applications but requiring expertise to program them. FPGAs are particularly well-suited for implementing SNNs due to their ability to be customized at the circuit level. This feature allows for the deployment of small to medium-sized SNNs with reduced power consumption and enhanced performance through optimizing SNN computations, efficient parallel processing, and low-latency operations [
45]. The work of [
46,
47] are two examples of SNNs on the order of thousands of neurons implemented onto FPGAs for real-time performance. Further development such as in [
48] enabled the inclusion of an on-FPGA STDP for online learning [
31]. Other works such as [
44,
46] investigated biologically plausible and biophysically meaningful SNN modeling on FPGAs. FPGAs may be seen as “in-between” typical von Neumann machines with more specialized hardware, similar to neuromorphic hardware, suitable for when a medium-sized CPU/GPU model needs accessing constrained resources.
Neuromorphic computing is an exemplary realization of In-Memory Computing (IMC) [
49], particularly in the context of embodied cognition and robotics. Neuromorphic hardware, integrating memory and processing units, mimics the behavior of biological neural networks. Executing computations directly within memory reduces data movement, resulting in low-latency, energy efficiency, and parallel processing [
50]. This approach offers substantial advantages for embodied cognition and robotics by enabling real-time, event-driven processing of sensory inputs, enhancing performance, and facilitating time-sensitive tasks. With efficient storage of synaptic weights and specialized memory technologies, neuromorphic architectures provide scalable and brain-inspired computing solutions. Tool development to evaluate this hardware is also an active field of research, one such example being the work of [
51] which introduced SpikeSim, a platform for an end-to-end compute-in-memory benchmarking tool to compare different SNN models on a chip for their power and latency efficiency.
As neuromorphic computing platforms are still nascent and unavailable [
52], the selection of software for simulating large-scale neural systems on digital hardware [
53] is a decision contingent upon research needs, as a co-design problem [
54]. Neuromorphic hardware is one type of specialized IMC hardware [
55] capable of realizing certain classes and sizes of SNNs, while simulations run on general-purpose computers (e.g., CPU and GPU) require the numerical integration of sparsely coupled neuronal equations which are limited by memory and bandwidth constraints [
56]. These limits can be overcome by a combination of software and hardware techniques, as in the work of [
57] which makes use of code-generation for SNN [
58], targeting GPUs; here, further software optimizations may be explored to accelerate SNN simulations on traditional hardware. Additionally, related work by [
59] compiles models to be run on an emulated Loihi chip [
1], allowing researchers to errorlessly simulate how their model would perform on the neuromorphic chip without actual access to one. This is possible because the Loihi chip can be shown to be equivalent to a leaky integrate-and-fire neuron with Euler time stepping integration [
59]. Different neuromorphic chips implement different neuronal models by varying the design and functionality of their circuitry. Some chips may focus on implementing simplified spiking neuron models, while others may aim for more biologically realistic models, incorporating detailed features such as dendritic processing, learning rules, or higher-order dynamics, allowing for a wide range of neuronal behaviors and capabilities. The selection of neuromorphic hardware depends on the trade-offs in specific experimental or computational research, as different chips may offer varying compromises between computational power, energy efficiency, scalability, ease of programming, and support for different neuronal models. A non-comprehensive table listing neuromorphic chips and their tradeoffs can be found in
Table A2,
Appendix B.
Overall, the implementation of SNNs on different computing platforms presents distinct challenges and benefits, particularly in terms of the scale of neural networks that can be implemented [
60]. CPUs provide flexibility in programming, making them suitable for small to moderate-sized SNNs. However, CPUs may struggle to handle large-scale SNN computations efficiently, resulting in slower performance and longer training times. GPUs excel in parallel processing and are capable of accelerating computations for medium to large-sized SNNs, although energy consumption may be a concern. With their high number of cores, GPUs can handle the massive parallelism inherent in SNNs, significantly speeding up training and inference processes. FPGAs offer low-latency and low-power operation, making them ideal for implementing small to medium-sized SNNs in resource-constrained environments [
61]. FPGAs can be programmed to optimize SNN computations, resulting in efficient and high-performance implementations. However, FPGAs may have limited resources compared to CPUs and GPUs, which may limit the scale of SNNs that can be deployed [
43]. Neuromorphic computing, leveraging specialized hardware like neuromorphic chips, provides an opportunity for implementing large-scale SNNs efficiently. Neuromorphic chips are designed to handle the unique characteristics of SNNs, allowing for the implementation of large-scale networks while offering energy efficiency and real-time processing capabilities. Therefore, the choice of platform ultimately depends on the scale of the neural network, available resources, and specific requirements of the application such as real-time computing.
Here, we compare two software platforms to highlight different types of interdisciplinary research needs: (i) Nengo [
62], and (ii) Brian2 [
63] (or Brian [
64]), both of which are developed in Python [
65]. For more information on different packages, consult with
Table A3 and
Table A4 in
Appendix C that respectively compare various SNN simulators and SNN-based frameworks to support various studies, ranging from cognitive neuroscience to brain modeling. While simulators and theoretical frameworks are often developed in tandem (as in the case of Nengo), it is important to recall that these theoretical frameworks can be implemented in other libraries that may be better optimized for performance and efficiency. For example, a dynamic field theory (DFT) model was re-implemented in the Nengo library by [
66]. Nengo is a functional-based simulator that includes a graphical user interface, allowing for interactive inspection and visualization of different values present in the neuronal population models. A user must implement their dynamical system equations in Python-like code within the namespace scope of the Nengo classes in order for the program to simulate the time evolution of the neural activities. These Nengo scripts may also be implemented without the interface as regular Python scripts when interactive model probing is not required. Brian instead uses code-generation [
58] to transform a user-specified spiking neural network model to optimized code in Python (which also supports C++) for execution. The advantage of code generation is its high computational efficiency due to the generation of low-level codes for resource-intensive parts of the simulation cycle. While research is ongoing, both simulators are able to compile SNN models onto neuromorphic chips [
67].
One constraint of Brian, absent in Nengo, is the implementation of dimensioned quantities. Brian applies the International System of Units (SI) for quantities declared in the model to check the correctness of the dimensionality of neuronal equations. This enforced sanity check of the physical consistency of neuronal models is advantageous especially when biological plausibility is a priority. However, whenever just the functionality of neural populations is concerned, the specific units of quantities may not be relevant. One example is the tuning-curve approach in Nengo, where the parameter values for a neural population are specified by a least-squares fit against the desired representational transformation for that population. Hence, as much as Brian is a suitable option for a neuroscientist who wishes to model biological neural networks, Nengo can help a roboticist reproduce traditional control systems with SNNs.
The high-level functional approach to brain modeling present in Nengo is particularly ideal in robotics applications, since the prescribed functionality (representation and logic) emergent in connectionist SNN models [
68,
69] allows for an abstraction of low-level neurochemical dynamics observed in vivo while achieving desired cognitive behaviors. This idea has been formalized using the Neural Engineering Framework (NEF) [
70] and the Semantic Pointer Architecture (SPA) [
71] that led to the development of the Spaun [
72]. The NEF consists of three principles of neural computation: (i) a neural population represents a vector acting as a nonlinear encoding of spike trains and facilitating their linear decoding; (ii) various linear transformations of such vectors are defined by alternative decoding weights; and (iii) the neural representations can be treated as control-theoretic state variables. These three NEF principles allow for the vectorial representation of mental activities captured by neuronal and synaptic dynamics. The orchestration of encoding, transforming, and transferring vector signals among neural populations forms a connectionist model, where each population’s representation is a state variable in a control system and synaptic connections provide information feedback.
The SPA is an extension of the NEF that is based on the semantic pointer hypothesis [
71] which states: high-level cognitive functions in biological systems are representable by operations on semantic pointers in a high-dimensional vector space. Semantic pointers are vector representations of neural networks that carry partial semantic contents and are composable into the representational structures necessary to support complex cognition. The mathematical method antecedent to the SPA, Holographic Reduced Representations (HRR) [
73,
74], allows for defining a convolutional algebra over a space of distributional (real-valued vectors) representations. In this algebra, two vectors representing partial semantic contents can be convolved to produce a third vector representing the binding of the partial contents. The addition of vectors is treated as a bundling of semantic representations. Both the SPA and HRR are examples of the Vector Symbolic Architectures (VSA), a survey of which can be found in [
75]. Vector Symbolic Architectures are a class of computational models that use high-dimensional vectors to represent complex cognitive concepts, such as words, objects, or actions. These vectors can be manipulated using mathematical operations, allowing for the creation of novel concepts through the combination of existing ones.
Table A5 in
Appendix D describes the main features of VSA and their relation to SNNs. The work of [
76] argued that VSA is an attractive approach to addressing problems in higher-order cognitive processes such as linguistics and analogical reasoning because they provide a way to represent and manipulate high-dimensional, distributed representations that are flexible and context-sensitive, while still preserving some of the key properties of symbolic representations. VSA has recently been generalized to the Vector Function Architecture (VFA) [
77] which extends the algebraic operations on vector spaces to functional spaces. In this architecture, vectors not only represent individual data points but also elements of a functional space that can be bound, bundled, and unbound. The contribution of the SPA is to achieve a similar convolutional algebra but using vectors from the space of neural representations, determined by the SNN encoding tuning curves and decoding weights. In practice, this amounts to the projection of desired SPA vocabularies into a high-dimensional vector space such that the vector resulting from the convolution of two vectors is always orthogonal to those two vectors. This vectorial representation transforms a SNN model to a control system, where the vectors form the state variables; thus, the problem of neural process (cognition) becomes a control problem of how to transition neural activities from one state (representing some semantic content) to another. This transition can be a complex combination of convolutional bindings and vector additions but the resulting vector nonetheless stays in the state space of the system. For example, a list structure in working memory can be realized by binding semantic contents to different semantic pointers and bundling those vectors together as a neural representation of the memory. Then querying a specific content is the deconvolution with the desired pointer that zeros all other semantic vectors in the binding due to the orthogonality property. As seen in the approach taken by NEF, constructing spiking neural networks whose spiking activities code the vector symbolic operations allows for the construction of higher-level cognitive algorithms capable of being deployed on energy-efficient neuromorphic hardware. SNNs are thought to be efficient for their exploitation of spatiotemporal sparsity: i.e., at any point, not all neurons are active, and therefore the modeling and hardware implementation of these networks may similarly be massively parallel and asynchronous. Thus, the realization of a VSA using SNNs suggests that spatiotemporally distributed spikes are able to encode the operations performed by conventional VSAs in a power-efficient manner.
Table A6 in
Appendix E summarizes different parallelisms possible in SNNs.
5. Coda: Trends, Limitations, and Prospective
In this paper, we have examined that striking the right balance between computational efficiency and biological realism is one of the challenges at the intersection of AI, robotics, and neuroscience, and there are ongoing debates on the accurate representation of neural processes versus the efficiency of network dynamics. At present, many of the debated points focus on the characteristics, limitations, and synergy of the hardware and the software. It can be said that initially biological plausibility was frequently sacrificed for computational efficiency, due to the fact that neural networks are basically a simulation of analog parallel processing on traditional Turing machines. In this sense, our endeavor to use neural networks has been nothing but an in-principle demonstration of what could be possibly performed given the limitations of the underlying hardware, which is not truly an analog to nervous tissues.
Therefore, in this first historical phase, the contribution of the research on neural networks and especially experimenting with deep networks have been mostly on the algorithmic side. To further the applications on complex systems embedded in challenging environments, the dominant AI research community awaits new advancements in quantum computation and hypercomplex multidimensional dynamic systems, whose formal transparency is the main dispute, which reaffirms that we are still dealing with implications of Godel’s incompleteness theorem. This de facto has been sidestepped in the last ten years as neural networks have been progressively exploited independently of their designs, given increased computational efficiency, speed, and power in this era. We have witnessed an explosion of hidden layers from single digits to over 50 in deep learning research and applications. However, there are no 50 layers of neurons anywhere in the brain, so any tenuous biological link is lost in such most recent developments. This state of affairs has an enormous downside in that these new networks create increasingly complex emergent computational dynamics, while scientific explainability and transparency tend to decrease asymptotically. It might be refreshing to use an example that is already old news in the field of AI: the complete domination of AlphaGo software [
158,
159,
160] in board games, already exhibiting behaviors that are perceived by champion gamers as “incomprehensible” and “alien” [
161].
In the last five years or so, it appears we have entered another historical phase of research on brain-inspired intelligence with the birth of neuromorphic hardware. Such hardware is designed starting from the principles of the material emulative constraints of neural tissues, such as memristive devices [
162]. As we have discussed in the previous sections, in this instance, the driving force of computational efficiency is increasing adherence and fidelity to biological reality (although as we argued ”reality” is still an abstract model). Even so, as our review suggests, the challenge of designing fitting software remains the same as in non-neuromorphic devices. As we have mentioned, the current practice is to develop algorithms that are adaptable to neuromorphic hardware and then test them. The issue of explainability is not resolved yet but simply redistributed.
We have discussed how some of the issues involved in biological plausibility have been dealt with in some of the applications, considering robotic vision as a paradigmatic example. This particular choice was dictated by the fact that there is a wide consensus that vision is one of the most and best studied and understood processing modalities in current computational neuroscience research [
85]. There are now several reviews that have addressed theory and application from the point of view of artificial intelligence and one of the trends has been to approach issues of explainability and transparency by reference to what we know about the brain and neural processes in vision [
80]. This, however, presents some of the same limitations we have raised regarding assumptions of biological plausibility given incomplete and debated knowledge in neuroscience. That is, we still do not know many details about vision as a biological process and less in relation to incorporating it into behavioral functions. Neurological facts are still maps (models) of reality rather than the thing itself (the territory).
An additional limitation in the current literature is that the applications are very specific and modular and rarely link, e.g., two different sensory modalities or cognitive functions. While it is important to consider the generalization of findings and methodologies to other cognitive functions and domains other than vision, this is performed in principle and very few applications and implementations transcend the original modules. We have highlighted some of the tools already available in the literature that could potentially be used to lay these bridges across modalities and functions.
In terms of the state-of-the-art, the marriage between biological plausibility and computational efficiency has no real compelling grounding. We argue that this changes when the relationship is referenced to embodied agents such as robots. The reason for this is that explainability and transparency can be shifted from the purely computational and algorithmic level to the behavioral and cognitive level. As Chen et al. [
163] argue, neurorobotics follows similar methodological logic to the field of neuroethology. Neuroscientists are faced with similar problems in understanding why some systems behave the way they do. The neuroethological approach attempts to address this issue by closely observing behavior while recording neurons or manipulating brain circuits. In a similar way, neurorobotics can be used to explain how neural network activities lead to a certain behavior. We submit that cognitive neurorobotics provides so far the best grounding to integrate biological plausibility, computational efficiency, and behavioral explainability. It further provides the best testbed to examine the ethical issues in interpreting emerging complex deep neural networks and machine learning behaviors. As neuromorphic systems become more complex, the interpretability of their behavior might become a challenge. Understanding how these systems make decisions and behave in complex real-world scenarios will be non-trivial.