Deep Medullary Vein and MRI Markers Were Related to Cerebral Hemorrhage Subtypes
Abstract
:1. Introduction
2. Methods and Materials
2.1. Patients
2.2. Magnetic Resonance Imaging Protocol
2.3. MRI Data Analysis and CSVD Score
2.4. ICH Subtype
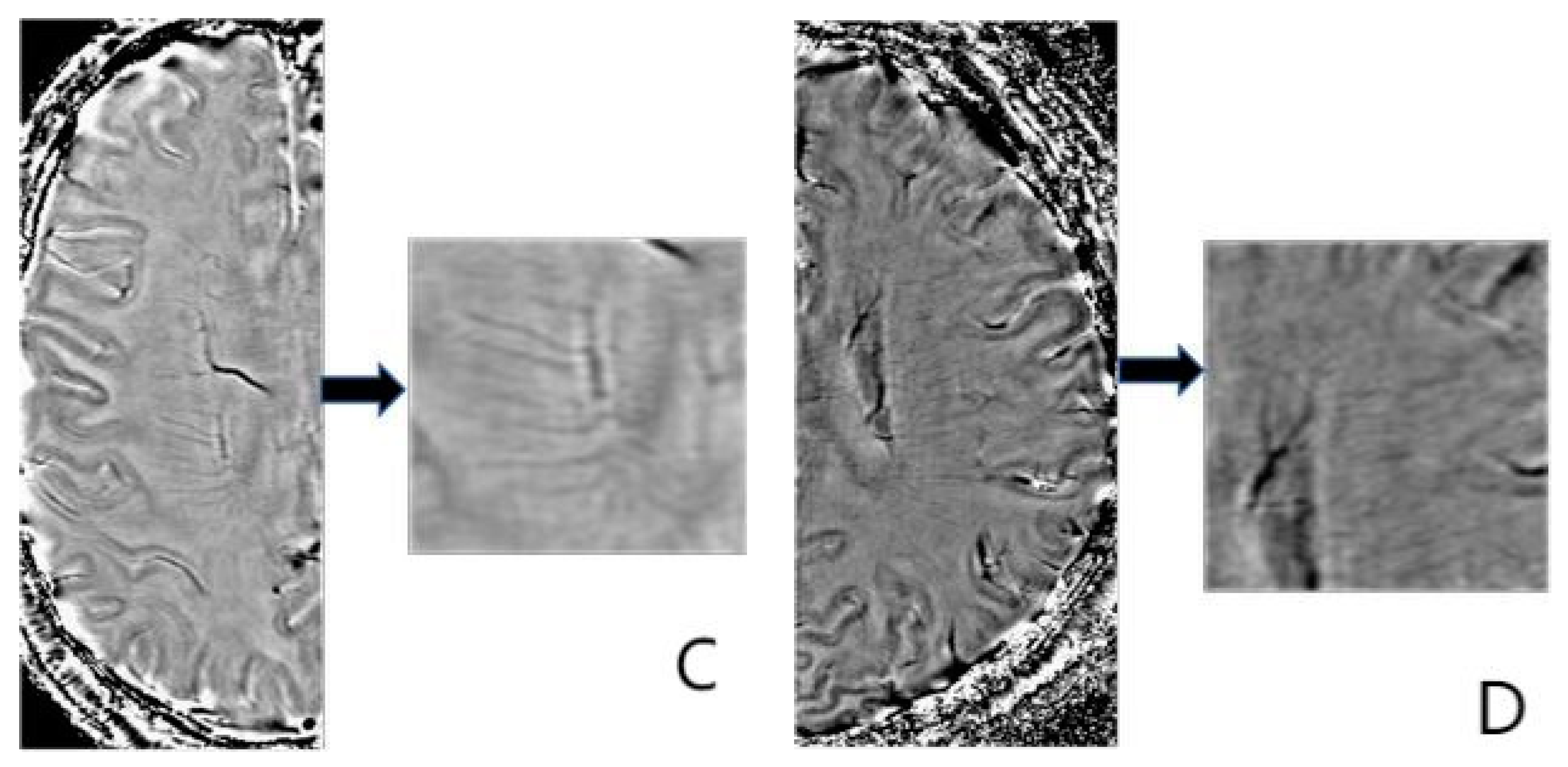
2.5. Deep Medullary Veins Score
2.6. Statistical Analysis
3. Result
3.1. Subject Characteristics in Different ICH Subtypes
3.2. Subject Characteristics in Different DMV Groups
3.3. Relationship between DMV and Other Variables
3.4. ROC in DMV Groups
3.5. Logistic Regression Analysis of ICH Subtypes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Zhang, D.D.; Cao, Y.; Mu, J.Y.; Liu, Y.M.; Gao, F.; Han, F.; Zhai, F.F.; Zhou, L.X.; Ni, J.; Yao, M.; et al. Inflammatory biomarkers and cerebral small vessel disease: A community-based cohort study. Stroke Vasc. Neurol. 2022, 7, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qin, R.R.; Chen, J.; Jiang, H.F.; Tang, P.; Wang, Y.J.; Xu, D.W.; Xu, T.; Yuan, T.F. Neuropsychiatric symptoms and altered sleep quality in cerebral small vessel disease. Front. Psychiatry 2022, 13, 882922. [Google Scholar] [CrossRef]
- Yang, Y.; Deng, G.; Wang, P.; Lv, G.; Mao, R.; Sun, Y.; Wang, B.; Liu, X.; Bian, L.; Zhou, D. A Selenium Nanocomposite Protects the Mouse Brain from Oxidative Injury Following Intracerebral Hemorrhage. Int. J. Nanomed. 2021, 16, 775–788. [Google Scholar] [CrossRef]
- Pasi, M.; Sugita, L.; Xiong, L.; Charidimou, A.; Boulouis, G.; Pongpitakmetha, T.; Singh, S.; Kourkoulis, C.; Schwab, K.; Greenberg, S.M.; et al. Association of Cerebral Small Vessel Disease and Cognitive Decline After Intracerebral Hemorrhage. Neurology 2021, 96, e182–e192. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.C.; Jensen-Kondering, U.; Sacco, S.; Posener, S.; Benzakoun, J.; Pallud, J.; Oppenheim, C.; Varlet, P.; Turc, G. Can novel CT-and MR-based neuroimaging biomarkers further improve the etiological diagnosis of lobar intra-cerebral hemorrhage? J. Neurol. 2023, 270, 582–588. [Google Scholar] [CrossRef]
- Xu, Z.; Li, F.; Xing, D.; Song, H.; Chen, J.; Duan, Y.; Yang, B. A Novel Imaging Biomarker for Cerebral Small Vessel Disease Associated with Cognitive Impairment: The Deep-Medullary-Veins Score. Front. Aging Neurosci. 2021, 13, 720481. [Google Scholar] [CrossRef]
- Xu, Z.; Li, F.; Wang, B.; Xing, D.; Pei, Y.; Yang, B.; Duan, Y. New Insights in Addressing Cerebral Small Vessel Disease: Association with the Deep Medullary Veins. Front. Aging Neurosci. 2020, 12, 597799. [Google Scholar] [CrossRef]
- Yu, C.; Lu, W.; Qiu, J.; Wang, F.; Li, J.; Wang, L. Alterations of the Whole Cerebral Blood Flow in Patients with Different Total Cerebral Small Vessel Disease Burden. Front. Aging Neurosci. 2020, 12, 175. [Google Scholar] [CrossRef]
- Dolui, S.; Tisdall, D.; Vidorreta, M.; Jacobs, D.R.; Nasrallah, I.M.; Bryan, R.N.; Wolk, D.A.; Detre, J.A. Characterizing a perfusion-based periventricular small vessel region of interest. Neuroimage Clin. 2019, 23, 101897. [Google Scholar] [CrossRef]
- Kim, H.G.; Choi, J.W.; Han, M.; Lee, J.H.; Lee, H.S. Texture analysis of deep medullary veins on susceptibility-weighted imaging in infants: Evaluating developmental and ischemic changes. Eur. Radiol. 2020, 30, 2594–2603. [Google Scholar] [CrossRef]
- Moran, C.; Phan, T.G.; Srikanth, V.K. Cerebral small vessel disease: A review of clinical, radiological, and histopathological phenotypes. Int. J. Stroke 2012, 7, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Ao, D.H.; Zhang, D.D.; Zhai, F.F.; Zhang, J.T.; Han, F.; Li, M.L.; Ni, J.; Yao, M.; Zhang, S.Y.; Cui, L.Y.; et al. Brain deep medullary veins on 3-T MRI in a population-based cohort. J. Cereb. Blood Flow. Metab. 2021, 41, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wei, L.; Wang, J.; Shan, Y.; Cai, W.; Men, X.; Liu, S.; Kang, Z.; Lu, Z.; Mok, V.C.T.; et al. Decreased visible deep medullary veins is a novel imaging marker for cerebral small vessel disease. Neurol. Sci. 2020, 41, 1497–1506. [Google Scholar] [CrossRef] [PubMed]
- Fazekas, F.; Barkhof, F.; Wahlund, L.; Pantoni, L.; Erkinjuntti, T.; Scheltens, P.; Schmidt, R. CT and MRI rating of white matter lesions. Cerebrovasc. Dis. 2002, 13 (Suppl. S2), 31–36. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Vernooij, M.W.; Cordonnier, C.; Viswanathan, A.; Salman, R.A.-S.; Warach, S.; Launer, L.J.; A Van Buchem, M.; Breteler, M.M. Cerebral microbleeds: A guide to detection and interpretation. Lancet Neurol. 2009, 8, 165–174. [Google Scholar] [CrossRef]
- Charidimou, A.; Boulouis, G.; Pasi, M.; Auriel, E.; van Etten, E.S.; Haley, K.; Ayres, A.; Schwab, K.M.; Martinez-Ramirez, S.; Goldstein, J.N.; et al. MRI-visible perivascular spaces in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology 2017, 88, 1157–1164. [Google Scholar] [CrossRef]
- Charidimou, A.; Boulouis, G.; Frosch, M.P.; Baron, J.-C.; Pasi, M.; Albucher, J.F.; Banerjee, G.; Barbato, C.; Bonneville, F.; Brandner, S.; et al. The Boston criteria version 2.0 for cerebral amyloid angiopathy: A multicentre, retrospective, MRI-neuropathology diagnostic accuracy study. Lancet Neurol. 2022, 21, 714–725. [Google Scholar] [CrossRef]
- Delavaran, H.; Jönsson, A.C.; Lövkvist, H.; Iwarsson, S.; Elmståhl, S.; Norrving, B.; Lindgren, A. Cognitive function in stroke survivors: A 10-year follow-up study. Acta Neurol. Scand. 2017, 136, 187–194. [Google Scholar] [CrossRef]
- Zhang, R.; Huang, P.; Jiaerken, Y.; Wang, S.; Hong, H.; Luo, X.; Xu, X.; Yu, X.; Li, K.; Zeng, Q.; et al. Venous disruption affects white matter integrity through increased interstitial fluid in cerebral small vessel disease. J. Cereb. Blood Flow. Metab. 2021, 41, 157–165. [Google Scholar] [CrossRef]
- Zhang, K.; Zhou, Y.; Zhang, W.; Li, Q.; Sun, J.; Lou, M. MRI-visible perivascular spaces in basal ganglia but not centrum semiovale or hippocampus were related to deep medullary veins changes. J. Cereb. Blood Flow. Metab. 2022, 42, 136–144. [Google Scholar] [CrossRef]
- Park, S.H.; Zong, X.; Gao, Y.; Lin, W.; Shen, D. Segmentation of perivascular spaces in 7T MR image using auto-context model with orientation-normalized features. Neuroimage 2016, 134, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Mestre, H.; Kostrikov, S.; Mehta, R.I.; Nedergaard, M. Perivascular spaces, glymphatic dysfunction, and small vessel disease. Clin. Sci. 2017, 131, 2257–2274. [Google Scholar] [CrossRef]
- Black, S.; Gao, F.; Bilbao, J. Understanding white matter disease: Imaging-pathological correlations in vascular cognitive impairment. Stroke 2009, 40 (Suppl. S3), S48–S52. [Google Scholar] [CrossRef] [PubMed]
- Nan, D.; Cheng, Y.; Feng, L.; Zhao, M.; Ma, D.; Feng, J. Potential Mechanism of Venous System for Leukoaraiosis: From post-mortem to in vivo Research. Neurodegener. Dis. 2019, 19, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Keith, J.; Gao, F.Q.; Noor, R.; Kiss, A.; Balasubramaniam, G.; Au, K.; Rogaeva, E.; Masellis, M.; Black, S.E. Collagenosis of the Deep Medullary Veins: An Underrecognized Pathologic Correlate of White Matter Hyperintensities and Periventricular Infarction? J. Neuropathol. Exp. Neurol. 2017, 76, 299–312. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, Y.; Yan, S.; Zhong, G.; Liu, C.; Jiaerken, Y.; Song, R.; Yu, X.; Zhang, M.; Lou, M. A Brain Region-Based Deep Medullary Veins Visual Score on Susceptibility Weighted Imaging. Front. Aging Neurosci. 2017, 9, 269. [Google Scholar] [CrossRef]
- Zhang, R.; Li, Q.; Zhou, Y.; Yan, S.; Zhang, M.; Lou, M. The relationship between deep medullary veins score and the severity and distribution of intracranial microbleeds. Neuroimage Clin. 2019, 23, 101830. [Google Scholar] [CrossRef]
- Fisher, M.; French, S.; Ji, P.; Kim, R.C. Cerebral microbleeds in the elderly: A pathological analysis. Stroke 2010, 41, 2782–2785. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Sandercock, P.A.; Dennis, M.S.; Starr, J. Is breakdown of the blood-brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke 2003, 34, 806–812. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Q.; Zhang, R.; Zhang, W.; Yan, S.; Xu, J.; Wang, S.; Zhang, M.; Lou, M. Role of deep medullary veins in pathogenesis of lacunes: Longitudinal observations from the CIRCLE study. J. Cereb. Blood Flow. Metab. 2020, 40, 1797–1805. [Google Scholar] [CrossRef]
- Pasi, M.; Charidimou, A.; Boulouis, G.; Auriel, E.; Ayres, A.; Schwab, K.M.; Goldstein, J.N.; Rosand, J.; Viswanathan, A.; Pantoni, L.; et al. Mixed-location cerebral hemorrhage/microbleeds: Underlying microangiopathy and recurrence risk. Neurology 2018, 90, e119–e126. [Google Scholar] [CrossRef] [PubMed]


| Clinical and Imaging Characters | ICH (n = 232) | p-Value | ||
|---|---|---|---|---|
| CAA-Related (n = 38) | HTNA-Related (n = 96) | Mixed (n = 98) | ||
| Age (years), mean (SD) | 65.00 ± 15.61 * | 56.45 ± 13.67 † | 64.32 ± 11.81 | <0.001 |
| Male, n(%) | 17(44.7) * | 73(76.0) | 63(64.3) # | 0.002 |
| Hypertension, n(%) | 19(50.0) * | 73(76.0) | 84(85.7) # | <0.001 |
| Alcohol use, n(%) | 32(84.2) * | 59(61.5) | 65(66.3) # | <0.05 |
| Tobacco use, n(%) | 28(73.7) * | 49(51.0) | 62(63.3) | <0.05 |
| Diabetes, n(%) | 5(13.2) | 22(22.9) | 28(28.6) | 0.167 |
| Hyperlipidemia, n(%) | 5(13.2) | 18(18.8) | 18(18.4) | 0.742 |
| Coronary heart disease, n(%) | 2(5.3) | 1(1.0) | 5(5.1) | 0.202 |
| Stroke history, n(%) | 4(10.5) | 5(5.2) | 11(11.2) | 0.280 |
| PVS, median (IQR) | 1.5(1, 2) | 1(1, 2) † | 2(1, 3) | <0.001 |
| CMB, median (IQR) | 1(0, 1.25) | 1(0, 1) † | 2(1, 2) # | <0.05 |
| Lacuna, n(%) | 12(31.6) | 39(40.6) † | 69(70.4) # | <0.001 |
| DWMH, median (IQR) | 1(0.75, 2) | 1(0, 2) † | 2(2, 3) # | <0.001 |
| PWMH, median (IQR) | 1(0, 2) | 1(0, 2) † | 2(2, 3) # | <0.001 |
| CSVD score, median (IQR) | 2(1, 3) | 2(1, 3) † | 5(3, 5) # | <0.001 |
| FDMV score, median (IQR) | 2(2, 3) | 2(2, 2) † | 2(2, 3) | 0.002 |
| PDMV score, median (IQR) | 2(1, 2) | 2(1, 2) | 2(1, 2) | 0.077 |
| ODMV score, median (IQR) | 2(1, 2) | 2(1, 2) † | 2(1, 2) | 0.002 |
| TDMV score, median (IQR) | 6(5, 6) | 5(4, 6) † | 6(5, 7) | 0.001 |
| Clinical and Imaging Characters | DMV Score | p-Value | ||
|---|---|---|---|---|
| Mild (0–3) (n = 23) | Moderate (4–6) (n = 143) | Severe (7–9) (n = 66) | ||
| Age (years), mean (SD) | 48.08 ± 13.07 * | 60.21 ± 13.10 † | 67.83 ± 11.68 | <0.001 |
| Male, n(%) | 16(69.6) | 95(66.4) | 42(63.6) | 0.858 |
| Hypertension, n(%) | 12(52.2) * | 110(76.9) | 54(81.8) # | <0.001 |
| Alcohol use, n(%) | 5(21.7) | 52(36.4) | 19(28.8) | 0.275 |
| Tobacco use, n(%) | 8(34.8) | 59(41.2) | 26(39.4) | 0.833 |
| Diabetes, n(%) | 5(21.7) | 34(23.8) | 16(24.2) | 0.97 |
| Hyperlipidemia, n(%) | 1(4.3) | 34(23.8) | 6(9.1) | 0.07 |
| Coronary heart disease, n(%) | 1(4.3) | 3(2.1) | 4(6.1) | 0.263 |
| Stroke history, n(%) | 0(0) | 10(7.0) | 10(15.2) ** | 0.045 |
| PVS, median (IQR) | 1(1, 2) | 2(1, 2) | 2(1, 2) # | <0.05 |
| CMB, median (IQR) | 0(0, 1) | 1(0,2) | 1(0, 2) # | <0.05 |
| Lacuna, n(%) | 14(60.9) | 71(49.7) | 27(40.9) | 0.223 |
| DWMH, median (IQR) | 0(0, 2) * | 2(1, 2) † | 2(1, 3) # | <0.001 |
| PWMH, median (IQR) | 1(0, 2) | 2(1, 2) † | 2(1, 3) # | <0.001 |
| CSVD score, median (IQR) | 1(0, 3) * | 3(1, 5) † | 3(2, 5) # | <0.001 |
| TDMV Score | Age | Hypertension | Stroke History | PVS | CMB | Lacuna | DWMH | PWMH | CSVD Score | |
|---|---|---|---|---|---|---|---|---|---|---|
| TDMV score | - | |||||||||
| Age | 0.480 # (p < 0.001) | - | ||||||||
| Hypertension | 0.202 * (p = 0.002) | 0.181 * (p = 0.006) | - | |||||||
| Stroke History | 0.194 * (p = 0.003) | 0.126 (p = 0.056) | 0.030 (p = 0.653) | - | ||||||
| PVS | 0.190 * (p = 0.004) | 0.211 * (p = 0.001) | 0.016 (p = 0.804) | 0.002 (p = 0.972) | - | |||||
| CMB | 0.211 * (p = 0.001) | 0.271 (p < 0.001) | 0.177 * (p = 0.007) | 0.197 * (p = 0.003) | 0.246 # (p < 0.001) | - | ||||
| Lacuna | 0.125 (p = 0.058) | 0.264 (p < 0.001) | 0.241 (p < 0.001) | 0.143 * (p = 0.029) | 0.286 # (p < 0.001) | 0.417 # (p < 0.001) | - | |||
| DWMH | 0.310 # (p < 0.001) | 0.501 # (p < 0.001) | 0.188 * (p = 0.004) | 0.200 * (p = 0.002) | 0.278 # (p < 0.001) | 0.516 # (p < 0.001) | 0.558 # (p < 0.001) | - | ||
| PWMH | 0.295 # (p < 0.001) | 0.528 # (p < 0.001) | 0.243 # (p < 0.001) | 0.279 # (p < 0.001) | 0.293 # (p < 0.001) | 0.496 # (p < 0.001) | 0.559 # (p < 0.001) | 0.830 # (p < 0.001) | - | |
| CSVD Score | 0.308 # (p < 0.001) | 0.424 # (p < 0.001) | 0.243 # (p < 0.001) | 0.224 * (p = 0.001) | 0.468 # (p < 0.001) | 0.793 # (p < 0.001) | 0.717 # (p < 0.001) | 0.811 # (p < 0.001) | 0.790 # (p < 0.001) | - |
| Groups | Parameter | Cut-off | AUC | Sensitivity | Specificity |
|---|---|---|---|---|---|
| Mild vs. Moderate | Age | 52.5 | 0.734 | 90.4 | 46.7 |
| PVS | 1.5 | 0.630 | 62.4 | 57.9 | |
| CMB | 0.5 | 0.633 | 77.6 | 37.4 | |
| DWMH | 1.5 | 0.678 | 70.4 | 100 | |
| PWMH | 1.5 | 0.630 | 65.6 | 51.4 | |
| CSVD score | 1.5 | 0.695 | 83.2 | 38.3 | |
| Mild vs. Severe | Age | 54.5 | 0.879 | 67.8 | 78.3 |
| PVS | 2.5 | 0.697 | 22.4 | 100 | |
| CMB | 0.5 | 0.695 | 69.9 | 52.2 | |
| DWMH | 0.5 | 0.789 | 80.4 | 52.2 | |
| PWMH | 1.5 | 0.747 | 52.4 | 69.6 | |
| CSVD score | 1.5 | 0.793 | 72.7 | 60.9 | |
| Moderate vs. Severe | Age | 54.5 | 0.668 | 86.7 | 78.3 |
| DWMH | 0.5 | 0.621 | 97.0 | 52.2 | |
| PWMH | 1.5 | 0.628 | 71.2 | 69.6 | |
| CSVD score | 1.5 | 0.617 | 86.4 | 60.9 |
| OR | OR Value (95% CI) | p-Value | |||
|---|---|---|---|---|---|
| Lowest | Highest | ||||
| CAA-related vs. Mixed ICH | age | 1.074 | 1.028 | 1.122 | p = 0.001 |
| hypertension | 5.169 | 1.969 | 13.567 | p = 0.001 | |
| HTNA-related vs. Mixed ICH | DWMH | 0.452 | 0.253 | 0.809 | p < 0.05 |
| PVS | 0.633 | 0.416 | 0.963 | p < 0.05 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Xiang, Y.; Peng, Y.; Zeng, P.; Zeng, B.; Chai, Y.; Li, Y. Deep Medullary Vein and MRI Markers Were Related to Cerebral Hemorrhage Subtypes. Brain Sci. 2023, 13, 1315. https://doi.org/10.3390/brainsci13091315
Wang D, Xiang Y, Peng Y, Zeng P, Zeng B, Chai Y, Li Y. Deep Medullary Vein and MRI Markers Were Related to Cerebral Hemorrhage Subtypes. Brain Sciences. 2023; 13(9):1315. https://doi.org/10.3390/brainsci13091315
Chicago/Turabian StyleWang, Dan, Yayun Xiang, Yuling Peng, Peng Zeng, Bang Zeng, Ying Chai, and Yongmei Li. 2023. "Deep Medullary Vein and MRI Markers Were Related to Cerebral Hemorrhage Subtypes" Brain Sciences 13, no. 9: 1315. https://doi.org/10.3390/brainsci13091315
APA StyleWang, D., Xiang, Y., Peng, Y., Zeng, P., Zeng, B., Chai, Y., & Li, Y. (2023). Deep Medullary Vein and MRI Markers Were Related to Cerebral Hemorrhage Subtypes. Brain Sciences, 13(9), 1315. https://doi.org/10.3390/brainsci13091315





