Moderate Hyperkalemia Regulates Autophagy to Reduce Cerebral Ischemia-Reperfusion Injury in a CA/CPR Rat Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Preparation and Animal Grouping
2.2. Study Protocol and Establishment of the CA/CPR Rat Model
2.3. Neurological Evaluation
2.4. Preparation of Brain Tissues
2.5. Biochemical Analysis: Serum (K+), ROS, MDA, SOD and GSH Levels
2.6. HE and Nissl Staining
2.7. Transmission Electron Microscopy
2.8. Immunofluorescence Staining
2.9. Western Blot (WB) Analysis
2.10. Statistical Analysis
3. Results
3.1. Baseline Characteristics
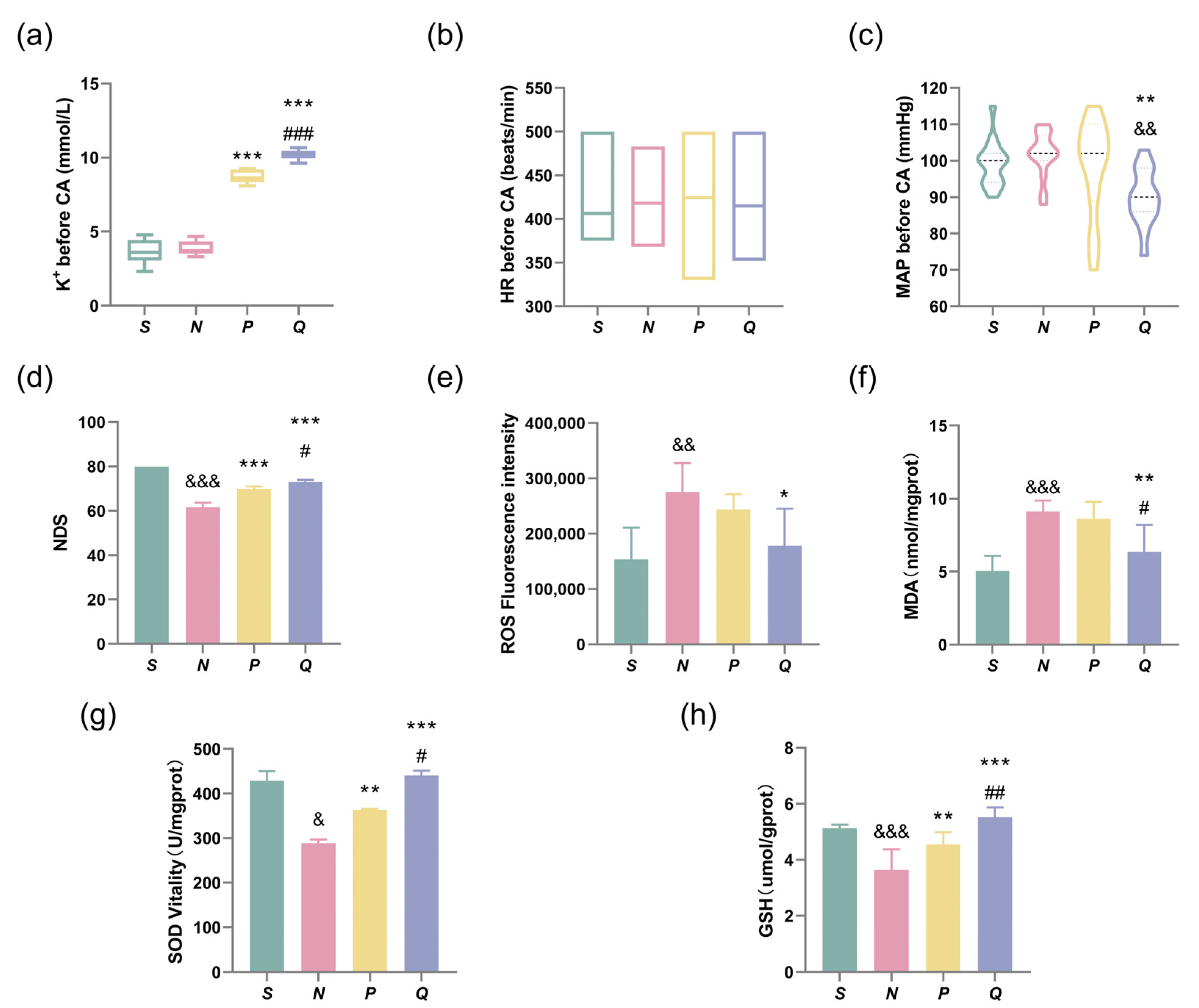
3.2. Elevating the Pre-CA Serum (K+) Levels in Rats Improves Neurological Function and Reduces ROSC-Induced Neuronal Damage
3.3. HE and Nissl Staining
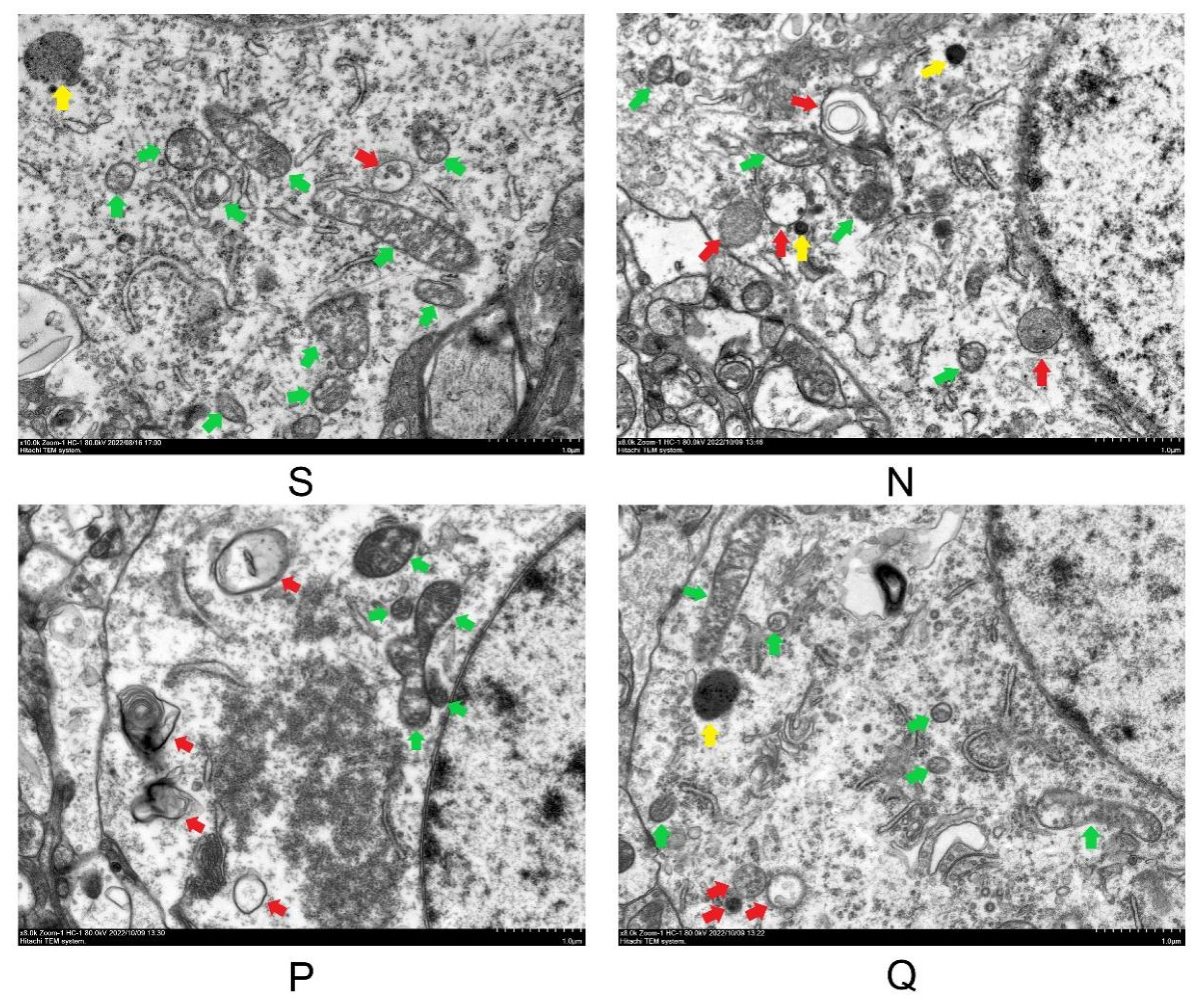
3.4. Scanning Electron Microscopy Was Performed on the Cerebral Cortex of Each Rat Group after 24 h of Reperfusion
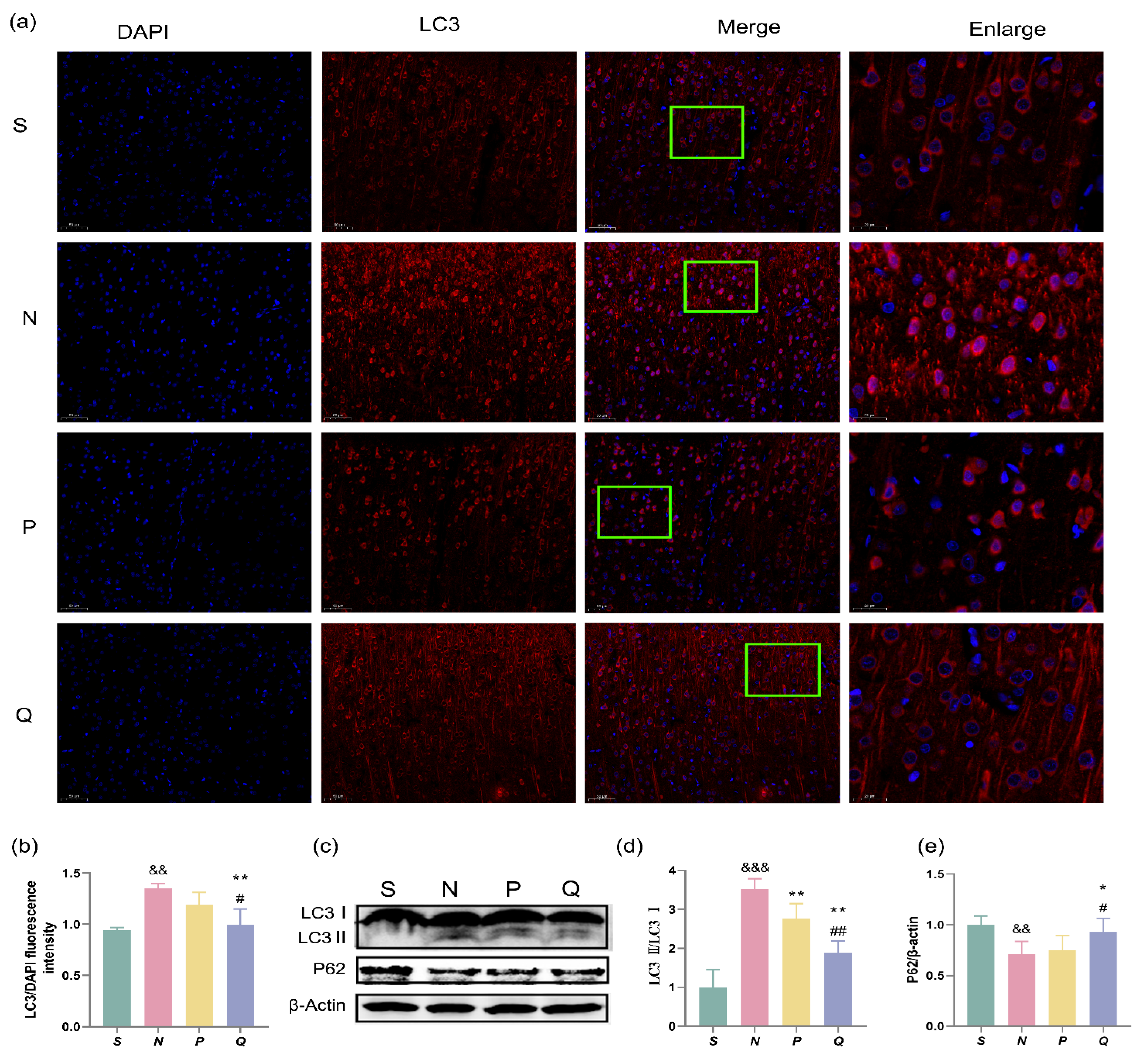
3.5. Expression of Autophagosome Membrane Protein LC3 and Autophagy Adaptor P62 in Cerebral Cortex of Rats
3.6. Lysosome Expression and Co-Expression with LC3 in Cerebral Cortex of CA/CPR Rats
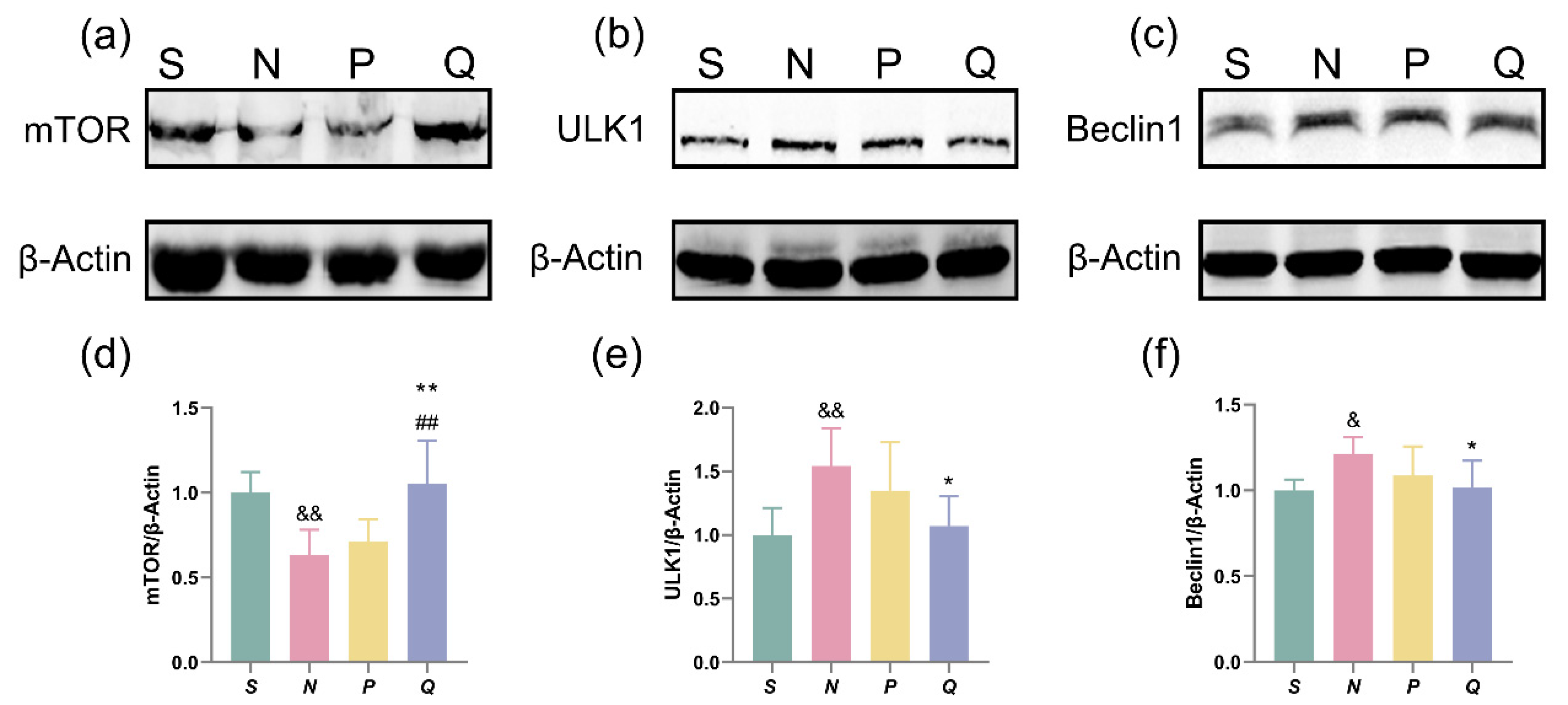
3.7. The Effect of Moderately Increasing Serum (K+) Levels on the mTOR-ULK1-Beclin1 Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Scoring Criteria | Score |
|---|---|
| (A) General behavioral deficit | |
| Consciousness | Normal [10], Stuporous [5], Comatose [0] |
| Arousal | Eyes open spontaneously [3], Eyes open to pain [1], No eye opening [0] |
| Respiration | Normal [6], Abnormal [0], Absent [0]: Total score = 19 |
| (B) Brain-stem function | |
| Olfaction | Present [3], Absent [0] |
| Vision | Present [3], Absent [0] |
| Pupillary reflex | Present [3], Absent [0] |
| Corneal reflex | Present [3], Absent [0] |
| Startle reflex | Present [3], Absent [0] |
| Whisker stimulation | Present [3], Absent [0] |
| Swallowing | Present [3], Absent [0]: Total score = 21 |
| (C) Motor assessment | |
| Strength (left and right side tested and scored separately) | Normal [3], Stiff/weak [1], No movement/paralyzed [0]: Total score = 6 |
| (D) Sensory assessment | |
| Pain (left and right side tested and scored separately) | Brisk withdrawal with pain [3], Weak or abnormal response [1], No withdrawal [0]: Total score = 6 |
| (E) Motor behavior | |
| Gait coordination | Normal [3], Abnormal [1], Absent [0] |
| Balance on beam | Normal [3], Abnormal [1], Absent [0]: Total score = 6 |
| (F) Behavior | |
| Righting reflex | Normal [3], Abnormal [1], Absent [0] |
| Negative geotaxis | Normal [3], Abnormal [1], Absent [0] |
| Visual placing | Normal [3], Abnormal [1], Absent [0] |
| Turning alley | Normal [3], Abnormal [1], Absent [0]: Total score = 12 |
| (G) Seizures | No Seizure [10], Focal Seizure [5], General Seizure [0], Total score = 10 |
References
- Wu, M.; Gu, X.; Ma, Z. Mitochondrial Quality Control in Cerebral Ischemia-Reperfusion Injury. Mol. Neurobiol. 2021, 58, 5253–5271. [Google Scholar] [CrossRef] [PubMed]
- Katz, A.; Brosnahan, S.B.; Papadopoulos, J.; Parnia, S.; Lam, J.Q. Pharmacologic neuroprotection in ischemic brain injury after cardiac arrest. Ann. N. Y. Acad. Sci. 2022, 1507, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Button, R.W.; Luo, S.; Rubinsztein, D.C. Autophagic activity in neuronal cell death. Neurosci. Bull. 2015, 31, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Kourtis, N.; Tavernarakis, N. Autophagy and cell death in model organisms. Cell Death Differ. 2009, 16, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Llambi, F. Cell Death Signaling. Cold Spring Harb Perspect Biol. 2015, 7, a006080. [Google Scholar] [CrossRef] [PubMed]
- Parzych, K.R.; Klionsky, D.J. An overview of autophagy: Morphology, mechanism, and regulation. Antioxid Redox Signal. 2014, 20, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Xia, Q.; Chu, K.T.; Pan, J.; Sun, L.N.; Zeng, B.; Zhu, Y.J.; Wang, Q.; Wang, K.; Luo, B.Y. Severe global cerebral ischemia-induced programmed necrosis of hippocampal CA1 neurons in rat is prevented by 3-methyladenine: A widely used inhibitor of autophagy. J. Neuropathol. Exp. Neurol. 2011, 70, 314–322. [Google Scholar] [CrossRef]
- Wang, W.Y.; Shi, J.X.; Chen, M.H.; Zhuge, X.Z.; Dai, C.G.; Xie, L. Calpain inhibitor MDL28170 alleviates cerebral ischemia-reperfusion injury by suppressing inflammation and autophagy in a rat model of cardiac arrest. Exp. Ther. Med. 2023, 25, 196. [Google Scholar] [CrossRef]
- Zheng, J.H.; Xie, L.; Li, N.; Fu, Z.Y.; Tan, X.F.; Tao, R.; Qin, T.; Chen, M.H. PD98059 protects the brain against mitochondrial-mediated apoptosis and autophagy in a cardiac arrest rat model. Life Sci. 2019, 232, 116618. [Google Scholar] [CrossRef]
- Degterev, A.; Huang, Z.; Boyce, M.; Li, Y.; Jagtap, P.; Mizushima, N.; Cuny, G.D.; Mitchison, T.J.; Moskowitz, M.A.; Yuan, J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 2005, 1, 112–119. [Google Scholar] [CrossRef]
- Liu, C.; Gao, Y.; Barrett, J.; Hu, B. Autophagy and protein aggregation after brain ischemia. J. Neurochem. 2010, 115, 68–78. [Google Scholar] [CrossRef]
- Wen, Y.D.; Sheng, R.; Zhang, L.S.; Han, R.; Zhang, X.; Zhang, X.D.; Han, F.; Fukunaga, K.; Qin, Z.H. Neuronal injury in rat model of permanent focal cerebral ischemia is associated with activation of autophagic and lysosomal pathways. Autophagy 2008, 4, 762–769. [Google Scholar] [CrossRef]
- Liu, W.; Shang, G.; Yang, S.; Huang, J.; Xue, X.; Lin, Y.; Zheng, Y.; Wang, X.; Wang, L.; Lin, R.; et al. Electroacupuncture protects against ischemic stroke by reducing autophagosome formation and inhibiting autophagy through the mTORC1-ULK1 complex-Beclin1 pathway. Int. J. Mol. Med. 2016, 37, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Qin, S.; Xie, L.; Qin, T.; Yang, Y.; Fang, W.; Chen, M. Elevated Serum Potassium Concentration Alleviates Cerebral Ischemia-Reperfusion Injury via Mitochondrial Preservation. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 48, 1664–1674. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Qin, T.; Li, N.; Yang, Y.; Zheng, J.; Xie, L.; Chen, M. High-potassium preconditioning enhances tolerance to focal cerebral ischemia-reperfusion injury through anti-apoptotic effects in male rats. J. Neurosci. Res. 2019, 97, 1253–1265. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, H. Mechanism of myocardial protection with potassium arrest in isolated ischemic rat hearts. Kokyu Junkan 1991, 39, 1151–1157. [Google Scholar]
- Yamamoto, H.; Magishi, K.; Goh, K.; Sasajima, T.; Yamamoto, F. Cardioprotective effects of normothermic reperfusion with oxygenated potassium cardioplegia: A possible mechanism. Interact. Cardiovasc. Thorac. Surg. 2009, 9, 598–604. [Google Scholar] [CrossRef]
- Hessel, E.A., 2nd. A Brief History of Cardiopulmonary Bypass. Semin. Cardiothorac. Vasc. Anesth. 2014, 18, 87–100. [Google Scholar] [CrossRef]
- Dittrich, K.L.; Walls, R.M. Hyperkalemia: ECG manifestations and clinical considerations. J. Emerg. Med. 1986, 4, 449–455. [Google Scholar] [CrossRef]
- Werner, C.; Lu, H.; Engelhard, K.; Unbehaun, N.; Kochs, E. Sevoflurane impairs cerebral blood flow autoregulation in rats: Reversal by nonselective nitric oxide synthase inhibition. Anesth. Analg. 2005, 101, 509–516. [Google Scholar] [CrossRef]
- Sun, N.; Luo, W.; Li, L.Z.; Luo, Q. Monitoring hemodynamic and metabolic alterations during severe hemorrhagic shock in rat brains. Acad. Radiol. 2014, 21, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Liu, T.W.; Xie, L.; Song, F.Q.; He, T.; Zeng, Z.Y.; Mo, S.R. A simpler cardiac arrest model in rats. Am. J. Emerg. Med. 2007, 25, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.S.; Xie, L.; Wang, W.Y.; Zhao, G.Y.; Tian, X.Y.; Chen, M.H. Pomelo peel oil alleviates cerebral NLRP3 inflammasome activation in a cardiopulmonary resuscitation rat model. Exp. Ther. Med. 2021, 21, 233. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Koenig, M.A.; Shin, H.C.; Zhen, G.; Pardo, C.A.; Hanley, D.F.; Thakor, N.V.; Geocadin, R.G. Improving neurological outcomes post-cardiac arrest in a rat model: Immediate hypothermia and quantitative EEG monitoring. Resuscitation 2008, 76, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Kalogeris, T.; Baines, C.; Krenz, M.; Korthuis, R. Ischemia/Reperfusion. Compr. Physiol. 2016, 7, 113–170. [Google Scholar]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef]
- Tanida, I.; Minematsu-Ikeguchi, N.; Ueno, T.; Kominami, E. Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy. Autophagy 2005, 1, 84–91. [Google Scholar] [CrossRef]
- Xu, F.; Gu, J.H.; Qin, Z.H. Neuronal autophagy in cerebral ischemia. Neurosci. Bull. 2012, 28, 658–666. [Google Scholar] [CrossRef]
- Itakura, E.; Mizushima, N. Characterization of autophagosome formation site by a hierarchical analysis of mammalian Atg proteins. Autophagy 2010, 6, 764–776. [Google Scholar] [CrossRef]
- Szwed, A.; Kim, E.; Jacinto, E. Regulation and metabolic functions of mTORC1 and mTORC2. Physiol. Rev. 2021, 101, 1371–1426. [Google Scholar] [CrossRef]
- Ganley, I.G.; Lam du, H.; Wang, J.; Ding, X.; Chen, S.; Jiang, X. ULK1.ATG13.FIP200 complex mediates mTOR signaling and is essential for autophagy. J. Biol. Chem. 2009, 284, 12297–12305. [Google Scholar] [CrossRef] [PubMed]
- Wirth, M.; Joachim, J.; Tooze, S.A. Autophagosome formation--the role of ULK1 and Beclin1-PI3KC3 complexes in setting the stage. Semin. Cancer Biol. 2013, 23, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Zachari, M.; Ganley, I.G. The mammalian ULK1 complex and autophagy initiation. Essays Biochem. 2017, 61, 585–596. [Google Scholar] [PubMed]
- Poillet-Perez, L.; Despouy, G.; Delage-Mourroux, R.; Boyer-Guittaut, M. Interplay between ROS and autophagy in cancer cells, from tumor initiation to cancer therapy. Redox Biol. 2015, 4, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Levine, B. Autosis and autophagic cell death: The dark side of autophagy. Cell Death Differ. 2015, 22, 367–376. [Google Scholar] [CrossRef]
- Balduini, W.; Carloni, S.; Buonocore, G. Autophagy in hypoxia-ischemia induced brain injury: Evidence and speculations. Autophagy 2009, 5, 221–223. [Google Scholar] [CrossRef]
- Xu, S.; Huang, P.; Yang, J.; Du, H.; Wan, H.; He, Y. Calycosin alleviates cerebral ischemia/reperfusion injury by repressing autophagy via STAT3/FOXO3a signaling pathway. Phytomedicine 2023, 115, 154845. [Google Scholar] [CrossRef]
- Zhao, F.; Peng, C.; Li, H.; Chen, H.; Yang, Y.; Ai, Q.; Chen, N.; Liu, F. Paeoniae Radix Rubra extract attenuates cerebral ischemia injury by inhibiting ferroptosis and activating autophagy through the PI3K/Akt signalling pathway. J. Ethnopharmacol. 2023, 315, 116567. [Google Scholar] [CrossRef]
- Tang, H.; Lü, Q.Y.; Wang, H.J.; Jiang, S.S.; Chen, C.T.; Tian, H.M.; Xiang, J.; Li, Y.P. Acupuncture regulates autophagy in hippocampal neurons of cerebral ischemia/reperfusion injured rats by activating type Ⅲ PI3K/Beclin-1 pathway. Zhen Ci Yan Jiu 2023, 48, 423–430. [Google Scholar]
- Kuang, Q.; Purhonen, P.; Hebert, H. Structure of potassium channels. Cell Mol. Life Sci. 2015, 72, 3677–3693. [Google Scholar] [CrossRef]
- Williams, A.; Sarkar, S.; Cuddon, P.; Ttofi, E.K.; Saiki, S.; Siddiqi, F.H.; Jahreiss, L.; Fleming, A.; Pask, D.; Goldsmith, P.; et al. Novel targets for Huntington’s disease in an mTOR-independent autophagy pathway. Nat. Chem. Biol. 2008, 4, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Caimmi, P.P.; Molinari, C.; Uberti, F.; Micalizzi, E.; Valente, G.; Mary, D.A.; Vacca, G.; Grossini, E. Intracoronary levosimendan prevents myocardial ischemic damages and activates survival signaling through ATP-sensitive potassium channel and nitric oxide. Eur. J. Cardiothorac. Surg. 2011, 39, e59–e67. [Google Scholar] [CrossRef] [PubMed]
- Samokhvalov, V.; Alsaleh, N.; El-Sikhry, H.E.; Jamieson, K.L.; Chen, C.B.; Lopaschuk, D.G.; Carter, C.; Light, P.E.; Manne, R.; Falck, J.R.; et al. Epoxyeicosatrienoic acids protect cardiac cells during starvation by modulating an autophagic response. Cell Death Dis. 2013, 4, e885. [Google Scholar] [CrossRef] [PubMed]
- Grossini, E.; Bellofatto, K.; Farruggio, S.; Sigaudo, L.; Marotta, P.; Raina, G.; De Giuli, V.; Mary, D.; Pollesello, P.; Minisini, R.; et al. Levosimendan inhibits peroxidation in hepatocytes by modulating apoptosis/autophagy interplay. PLoS ONE 2015, 10, e0124742. [Google Scholar] [CrossRef]
- Fernández, Á.F.; Liu, Y.; Ginet, V.; Shi, M.; Nah, J.; Zou, Z.; Zhou, A.; Posner, B.A.; Xiao, G.; Tanguy, M.; et al. Interaction between the autophagy protein Beclin 1 and Na+,K+-ATPase during starvation, exercise, and ischemia. JCI Insight. 2020, 5, e133282. [Google Scholar] [CrossRef]
- Fazan, F.; Colombari, D.S.A.; Menani, J.V.; Fazan, R., Jr.; Colombari, E. Electrocardiographic changes in the acute hyperkalaemia produced by intragastric KCl load in rats. Exp. Physiol. 2021, 106, 1263–1271. [Google Scholar] [CrossRef]
- Konopelski, P.; Ufnal, M. Electrocardiography in rats: A comparison to human. Physiol. Res. 2016, 65, 717–725. [Google Scholar] [CrossRef]
- Weiss, J.N.; Qu, Z.; Shivkumar, K. Electrophysiology of Hypokalemia and Hyperkalemia. Circ. Arrhythmia Electrophysiol. 2017, 10, e004667. [Google Scholar] [CrossRef]



| Baseline Characteristics | S (n = 15) | N (n = 15) | P (n = 15) | Q (n = 15) |
|---|---|---|---|---|
| BW (g) (I) | 228 ± 8 | 231 ± 12 | 228 ± 15 | 228 ± 11 |
| K+ (mmol/L) | 3.71 ± 0.41 | 3.5 ± 0.47 | 3.51 ± 0.36 | 3.62 ± 0.50 |
| HR (Beats/min) | 402 ± 36 | 400 ± 27 | 422 ± 40 | 423 ± 39 |
| MAP (mmHg) | 101 ± 9 | 104 ± 7 | 103 ± 15 | 104 ± 12 |
| P wave amplitude (mV) | 0.21 ± 0.02 | 0.20 ± 0.01 | 0.20 ± 0.04 | 0.21 ± 0.02 |
| QRS complex duration (ms) | 23 ± 1.10 | 24 ± 1.0 | 22 ± 1.2 | 24 ± 1.0 |
| BW (g) (ROSC 24 h) | 227 ± 7 | 213 ± 12 | 212 ± 15 | 211 ± 12 |
| Pre-CA | S (n = 15) | N (n = 15) | P (n = 15) | Q (n = 15) |
|---|---|---|---|---|
| T (min) | 10 | 10 | 8.9 ± 0.5 | 13.04 ± 1.04 |
| M (KCl) (mg) | 0 | 0 | 43.58 ± 3.88 | 63.89 ± 6.66 |
| K+ (mmol/L) | 3.66 ± 0.78 | 3.85 ± 0.47 | 8.76 ± 0.45 *** | 10.18 ± 0.30 ***### |
| HR (Beats/min) | 406 ± 35 | 418 ± 35 | 425 ± 53 | 415 ± 49 |
| MAP (mmHg) | 99 ± 6 | 101 ± 6 | 98 ± 13 | 90 ± 8 **&& |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Tian, X.; Shen, H.; Zhang, X.; Xie, L.; Chen, M. Moderate Hyperkalemia Regulates Autophagy to Reduce Cerebral Ischemia-Reperfusion Injury in a CA/CPR Rat Model. Brain Sci. 2023, 13, 1285. https://doi.org/10.3390/brainsci13091285
Wang X, Tian X, Shen H, Zhang X, Xie L, Chen M. Moderate Hyperkalemia Regulates Autophagy to Reduce Cerebral Ischemia-Reperfusion Injury in a CA/CPR Rat Model. Brain Sciences. 2023; 13(9):1285. https://doi.org/10.3390/brainsci13091285
Chicago/Turabian StyleWang, Xiaoqin, Xinyue Tian, Haiying Shen, Xiaohua Zhang, Lu Xie, and Menghua Chen. 2023. "Moderate Hyperkalemia Regulates Autophagy to Reduce Cerebral Ischemia-Reperfusion Injury in a CA/CPR Rat Model" Brain Sciences 13, no. 9: 1285. https://doi.org/10.3390/brainsci13091285
APA StyleWang, X., Tian, X., Shen, H., Zhang, X., Xie, L., & Chen, M. (2023). Moderate Hyperkalemia Regulates Autophagy to Reduce Cerebral Ischemia-Reperfusion Injury in a CA/CPR Rat Model. Brain Sciences, 13(9), 1285. https://doi.org/10.3390/brainsci13091285





