Abstract
(1) Background: The treatment of substance addiction is challenging and has persisted for decades, with only a few therapeutic options. Although there are some recommendations for specific treatments for Alcohol Use Disorder (AUD), there is no specific medication used to treat alcohol cravings, which could benefit millions of patients that are suffering from alcoholism. Cravings, or the urge to use drugs, refer to the desire to experience the effects of a previously experienced psychoactive substance. (2) Methods: We included original studies of alcohol abuse or dependence extracted from a controlled, blind, pharmacological treatment study which presented measures and outcomes related to alcohol cravings. (3) Results: Specific drugs used for the treatment of alcoholism, such as Naltrexone and Acamprosate, have had the best results in relieving craving symptoms, as well as promoting abstinence. Baclofen and anticonvulsants such as Gabapentin and Topiramate have shown good results in promoting abstinence and the cessation of cravings. (4) Conclusions: Specific drugs used for the treatment of alcoholism to obtain the best results can be considered the gold standard for promoting abstinence and relieving cravings. Anticonvulsants and Baclofen also had good results, with these medications being considered as second-line ones. Varenicline is an option for alcohol dependents who also concomitantly ingest tobacco.
1. Introduction
Alcohol Use Disorder (AUD) is a worldwide disease with strong impacts not only on people’s health, but also on the economy and society. The result is a greater number of sick patients, with consequent expenses due to hospitalizations and the involvement of a portion of the economically active population. According to the WHO (World Health Organization) surveys, worldwide, three million deaths a year result from the harmful use of alcohol, representing 5.3% of all deaths. The harmful use of alcohol is a causal factor for over 200 illnesses and injuries. Overall, 5.1% of the global burden of diseases and injuries is attributable to alcohol consumption, as calculated in terms of Disability-Adjusted Life Years Lost (DALY). Alcohol consumption causes death and disability relatively early in life. In the 20–39 age group, approximately 13.5% of the total deaths are attributable to alcohol. Unfortunately, treatment options aimed at abstinence are scarce and have poor results [1].
Despite this, there are numerous outcomes beyond abstinence that can improve an individual’s quality of life, given the immense difficulty for addicted patients to remain abstinent. An alternative to reducing their alcohol consumption or even achieving abstinence would be offered to treat the cravings.
Cravings were recognized for the first time by Jellinek and colleagues in 1955 as a central component of alcohol dependence syndrome [2]. In the most specific sense, cravings refers to the subjective longing or desire to use drugs. Within this perspective, the subjective experience of cravings is considered to be the primary aspect of and driving force behind drug use [2].
The contemporary theoretical framework regarding the phenomenon of cravings incorporates behavioral theories of classical conditioning, cognitive and motivational mechanisms, as well as anatomofunctional and neurobiochemical factors [3]. According to the Diagnostic and Statistical Manual of Mental Disorders (DSM) 5 cravings are one of the diagnostic criteria for alcohol dependence syndrome and is defined as “the desire to experience the effects of a previously experienced psychoactive substance” [4].
Therefore, in treating the impending and often uncontrollable urge to use drugs, the patient will use the substance less often, causing less harm. Specific medications for alcoholism continue to be the first options for both curbing cravings and maintaining abstinence. Opioid antagonists, especially Naltrexone, are drugs with the best responses [5,6,7,8,9,10].
There are several medications studies used for the treatment of AUD, including anticonvulsants, psychotropic drugs, and anti-smoking drugs. Other authors have presented some important results, and they can be considered as second-line medications or in refractory cases [11,12,13,14,15,16,17,18,19,20,21].
Unfortunately, there are no summaries or guidelines for the effective pharmacological treatment of cravings. Clinical trials are usually designed with an abstinence endpoint, but many of them collect data on secondary endpoints, such as cravings. Perhaps this explains the reason for there being only a few studies on this pathology, which requires attention and specifies treatment.
The objective of this review is to correlate the possible treatments that cause a response to cravings, as well as to promote abstinence.
2. Materials and Methods
2.1. Eligibility
Original clinical trials testing a pharmacological treatment for cravings in alcohol abusers or dependents were included in this review. We excluded non-original studies (i.e., reviews, meta-analysis, case reports, discussion articles, and study protocols) in this review. We also excluded those which were not randomized, controlled, or double-blinded; studies in languages other than English; those that used animals; those that did not include alcohol abusers or dependents; those analyzing the non-pharmacological treatment of alcohol cravings; and studies evaluating other alcohol measures than cravings.
2.2. Information Sources
Databases from Pubmed of the US National Library of Medicine and PsycINFO were used to identify relevant studies.
2.3. Search Strategy
The keywords used in the search were ‘craving’ and ‘alcohol’ and ‘randomi* controlled Blind*’. ‘Clinical trial’ and ‘humans’ filters were used in the Pubmed and PsycINFO databases.
2.4. Study Selection
To select articles for this review, the first and the last authors read the abstracts of all the studies found in the search. Duplicated articles and studies not related to alcohol were excluded. In the last step, the first author read the remaining studies. Inclusion and exclusion criteria were applied. The present review followed the PRISMA Statement for the transparent reporting of systematic reviews and meta-analyses [22] (Table S1).
2.5. Data Collection Process
The first and last authors independently included studies. The quality assessment was performed using the Study Quality Guide from the Cochrane Consumers and Communication Review Group (2013), considering six domains of risk of bias: (1) random sequence generation; (2) allocation concealment; (3) the blinding of participants and personnel; (4) the blinding of the outcome assessment; (5) an incomplete data outcome; and (6) a selective report. In cases of disagreement between the first and the last authors on the information presented in the review, the second one decided on the best way to present the data.
2.6. Data Items
In this review, the authors searched for information on the following variables: the title of the study; author(s); year of publication; sample size; mean age; sample characteristics; setting; search for treatment; design of the study; craving measures and other relevant measures; intervention; outcomes; and drop-out rates.
2.7. Inclusion and Exclusion Criteria
We selected randomized the articles on double-blind, placebo-controlled clinical trials on patients with alcohol disorder who are dependent. Animal subjects, the use of other drugs, the presence of psychiatric comorbidities, and studies that did not assess cravings were excluded.
2.8. Selection Articles
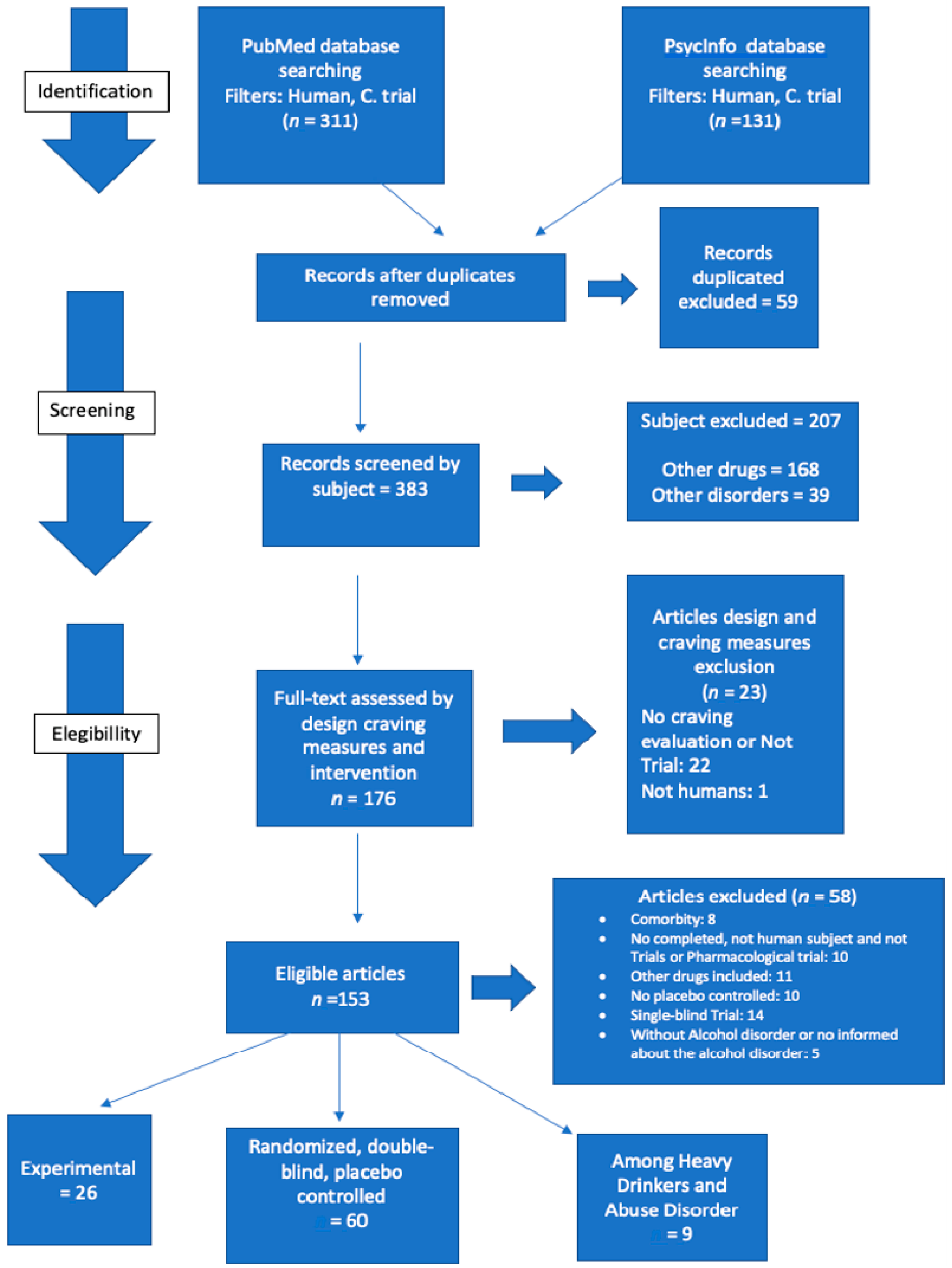
A total of 442 articles were identified. First, we read the title of the article, the publication of the journal, and their summaries, excluding repeated studies, studies that did not meet the criteria, and those did not address the relevant issue. A total of 59 articles were excluded. For a second time, we read the remaining 383 studies. Among these, 10 articles were incompleted, 22 did not analyze cravings or were not clinical trials, 1 included animal subjects; 5 were not related to Alcohol Use Disorder; 14 studies were single-blind trials; 11 included other drugs than alcohol; 10 were non-placebo-controlled articles; and 8 involved psychiatric comorbidities. Due to the large volume, we divided them into 3 groups: 60 randomized, double-blind, placebo-controlled articles on alcohol dependents, 26 articles were on cue-induced cravings, and 9 articles on heavy drinkers or subjects who misuse alcohol. In this review, we only cover double-blind placebo-controlled studies, excluding articles that did not study alcohol dependents and experimental/cue-induced-cravings-related articles (Appendix A) [23].
2.9. Qualitative Analysis of Studies
The selected articles were qualitatively analyzed through the evaluation of 13 items following the Checklist for Randomized Controlled Trials (Critical Appraisal tools for use in Joanna Briggs Institute Systematic Reviews). Thirteen questions were applied to each article, with the following answers: Yes (with a value of 1 point), No (0), Unclear (0.5), and Not Applicable (1) (Table S2).
A cut-off point of 10 was considered as the limit. Only one article had obtained a score below 10, and one article had a limit score of 10. All the other articles had a score above 10, with the average score of all studies being 12.7. These results demonstrate that the articles analyzed in this review are relevant and have only a few biases.
3. Results
There was a wide range of focuses among the selected studies. The studies use a population sample ranging from 11 and up to 493 participants, with an average n = 109 participants. The majority were outpatients, male, aged between 18 and 70 years, with an average age in fourth decade of life. All the participants had the diagnosis of alcohol dependence, following the criteria of the DSM-III, DSM-IV, and International Classification of Diseases (ICD-10). The oldest study found was in 1983, and the most recent one was conducted in 2020, with most of the studies coming from North America. Most of the studies, 47.6% (48), are from the US, 28.5% are European, 12.7% are from Asia and Russia, and 4.76% are Australian. Studies from Brazil and Canada account for the remaining 4.76%.
Most studies used the Obsessive Compulsive Drinking Scale (OCDS) as a measure (31.7%), followed by the Visual Analogue Scale (VAS) (28.5%), and then the Pennsylvania Alcohol Craving Scale (PACS) (19%). There were other scales that were used to a lesser extent, such as the Desire for Alcohol Questionnaire (Short DAQ), Clinical Institute Withdrawal Assessment for Alcohol Scale (CIWA-Ar), Tiffany Craving Questionnaire, Alcohol Craving Scale–Short Form, and self-reported craving scores.
The studies lasted for 3 days, and some lasted for up to 6 months, with the vast majority having 12 weeks of follow-up (41.2%) and a mean of 8.47 weeks per study. About dropouts, the average rate was 27.5%. Six studies had no dropouts, and three did not report the dropouts. The study with the highest number of dropouts had a rate of 85%.
The goal of this review is to assess which treatments obtained better results regarding cravings and to associate which ones contributed to a longer period of abstinence. By analyzing the studies qualitatively, 33 different drugs were tested, obtaining positive results concerning the control of cravings via the use of 15 drugs. In terms of abstinence, in 60 articles, 16 showed a longer period of abstinence compared to that of the placebo group, 21 studies showed no difference between the control and placebo groups, and in 13 articles, abstinence was not evaluated, only cravings.
3.1. Specific Drugs for the Treatment of Alcoholism
Specific medications used for the treatment of alcoholism comprised the vast majority of interventions used in the studies (about 18 studies) (Table 1). Among them were Naltrexone (eleven), Acamprosate (four), Naltrexone and Acamprosate (two), and Nalmefene (one).

Table 1.
Specific drugs used for the treatment of alcoholism.
Naltrexone, a pharmacological agent acting as an antagonist on the opioid receptors, has demonstrated a notable efficacy in reducing craving when administered as a monotherapy, as evidenced by the more positive outcomes in six out of seven studies compared to those of the placebo groups [5,6,7,29,32,34,38]. It is important to consider the pharmacokinetic properties of Naltrexone, including its half-life of 3.9–10.3 h and prolonged terminal elimination-phase half-life of 96 h [39]. Notably, the major metabolite of Naltrexone, 6-beta-naltrexol, exhibits higher plasma concentrations than the parent drug does. Understanding these pharmacokinetic characteristics provides insights into the sustained effects and potential mechanisms of action of Naltrexone in cravings reduction. In five studies associated with other interventions, there was a favorable result for craving cessation in only one when associated with Ondansetron [24]. In the other studies: PUFAS (Polyunsaturated Fatty Acids) [28], Cognitive Behavioral Therapy (CBT) [25], CBT and Supportive Therapy (ST) [27], and Acamprosate [33] were combined, but they were not superior to the placebo treatment applied to reduce the patients’ cravings.
Acamprosate, a structural analogue of gamma-aminobutyric acid (GABA), is believed to exert effects on alcohol intake by influencing the calcium channels and modulating transmission along GABA and decreasing the level of glutamate activity in NMDA receptors. These actions are hypothesized to lead to the reduced positive reinforcement of alcohol consumption and attenuated cravings during withdrawal [8,36]. Despite these proposed mechanisms, the precise mode of action of Acamprosate remain unknown. Acamprosate was focused on in six articles, where it reduced the patients’ cravings more than the placebo in two articles [30,31,36,37]. Nalmefene, an antagonist at the mu- and delta-opioid receptors [40], exhibited a non-significant reduction in cravings compared to that of a placebo in one study [26]. Nalmefene shares a similar chemical structure to Naltrexone, but it may possess unique properties that allow tighter binding to the opioid receptors [41].
As for abstinence, opioid receptor antagonists such as Naltrexone and Nalmefene were analyzed in 12 studies. In 5 out of 11 studies, Naltrexone was superior to the placebo [25,27,33,38,42]. Nalmefene did not result in a better response than that of the placebo in the only study included. It is worth mentioning that for Naltrexone, despite not having presented a positive response for the reduction of cravings when associated with CBT or ST, there was a positive response in terms of maintaining abstinence [25,27]. Acamprosate was successful in maintaining abstinence in two out of four articles [31,33].
In two studies, Naltrexone was compared to Acamprosate and a placebo. In one article, Naltrexone was found to be superior at reducing cravings, while Acamprosate did not differ from the placebo [35]. In the other paper, a comparison was made between Naltrexone, Acamprosate, placebo, and a combination of Naltrexone and Acamprosate, with the aim to correlate it to an increasing leptin plasma concentration. There was no association between the baseline plasma leptin concentration and cravings. However, in the group with the combined medication, decreasing leptin concentrations correlated significantly with the duration of abstinence [33].
3.2. Anticonvulsants
Another important group of drugs that is widely used in psychiatry is anticonvulsants. These drugs have three mechanisms of action: (a) the potentiation of GABA action; (b) the inhibition of sodium channel function; and (c) the inhibition of calcium channel function. By promoting central nervous system depression, these drugs act on one of the receptors that alcohol acts on, simulating its effect and attenuating the desire or cravings. Ten articles which used anticonvulsants were found: Gabapentin (three), Topiramate (three), Gamma-hydroxybutyric acid (GHB) (one), Pregabalin (one), Oxcarbazepine (one), and Levetiracetam (one) (Table 2).

Table 2.
Anticonvulsants.
Gabapentin is a synthetic amino acid that serves as a structural analog of GABA. It acts by binding to the alpha-2-delta type 1 subunit of voltage-gated calcium channels. Through this mechanism, Gabapentin selectively inhibits the influx of calcium ions (Ca2+) via these channels. By reducing the post-synaptic excitability and modulating the release of excitatory neurotransmitters, Gabapentin can decrease the level of neuronal excitability. Moreover, Gabapentin is believed to increase the concentration of GABA in the brain, possibly by blocking excitatory neurotransmission [52,53,54,55]. About Gabapentin, two articles were found to have evaluated cravings, showing a benefit superior to that of the placebo, and in one of them, there was the maintenance of abstinence, while the other article did not evaluate abstinence [14,47]. An important fact is that Gabapentin enacarbyl extended release was used in one study, which had negative results in an attempt to reduce the cravings and promote abstinence [13].
Topiramate was analyzed in three articles, demonstrating a favorable response in two to the cessation of cravings and in maintaining abstinence [16,51]. Topiramate, initially developed as an anti-diabetic drug and later used as an anticonvulsant, shares structural similarities with acetazolamide. It acts as a positive allosteric modulator at the GABA A receptors, promoting the increased influx of chloride ions into neurons and enhancing the overall GABA-mediated inhibition. These effects are likely mediated through non-benzodiazepine binding sites on GABA A receptors [9,56].
GHB showed positive results for abstinence and craving reduction in the only article that was found. GHB is a short-chain fatty acid that occurs naturally in the central nervous system (CNS) in humans and is utilized for both recreational and therapeutic purposes [57,58].
Pregabalin, an α2δ voltage-gated calcium channel subunit ligand, and Levetiracetam, a second-generation antiepileptic drug, which exhibit their effects through various mechanisms that are not fully understood [52,53,59,60], showed no benefit over the placebo to reduce the cravings [16,51]. About abstinence, this was analyzed in a study on Pregabalin, which was favorable in this article [3,46].
A drug that is not an anticonvulsant, but has a similar action, is Baclofen. This medication is responsible for the treatment of muscle spasticity with a highly effective medullary action, and it depresses the transmission of the monosynaptic and polysynaptic reflexes through the stimulation of GABA B receptors. This stimulation, in turn, inhibits the release of excitatory amino acids, glutamate and aspartate. In six articles, Baclofen showed a significant reduction in cravings compared to the placebo in three articles [43,45,49]. About abstinence, there was a positive response in three out of six articles [43,48,50].
Oxcarbazepine is an anticonvulsant that blocks voltage-gated sodium channels, resulting in the stabilization of hyperexcited neural membranes, the inhibition of repetitive neuronal discharge, and the decreased propagation of synaptic impulses. Like other anticonvulsants, it could reduce cravings by reducing the level of neuronal excitability. However, in only one study, Oxcarbazepine did not differ from the placebo in reducing cravings [44].
3.3. Varenicline
As an important drug and the gold standard in the treatment of smoking, Varenicline binds with a high affinity and selectivity to the α4β2 neuronal nicotinic acetylcholine receptors, where it acts as a partial agonist. Since many alcoholic patients also smoke, which is an associated comorbidity, the hypothesis of a possible treatment for smokers who are also alcohol-dependent was raised. Varenicline was effective in reducing alcohol cravings in alcohol-dependent smokers in the two selected studies. Among them, only one study evaluated the possible benefit of abstinence, with a favorable result when it was compared with that of the placebo [12,61] (Table 3).

Table 3.
Varenicline and Mecamylamine.
Mecamylamine, a nicotine antagonist, is a ganglionic cholinergic blocker, which was originally marketed for blood pressure lowering, and its use in smoking cessation blocks the rewarding effects of nicotine, and therefore, reduces the urge to smoke. The studied paper had a different result compared to that of Varenicline, showing a larger non-significant reduction in cravings than that of the placebo and no effect on promoting abstinence among smokers with alcohol dependence [62] (Table 3).
3.4. Other Psychotropic Drugs
Memantine, Fluoxetine, Olanzapine, Samidorphan, Amisulpride, Buspirone, Ritanserin, Citalopram, and Quetiapine were all used in one individualized study for each (Table 4). Memantine acts on the glutamatergic system by blocking the N-methyl-d-aspartate-type glutamate receptors (NMDARs) [63]. Fluoxetine and Citalopram are selective serotonin reuptake inhibitors (SSRIs) and antidepressants used for the treatment of people with co-occurring depression and alcohol dependence [64,65]. Samidorphan is a new chemical entity that, in vivo, has been demonstrated to function as a μ-opioid antagonist [66]. Olanzapine, Amisulpride, and Quetipine are atypical antipsychotic drugs used in dual disorders [21,67]. Buspirone acts as a 5-HT1A receptor agonist for anxiety symptoms [68], and ritanserin is a serotonin antagonist drug described as an anxiolytic, antidepressant, antiparkinsonian, and antihypertensive drug. However, ritanserin has never been marketed for medical use due to safety issues, but it is currently used in scientific research [17,69].

Table 4.
Other psychotropic drugs.
The ones that promoted the increased reduction of cravings compared to that of the placebo were Fluoxetine, Samidorphan, and Quetiapine [66,72,73], and Quetiapine was favorable in maintaining abstinence [66].
Memantine had inferior results in maintaining abstinence and a larger non-significant reduction in cravings than that of the placebo. Additionally, it resulted in numerous side effects, which led to a drug dose reduction or discontinuation [70]. Olanzapine did not differ from the placebo in reducing cravings or maintaining abstinence [71].
3.5. Other Drugs
Numerous drugs which are not specifically used for the treatment of AUD are used in psychiatry with a certain frequency due to the scarcity of therapeutic options.
In view of this, eight different drugs used widely in medicine, but are not previously indicated for substance use disorder (SUD) were tested in different 10 studies: Bromocriptine (2), Prazosin (2), N2O (1), Acetyl-L-carnitine (1), Atenolol (1), Dextromethorphan (1), Doxazosin (1), Intranasal Oxytocin (1), Ondansetron (1), Kudzu Root Extract, (1) and Enalapril (1) (Table 5).

Table 5.
Other drugs.
Bromocriptine, a derivative of ergot alkaloid, exhibits dual properties as both a dopamine agonist and antagonist. It has been extensively investigated in various psychiatric disorders, covering a wide range of conditions [85]. Prazosin, which functions as an alpha1-adrenergic antagonist, effectively mitigates adrenergic hyperactivity and excessive drinking associated with alcohol withdrawal in laboratory animals. Moreover, it has demonstrated the ability to reduce the rate of alcohol seeking triggered by stress and lower the risk of relapse in individuals with AUD [10,20,86]. Carnitine plays a crucial role in facilitating the transportation of long-chain fatty acids (FAs) into mitochondria for β-oxidation. This process is mediated by the enzyme carnitine palmitoyltransferase-1 (CPT1) [87]. Atenolol and Enalapril are both antihypertensive medications. Atenolol is a hydrophilic beta-receptor blocking drug, while Enalapril is a long-acting, nonsulfhydryl Angiotensin-Converting Enzyme (ACE) inhibitor [88,89]. Dextromethorphan (DXM), which is commonly used as an antitussive medication, acts as a non-competitive, low-affinity antagonist of the NMDA receptor and exhibits potential neuroprotective properties [77]. Prazosin is a selective α1-adrenergic antagonist, promoting the idea that theoretical models and preclinical findings support the involvement of noradrenergic circuits in alcohol reinforcement and the propensity for relapses [84]. Oxytocin is a neuropeptide that is widely distributed within the brain and alters neuroadaptation to addictive substances, including alcohol [82].
Ondansetron, a highly selective 5HT3 receptor antagonist, has been shown to reduce cravings when it is combined with Naltrexone [24]. In one study, where it was used as monotherapy, Ondansetron significantly reduced the overall cravings among early-onset alcoholics. In contrast, it increased the cravings in late-onset alcoholics [18].
The highlight of those was Bromocriptine, which in the two studies that were evaluated, showed a positive result for cravings reduction [11,79]. Acetyl-L-carnitine and Intranasal Oxytocin also showed cravings reduction in the only studies that were found [80,82]. Prazosin was focused on in two studies; one of them showed a better result for cravings [75], while in the other study, its use did not differ from that of the placebo [84]. The other drugs did not show a positive result regarding abstinence [74,76,77,78,81,84].
As for abstinence, Bromocriptine also had a favorable response in two articles that were studied [11,79]. Enalapril showed a more favorable result for abstinence compared to that of the placebo at a dose of 20 mg/day, but did not differ at a dose of 10 mg [81]. Acetyl-L-carnitine showed a good response to abstinence [80]. In the studies of the other drugs, abstinence was not evaluated or not shown to benefit the patients more than the placebo did.
In contrast to all the studies that used pharmacological interventions, some used Kudzu, a climbing vine native to Asia. The roots, flowers, and leaves are used as medicine in China to treat variety of disorders, including neck pain, eye pain, fever, and measles. More recently, Chinese people have used Kudzu in the treatment of alcohol addiction. In only one study, there was no difference between the use of this and the placebo in terms of maintaining abstinence and cravings reduction [83].
4. Discussion
The treatment of AUD remains one of the greatest challenges in psychiatry due to the scarcity of specific and recent treatments as well as the difficulty in carrying out long-term studies with large samples due to the complexity of the population that serves as a sample. The main objective of this review was to list the possible treatments for alcohol cravings, but also to assess whether there was a satisfactory long-term response in maintaining abstinence. The drugs with the highest number of studies were Naltrexone (thirteen trials; eight monotherapy and five combined with other interventions), Baclofen (six), and Acamprosate (six). Among the eight trials in which Naltrexone was tested alone, there was a favorable response to reducing cravings in seven trials. However, when Naltrexone was associated with another intervention, it did not show a reduction in craving in four out of five analyzed studies. Acamprosate and Baclofen, in monotherapies, showed positive results in reducing cravings in two out of five and three out of six trials, respectively.
Eleven different substances associated with a few studies were beneficial in all the trials: Varenicline (two), Bromocriptine (two), Gabapentin (two), Acetyl-L-carnitine (one), Fluoxetine (one), Gamma-hydroxybutyric Acid (one), Quetiapine (one), Samidorphan (one), Ondansetron (one), and Intranasal Oxytocin. Although these drugs are widely used in psychiatry, there were only a few studies. This demonstrates the need for more targeted Randomized Controlled Trials (RCT) to assess the cravings reduction.
Among the specific drugs used for the treatment of alcoholism, Naltrexone was still the best option for reducing alcohol cravings, followed by Acamprosate. In one study where Naltrexone, Acamprosate, and a placebo were evaluated, only Naltrexone showed a reduction of cravings, while Acamprosate had no effect. When it was combined with other therapies, such as PUFAS (Polyunsaturated Fatty Acids) and Cognitive Behavioral Therapy, Naltrexone did not differ from the placebo [25,27,28]. However, when Naltrexone was associated with psychotherapeutic approaches, such as CBT and/or ST, there was a more positive response in terms of abstinence compared to that of the placebo associated with these interventions. This demonstrates how much more Naltrexone can contribute to multidisciplinary treatments compared to that of isolated psychotherapy.
When combined with other pharmacological interventions, Naltrexone plus Acamprosate were superior to the placebo in maintaining abstinence, while, when they were associated with Ondansetron, it promoted cravings reduction [24,33].
To promote abstinence, Naltrexone, Acamprosate, and Nalmefene, acting in a monotherapy, were effective in 30% (three) of the studies, showing that there are beneficial results not only to attenuate the symptoms of craving, but also to maintain long-term abstinence.
Out of the anticonvulsants, Gabapentin is one of the drugs widely used in the treatment of dependences; it has shown good results both in reducing cravings and maintaining abstinence among alcohol-dependent patients. Topiramate, GHB, Pregabalin, and Levetiracetam were present in a few studies, but demonstrate some results both for abstinence and for reducing cravings in relation to the placebo. This class of drugs thus represents a possible second line of treatment for AUD.
Baclofen, which is already a drug used by those with alcoholism, especially those who suffer from a certain chronic liver disease resulting from alcohol use, showed promising results in the five articles that were found in this review, with cravings reduction in two and favorable abstinence results in three. This drug may be used as an adjuvant due to these favorable results, with only a few side effects; this drug may be used as an as a therapeutic option due to its favorable results with, only a few side effects, as well as Gabapentin, especially for patients with already compromised liver function.
Varenicline, which is a drug widely used in the treatment of smoking cessation, had a significant impact. Two studies had a 100% response rate to cravings, which was compared to that of the placebo, for those who use tobacco and alcohol at the same time; for those with dependences, there was one positive result for abstinence for both substances.
Furthermore, psychotropic drugs that are frequently used in the treatment of AUD, despite there only being a few studies, only Fluoxetine and Quetiapine were beneficial in reducing cravings; the latter was also effective for abstinence. Such medications could perhaps be used as adjunctive treatments for those with addictions, especially in patients with psychiatric comorbidities.
Of the drugs that are not frequently associated with the treatment of mental disorders, including AUD, Bromocriptine, a well-known dopamine agonist, which is used more frequently to treat Parkinson’s disease, showed a favorable result in terms of both abstinence and cravings, as well as Acetyl-L-carnitine. Additionally, Prazosin was included in one study, showing a positive result in reducing cravings. Such drugs could perhaps be used as a third-line treatment when all other alternatives have been used.
Of the 60 articles, 47 assessed both the outcomes of cravings reduction and abstinence. Six articles only analyzed cravings. Of the 47 studies, 23 articles showed that abstinence was favored by the control group, and 29 articles showed that there was a larger reduction of cravings in the control group compared to that of the placebo group.
Fourteen studies were successful in both reducing cravings and maintaining abstinence, including those on: Naltrexone (two out of seven studies), Acamprosate (one out of four), Gabapentin (two out of three), Topiramate (two out of three), Baclofen (one out of six), Bromocriptine (two out of two), Acetyl-L-carnitine (one out of one), Quetiapine (one out of one), Ondansetron (one out of one), Varenicline (in two studies where both outcomes were evaluated, it was positive in both in only one, and in the other article, it was positive for craving reduction and showed no difference compared to the placebo in maintaining abstinence).
Regarding the specific drugs used for an alcohol abuse treatment, Naltrexone proved to be quite effective in reducing cravings (85.7%); however, the authors obtained worse abstinence results (33.33%) for both criteria (28.5%). Acamprosate, which only showed the benefits in both abstinence and cravings reduction in one article, did not have a positive result for both criteria in the other two studies found. Nalmefene, on the other hand, did not show a significant difference compared to the placebo in one article.
About anticonvulsants, from 14 articles found where abstinence and cravings reduction were evaluated, five articles showed benefits in both criteria, while eight included the cessation of abstinence. Interestingly, Baclofen resulted in the patients comprising a higher proportion of the abstinence maintenance group (three out of six articles), but a lower proportion of the cravings reduction group (two out of six). Both Gabapentin and Topiramate were focused on in two studies each with consistent results in promoting abstinence. Additionally, there were three studies on each drug that demonstrate concordant results in reducing cravings. However, for Topiramate, out of the three studies, only two evaluated abstinence, and these showed favorable results in compared to those of the placebo.
Out of the fourteen studies involving anticonvulsant drugs, eight obtained positive results for abstinence maintenance. The results were better than those observed for specific drugs used for the treatment of alcoholism, which resulted in a higher proportion of patients with cravings reduction.
Furthermore, psychotropic drugs are frequently employed in the treatment of AUD, despite there only being a few studies; only Fluoxetine, Samidorphan, and Quetiapine were beneficial in reducing cravings; the latter was also effective for abstinence. These medications may hold potential as adjunctive treatments, especially for patients with psychiatric comorbidities.
Among the drugs not typically associated with mental disorders or AUD treatment, Bromocriptine, a well-known dopamine agonist, used primarily to treat Parkinson’s disease, exhibited favorable results in both abstinence and cravings reduction, as well as Acetyl-L-carnitine. Additionally, one study demonstrated the positive effect of Prazosin in reducing cravings. These drugs could perhaps be used as a third-line treatment option when all other alternatives have been exhausted.
Despite providing valuable insights, the analyzed studies have certain limitations. Firstly, there is a paucity of research on psychotropic drugs for AUD. Secondly, not all studies that evaluated cravings also assessed abstinence, making it difficult to establish a correlation between these two outcomes. Thirdly, there is a substantial disparity in the study durations with some being long, and other being short. Furthermore, approximately one-quarter of the studies did not report the number of dropouts, and a publication bias may have influenced the reported outcomes. Moreover, the heterogeneity of the cravings measures precluded a meta-analysis of standardized instruments. Lastly, this review relied on articles from English language databases, and no studies from African and Middle Eastern countries were found, possibly due to there being limited research in these regions and less alcohol consumption in Muslim countries.
5. Conclusions
In conclusion, this review underscores the ongoing challenges in treating AUD and highlights the potential of various medications in reducing cravings and maintaining long-term abstinence. Naltrexone and Acamprosate emerged as the most effective options, with other substances showing promise, but requiring future investigation through targeted RCTs. Additionally, anticonvulsants and certain psychotropic drugs may offer viable treatment alternatives. However, it is crucial to address the limitations of the mentioned studies and conduct comprehensive research to advance our understanding of cravings and optimize their assessment and treatment approaches.
Despite the fact that specific drugs used for the treatment of alcoholism are the gold standard for reducing cravings (especially Naltrexone), not all studies have shown a good response for maintaining abstinence. About 1/3 of the studies demonstrated a reduction of cravings and the maintenance of abstinence compared to those of the placebo.
As for anticonvulsants, more than half of the studies had a favorable result in the cessation of cravings and in maintaining abstinence. A total of 57% of studies using anticonvulsants obtained a favorable response to abstinence. A possible therapeutic alternative would be not only to switch specific drugs for the treatment of alcoholism, such as Naltrexone, to anticonvulsants, but also to associate them for refractory cases or situations where the expected response was not achieved, particularly when the goal is to reach total abstinence.
There are promising results with anti-smoking drugs for those who suffer from a comorbidity associated with alcohol and tobacco dependence. Psychotropics, such as antidepressants and antipsychotics, and drugs that are not regularly used for the treatment of AUD could be used as a third line for a possible treatment of some cases. Such a review may direct the development of a possible algorithm for the treatment of alcoholism in the future and open perspectives for future studies on the aforementioned medications, further increasing the level of evidence for each treatment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/brainsci13081206/s1, Table S1: Checklist PISMA; Table S2: JBI Systematic Review.
Author Contributions
Conceptualization, M.C.D.M. and M.O.P.P.; methodology, M.C.D.M., M.O.P.P. and J.M.C.-M.; validation, M.C.D.M., M.O.P.P. and J.M.C.-M.; formal analysis, M.C.D.M., M.O.P.P. and J.M.C.-M.; investigation, M.C.D.M., M.O.P.P. and J.M.C.-M.; resources, M.C.D.M., M.O.P.P. and J.M.C.-M.; data curation, M.C.D.M., M.O.P.P. and J.M.C.-M.; writing—original draft preparation, M.C.D.M., M.O.P.P. and J.M.C.-M.; writing—review and editing, J.T., M.W.C., G.T.K., M.B.B.d.S., F.I.C., I.K.B., D.L.S.L., A.S.M.-d.-S. and G.P.; visualization, J.T., M.W.C. and G.T.K., Santos, M.B.B.d.S., F.I.C., I.K.B., D.L.S.L., A.S.M.-d.-S. and G.P.; supervision, J.M.C.-M., A.M., A.G.d.A., K.L., A.B.N., C.d.A.-M.P. and A.V.; project administration, J.M.C.-M., A.M., A.G.d.A., K.L., A.B.N., C.d.A.-M.P. and A.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
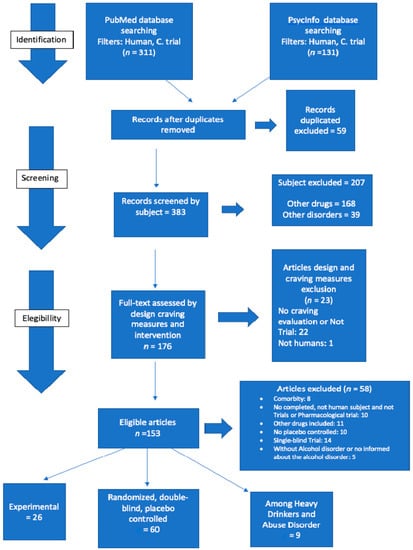
Figure A1.
Selection articles.
Figure A1.
Selection articles.
References
- World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/alcohol (accessed on 12 July 2022).
- Likhitsathian, S.; Uttawichai, K.; Booncharoen, H.; Wittayanookulluk, A.; Angkurawaranon, C.; Srisurapanont, M. Topiramate treatment for alcoholic outpatients recently receiving residential treatment programs: A 12-week, randomized, placebo-controlled trial. Drug Alcohol Depend. 2013, 133, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Richter, C.; Effenberger, S.; Bschor, T.; Bonnet, U.; Haasen, C.; Preuss, U.W.; Heinz, A.; Förg, A.; Volkmar, K.; Glauner, T.; et al. Efficacy and safety of levetiracetam for the prevention of alcohol relapse in recently detoxified alcohol-dependent patients: A randomized trial. J. Clin. Psychopharmacol. 2012, 32, 558–562. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Chick, J.; Anton, R.; Checinski, K.; Croop, R.; Drummond, D.C.; Farmer, R.; Labriola, D.; Marshall, J.; Moncrieff, J.; Morgan, M.Y.; et al. A multicentre, randomized, double-blind, placebo-controlled trial of naltrexone in the treatment of alcohol dependence or abuse. Alcohol Alcohol. 2000, 35, 587–593. [Google Scholar] [CrossRef]
- Garbutt, J.C.; Kampov-Polevoy, A.B.; Kalka-Juhl, L.S.; Gallop, R.J. Association of the Sweet-Liking Phenotype and Craving for Alcohol with the Response to Naltrexone Treatment in Alcohol Dependence: A Randomized Clinical Trial. JAMA Psychiatry 2016, 73, 1056–1063. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guardia, J.; Caso, C.; Arias, F.; Gual, A.; Sanahuja, J.; Ramírez, M.; Mengual, I.; Gonzalvo, B.; Segura, L.; Trujols, J.; et al. A double-blind, placebo-controlled study of naltrexone in the treatment of alcohol-dependence disorder: Results from a multicenter clinical trial. Alcohol. Clin. Exp. Res. 2002, 26, 1381–1387. [Google Scholar] [CrossRef]
- Hunter, K.; Ochoa, R. Acamprosate (campral) for treatment of alcoholism. Am. Fam. Physician 2006, 74, 645–646. [Google Scholar]
- Manhapra, A.; Chakraborty, A.; Arias, A.J. Topiramate Pharmacotherapy for Alcohol Use Disorder and Other Addictions: A Narrative Review. J. Addict. Med. 2019, 13, 7–22. [Google Scholar] [CrossRef]
- Rasmussen, D.D.; Alexander, L.L.; Raskind, M.A.; Froehlich, J.C. The alpha1-adrenergic receptor antagonist, prazosin, reduces alcohol drinking in alcohol-preferring (P) rats. Alcohol. Clin. Exp. Res. 2009, 33, 264–272. [Google Scholar] [CrossRef]
- Borg, V. Bromocriptine in the prevention of alcohol abuse. Acta Psychiatr. Scand. 1983, 68, 100–110. [Google Scholar] [CrossRef]
- de Bejczy, A.; Löf, E.; Walther, L.; Guterstam, J.; Hammarberg, A.; Asanovska, G.; Franck, J.; Isaksson, A.; Söderpalm, B. Varenicline for treatment of alcohol dependence: A randomized, placebo-controlled trial. Alcohol. Clin. Exp. Res. 2015, 39, 2189–2199. [Google Scholar] [CrossRef]
- Falk, D.E.; Ryan, M.L.; Fertig, J.B.; Devine, E.G.; Cruz, R.; Brown, E.S.; Burns, H.; Salloum, I.M.; Newport, D.J.; Mendelson, J.; et al. Gabapentin Enacarbil Extended-Release for Alcohol Use Disorder: A Randomized, Double-Blind, Placebo-Controlled, Multisite Trial assessing Efficacy and Safety. Alcohol. Clin. Exp. Res. 2019, 43, 158. [Google Scholar] [CrossRef]
- Furieri, F.A.; Nakamura-Palacios, E.M. Gabapentin reduces alcohol consumption and craving: A randomized, double-blind, placebo-controlled trial. J. Clin. Psychiatry 2007, 68, 1691–1700. [Google Scholar] [CrossRef]
- Gallimberti, L.; Ferri, M.; Ferrara, S.D.; Fadda, F.; Gessa, G.L. Gamma-Hydroxybutyric acid in the treatment of alcohol dependence: A double-blind study. Alcohol. Clin. Exp. Res. 1992, 16, 673–676. [Google Scholar] [CrossRef]
- Johnson, B.A.; Ait-Daoud, N.; Bowden, C.L.; DiClemente, C.C.; Roache, J.D.; Lawson, K.; Javors, M.A.; Ma, J.Z. Oral topiramate for treatment of alcohol dependence: A randomised controlled trial. Lancet 2003, 361, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.A.; Jasinski, D.R.; Galloway, G.P.; Kranzler, H.; Weinreib, R.; Anton, R.F.; Mason, B.J.; Bohn, M.J.; Pettinati, H.M.; Rawson, R.; et al. Ritanserin in the treatment of alcohol dependence—A multi-center clinical trial. Psychopharmacology 1996, 128, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.A.; Roache, J.D.; Ait-Daoud, N.; Zanca, N.A.; Velazquez, M. Ondansetron reduces the craving of biologically predisposed alcoholics. Psychopharmacology 2002, 160, 408–413. [Google Scholar] [CrossRef]
- Khemiri, L.; Brynte, C.; Stunkel, A.; Klingberg, T.; Jayaram-Lindström, N. Working Memory Training in Alcohol Use Disorder: A Randomized Controlled Trial. Alcohol. Clin. Exp. Res. 2019, 43, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Lê, A.D.; Funk, D.; Juzytsch, W.; Coen, K.; Navarre, B.M.; Cifani, C.; Shaham, Y. Effect of prazosin and guanfacine on stress-induced reinstatement of alcohol and food seeking in rats. Psychopharmacology 2011, 218, 89–99. [Google Scholar] [CrossRef]
- Martinotti, G.; Chiappini, S.; Mosca, A.; Miuli, A.; Santovito, M.C.; Pettorruso, M.; Skryabin, V.; Sensi, S.L.; Giannantonio, M.D. Atypical Antipsychotic Drugs in Dual Disorders: Current Evidence for Clinical Practice. Curr. Pharm. Des. 2022, 28, 2241–2259. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- The Joanna Briggs Institute Critical Appraisal Tools for Use in JBI Systematic Reviews—Checklist for Randomized Controlled Trials. Available online: https://view.officeapps.live.com/op/view.aspx?src=https%3A%2F%2Fjbi.global%2Fsites%2Fdefault%2Ffiles%2F2021-10%2FChecklist_for_RCTs.docx&wdOrigin=BROWSELINK (accessed on 27 September 2022).
- Ait-Daoud, N.; Johnson, B.A.; Prihoda, T.J.; Hargita, I.D. Combining ondansetron and naltrexone reduces craving among biologically predisposed alcoholics: Preliminary clinical evidence. Psychopharmacology 2001, 154, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Anton, R.F.; Moak, D.H.; Waid, L.R.; Latham, P.K.; Malcolm, R.J.; Dias, J.K. Naltrexone and cognitive behavioral therapy for the treatment of outpatient alcoholics: Results of a placebo-controlled trial. Am. J. Psychiatry 1999, 156, 1758–1764. [Google Scholar] [CrossRef]
- Anton, R.F.; Pettinati, H.; Zweben, A.; Kranzler, H.R.; Johnson, B.; Bohn, M.J.; McCaul, M.E.; Anthenelli, R.; Salloum, I.; Galloway, G.; et al. A multi-site dose ranging study of nalmefene in the treatment of alcohol dependence. J. Clin. Psychopharmacol. 2004, 24, 421–428. [Google Scholar] [CrossRef]
- Balldin, J.; Berglund, M.; Borg, S.; Månsson, M.; Bendtsen, P.; Franck, J.; Gustafsson, L.; Halldin, J.; Nilsson, L.H.; Stolt, G.; et al. A 6-month controlled naltrexone study: Combined effect with cognitive behavioral therapy in outpatient treatment of alcohol dependence. Alcohol. Clin. Exp. Res. 2003, 27, 1142–1149. [Google Scholar] [CrossRef]
- Fogaça, M.N.; Santos-Galduróz, R.F.; Eserian, J.K.; Galduróz, J.C. The effects of polyunsaturated fatty acids in alcohol dependence treatment--a double-blind, placebo-controlled pilot study. BMC Clin. Pharmacol. 2011, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Gastpar, M.; Bonnet, U.; Böning, J.; Mann, K.; Schmidt, L.G.; Soyka, M.; Wetterling, T.; Kielstein, V.; Labriola, D.; Croop, R. Lack of efficacy of naltrexone in the prevention of alcohol relapse: Results from a German multicenter study. J. Clin. Psychopharmacol. 2002, 22, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Hammarberg, A.; Nylander, I.; Zhou, Q.; Jayaram-Lindström, N.; Reid, M.S.; Franck, J. The effect of acamprosate on alcohol craving and correlation with hypothalamic pituitary adrenal (HPA) axis hormones and beta-endorphin. Brain Res. 2009, 1305 (Suppl. S2), S2–S6. [Google Scholar] [CrossRef]
- Higuchi, S. Efficacy of acamprosate for the treatment of alcohol dependence long after recovery from withdrawal syndrome: A randomized, double-blind, placebo-controlled study conducted in Japan (sunrise study). J. Clin. Psychiatry 2015, 76, 181–188. [Google Scholar] [CrossRef]
- Huang, M.C.; Chen, C.H.; Yu, J.M.; Chen, C.C. A double-blind, placebo-controlled study of naltrexone in the treatment of alcohol dependence in Taiwan. Addict. Biol. 2005, 10, 289–292. [Google Scholar] [CrossRef]
- Kiefer, F.; Jahn, H.; Otte, C.; Demiralay, C.; Wolf, K.; Wiedemann, K. Increasing leptin precedes craving and relapse during pharmacological abstinence maintenance treatment of alcoholism. J. Psychiatr. Res. 2005, 39, 545–551. [Google Scholar] [CrossRef]
- Monterosso, J.R.; Flannery, B.A.; Pettinati, H.M.; Oslin, D.W.; Rukstalis, M.; O’Brien, C.P.; Volpicelli, J.R. Predicting treatment response to naltrexone: The influence of craving and family history. Am. J. Addict. 2001, 10, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Morley, K.C.; Teesson, M.; Reid, S.C.; Sannibale, C.; Thomson, C.; Phung, N.; Weltman, M.; Bell, J.R.; Richardson, K.; Haber, P.S. Naltrexone versus acamprosate in the treatment of alcohol dependence: A multi-centre, randomized, double-blind, placebo-controlled trial. Addiction 2006, 101, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Namkoong, K.; Lee, B.O.; Lee, P.G.; Choi, M.J.; Lee, E. Acamprosate in Korean alcohol-dependent patients: A multi-centre, randomized, double-blind, placebo-controlled study. Alcohol Alcohol. 2003, 38, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Umhau, J.C.; Schwandt, M.L.; Usala, J.; Geyer, C.; Singley, E.; George, D.T.; Heilig, M. Pharmacologically induced alcohol craving in treatment seeking alcoholics correlates with alcoholism severity, but is insensitive to acamprosate. Neuropsychopharmacology 2011, 36, 1178–1186. [Google Scholar] [CrossRef]
- Volpicelli, J.R.; Alterman, A.I.; Hayashida, M.; O’brien, C.P. Naltrexone in the treatment of alcohol dependence. Arch. Gen. Psychiatry 1992, 49, 876–880. [Google Scholar] [CrossRef]
- Crabtree, B.L. Review of naltrexone, a long-acting opiate antagonist. Clin. Pharm. 1984, 3, 273–280. [Google Scholar]
- Michel, M.E.; Bolger, G.; Weissman, B.A. Binding of a new opiate antagonist, nalmefene, to rat brain membranes. Methods Find Exp. Clin. Pharmacol. 1985, 7, 175–177. [Google Scholar]
- Ingman, K.; Hagelberg, N.; Aalto, S.; Nagren, K.; Juhakoski, A.; Karhuvaara, S.; Kallio, A.; Oikonen, V.; Hietala, J.; Scheinin, H. Prolonged central mu-opioid receptor occupancy after single and repeated nalmefene dosing. Neuropsychopharmacology 2005, 30, 2245–2253. [Google Scholar] [CrossRef]
- Garbutt, J.C.; Kampov-Polevoy, A.B.; Gallop, R.; Kalka-Juhl, L.; Flannery, B.A. Efficacy and safety of baclofen for alcohol dependence: A randomized, double-blind, placebo-controlled trial. Alcohol. Clin. Exp. Res. 2010, 34, 1849–1857. [Google Scholar] [CrossRef]
- Addolorato, G.; Caputo, F.; Capristo, E.; Domenicali, M.; Bernardi, M.; Janiri, L.; Agabio, R.; Colombo, G.; Gessa, G.L.; Gasbarrini, G. Baclofen efficacy in reducing alcohol craving and intake a preliminary double-blind randomized controlled study. Alcohol Alcohol. 2002, 37, 504–508. [Google Scholar] [CrossRef]
- Koethe, D.; Juelicher, A.; Nolden, B.M.; Braunwarth, W.D.; Klosterkötter, J.; Niklewski, G.; Wodarz, N.; Klatt, J.; Burtscheidt, W.; Gaebel, W.; et al. Oxcarbazepine—Efficacy and tolerability during treatment of alcohol withdrawal: A double-blind, randomized, placebo-controlled multicenter pilot study. Alcohol. Clin. Exp. Res. 2007, 31, 1188–1194. [Google Scholar] [CrossRef]
- Krupitsky, E.M.; Rybakova, K.V.; Kiselev, A.S.; Alexeeva, Y.V.; Berntsev, V.A.; Chekhlaty, E.I.; Zubova, E.Y.; Popov, Y.V.; Neznanov, N.G. Double blind placebo controlled randomized pilot clinical trial of baclofen (Baclosan®) for alcohol dependence. Zhurnal Nevrol. I Psikhiatrii Im. SS Korsakova 2015, 115, 53–62. (In Russian) [Google Scholar] [CrossRef] [PubMed]
- Krupitsky, E.M.; Rybakova, K.V.; Skurat, E.P.; Semenova, N.V.; Neznanov, N.G. A double-blind placebo controlled randomized clinical trial of the efficacy and safety of pregabalin in induction of remission in patients with alcohol dependence. Zhurnal Nevrol. I Psikhiatrii Im. SS Korsakova 2020, 120, 33–43. [Google Scholar] [CrossRef]
- Mason, B.J.; Quello, S.; Goodell, V.; Shadan, F.; Kyle, M.; Begovic, A. Gabapentin treatment for alcohol dependence: A randomized clinical trial. JAMA Intern. Med. 2014, 174, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Morley, K.C.; Luquin, N.; Baillie, A.; Fraser, I.; Trent, R.J.; Dore, G.; Phung, N.; Haber, P.S. Moderation of baclofen response by a GABAB receptor polymorphism: Results from the BacALD randomized controlled trial. Addiction 2018, 113, 2205–2213. [Google Scholar] [CrossRef] [PubMed]
- Ponizovsky, A.M.; Rosca, P.; Aronovich, E.; Weizman, A.; Grinshpoon, A. Baclofen as add-on to standard psychosocial treatment for alcohol dependence: A randomized, double-blind, placebo-controlled trial with 1 year follow-up. J. Subst. Abus. Treat. 2014, 52, 24–30. [Google Scholar] [CrossRef]
- Rombouts, S.A.; Baillie, A.; Haber, P.S.; Morley, K.C. Clinical Predictors of Response to Baclofen in the Treatment of Alcohol Use Disorder: Results from the BacALD Trial. Alcohol Alcohol. 2019, 54, 272–278. [Google Scholar] [CrossRef]
- Rubio, G.; Martínez-Gras, I.; Manzanares, J. Modulation of impulsivity by topiramate: Implications for the treatment of alcohol dependence. J. Clin. Psychopharmacol. 2009, 29, 584–589. [Google Scholar] [CrossRef]
- Cunningham, M.O.; Woodhall, G.L.; Thompson, S.E.; Dooley, D.J.; Jones, R.S. Dual effects of gabapentin and pregabalin on glutamate release at rat entorhinal synapses in vitro. Eur. J. Neurosci. 2004, 20, 1566–1576. [Google Scholar] [CrossRef]
- Sills, G.J. The mechanisms of action of gabapentin and pregabalin. Curr. Opin. Pharmacol. 2006, 6, 108–113. [Google Scholar] [CrossRef]
- Taylor, C.P.; Gee, N.S.; Su, T.Z.; Kocsis, J.D.; Welty, D.F.; Brown, J.P.; Dooley, D.J.; Boden, P.; Singh, L. A summary of mechanistic hypotheses of gabapentin pharmacology. Epilepsy Res. 1998, 29, 233–249. [Google Scholar] [CrossRef] [PubMed]
- Uchitel, O.D.; Di Guilmi, M.N.; Urbano, F.J.; Gonzalez-Inchauspe, C. Acute modulation of calcium currents and synaptic transmission by gabapentinoids. Channels 2010, 4, 490–496. [Google Scholar] [CrossRef] [PubMed]
- White, H.S. Mechanism of action of newer anticonvulsants. J. Clin. Psychiatry 2003, 64 (Suppl. 8), 5–8. [Google Scholar] [PubMed]
- Caputo, F.; Vignoli, T.; Maremmani, I.; Bernardi, M.; Zoli, G. Gamma hydroxybutyric acid (GHB) for the treatment of alcohol dependence: A review. Int. J. Environ. Res. Public Health 2009, 6, 1917–1929. [Google Scholar] [CrossRef]
- Trombley, T.A.; Capstick, R.A.; Lindsley, C.W. DARK Classics in Chemical Neuroscience: Gamma-Hydroxybutyrate (GHB). ACS Chem. Neurosci. 2020, 11, 3850–3859. [Google Scholar] [CrossRef] [PubMed]
- Le Strat, Y. Levetiracetam in the treatment of alcohol dependence: Toward the end of the story? Alcohol. Clin. Exp. Res. 2012, 36, 1309–1310. [Google Scholar] [CrossRef]
- Palma, E.; Ragozzino, D.; Di Angelantonio, S.; Mascia, A.; Maiolino, F.; Manfredi, M.; Cantore, G.; Esposito, V.; Di Gennaro, G.; Quarato, P.; et al. The antiepileptic drug levetiracetam stabilizes the human epileptic GABAA receptors upon repetitive activation. Epilepsia 2007, 48, 1842–1849. [Google Scholar] [CrossRef]
- Litten, R.Z.; Ryan, M.L.; Fertig, J.B.; Falk, D.E.; Johnson, B.; Dunn, K.E.; Green, A.I.; Pettinati, H.M.; Ciraulo, D.A.; Sarid-Segal, O.; et al. A double-blind, placebo-controlled trial assessing the efficacy of varenicline tartrate for alcohol dependence. J. Addict. Med. 2013, 7, 277–286. [Google Scholar] [CrossRef]
- Petrakis, I.L.; Ralevski, E.; Gueorguieva, R.; O’Malley, S.S.; Arias, A.; Sevarino, K.A.; Jane, J.S.; O’Brien, E.; Krystal, J.H. Mecamylamine treatment for alcohol dependence: A randomized controlled trial. Addiction 2018, 113, 6–14. [Google Scholar] [CrossRef]
- Yuanyuan, J.; Junyan, Z.; Cuola, D.; Jingjing, C.; Yuhui, S.; Dan, X.; Wei, D.; Yongsheng, Z. Memantine attenuated alcohol withdrawal-induced anxiety-like behaviors through down-regulating NR1-CaMKII-ERK signaling pathway. Neurosci. Lett. 2018, 686, 133–139. [Google Scholar] [CrossRef]
- Agabio, R.; Trogu, E.; Pani, P.P. Antidepressants for the treatment of people with co-occurring depression and alcohol dependence. Cochrane Database Syst. Rev. 2018, 4, CD008581. [Google Scholar] [CrossRef]
- Wong, W.M.; Hasemann, S.; Schwarz, M.; Zill, P.; Koller, G.; Soyka, M.; Preuss, U.W. Citalopram neuropharmacological challenge in alcohol-dependent patients and controls: Pharmacogenetic, endocrine and psychobehavioral results. Pharmacopsychiatry 2008, 41, 72–78. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, S.S.; Todtenkopf, M.S.; Du, Y.; Ehrich, E.; Silverman, B.L. Effects of the Opioid System Modulator, Samidorphan, on Measures of Alcohol Consumption and Patient-Reported Outcomes in Adults with Alcohol Dependence. Alcohol. Clin. Exp. Res. 2018, 42, 2011–2021. [Google Scholar] [CrossRef] [PubMed]
- Marra, D.; Warot, D.; Berlin, I.; Hispard, E.; Notides, C.; Tilikete, S.; Payan, C.; Lépine, J.P.; Dally, S.; Aubin, H.J. Amisulpride does not prevent relapse in primary alcohol dependence: Results of a pilot randomized, placebo-controlled trial. Alcohol. Clin. Exp. Res. 2002, 26, 1545–1552. [Google Scholar] [CrossRef]
- Malec, E.; Malec, T.; Gagné, M.A.; Dongier, M. Buspirone in the treatment of alcohol dependence: A placebo-controlled trial. Alcohol. Clin. Exp. Res. 1996, 20, 307–312. [Google Scholar] [CrossRef]
- Wiesbeck, G.A.; Weijers, H.G.; Chick, J.; Naranjo, C.A.; Boening, J.; Behalf of the Ritanserin in Alcoholism Work Group. Ritanserin in relapse prevention in abstinent alcoholics: Results from a placebo-controlled double-blind international multicenter trial. Alcohol. Clin. Exp. Res. 1999, 23, 230–235. [Google Scholar] [CrossRef]
- Evans, S.M.; Levin, F.R.; Brooks, D.J.; Garawi, F. A pilot double-blind treatment trial of memantine for alcohol dependence. Alcohol. Clin. Exp. Res. 2007, 31, 775–782. [Google Scholar] [CrossRef]
- Guardia, J.; Segura, L.; Gonzalvo, B.; Iglesias, L.; Roncero, C.; Cardús, M.; Casas, M. A double-blind, placebo-controlled study of olanzapine in the treatment of alcohol-dependence disorder. Alcohol. Clin. Exp. Res. 2004, 28, 736–745. [Google Scholar] [CrossRef]
- Kabel, D.I.; Petty, F. A Placebo-Controlled, Double-Blind Study of Fluoxetine in Severe Alcohol Dependence: Adjunctive Pharmacotherapy During and After Inpatient Treatment. Alcohol. Clin. Exp. Res. 1996, 20, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Kampman, K.M.; Pettinati, H.M.; Lynch, K.G.; Whittingham, T.; Macfadden, W.; Dackis, C.; Tirado, C.; Oslin, D.W.; Sparkman, T.; O’Brien, C.P. A double-blind, placebo-controlled pilot trial of quetiapine for the treatment of Type A and Type B alcoholism. J. Clin. Psychopharmacol. 2007, 27, 344–351. [Google Scholar] [CrossRef]
- Alho, H.; Methuen, T.; Paloheimo, M.; Strid, N.; Seppä, K.; Tiainen, J.; Salaspuro, M.; Roine, R. Long-term effects of and physiological responses to nitrous oxide gas treatment during alcohol withdrawal: A double-blind, placebo-controlled trial. Alcohol. Clin. Exp. Res. 2002, 26, 1816–1822. [Google Scholar] [CrossRef]
- Fox, H.C.; Anderson, G.M.; Tuit, K.; Hansen, J.; Kimmerling, A.; Siedlarz, K.M.; Morgan, P.T.; Sinha, R. Prazosin effects on stress- and cue-induced craving and stress response in alcohol-dependent individuals: Preliminary findings. Alcohol. Clin. Exp. Res. 2012, 36, 351–360. [Google Scholar] [CrossRef]
- Gottlieb, L.D.; Horwitz, R.I.; Kraus, M.L.; Segal, S.R.; Viscoli, C.M. Randomized controlled trial in alcohol relapse prevention: Role of atenolol, alcohol craving, and treatment adherence. J. Subst. Abus. Treat. 1994, 11, 253–258. [Google Scholar] [CrossRef]
- Huang, M.C.; Chen, C.H.; Pan, C.H.; Lin, S.K. Lack of efficacy of dextromethorphan in managing alcohol withdrawal: A preliminary report of a randomized, double-blind, placebo-controlled trial. J. Clin. Psychopharmacol. 2014, 34, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Kenna, G.A.; Haass-Koffler, C.L.; Zywiak, W.H.; Edwards, S.M.; Brickley, M.B.; Swift, R.M.; Leggio, L. Role of the α1 blocker doxazosin in alcoholism: A proof-of-concept randomized controlled trial. Addict. Biol. 2016, 21, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Lawford, B.R.; Young, R.M.; Rowell, J.A.; Qualichefski, J.; Fletcher, B.H.; Syndulko, K.; Ritchie, T.; Noble, E.P. Bromocriptine in the treatment of alcoholics with the D2 dopamine receptor A1 allele. Nat. Med. 1995, 1, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Martinotti, G.; Reina, D.; di Nicola, M.; Andreoli, S.; Tedeschi, D.; Ortolani, I.; Pozzi, G.; Iannoni, E.; D’Iddio, S.; Janiri, L. Acetyl-L-carnitine for alcohol craving and relapse prevention in anhedonic alcoholics: A randomized, double-blind, placebo-controlled pilot trial. Alcohol Alcohol. 2010, 45, 449–455. [Google Scholar] [CrossRef]
- Naranjo, C.A.; Kadlec, K.E.; Sanhueza, P.; Woodley-Remus, D.; Sellers, E.M. Enalapril effects on alcohol intake and other consummatory behaviors in alcoholics. Clin. Pharmacol. Ther. 1991, 50, 96–106. [Google Scholar] [CrossRef]
- Pedersen, C.A.; Smedley, K.L.; Leserman, J.; Jarskog, L.F.; Rau, S.W.; Kampov-Polevoi, A.; Casey, R.L.; Fender, T.; Garbutt, J.C. Intranasal oxytocin blocks alcohol withdrawal in human subjects. Alcohol. Clin. Exp. Res 2013, 37, 484–489. [Google Scholar] [CrossRef]
- Shebek, J.; Rindone, J.P. A pilot study exploring the effect of kudzu root on the drinking habits of patients with chronic alcoholism. J. Altern. Complement. Med. 2000, 6, 45–48. [Google Scholar] [CrossRef]
- Simpson, T.L.; Saxon, A.J.; Meredith, C.W.; Malte, C.A.; McBride, B.; Ferguson, L.C.; Gross, C.A.; Hart, K.L.; Raskind, M. A pilot trial of the alpha-1 adrenergic antagonist, prazosin, for alcohol dependence. Alcohol. Clin. Exp. Res. 2009, 33, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Sitland-Marken, P.A.; Wells, B.G.; Froemming, J.H.; Chu, C.C.; Brown, C.S. Psychiatric applications of bromocriptine therapy. J. Clin. Psychiatry 1990, 51, 68–82. [Google Scholar] [PubMed]
- Sinha, R.; Fogelman, N.; Wemm, S.; Angarita, G.; Seo, D.; Hermes, G. Alcohol withdrawal symptoms predict corticostriatal dysfunction that is reversed by prazosin treatment in alcohol use disorder. Addict. Biol. 2022, 27, e13116. [Google Scholar] [CrossRef]
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta 2016, 1863, 2422–2435. [Google Scholar] [CrossRef] [PubMed]
- Gomez, H.J.; Cirillo, V.J.; Irvin, J.D. Enalapril: A review of human pharmacology. Drugs 1985, 30 (Suppl. 1), 13–24. [Google Scholar] [CrossRef] [PubMed]
- Kirch, W.; Görg, K.G. Clinical pharmacokinetics of atenolol—A review. Eur. J. Drug Metab. Pharmacokinet. 1982, 7, 81–91. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
