Development and Validation of CXCR4 Nomogram-Based Immune Infiltration/Tumor Inflammation in Primary Glioblastoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Datasets Download
2.3. Functional Enrichment Analysis
2.4. Tumor Immunology Analysis
2.5. Cancer Single-Cell State Atlas
2.6. Model Construction and Evaluation
2.7. Immunohistochemistry Staining (IHC) and Quantification
2.8. Cell Culture with CXCR4 Knockdown
2.9. Western Blot
2.10. Enzyme-Linked Immunosorbent Assay (ELISA)
2.11. Statistical Analysis
3. Result
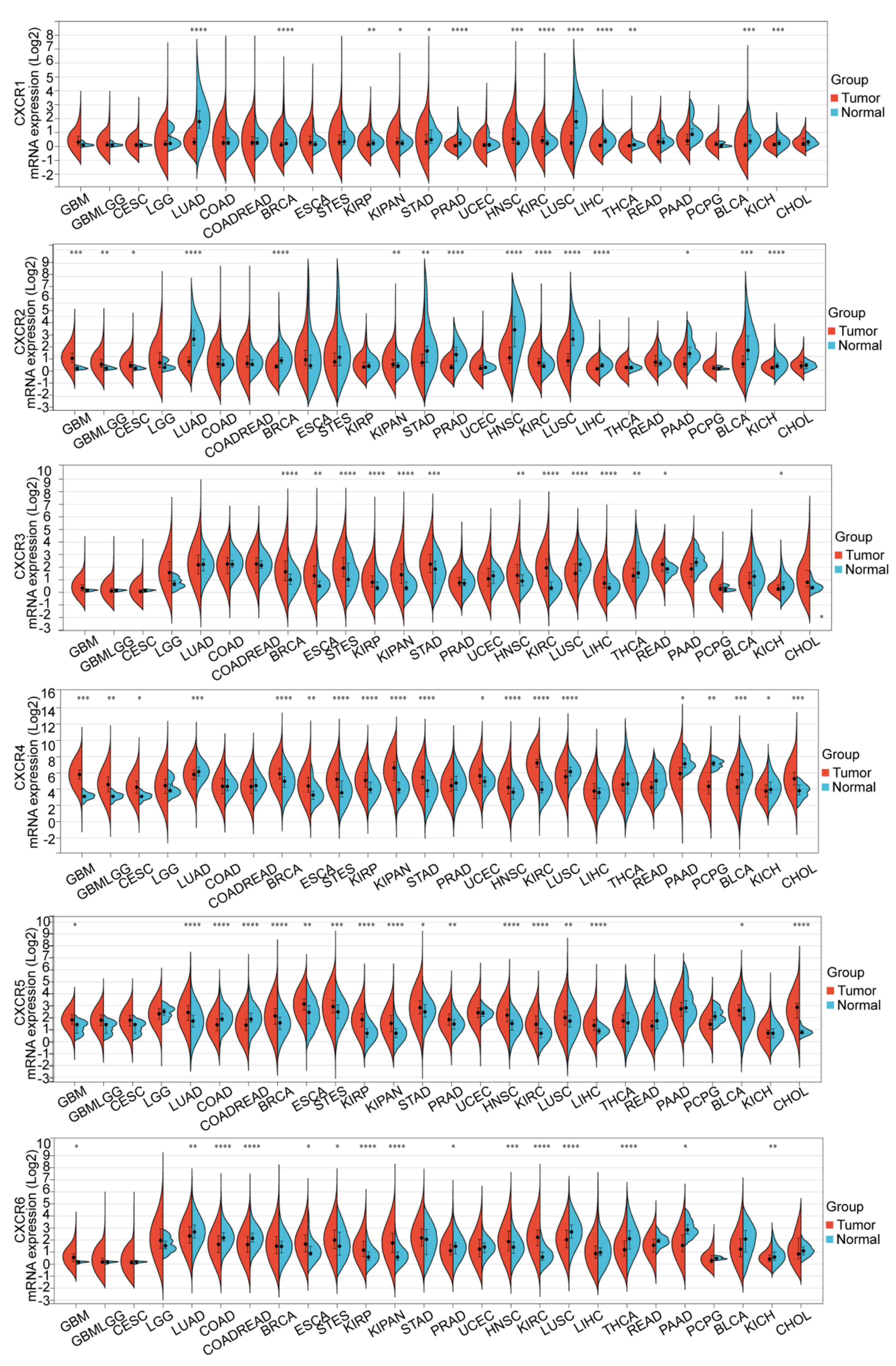
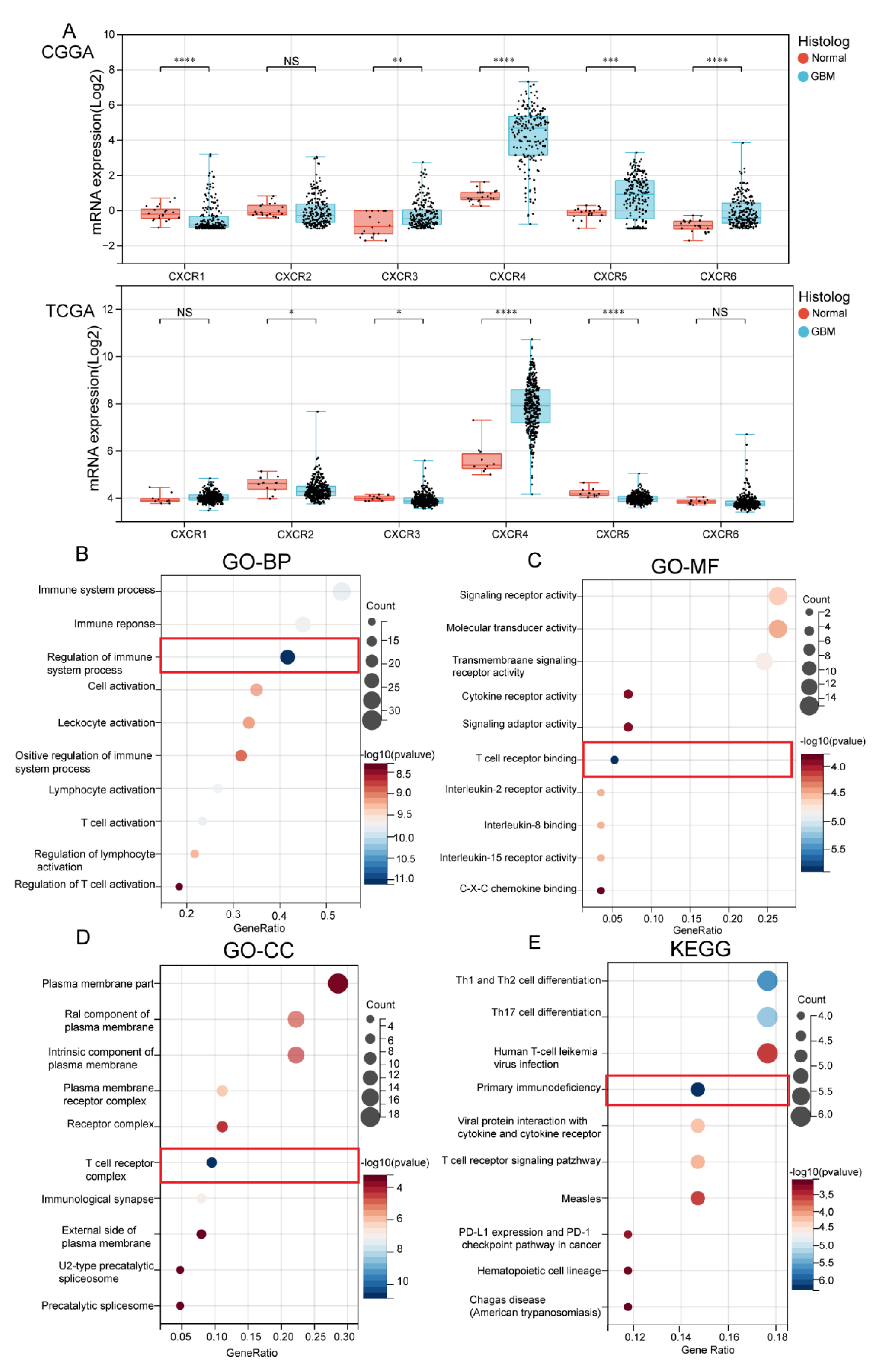
3.1. Transcription Level of CXCRs in Patients with GBM
3.2. Biological Functional Analysis of CXCR Members
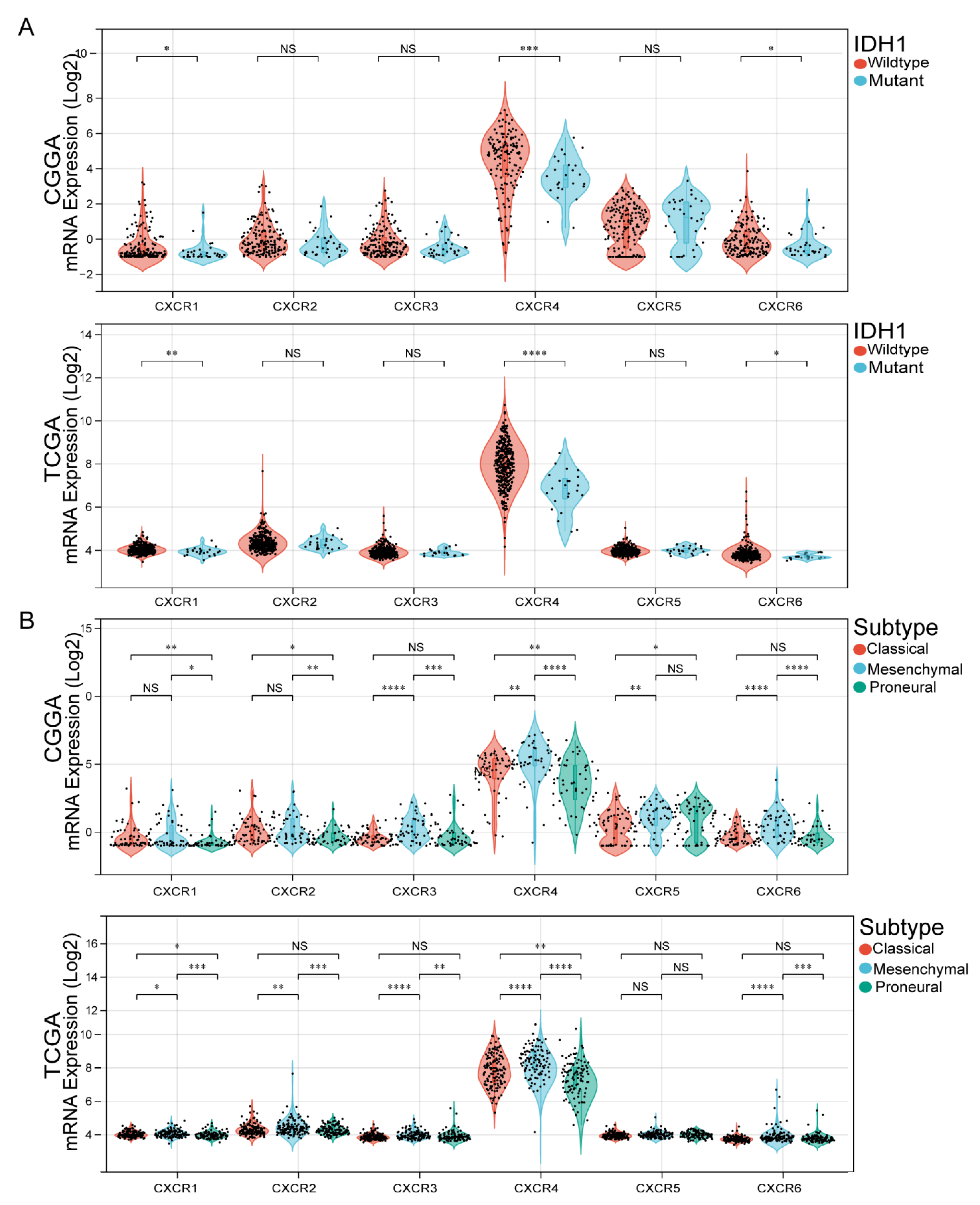
3.3. Association of CXCR Members with IDH1 Mutation Status and GBM Subtypes
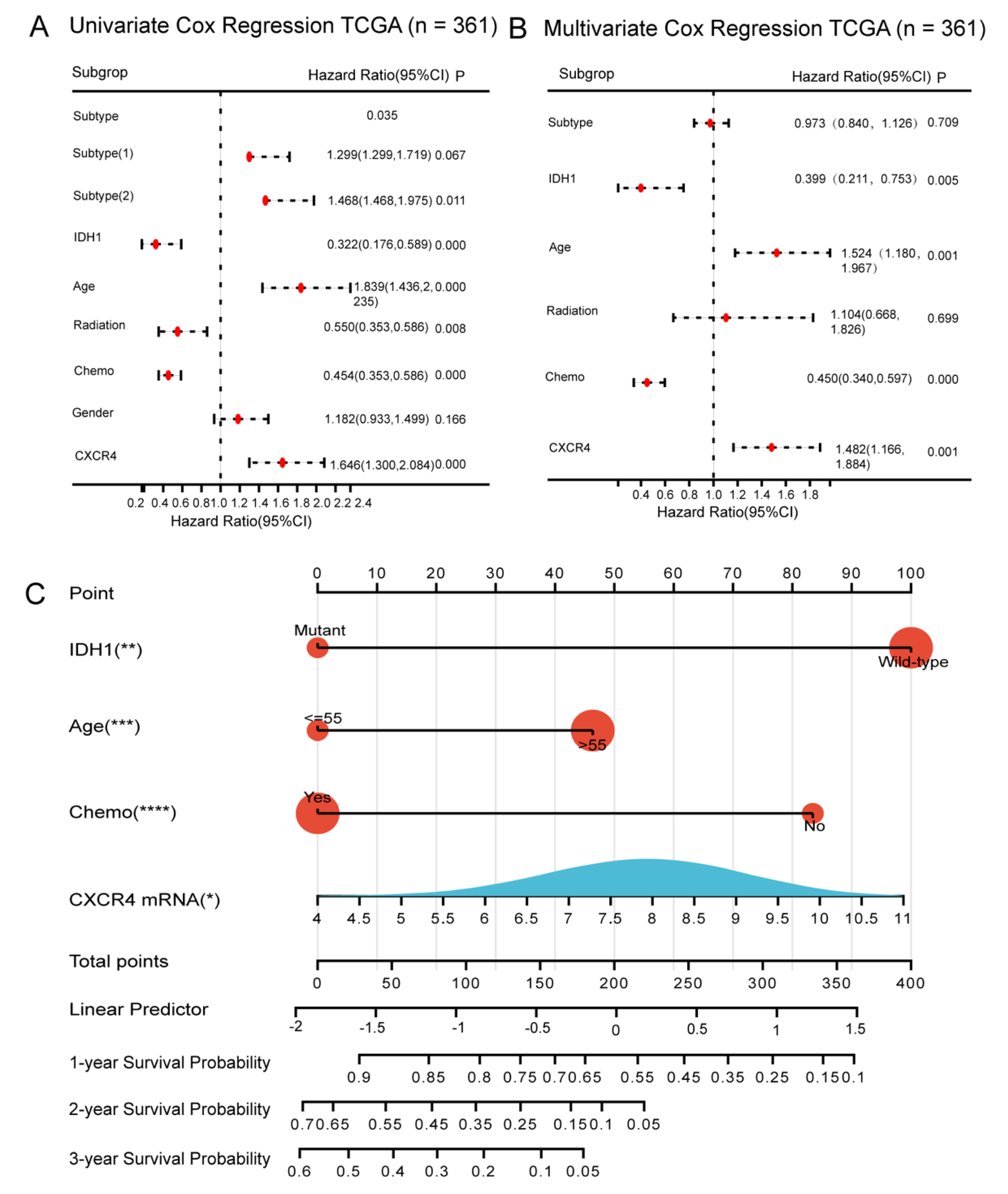
3.4. Relationship between Expression of CXCR Members and Prognosis of GBM Patients
3.5. Construction of CXCR4-Based Nomogram and Risk Score Models for Predicting Survival of GBM Patients
3.6. Evaluation of CXCR4-Based Risk Scoring Models in Primary GBM
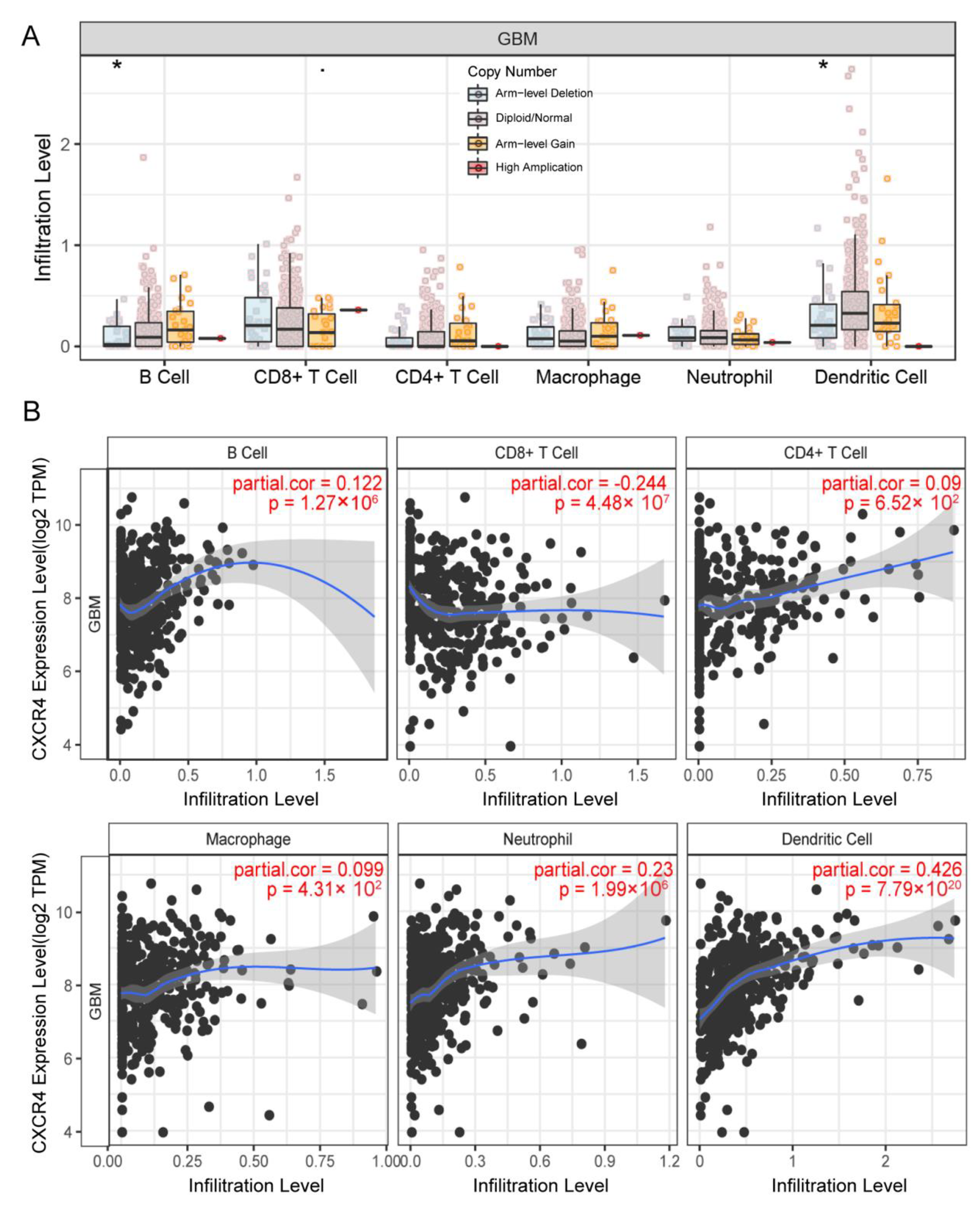
3.7. CXCR4 Expression in the Infiltration Level of Immune Cells
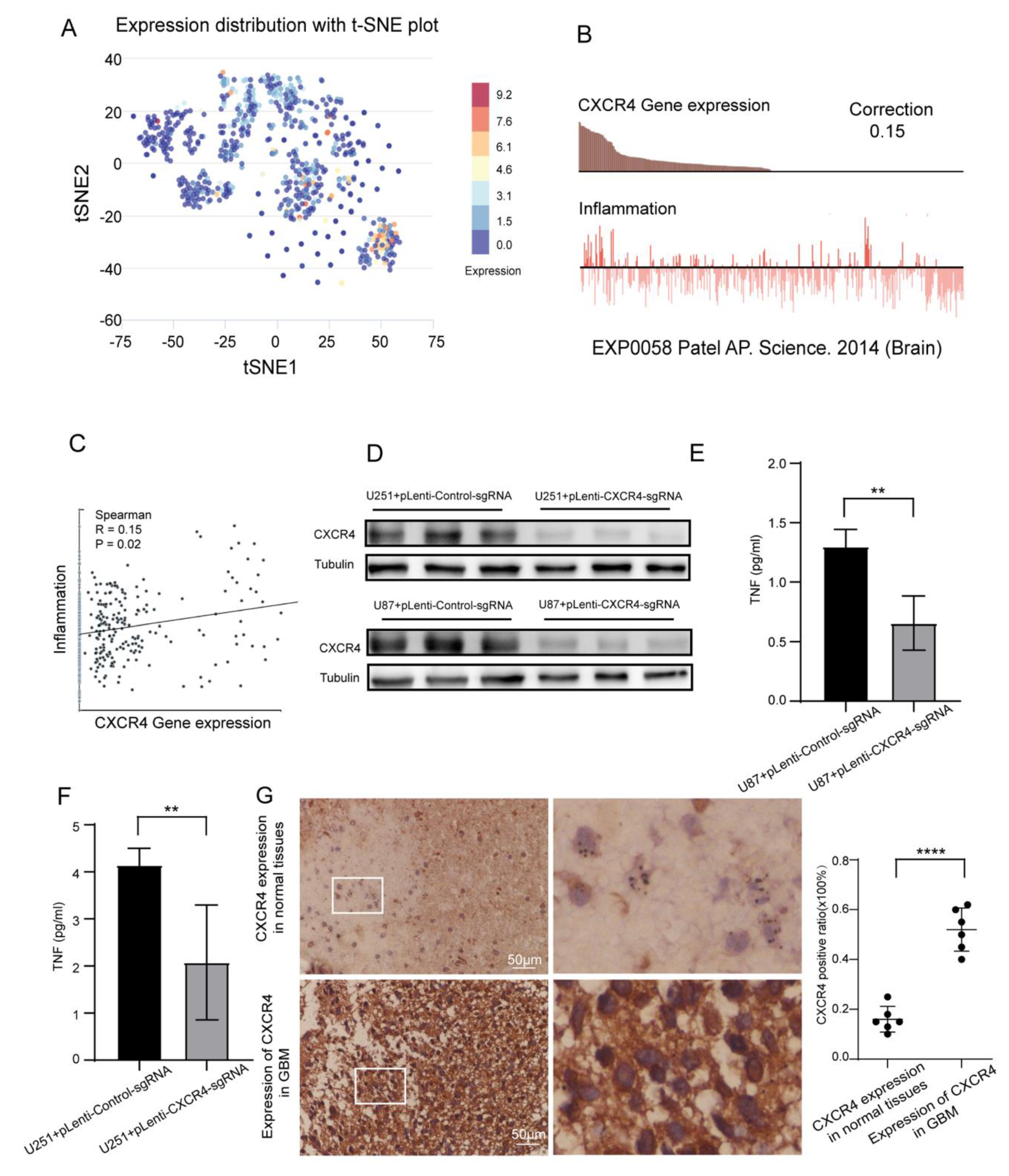
3.8. Single Cell Analysis and Verification of Inflammatory Correlation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Presti, M.; Mazzon, E.; Basile, M.S.; Petralia, M.C.; Bramanti, A.; Colletti, G.; Bramanti, P.; Nicoletti, F.; Fagone, P. Overexpression of macrophage migration inhibitory factor and functionally-related genes, D-DT, CD74, CD44, CXCR2 and CXCR4, in glioblastoma. Oncol. Lett. 2018, 16, 2881–2886. [Google Scholar] [CrossRef] [PubMed]
- Hira, V.V.V.; Van Noorden, C.J.F.; Molenaar, R.J. CXCR4 Antagonists as Stem Cell Mobilizers and Therapy Sensitizers for Acute Myeloid Leukemia and Glioblastoma? Biology 2020, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Yan, T.; Han, Y.; Wang, Q.; Zhang, G.; Zhang, L.; Zhu, W.; Xie, L.; Guo, X. Nomograms for prognostic risk assessment in glioblastoma multiforme: Applications and limitations. Clin. Genet. 2022, 102, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Alghamri, M.S.; Banerjee, K.; Mujeeb, A.A.; Mauser, A.; Taher, A.; Thalla, R.; McClellan, B.L.; Varela, M.L.; Stamatovic, S.M.; Martinez-Revollar, G.; et al. Systemic Delivery of an Adjuvant CXCR4-CXCL12 Signaling Inhibitor Encapsulated in Synthetic Protein Nanoparticles for Glioma Immunotherapy. ACS Nano 2022, 16, 8729–8750. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.W.; Yang, S.X.; Chen, J.H.; Ping, Y.F.; Zhou, X.D.; Wang, Q.L.; Jiang, X.F.; Gong, W.; Xiao, H.L.; Du, L.L.; et al. Preferential expression of chemokine receptor CXCR4 by highly malignant human gliomas and its association with poor patient survival. Neurosurgery 2007, 61, 570–578; discussion 578–579. [Google Scholar] [CrossRef] [PubMed]
- Urbantat, R.M.; Vajkoczy, P.; Brandenburg, S. Advances in Chemokine Signaling Pathways as Therapeutic Targets in Glioblastoma. Cancers 2021, 13, 2983. [Google Scholar] [CrossRef] [PubMed]
- Urbantat, R.M.; Blank, A.; Kremenetskaia, I.; Vajkoczy, P.; Acker, G.; Brandenburg, S. The CXCL2/IL8/CXCR2 Pathway Is Relevant for Brain Tumor Malignancy and Endothelial Cell Function. Int. J. Mol. Sci. 2021, 22, 2634. [Google Scholar] [CrossRef]
- Chien, Y.C.; Chen, J.N.; Chen, Y.H.; Chou, R.H.; Lee, H.C.; Yu, Y.L. Epigenetic Silencing of miR-9 Promotes Migration and Invasion by EZH2 in Glioblastoma Cells. Cancers 2020, 12, 1781. [Google Scholar] [CrossRef]
- Hattermann, K.; Held-Feindt, J.; Lucius, R.; Müerköster, S.S.; Penfold, M.E.; Schall, T.J.; Mentlein, R. The chemokine receptor CXCR7 is highly expressed in human glioma cells and mediates antiapoptotic effects. Cancer Res. 2010, 70, 3299–3308. [Google Scholar] [CrossRef] [PubMed]
- Vandercappellen, J.; Van Damme, J.; Struyf, S. The role of CXC chemokines and their receptors in cancer. Cancer Lett. 2008, 267, 226–244. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, C.; Sanmamed, M.F.; Rodríguez-Ruiz, M.E.; Teijeira, Á.; Oñate, C.; González, Á.; Ponz, M.; Schalper, K.A.; Pérez-Gracia, J.L.; Melero, I. Interleukin-8 in cancer pathogenesis, treatment and follow-up. Cancer Treat. Rev. 2017, 60, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Sallusto, F.; Lenig, D.; Mackay, C.R.; Lanzavecchia, A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J. Exp. Med. 1998, 187, 875–883. [Google Scholar] [CrossRef]
- Scotton, C.J.; Wilson, J.L.; Scott, K.; Stamp, G.; Wilbanks, G.D.; Fricker, S.; Bridger, G.; Balkwill, F.R. Multiple actions of the chemokine CXCL12 on epithelial tumor cells in human ovarian cancer. Cancer Res. 2002, 62, 5930–5938. [Google Scholar]
- Hussain, M.; Adah, D.; Tariq, M.; Lu, Y.; Zhang, J.; Liu, J. CXCL13/CXCR5 signaling axis in cancer. Life Sci. 2019, 227, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Lepore, F.; D’Alessandro, G.; Antonangeli, F.; Santoro, A.; Esposito, V.; Limatola, C.; Trettel, F. CXCL16/CXCR6 Axis Drives Microglia/Macrophages Phenotype in Physiological Conditions and Plays a Crucial Role in Glioma. Front. Immunol. 2018, 9, 2750. [Google Scholar] [CrossRef] [PubMed]
- Naumann, U.; Cameroni, E.; Pruenster, M.; Mahabaleshwar, H.; Raz, E.; Zerwes, H.G.; Rot, A.; Thelen, M. CXCR7 functions as a scavenger for CXCL12 and CXCL11. PLoS ONE 2010, 5, e9175. [Google Scholar] [CrossRef] [PubMed]
- Bowman, R.L.; Wang, Q.; Carro, A.; Verhaak, R.G.; Squatrito, M. GlioVis data portal for visualization and analysis of brain tumor expression datasets. Neuro-Oncology 2017, 19, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, K.N.; Wang, Q.; Li, G.; Zeng, F.; Zhang, Y.; Wu, F.; Chai, R.; Wang, Z.; Zhang, C.; et al. Chinese Glioma Genome Atlas (CGGA): A Comprehensive Resource with Functional Genomic Data from Chinese Glioma Patients. Genom. Proteom. Bioinform. 2021, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dell’albani, P.; Rodolico, M.; Pellitteri, R.; Tricarichi, E.; Torrisi, S.A.; D’Antoni, S.; Zappia, M.; Albanese, V.; Caltabiano, R.; Platania, N.; et al. Differential patterns of NOTCH1-4 receptor expression are markers of glioma cell differentiation. Neuro-Oncology 2014, 16, 204–216. [Google Scholar] [CrossRef]
- Yang, W.; Ding, N.; Luo, R.; Zhang, Q.; Li, Z.; Zhao, F.; Zhang, S.; Zhang, X.; Zhou, T.; Wang, H.; et al. Exosomes from young healthy human plasma promote functional recovery from intracerebral hemorrhage via counteracting ferroptotic injury. Bioact. Mater. 2023, 27, 1–14. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Sokratous, G.; Polyzoidis, S.; Ashkan, K. Immune infiltration of tumor microenvironment following immunotherapy for glioblastoma multiforme. Hum. Vaccin. Immunother. 2017, 13, 2575–2582. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, L.; Ajmone-Cat, M.A.; Cecchetti, S.; Ricci, A.; Bozzuto, G.; Molinari, A.; Manni, I.; Pollo, B.; Scala, S.; Carpinelli, G.; et al. Targeting CXCR4 by a selective peptide antagonist modulates tumor microenvironment and microglia reactivity in a human glioblastoma model. J. Exp. Clin. Cancer Res. 2016, 35, 55. [Google Scholar] [CrossRef]
- Gjorgjevski, M.; Hannen, R.; Carl, B.; Li, Y.; Landmann, E.; Buchholz, M.; Bartsch, J.W.; Nimsky, C. Molecular profiling of the tumor microenvironment in glioblastoma patients: Correlation of microglia/macrophage polarization state with metalloprotease expression profiles and survival. Biosci. Rep. 2019, 39, BSR20182361. [Google Scholar] [CrossRef] [PubMed]
- Mollica Poeta, V.; Massara, M.; Capucetti, A.; Bonecchi, R. Chemokines and Chemokine Receptors: New Targets for Cancer Immunotherapy. Front. Immunol. 2019, 10, 379. [Google Scholar] [CrossRef]
- He, J.; Jiang, Z.; Lei, J.; Zhou, W.; Cui, Y.; Luo, B.; Zhang, M. Prognostic Value and Therapeutic Perspectives of CXCR Members in the Glioma Microenvironment. Front. Genet. 2022, 13, 787141. [Google Scholar] [CrossRef]
- Hsieh, H.T.; Huang, H.C.; Chung, C.W.; Chiang, C.C.; Hsia, T.; Wu, H.F.; Huang, R.L.; Chiang, C.S.; Wang, J.; Lu, T.T.; et al. CXCR4-targeted nitric oxide nanoparticles deliver PD-L1 siRNA for immunotherapy against glioblastoma. J. Control. Release 2022, 352, 920–930. [Google Scholar] [CrossRef]
- Baron, N.; Deuster, O.; Noelker, C.; Stüer, C.; Strik, H.; Schaller, C.; Dodel, R.; Meyer, B.; Bacher, M. Role of macrophage migration inhibitory factor in primary glioblastoma multiforme cells. J. Neurosci. Res. 2011, 89, 711–717. [Google Scholar] [CrossRef]
- Yi, L.; Tong, L.; Li, T.; Hai, L.; Abeysekera, I.R.; Tao, Z.; Ma, H.; Liu, P.; Xie, Y.; Li, J.; et al. Bioinformatic analyses reveal the key pathways and genes in the CXCR4 mediated mesenchymal subtype of glioblastoma. Mol. Med. Rep. 2018, 18, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Hoelzinger, D.B.; Demuth, T.; Berens, M.E. Autocrine factors that sustain glioma invasion and paracrine biology in the brain microenvironment. J. Natl. Cancer Inst. 2007, 99, 1583–1593. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, G.; Zheng, Z.-Q.; Zhang, J.; Tian, Z.; Li, X.; Yu, Z.; Wang, Z.; You, W.; Chen, G. Development and Validation of CXCR4 Nomogram-Based Immune Infiltration/Tumor Inflammation in Primary Glioblastoma. Brain Sci. 2023, 13, 1152. https://doi.org/10.3390/brainsci13081152
Jiang G, Zheng Z-Q, Zhang J, Tian Z, Li X, Yu Z, Wang Z, You W, Chen G. Development and Validation of CXCR4 Nomogram-Based Immune Infiltration/Tumor Inflammation in Primary Glioblastoma. Brain Sciences. 2023; 13(8):1152. https://doi.org/10.3390/brainsci13081152
Chicago/Turabian StyleJiang, Guannan, Zong-Qing Zheng, Jie Zhang, Zhichao Tian, Xiang Li, Zhengquan Yu, Zhong Wang, Wanchun You, and Gang Chen. 2023. "Development and Validation of CXCR4 Nomogram-Based Immune Infiltration/Tumor Inflammation in Primary Glioblastoma" Brain Sciences 13, no. 8: 1152. https://doi.org/10.3390/brainsci13081152
APA StyleJiang, G., Zheng, Z.-Q., Zhang, J., Tian, Z., Li, X., Yu, Z., Wang, Z., You, W., & Chen, G. (2023). Development and Validation of CXCR4 Nomogram-Based Immune Infiltration/Tumor Inflammation in Primary Glioblastoma. Brain Sciences, 13(8), 1152. https://doi.org/10.3390/brainsci13081152






