The Impact of Dance Movement Interventions on Psychological Health in Older Adults without Dementia: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Material and Methods
2.1. Protocol and Registration
2.2. Criteria for Considering Studies for this Review
2.2.1. Types of Studies
2.2.2. Types of Participants
2.2.3. Types of Interventions
2.2.4. Types of Outcome Measures
Primary Outcome
Additional Outcome
2.3. Search Methods for Identification of Studies
2.3.1. Selection of Studies
2.3.2. Data extraction and Management
2.3.3. Assessment of Risk of Bias in Included Studies
2.3.4. Assessment of Heterogeneity
2.3.5. Assessment of Reporting Bias
2.4. Data Synthesis
2.4.1. Accounting for Dependencies
2.4.2. Further Analyses
3. Results
3.1. Study Selection
3.2. Study Characteristics
| Study | Country | Participants | Intervention | Outcomes | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N (EXP/AC/PC) | Mean Age | Descriptions | Proportion of Females | Type of DMI | Period and Frequencies | Intensity | Control Type | |||
| Alves, 2013 [66] | Brazil | 65 (25/15/25) | 68.1 | non-clinical | 92.3% | Ballroom Dance | 16 weeks, 2/week, 120 min, | - | AC: Walking PC: no contact | (1) Ryff’s PWBS (2) PSQI (3) BAI (4) PSS (5) Raven’s Advanced Matrices |
| Bisbe et al., 2020 [75] | Spain | 31 (17/14/-) | 75.1 | MCI | 48.4% | Choreography | 12 weeks, 2/week, 60 min | light–moderate | AC: Physical Therapy | (1) SF-36 (2) HADS-A (3) HADS-D (4) MMSE |
| Chang et al., 2021 [73] | China | 109 (62/47/-) | 76.3 | MCI/SCD | 100% | Square Dance | 18 weeks, 3/week, 30 min | low | PC: Usual Care | (1) SF-12 (2) GDS-15 (3) MoCA |
| Cruz-Ferreira et al., 2015 [76] | Portugal | 57 (32/-/25) | 72.0 | non-clinical | 100% | Creative Dance | 24 weeks, 2/week, 50 min | - | PC: Waitlist | (1) LSS |
| Esmail et al., 2020 [71] | Canada | 41 (12/15/14) | 67.5 | non-clinical | 75.6% | Dance Movement | 12 weeks, 3/week, 60 min | - | AC: Aerobic Exercise PC: Waitlist | (1) SF-12 (2) HPLP2 (3) MHC (4) LSNS (5) STAI-Trait (6) BPI (7) MoCA |
| Eyigor et al., 2009 [77] | Turkey | 37 (19/-/18) | 72.4 | non-clinical (depression included) | 100% | Folkloric Dance | 8 weeks, 3/week, 60 min | - | PC: no intervention | (1) SF-36 (2) GDS |
| Hars et al., 2014 [67] | Switzerland | 134 (66/-/68) | 75.5 | non-clinical (age-related medical conditions included) | 96.3% | Eurythmy | 25 weeks, 1/week, 60 min | - | PC: Waitlist | (1) HADS-A (2) HADS-D (3) MMSE |
| Hui et al., 2009 [70] | China | 97 (52/- /45) | 68.0 | non-clinical | 96.9% | Aerobic dance | 12 weeks, 2/week, 50 min | low | PC: No intervention | (1) SF-36 |
| Kosmat and Vranic, 2017 [74] | Croatia | 24 (12/12/-) | 80.8 | non-clinical | 62.5% | Standard Dance | 10 weeks, 1/week, 45 min | - | AC: Social discussion | (1) SWLS (2) GSE |
| Lazarou et al., 2017 [78] | Greece | 129 (66/-/63) | 66.8 | MCI | 78.3% | Ballroom Dance | 40 weeks, 2/week, 60 min | - | PC: No intervention | (1) NPI (2) MMSE, MoCA |
| Liao et al., 2018 [69] | China | 107 (55/52/-) | 71.8 | non-clinical (mild–moderate depressive symptoms) | 61.7% | Music and Tai-Chi (combined) | 12 weeks, 3/week, 50 min | moderate | AC: Routine health education | (1) GDS |
| Mishra et al., 2022 [79] | India | 40 (20/20/-) | 65.7 | non-clinical | 80% | Folkloric Dance | 6 weeks, 5/week, 60 min | moderate | AC: Exercise program | (1) SF-36 (2) MoCA |
| Serrano-Guzmán et al., 2016 [80] | Spain | 52 (27/25/-) | 69.3 | non-clinical | 100% | Dance Therapy (Flamenco) | 8 weeks, 3/week, 50 min | low impact | AC: Self-care treatment advice | (1) SF-12 |
| Zhu et al., 2018 [72] | China | 60 (29/-/31) | 69.6 | MCI | 60% | Aerobic Dance | 12 weeks, 3/week, 35 min | moderate | PC: No intervention | (1) GDS-15 (2) SF-36 (3) MoCA |
3.3. Participant Characteristics
3.4. Intervention Characteristics
3.4.1. Intervention Type/Types of DMI
3.4.2. Intervention Period, Duration and Frequency
3.4.3. Intervention Setting
3.4.4. Intervention Adherence
3.4.5. Control Condition
3.5. Neurophysiological Measures
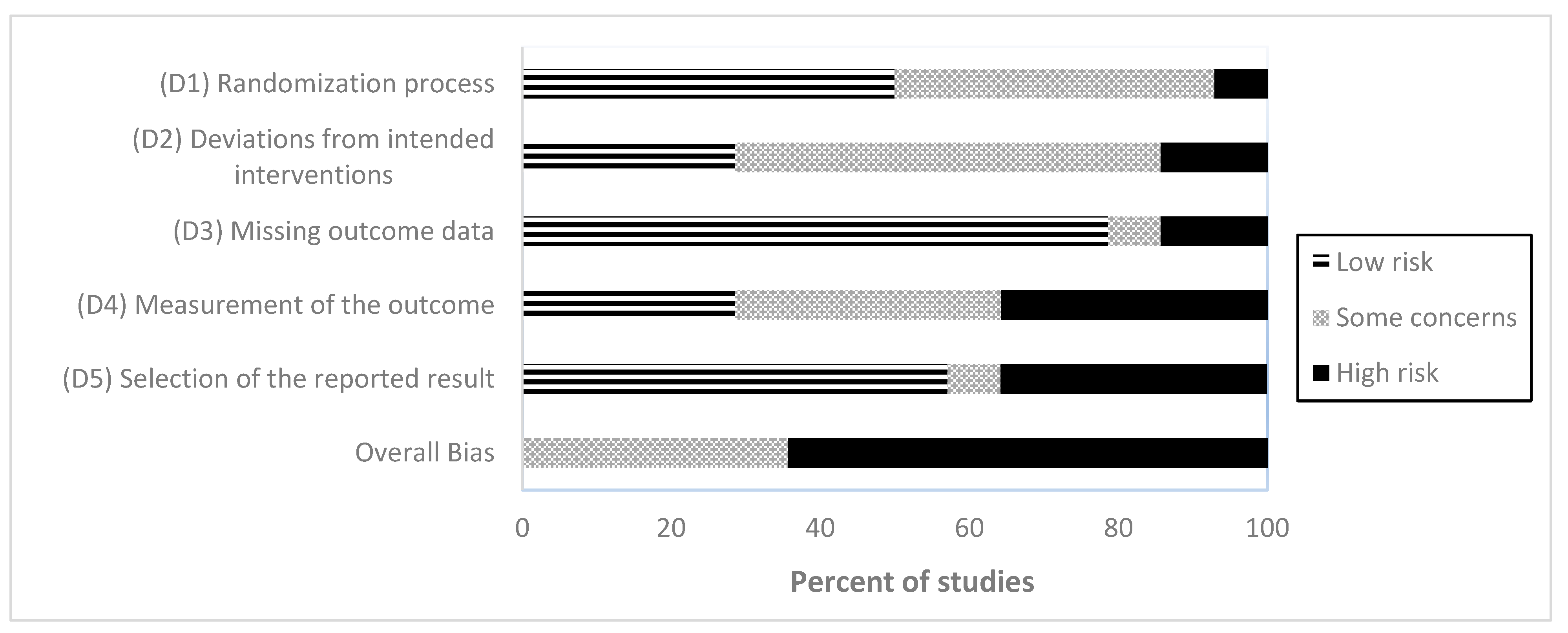
3.6. Risk of Bias
3.7. Publication Bias
3.8. Quantitative Synthesis of Results
3.8.1. Primary Outcome
3.8.2. Additional Outcome: Cognitive Function
3.8.3. Additional Analyses
4. Discussion
4.1. Summary of Main Findings
4.2. DMI and Psychological Health as Primary Outcome
4.3. DMI and Cognitive Function as Additional Outcome
4.4. Putative Neurophysiological Mechanisms
4.5. Recommendations for Future Studies
4.6. Synopsis and Outlook
4.7. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. World Population Ageing 2019. 2020. Available online: https://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2019-Report.pdf (accessed on 1 February 2022).
- Michalowsky, B.; Kaczynski, A.; Hoffmann, W. Ökonomische und gesellschaftliche Herausforderungen der Demenz in Deutschland—Eine Metaanalyse. Bundesgesundheitsblatt-Gesundh.-Gesundheitsschutz. 2019, 62, 981–992. [Google Scholar] [CrossRef]
- Hurd, M.D.; Martorell, P.; Delavande, A.; Mullen, K.J.; Langa, K.M. Monetary Costs of Dementia in the United States. N. Engl. J. Med. 2013, 368, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Connell, C.M.; Janevic, M.R.; Gallant, M.P. The Costs of Caring: Impact of Dementia on Family Caregivers. J. Geriatr. Psychiatry Neurol. 2001, 14, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef] [PubMed]
- Norton, S.; Matthews, F.E.; Barnes, D.E.; Yaffe, K.; Brayne, C. Potential for primary prevention of Alzheimer’s disease: An analysis of population-based data. Lancet Neurol. 2014, 13, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Marchant, N.L.; Howard, R.J. Cognitive debt and Alzheimer’s disease. J. Alzheimer’s Dis. 2015, 44, 755–770. [Google Scholar] [CrossRef]
- Rosenberg, A.; Mangialasche, F.; Ngandu, T.; Solomon, A.; Kivipelto, M. Multidomain Interventions to Prevent Cognitive Impairment, Alzheimer’s Disease, and Dementia: From FINGER to World-Wide FINGERS. J. Prev. Alzheimers Dis. 2020, 7, 29–36. [Google Scholar] [CrossRef]
- World Health Organization. Promoting Mental Health: Concepts, Emerging Evidence, Practice: A report of the World Health Organization, Department of Mental Health and Substance Abuse in collaboration with the Victorian Health Promotion Foundation and the University of Melbourne; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- López, J.; Perez-Rojo, G.; Noriega, C.; Carretero, I.; Velasco, C.; Martinez-Huertas, J.A.; López-Frutos, P.; Galarraga, L. Psychological well-being among older adults during the COVID-19 outbreak: A comparative study of the young–old and the old–old adults. Int. Psychogeriatr. 2020, 32, 1365–1370. [Google Scholar] [CrossRef]
- Marchant, N.L.; Lovland, L.R.; Jones, R.; Binette, A.P.; Gonneaud, J.; Arenaza-Urquijo, E.M.; Chételat, G.; Villeneuve, S. Repetitive negative thinking is associated with amyloid, tau, and cognitive decline. Alzheimer’s Dement. 2020, 16, 1054–1064. [Google Scholar] [CrossRef] [PubMed]
- Fredericks, C.A.; Sturm, V.E.; Brown, J.A.; Hua, A.Y.; Bilgel, M.; Wong, D.F.; Resnick, S.M.; Seeley, W.W. Early affective changes and increased connectivity in preclinical Alzheimer’s disease. Alzheimer’s Dement. 2018, 10, 471–479. [Google Scholar] [CrossRef]
- Schwarz, C.; Benson, G.S.; Antonenko, D.; Horn, N.; Köbe, T.; Klimecki, O.; Sommer, W.; Wirth, M.; Flöel, A. Negative affective burden is associated with higher resting-state functional connectivity in subjective cognitive decline. Sci. Rep. 2022, 12, 6212. [Google Scholar] [CrossRef]
- Terracciano, A.; Stephan, Y.; Luchetti, M.; Albanese, E.; Sutin, A.R. Personality traits and risk of cognitive impairment and dementia. J. Psychiatr. Res. 2017, 89, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.S.; Evans, D.A.; Bienias, J.L.; Mendes de Leon, C.F.; Schneider, J.A.; Bennett, D.A. Proneness to psychological distress is associated with risk of Alzheimer’s disease. Neurology 2003, 61, 1479–1485. [Google Scholar] [CrossRef] [PubMed]
- Borglin, G.; Jakobsson, U.; Edberg, A.-K.; Hallberg, I.R. Older people in Sweden with various degrees of present quality of life: Their health, social support, everyday activities and sense of coherence. Health Soc. Care Community 2006, 14, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Flicker, L.; Lautenschlager, N.T.; Almeida, O.P. Healthy mental ageing. J. Br. Menopause Soc. 2006, 12, 92–96. [Google Scholar] [CrossRef]
- Kim, E.S.; James, P.; Zevon, E.S.; Trudel-Fitzgerald, C.; Kubzansky, L.D.; Grodstein, F. Optimism and Healthy Aging in Women and Men. Am. J. Epidemiol. 2019, 188, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Kubzansky, L.D.; Soo, J.; Boehm, J.K. Maintaining Healthy Behavior: A Prospective Study of Psychological Well-Being and Physical Activity. Ann. Behav. Med. 2017, 51, 337–347. [Google Scholar] [CrossRef]
- Ryff, C.D. Psychological Well-Being Revisited: Advances in the Science and Practice of Eudaimonia. Psychother. Psychosom. 2014, 83, 10–28. [Google Scholar] [CrossRef]
- Steptoe, A.; Deaton, A.; Stone, A.A. Subjective wellbeing, health, and ageing. Lancet 2015, 385, 640–648. [Google Scholar] [CrossRef]
- Cohen, R.; Bavishi, C.; Rozanski, A. Purpose in Life and Its Relationship to All-Cause Mortality and Cardiovascular Events: A Meta-Analysis. Psychosom. Med. 2016, 78, 122–133. [Google Scholar] [CrossRef]
- Martín-María, N.; Miret, M.; Caballero, F.F.; Rico-Uribe, L.A.; Steptoe, A.; Chatterji, S.; Ayuso-Mateos, J.L. The Impact of Subjective Well-being on Mortality: A Meta-Analysis of Longitudinal Studies in the General Population. Psychosom. Med. 2017, 79, 565–575. [Google Scholar] [CrossRef]
- Felix, C.; Rosano, C.; Zhu, X.; Flatt, J.D.; Rosso, A.L. Greater Social Engagement and Greater Gray Matter Microstructural Integrity in Brain Regions Relevant to Dementia. J. Gerontol. Ser. B 2020, 76, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Demnitz-King, H.; Gonneaud, J.; Klimecki, O.M.; Chocat, A.; Collette, F.; Dautricourt, S.; Jessen, F.; Krolak-Salmon, P.; Lutz, A.; Morse, R.M.; et al. Association Between Self-Reflection, Cognition, and Brain Health in Cognitively Unimpaired Older Adults. Neurology 2022, 99, e1422–e1431. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, T.; Barnhofer, T.; Acabchuk, R.; Cohen, A.; Lee, M.; Schlosser, M.; Arenaza-Urquijo, E.M.; Böttcher, A.; Britton, W.; Coll-Padros, N.; et al. The Effect of Mindfulness-based Programs on Cognitive Function in Adults: A Systematic Review and Meta-analysis. Neuropsychol. Rev. 2021, 32, 677–702. [Google Scholar] [CrossRef]
- Duffner, L.A.; Deckers, K.; Cadar, D.; Steptoe, A.; de Vugt, M.; Köhler, S. The role of cognitive and social leisure activities in dementia risk: Assessing longitudinal associations of modifiable and non-modifiable risk factors. Epidemiol. Psychiatr. Sci. 2022, 31, e5. [Google Scholar] [CrossRef]
- Boyle, P.A.; Buchman, A.S.; Barnes, L.L.; Bennett, D.A. Effect of a Purpose in Life on Risk of Incident Alzheimer Disease and Mild Cognitive Impairment in Community-Dwelling Older Persons. Arch. Gen. Psychiatry 2010, 67, 304. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Tkatch, R.; Martin, D.; Macleod, S.; Sandy, L.; Yeh, C. Resilient Aging: Psychological Well-Being and Social Well-Being as Targets for the Promotion of Healthy Aging. Gerontol. Geriatr. Med. 2021, 7, 233372142110029. [Google Scholar] [CrossRef]
- Kubzansky, L.D.; Huffman, J.C.; Boehm, J.K.; Hernandez, R.; Kim, E.S.; Koga, H.K.; Feig, E.H.; Lloyd-Jones, D.M.; Seligman, M.E.P.; Labarthe, D.R. Positive Psychological Well-Being and Cardiovascular Disease: JACC Health Promotion Series. J. Am. Coll. Cardiol. 2018, 72, 1382–1396. [Google Scholar] [CrossRef]
- Kempermann, G. Environmental enrichment, new neurons and the neurobiology of individuality. Nat. Rev. Neurosci. 2019, 20, 235–245. [Google Scholar] [CrossRef]
- Kempermann, G. Embodied Prevention. Front. Psychol. 2022, 13, 841393. [Google Scholar] [CrossRef]
- de Witte, M.; Orkibi, H.; Zarate, R.; Karkou, V.; Sajnani, N.; Malhotra, B.; Ho, R.T.H.; Kaimal, G.; Baker, F.A.; Koch, S.C. From Therapeutic Factors to Mechanisms of Change in the Creative Arts Therapies: A Scoping Review. Front. Psychol. 2021, 12, 678397. [Google Scholar] [CrossRef]
- Herold, F.; Hamacher, D.; Schega, L.; Müller, N.G. Thinking While Moving or Moving While Thinking—Concepts of Motor-Cognitive Training for Cognitive Performance Enhancement. Front. Aging Neurosci. 2018, 10, 228. [Google Scholar] [CrossRef] [PubMed]
- Basso, J.C.; Satyal, M.K.; Rugh, R. Dance on the Brain: Enhancing Intra- and Inter-Brain Synchrony. Front. Hum. Neurosci. 2021, 14, 584312. [Google Scholar] [CrossRef] [PubMed]
- Verghese, J.; Lipton, R.B.; Katz, M.J.; Hall, C.B.; Derby, C.A.; Kuslansky, G.; Ambrose, A.F.; Sliwinski, M.; Buschke, H. Leisure activities and the risk of dementia in the elderly. N. Engl. J. Med. 2003, 348, 2508–2516. [Google Scholar] [CrossRef]
- Hewston, P.; Kennedy, C.C.; Borhan, S.; Merom, D.; Santaguida, P.; Ioannidis, G.; Marr, S.; Santesso, N.; Thabane, L.; Bray, S.; et al. Effects of dance on cognitive function in older adults: A systematic review and meta-analysis. Age Ageing 2020, 50, 1084–1092. [Google Scholar] [CrossRef]
- Meng, X.; Li, G.; Jia, Y.; Liu, Y.; Shang, B.; Liu, P.; Bao, X.; Chen, L. Effects of dance intervention on global cognition, executive function and memory of older adults: A meta-analysis and systematic review. Aging Clin. Exp. Res. 2019, 32, 7–19. [Google Scholar] [CrossRef]
- Chan, J.S.Y.; Wu, J.; Deng, K.; Yan, J.H. The Effectiveness of Dance Interventions on Cognition in Patients with Mild Cognitive Impairment: A Meta-Analysis of Randomized Controlled Trials. Elsevier Ltd.: Amsterdam, The Netherlands, 2020; pp. 80–88. [Google Scholar]
- Fong Yan, A.; Cobley, S.; Chan, C.; Pappas, E.; Nicholson, L.L.; Ward, R.E.; Murdoch, R.E.; Gu, Y.; Trevor, B.L.; Vassallo, A.J.; et al. The Effectiveness of Dance Interventions on Physical Health Outcomes Compared to Other Forms of Physical Activity: A Systematic Review and Meta-Analysis. Sports Med. 2018, 48, 933–951. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shen, P.L.; Tsai, Y.S. Dance intervention effects on physical function in healthy older adults: A systematic review and meta-analysis. Aging Clin. Exp. Res. 2021, 33, 253–263. [Google Scholar] [CrossRef]
- Mattle, M.; Chocano-Bedoya, P.O.; Fischbacher, M.; Meyer, U.; Abderhalden, L.A.; Lang, W.; Mansky, R.; Kressig, R.W.; Steurer, J.; Orav, E.J.; et al. Association of Dance-Based Mind-Motor Activities with Falls and Physical Function Among Healthy Older Adults. JAMA Netw. Open 2020, 3, e2017688. [Google Scholar] [CrossRef]
- Koch, S.; Kunz, T.; Lykou, S.; Cruz, R. Effects of dance movement therapy and dance on health-related psychological outcomes: A meta-analysis. Arts Psychother. 2014, 41, 46–64. [Google Scholar] [CrossRef]
- Koch, S.C.; Riege, R.F.F.; Tisborn, K.; Biondo, J.; Martin, L.; Beelmann, A. Effects of dance movement therapy and dance on health-related psychological outcomes A meta-analysis update. Front. Psychol. 2019, 10, 1806. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, M.; Tan, Y.; Dong, Z.; Wu, J.; Cui, H.; Shen, D.; Chi, I. Effectiveness of Dance-Based Interventions on Depression for Persons with MCI and Dementia: A Systematic Review and Meta-Analysis. Front. Psychol. 2022, 12, 709208. [Google Scholar] [CrossRef]
- Wu, V.X.; Chi, Y.; Lee, J.K.; Goh, H.S.; Chen, D.Y.M.; Haugan, G.; Chao, F.F.T.; Klainin-Yobas, P. The effect of dance interventions on cognition, neuroplasticity, physical function, depression, and quality of life for older adults with mild cognitive impairment: A systematic review and meta-analysis. Int. J. Nurs. Stud. 2021, 122, 104025. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Su, M.; Jiao, Y.; Ji, Y.; Zhu, S. Effects of Dance Interventions on Cognition, Psycho-Behavioral Symptoms, Motor Functions, and Quality of Life in Older Adult Patients with Mild Cognitive Impairment: A Meta-Analysis and Systematic Review. Front. Aging Neurosci. 2021, 13, 706609. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Raven, J.; Raven, J. Raven Progressive Matrices. Handbook of Nonverbal Assessment; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2003; pp. 223–237. [Google Scholar]
- Wu, C.X.; Yi, Q.; Zheng, X.Y.; Cui, S.Y.; Chen, B.; Lu, L.M.; Tang, C. Effects of Mind-Body Exercises on Cognitive Function in Older Adults: A Meta-Analysis. J. Am. Geriatr. Soc. 2018, 67, 749–758. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Zou, L.; Liu, X.; Song, W. The Effects of Mind-Body Exercise on Cognitive Performance in Elderly: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2018, 15, 2791. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C.J.B. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. Introduction to Meta-Analysis; John Wiley Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Hedges, L.V. Distribution Theory for Glass’s Estimator of Effect size and Related Estimators. J. Educ. Stat. 1981, 6, 107–128. [Google Scholar] [CrossRef]
- Morris, S.B. Estimating Effect Sizes from Pretest-Posttest-Control Group Designs. Organ. Res. Methods 2008, 11, 364–386. [Google Scholar] [CrossRef]
- Tanner-Smith, E.E.; Tipton, E.; Polanin, J.R. Handling Complex Meta-analytic Data Structures Using Robust Variance Estimates: A Tutorial in R. J. Dev. Life-Course Criminol. 2016, 2, 85–112. [Google Scholar] [CrossRef]
- Matt, G.E.; Cook, T.D. Threats to the Validity of Research Syntheses. In The Handbook of Research Synthesis; Russell Sage Foundation: Manhattan, NY, USA, 1994. [Google Scholar]
- Hedges, L.V.; Tipton, E.; Johnson, M.C. Robust variance estimation in meta-regression with dependent effect size estimates. Res. Synth. Methods 2010, 1, 39–65. [Google Scholar] [PubMed]
- Fisher, Z.; Tipton, E. Robumeta: An R-package for robust variance estimation in meta-analysis. arXiv 2015, arXiv:1503.02220. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Viechtbauer, W.; Cheung, M.W.-L. Outlier and influence diagnostics for meta-analysis. Res. Synth. Methods 2010, 1, 112–125. [Google Scholar] [CrossRef]
- Alves, H.V.D. Dancing and the Aging Brain: The Effects of a 4-Month Ballroom Dance Intervention on the Cognition of Healthy Older Adults: ProQuest Information Learning; University of Illinois: Champaign, IL, USA, 2014. [Google Scholar]
- Hars, M.; Herrmann, F.; Gold, G.; Rizzoli, R.; Trombetti, A. Effect of music-based multitask training on cognition and mood in older adults. Age Ageing 2014, 43, 196–200. [Google Scholar] [CrossRef]
- Trombetti, A.; Hars, M.; Herrmann, F.R.; Kressig, R.W.; Ferrari, S.; Rizzoli, R. Effect of Music-Based Multitask Training on Gait, Balance, and Fall Risk in Elderly People. Arch. Intern. Med. 2011, 171, 525–533. [Google Scholar] [CrossRef]
- Liao, S.J.; Tan, M.P.; Chong, M.C.; Chua, Y.P. The impact of combined music and tai chi on depressive symptoms among community-dwelling older persons: A cluster randomized controlled trial. Issues Ment. Health Nurs. 2018, 39, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Hui, E.; Chui, B.T.-K.; Woo, J. Effects of dance on physical and psychological well-being in older persons. Arch. Gerontol. Geriatr. 2009, 49, e45–e50. [Google Scholar] [CrossRef] [PubMed]
- Esmail, A.; Vrinceanu, T.; Lussier, M.; Predovan, D.; Berryman, N.; Houle, J.; Karelis, A.; Grenier, S.; Vu, T.T.M.; Villalpando, J.M.; et al. Effects of Dance/Movement Training vs. Aerobic Exercise Training on cognition, physical fitness and quality of life in older adults: A randomized controlled trial. J. Bodyw. Mov. Ther. 2020, 24, 212–220. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, H.; Qi, M.; Wang, S.; Zhang, Q.; Zhou, L.; Wang, S.; Wang, W.; Wu, T.; Xiao, M.; et al. Effects of a specially designed aerobic dance routine on mild cognitive impairment. Clin. Interv. Aging 2018, 13, 1691–1700. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Chen, Y.; Liu, C.; Yong, L.; Yang, M.; Zhu, W.; Wang, J.; Yan, J. Effect of Square Dance Exercise on Older Women With Mild Mental Disorders. Front. Psychiatry 2021, 12, 699778. [Google Scholar] [CrossRef] [PubMed]
- Kosmat, H.; Vranic, A. The Efficacy of a Dance Intervention as Cognitive Training for the Old-Old. J. Aging Phys. Act. 2017, 25, 32–40. [Google Scholar] [CrossRef]
- Bisbe, M.; Fuente-Vidal, A.; López, E.; Moreno, M.; Naya, M.; de Benetti, C.; Milà, R.; Bruna, O.; Boada, M.; Alegret, M. Comparative Cognitive Effects of Choreographed Exercise and Multimodal Physical Therapy in Older Adults with Amnestic Mild Cognitive Impairment: Randomized Clinical Trial. J. Alzheimer’s Dis. 2020, 73, 769–783. [Google Scholar] [CrossRef]
- Cruz-Ferreira, A.; Marmeleira, J.; Formigo, A.; Gomes, D.; Fernandes, J. Creative Dance Improves Physical Fitness and Life Satisfaction in Older Women. Res. Aging 2015, 37, 837–855. [Google Scholar] [CrossRef]
- Eyigor, S.; Karapolat, H.; Durmaz, B.; Ibisoglu, U.; Cakir, S. A randomized controlled trial of Turkish folklore dance on the physical performance, balance, depression and quality of life in older women. Arch. Gerontol. Geriatr. 2009, 48, 84–88. [Google Scholar] [CrossRef]
- Lazarou, I.; Parastatidis, T.; Tsolaki, A.; Gkioka, M.; Karakostas, A.; Douka, S.; Tsolaki, M. International Ballroom Dancing Against Neurodegeneration: A Randomized Controlled Trial in Greek Community-Dwelling Elders with Mild Cognitive impairment. Am. J. Alzheimers Dis. Other Dement. 2017, 32, 489–499. [Google Scholar] [CrossRef]
- Mishra, S.S.; Shukla, S. Effect of Indian folk-dance therapy on physical performances and quality of life in elderly. Biomed. Hum. Kinet. 2022, 14, 244–251. [Google Scholar] [CrossRef]
- Serrano-Guzmán, M.; Aguilar-Ferrándiz, M.E.; Valenza, C.M.; Ocaña-Peinado, F.M.; Valenza-Demet, G.; Villaverde-Gutiérrez, C. Effectiveness of a flamenco and sevillanas program to enhance mobility, balance, physical activity, blood pressure, body mass, and quality of life in postmenopausal women living in the community in Spain: A randomized clinical trial. Menopause 2016, 23, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Guzman, M.; Valenza-Pena, C.M.; Serrano-Guzman, C.; Aguilar-Ferrandiz, E.; Valenza-Demet, G.; Villaverde-Gutierrez, C. Effects of a dance therapy programme on quality of life, sleep and blood pressure in middle-aged women: A randomised controlled trial. Med. Clin. 2016, 147, 334–339. [Google Scholar] [CrossRef]
- Alpert, P.T.; Miller, S.K.; Wallmann, H.; Havey, R.; Cross, C.; Chevalia, T.; Gillis, C.B.; Kodandapari, K. The effect of modified jazz dance on balance, cognition, and mood in older adults. J. Am. Acad. Nurse Pract. 2009, 21, 108–115. [Google Scholar] [CrossRef]
- Jeon, M.Y.; Bark, E.S.; Lee, E.G.; Im, J.S.; Jeong, B.S.; Choe, E.S. The Effects of a Korean Traditional Dance Movement Program in Elderly Women. J. Korean Acad. Nurs. 2005, 35, 1268. [Google Scholar] [CrossRef] [PubMed]
- Crumbie, V.; Olmos, F.; Watts, C.; Avery, J.; Nelson, R. The Impact of Dance Interventions on Mood and Depression in Older Adults. Ther. Recreat. J. 2015, 49, 187–190. [Google Scholar]
- Ho, R.T.H.; Fong, T.C.T.; Chan, W.C.; Kwan, J.S.K.; Chiu, P.K.C.; Yau, J.C.Y.; Lam, L.C.W. Psychophysiological Effects of Dance Movement Therapy and Physical Exercise on Older Adults with Mild Dementia: A Randomized Controlled Trial. J. Gerontol. Ser. B-Psychol. Sci. Soc. Sci. 2020, 75, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Humphries, A.; Tasnim, N.; Rugh, R.; Patrick, M.; Basso, J.C. Acutely enhancing affective state and social connection following an online dance intervention during the COVID-19 social isolation crisis. BMC Psychology. 2023, 11, 13. [Google Scholar] [CrossRef]
- Chao, L.L.; Lee, J.A.; Martinez, S.; Barlow, C.; Chesney, M.A.; Mehling, W.E.; Barnes, D.E. Preventing Loss of Independence through Exercise (PLIÉ): A Pilot Trial in Older Adults with Subjective Memory Decline and Mild Cognitive Impairment. J. Alzheimer’s Dis. 2021, 82, 1543–1557. [Google Scholar] [CrossRef]
- Noguera, C.; Carmona, D.; Rueda, A.; Fernández, R.; Cimadevilla, J.M. Shall we dance? Dancing modulates executive functions and spatial memory. Int. J. Environ. Res. Public Health 2020, 17, 1960. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhong, Q.; Ji, J.; Ma, J.; Wu, H.; Gao, Y.; Ali, N.; Wang, T. Effects of Aerobic Dance on Cognition in Older Adults with Mild Cognitive Impairment: A Systematic Review and Meta-Analysis. J. Alzheimer’s Dis. 2020, 74, 679–690. [Google Scholar] [CrossRef]
- Teixeira-Machado, L.; Arida, R.M.; de Jesus Mari, J. Dance for neuroplasticity: A descriptive systematic review. Neurosci. Biobehav. Rev. 2019, 96, 232–240. [Google Scholar] [CrossRef]
- Elst, O.F.V.; Foster, N.H.; Vuust, P.; Keller, P.E.; Kringelbach, M.L. The Neuroscience of Dance: A Conceptual Framework and Systematic Review. Neurosci. Biobehav. Rev. 2023, 150, 105197. [Google Scholar] [CrossRef]
- Balbim, G.M.; Ajilore, O.A.; Erickson, K.I.; Lamar, M.; Aguiñaga, S.; Bustamante, E.E.; Marquez, D.X. The Impact of the BAILAMOS™ Dance Program on Brain Functional Connectivity and Cognition in Older Latino Adults: A Pilot Study. J. Cogn. Enhanc. 2020, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Balazova, Z.; Marecek, R.; Novakova, L.; Nemcova-Elfmarkova, N.; Kropacova, S.; Brabenec, L.; Grmela, R.; Vaculíková, P.; Svobodova, L.; Rektorova, I. Dance Intervention Impact on Brain Plasticity: A Randomized 6-Month fMRI Study in Non-expert Older Adults. Front. Aging Neurosci. 2021, 13, 724064. [Google Scholar] [CrossRef]
- Burzynska, A.Z.; Jiao, Y.; Knecht, A.M.; Fanning, J.; Awick, E.A.; Chen, T.; Gothe, N.; Voss, M.W.; McAuley, E.; Kramer, A.F. White Matter Integrity Declined Over 6-Months, but Dance Intervention Improved Integrity of the Fornix of Older Adults. Front. Aging Neurosci. 2017, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Rehfeld, K.; Müller, P.; Aye, N.; Schmicker, M.; Dordevic, M.; Kaufmann, J.; Hökelmann, A.; Müller, N.G. Dancing or Fitness Sport? The Effects of Two Training Programs on Hippocampal Plasticity and Balance Abilities in Healthy Seniors. Front. Hum. Neurosci. 2017, 11, 305. [Google Scholar] [CrossRef]
- Guzman, J.; Aguiñaga, S.; Balbim, G.M.; Lamar, M.; Marques, I.G.; Marquez, D.X. The effects of the BAILAMOS Dance Program on hippocampal volume in older Latinos: A randomized controlled pilot study. Transl. Behav. Med. 2021, 11, 1857–1862. [Google Scholar] [CrossRef] [PubMed]
- Vrinceanu, T.; Esmail, A.; Berryman, N.; Predovan, D.; Vu, T.T.M.; Villalpando, J.M.; Pruessner, J.C.; Bherer, L. Dance your stress away: Comparing the effect of dance/movement training to aerobic exercise training on the cortisol awakening response in healthy older adults. Stress-Int. J. Biol. Stress 2019, 22, 687–695. [Google Scholar] [CrossRef]
- Perrotin, A.; La Joie, R.; La Sayette, V.; Barré, L.; Mézenge, F.; Mutlu, J.; Guilloteau, D.; Egret, S.; Eustache, F.; Chételat, G. Subjective cognitive decline in cognitively normal elders from the community or from a memory clinic: Differential affective and imaging correlates. Alzheimer’s Dement. 2017, 13, 550–560. [Google Scholar] [CrossRef]
- Sannemann, L.; Schild, A.-K.; Altenstein, S.; Bartels, C.; Brosseron, F.; Buerger, K.; Cosma, N.C.; Fliessbach, K.; Freiesleben, S.D.; Glanz, W.; et al. Neuropsychiatric symptoms in at-risk groups for AD dementia and their association with worry and AD biomarkers-results from the DELCODE study. Alzheimer’s Res. Ther. 2020, 12, 131. [Google Scholar] [CrossRef]
- Jessen, F.; Amariglio, R.E.; van Boxtel, M.; Breteler, M.; Ceccaldi, M.; Chetelat, G.; Dubois, B.; Dufouil, C.; Ellis, K.A.; van der Flier, W.M.; et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement. 2014, 10, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Smart, C.M.; Karr, J.E.; Areshenkoff, C.N.; Rabin, L.A.; Hudon, C.; Gates, N.; Hampel, H. Non-Pharmacologic Interventions for Older Adults with Subjective Cognitive Decline: Systematic Review, Meta-Analysis, and Preliminary Recommendations. Neuropsychol. Rev. 2017, 27, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Marchant, N.L.; Barnhofer, T.; Coueron, R.; Wirth, M.; Lutz, A.; Arenaza-Urquijo, E.M.; Collette, F.; Poisnel, G.; Demnitz-King, H.; Schild, A.K.; et al. Effects of a Mindfulness-Based Intervention versus Health Self-Management on Subclinical Anxiety in Older Adults with Subjective Cognitive Decline: The SCD-Well Randomized Superiority Trial. Psychother. Psychosom. 2021, 90, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Chétela, G.; Lutz, A.; Klimecki, O.; Frison, E.; Asselineau, J.; Schlosser, M.; Arenaza-Urquijo, E.M.; Mézenge, F.; Kuhn, E.; Moulinet, I.; et al. Effect of an 18-Month Meditation Training on Regional Brain Volume and Perfusion in Older Adults: The Age-Well Randomized Clinical Trial. JAMA Neurol. 2022, 79, 1165–1174. [Google Scholar] [CrossRef]
- Gerino, E.; Rollè, L.; Sechi, C.; Brustia, P. Loneliness, Resilience, Mental Health, and Quality of Life in Old Age: A Structural Equation Model. Front. Psychol. 2017, 8, 2003. [Google Scholar] [CrossRef]
- Fancourt, D.; Finn, S. What is the Evidence on the Role of the Arts in Improving Health and Well-Being? A Scoping Review; WHO Regional Office for Europe: Copemhagen, Denmark, 2019. [Google Scholar]
- Haboush, A.; Floyd, M.; Caron, J.; LaSota, M.; Alvarez, K. Ballroom dance lessons for geriatric depression: An exploratory study. Arts Psychother. 2006, 33, 89–97. [Google Scholar] [CrossRef]
- Vankova, H.; Holmerova, I.; Machacova, K.; Volicer, L.; Veleta, P.; Celko, A.M. The Effect of Dance on Depressive Symptoms in Nursing Home Residents. J. Am. Med Dir. Assoc. 2014, 15, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Cheung, M.W.L.; Vijayakumar, R. A Guide to Conducting a Meta-Analysis. Neuropsychol. Rev. 2016, 26, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Becker, B.J. Synthesizing standardized mean-change measures. Br. J. Math. Stat. Psychol. 1988, 41, 178–257. [Google Scholar] [CrossRef]

| Construct | k (N ES) | ES | 95% CI | SE | df | p-Value | I2% | |
|---|---|---|---|---|---|---|---|---|
| Overall psychological health | All combined | 14 (33) | 0.30 | [0.06, 0.53] | 0.11 | 12 | 0.02 | 65.04 |
| Positive domain | Well-being | 9 (16) | 0.30 | [−0.03, 0.63] | 0.14 | 8 | 0.07 | 62.80 |
| Social Integration | 3 (3) | 0.85 | [−1.59, 3.29] | 0.57 | 2 * | * | 85.17 | |
| Negative domain | Anxiety/Stress | 4 (5) | 0.30 | [−0.54, 1.14] | 0.26 | 3 * | * | 66.22 |
| Depression | 5 (5) | 0.22 | [−0.25, 0.68] | 0.16 | 4 | 0.26 | 60.81 | |
| Quality of Life | All combined | 9 (22) | 0.29 | [−0.14, 0.73] | 0.19 | 8 | 0.16 | 76.10 |
| General cognitive function | All combined | 8 (9) | 0.50 | [0.12, 0.89] | 0.16 | 7 | 0.02 | 79.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podolski, O.S.; Whitfield, T.; Schaaf, L.; Cornaro, C.; Köbe, T.; Koch, S.; Wirth, M. The Impact of Dance Movement Interventions on Psychological Health in Older Adults without Dementia: A Systematic Review and Meta-Analysis. Brain Sci. 2023, 13, 981. https://doi.org/10.3390/brainsci13070981
Podolski OS, Whitfield T, Schaaf L, Cornaro C, Köbe T, Koch S, Wirth M. The Impact of Dance Movement Interventions on Psychological Health in Older Adults without Dementia: A Systematic Review and Meta-Analysis. Brain Sciences. 2023; 13(7):981. https://doi.org/10.3390/brainsci13070981
Chicago/Turabian StylePodolski, Odile Sophie, Tim Whitfield, Leah Schaaf, Clara Cornaro, Theresa Köbe, Sabine Koch, and Miranka Wirth. 2023. "The Impact of Dance Movement Interventions on Psychological Health in Older Adults without Dementia: A Systematic Review and Meta-Analysis" Brain Sciences 13, no. 7: 981. https://doi.org/10.3390/brainsci13070981
APA StylePodolski, O. S., Whitfield, T., Schaaf, L., Cornaro, C., Köbe, T., Koch, S., & Wirth, M. (2023). The Impact of Dance Movement Interventions on Psychological Health in Older Adults without Dementia: A Systematic Review and Meta-Analysis. Brain Sciences, 13(7), 981. https://doi.org/10.3390/brainsci13070981





