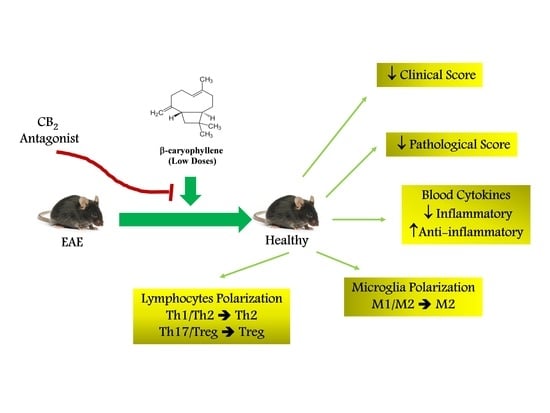
Low Doses of β-Caryophyllene Reduced Clinical and Paraclinical Parameters of an Autoimmune Animal Model of Multiple Sclerosis: Investigating the Role of CB2 Receptors in Inflammation by Lymphocytes and Microglial
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Kits
2.2. Animals and Husbandry
2.3. Induction of EAE and Clinical Evaluation
2.4. Experimental Group and Study Design
2.4.1. Experiment One
2.4.2. Experiment Two
2.5. Histological Evaluation
2.6. Serum Preparation and Inspection of the Cytokines Profile
2.7. Separation of Lymphocyte from Spleen and Cell Culture
2.7.1. BrdU Cell Proliferation Assay
2.7.2. Examination of the Cytokines Profile
2.7.3. Evaluation of Intracellular Levels of Transcription Factors
2.8. Microglia Isolation
2.8.1. Cell Proliferation Assay
2.8.2. Evaluation of the Cytokine Levels
2.8.3. Evaluation of the Intracellular Levels of Arg-1 and iNOS
2.8.4. Evaluation of Urea and NO Levels
2.9. Statistical Analysis
3. Results
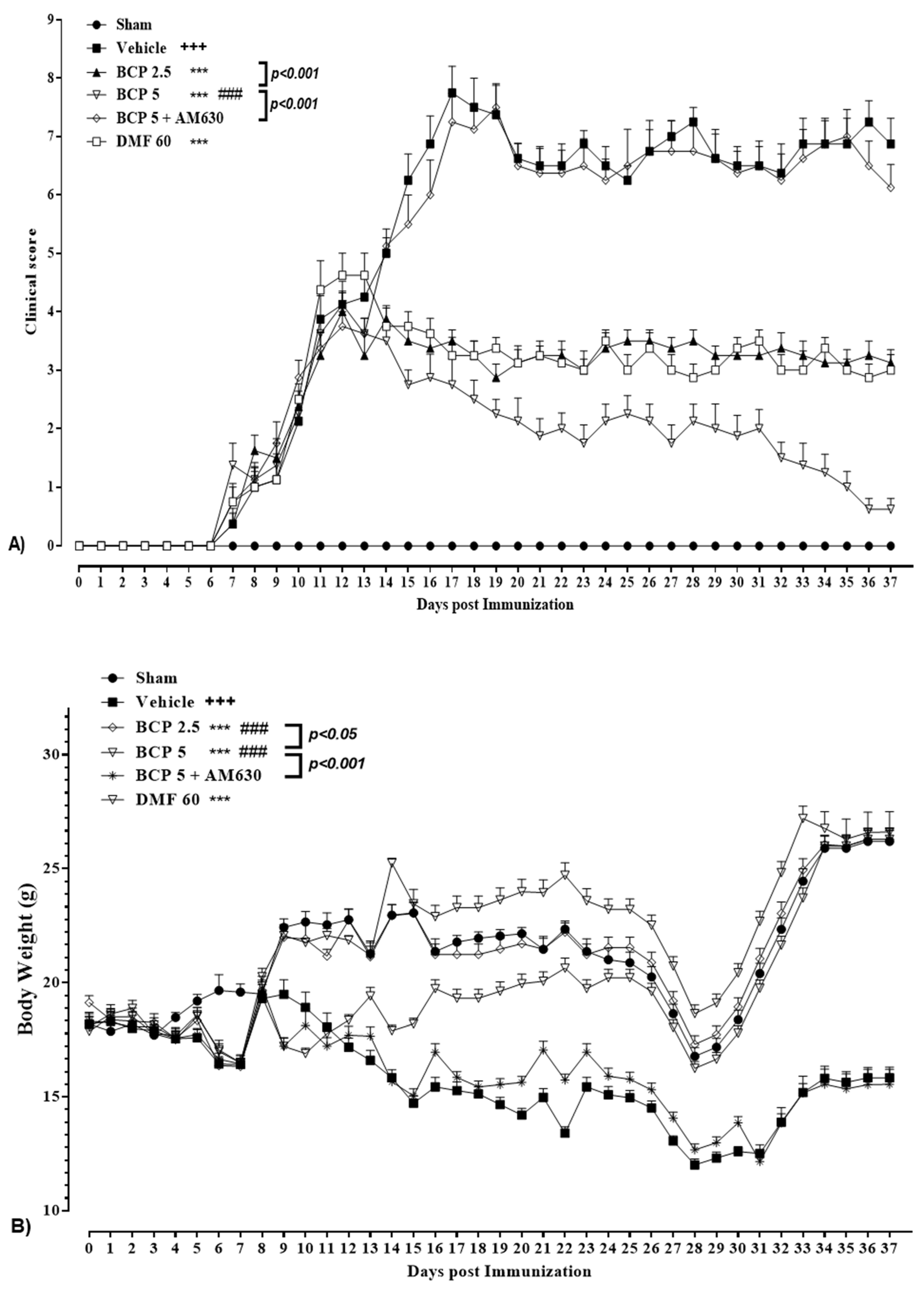
3.1. BCP Effect on Body Weight and the Clinical Score of EAE Mice
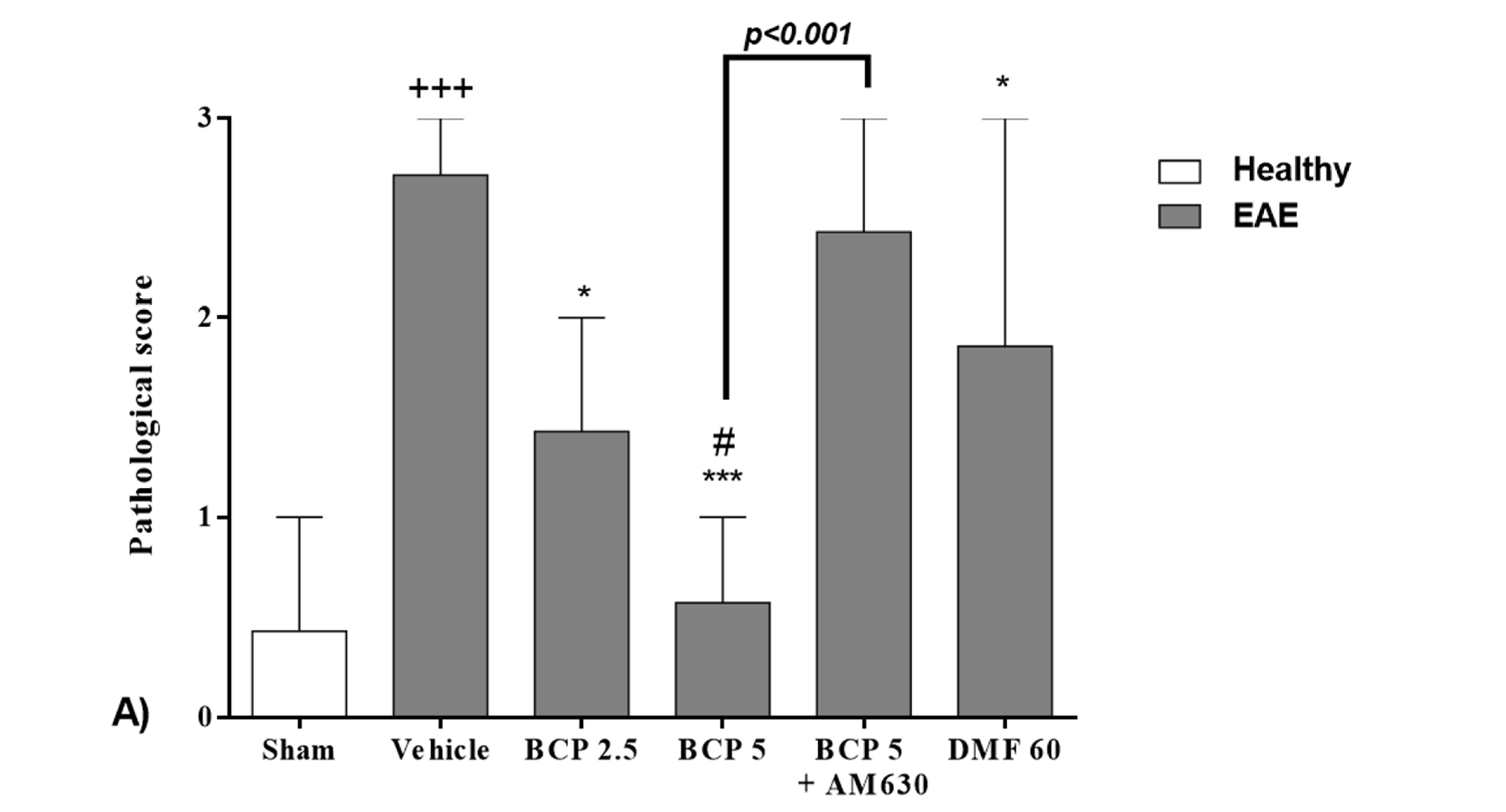
3.2. The BCP Effects on the Level of the Histopathological Score in EAE Mice
3.3. The BCP Effects on Serum Levels of Cytokines in EAE Mice
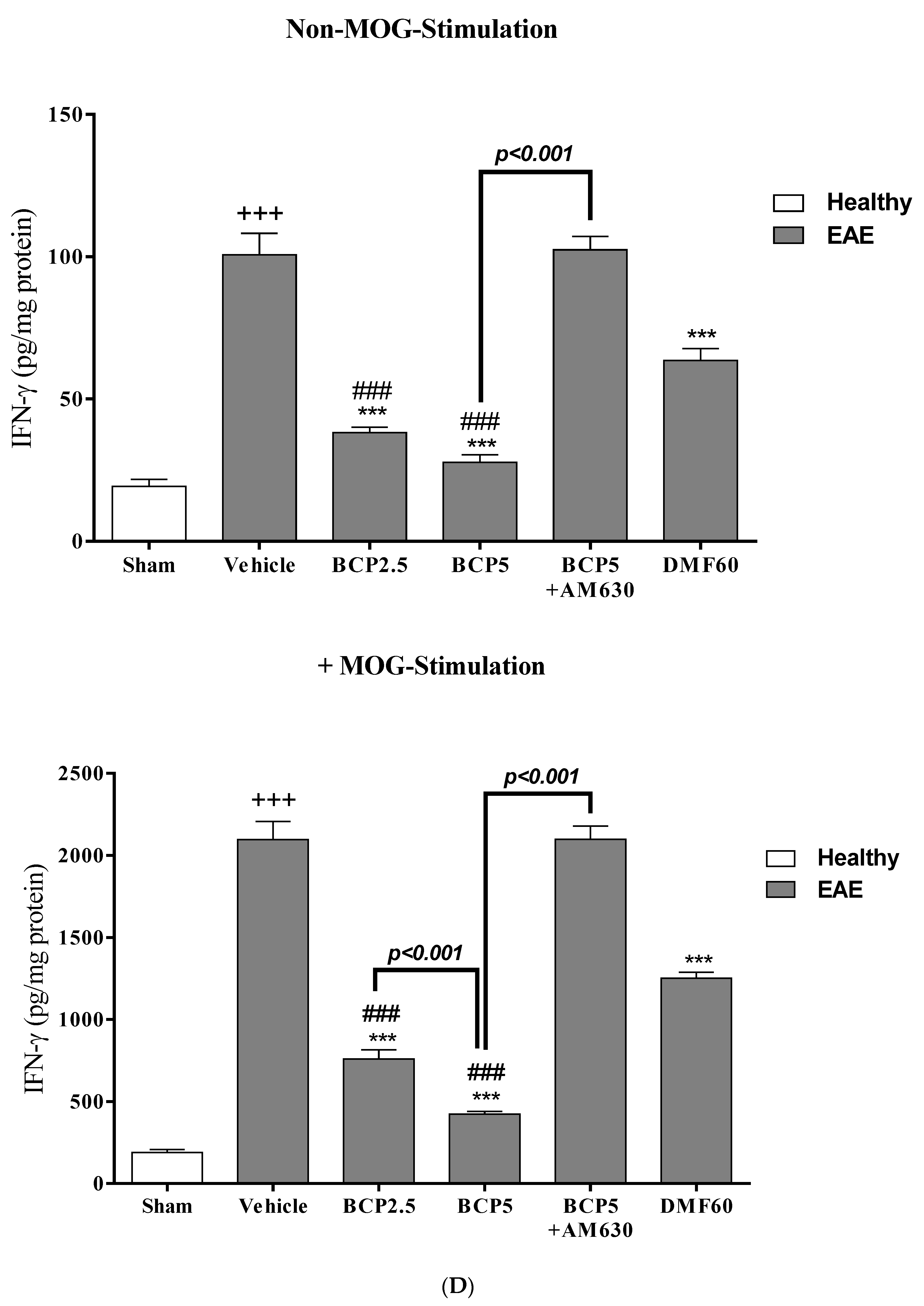
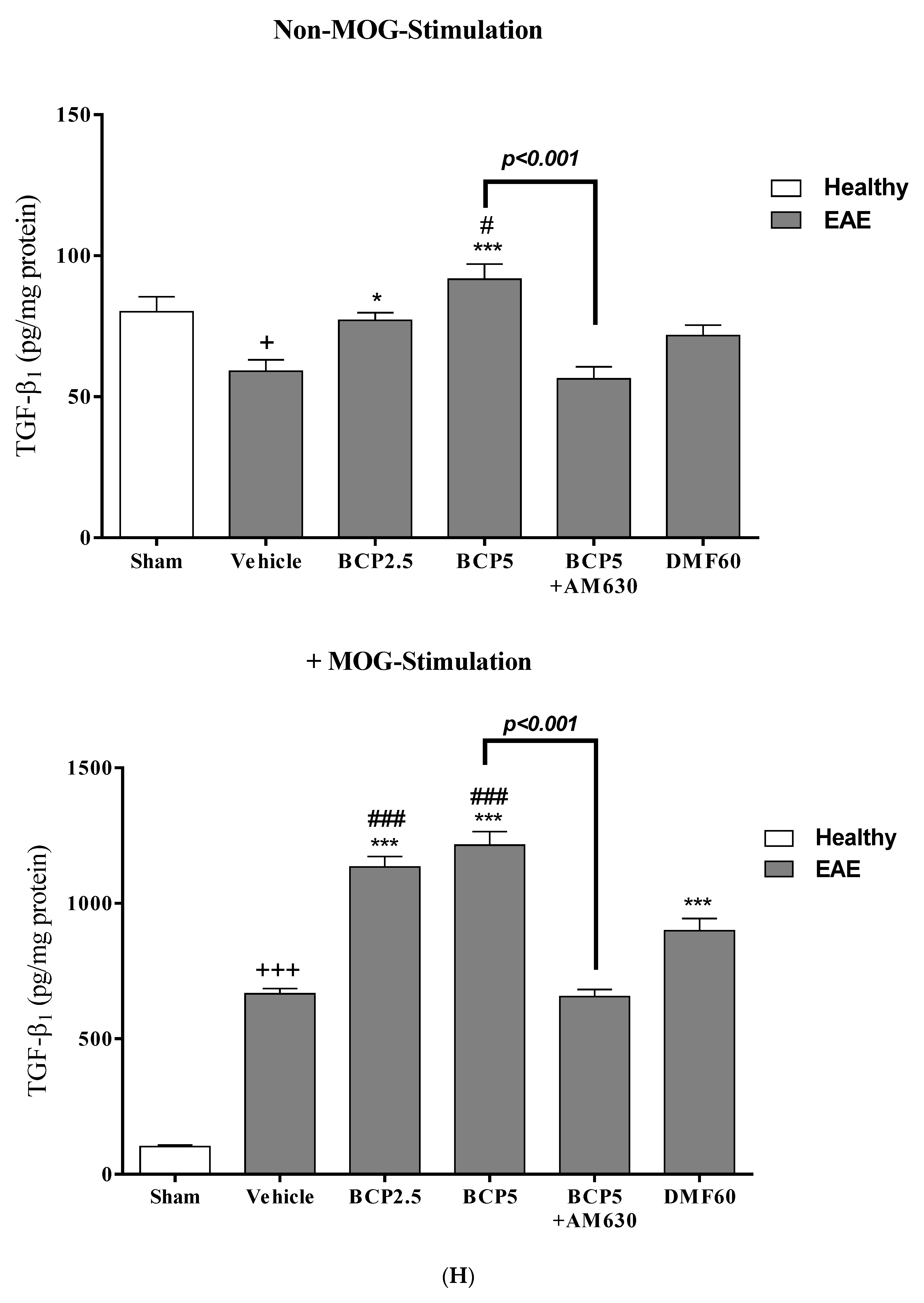
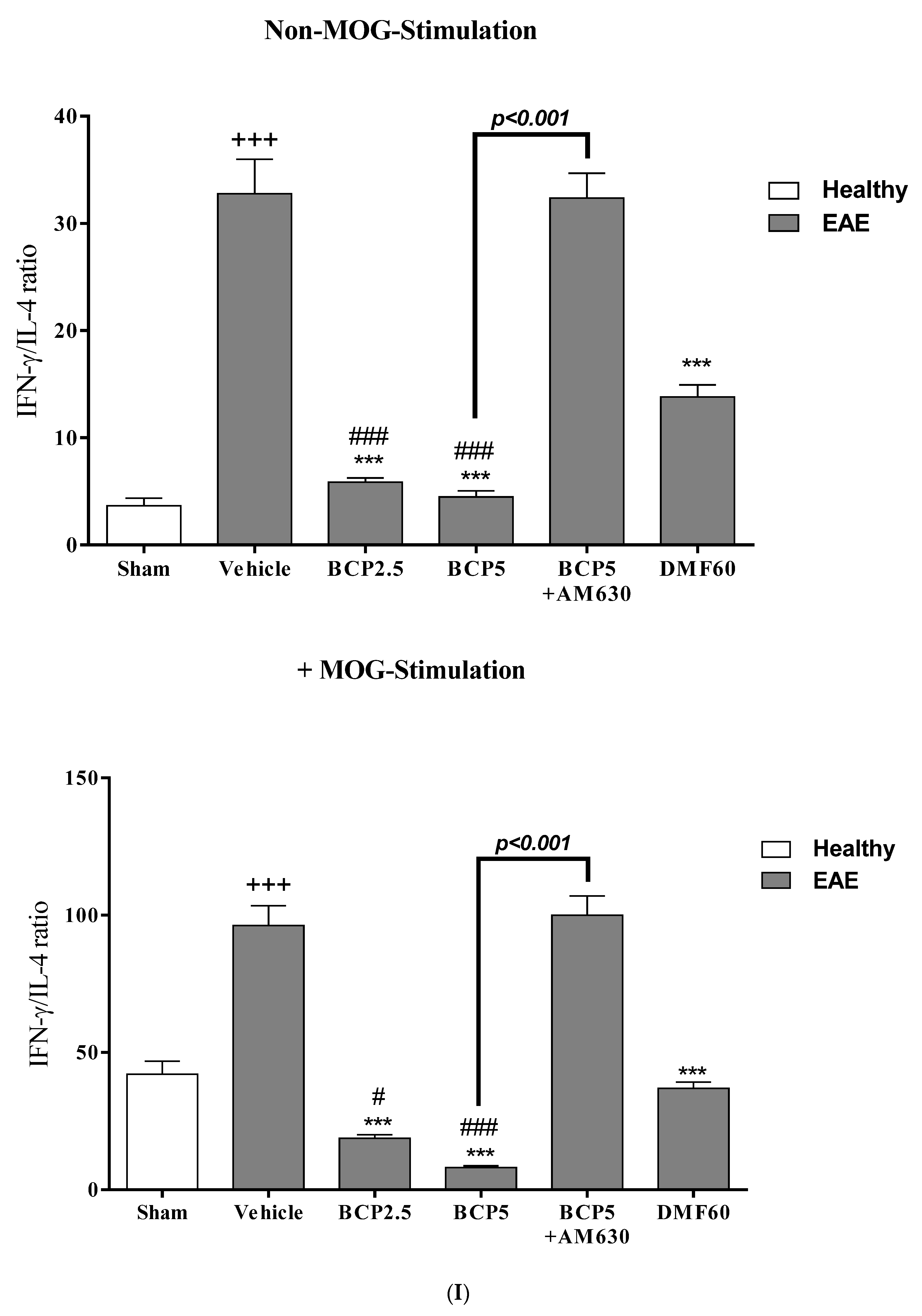
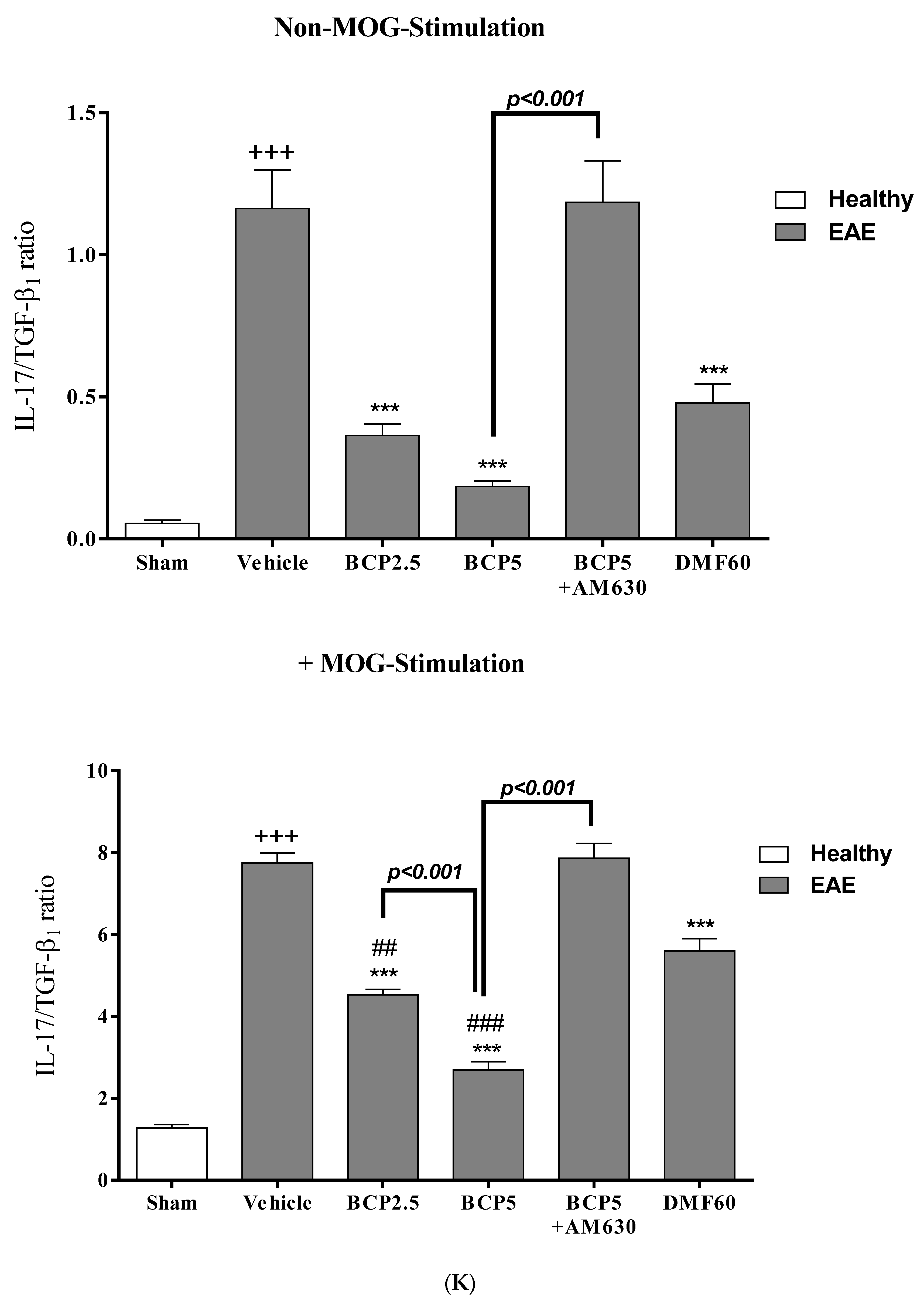
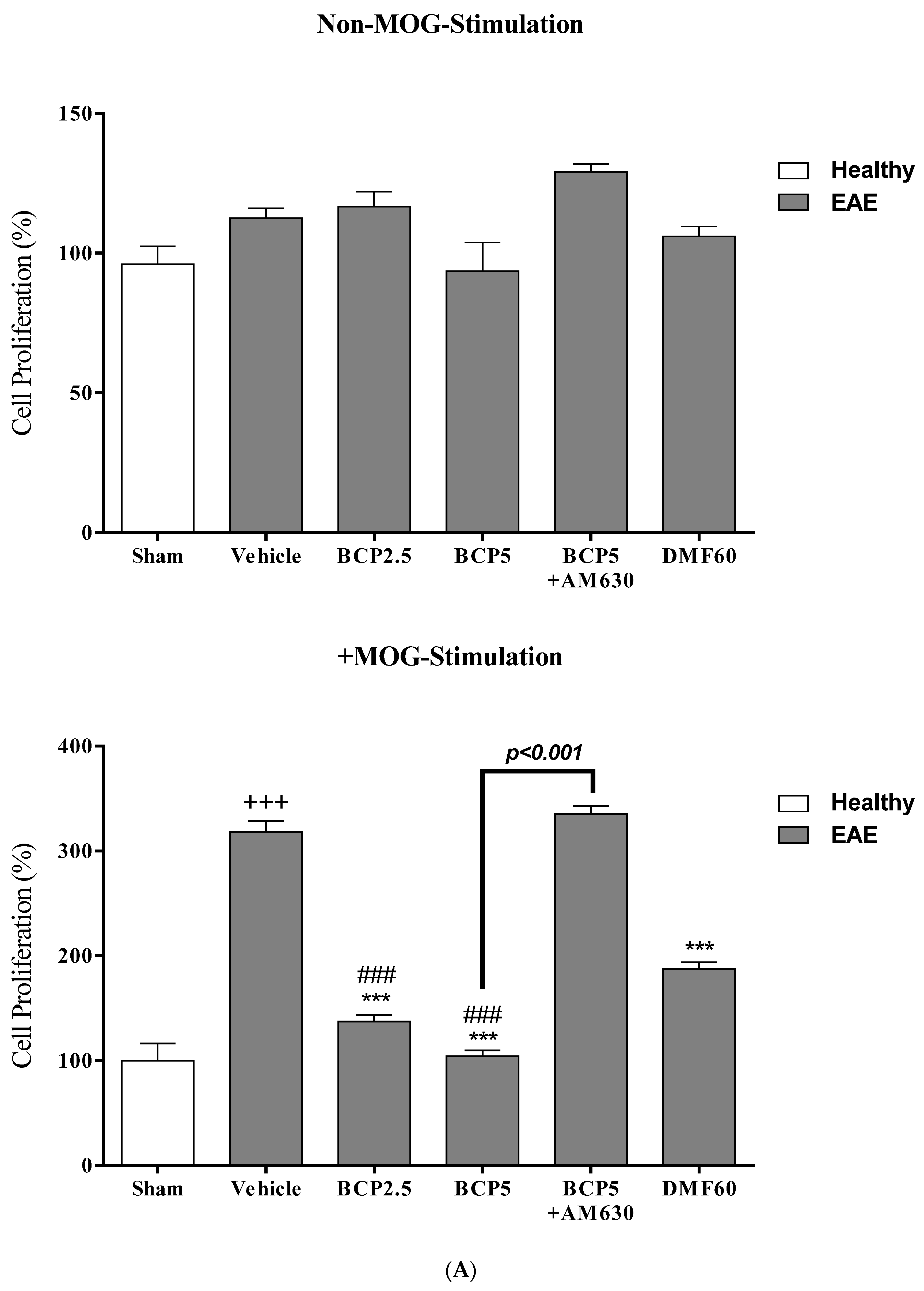
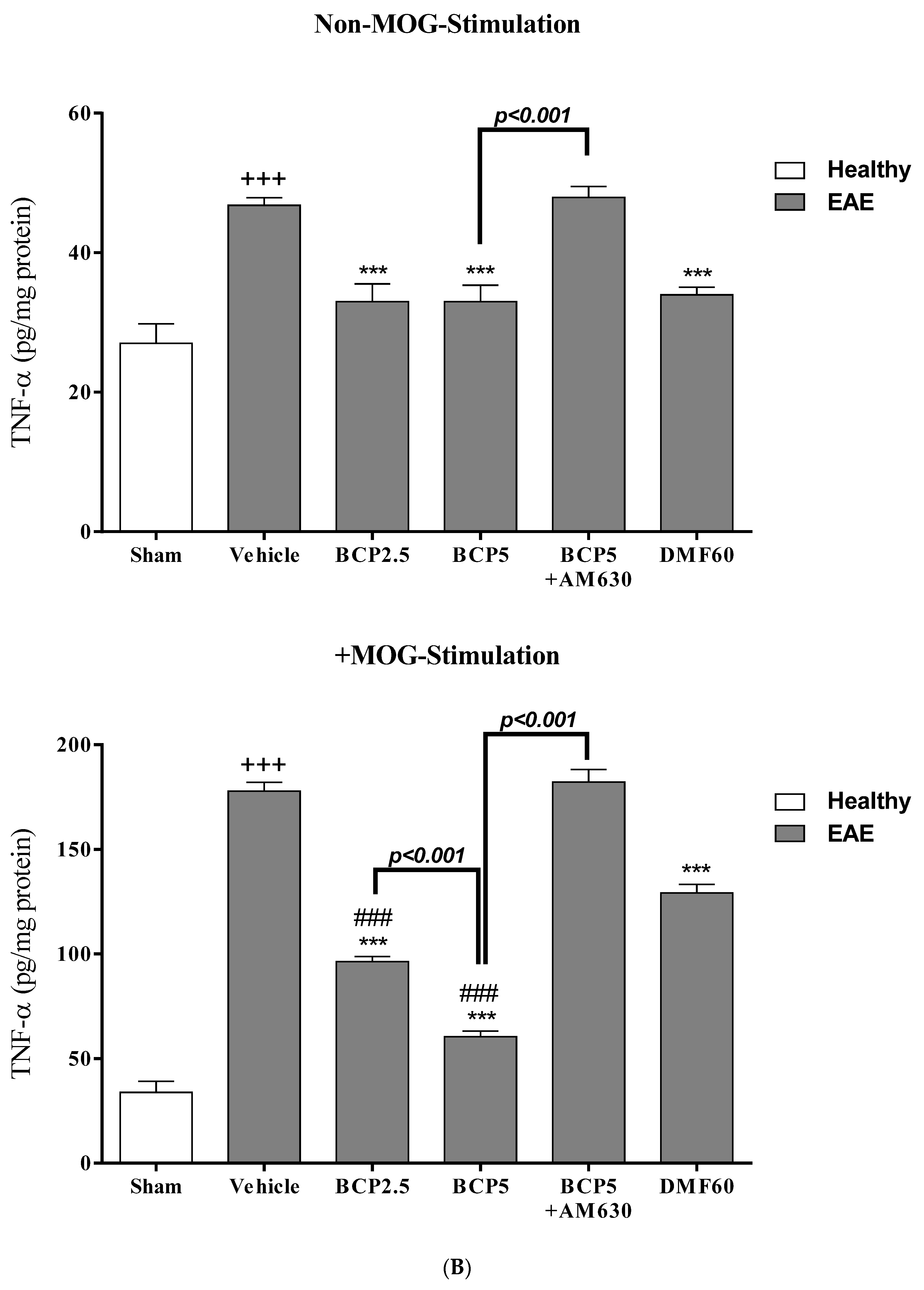
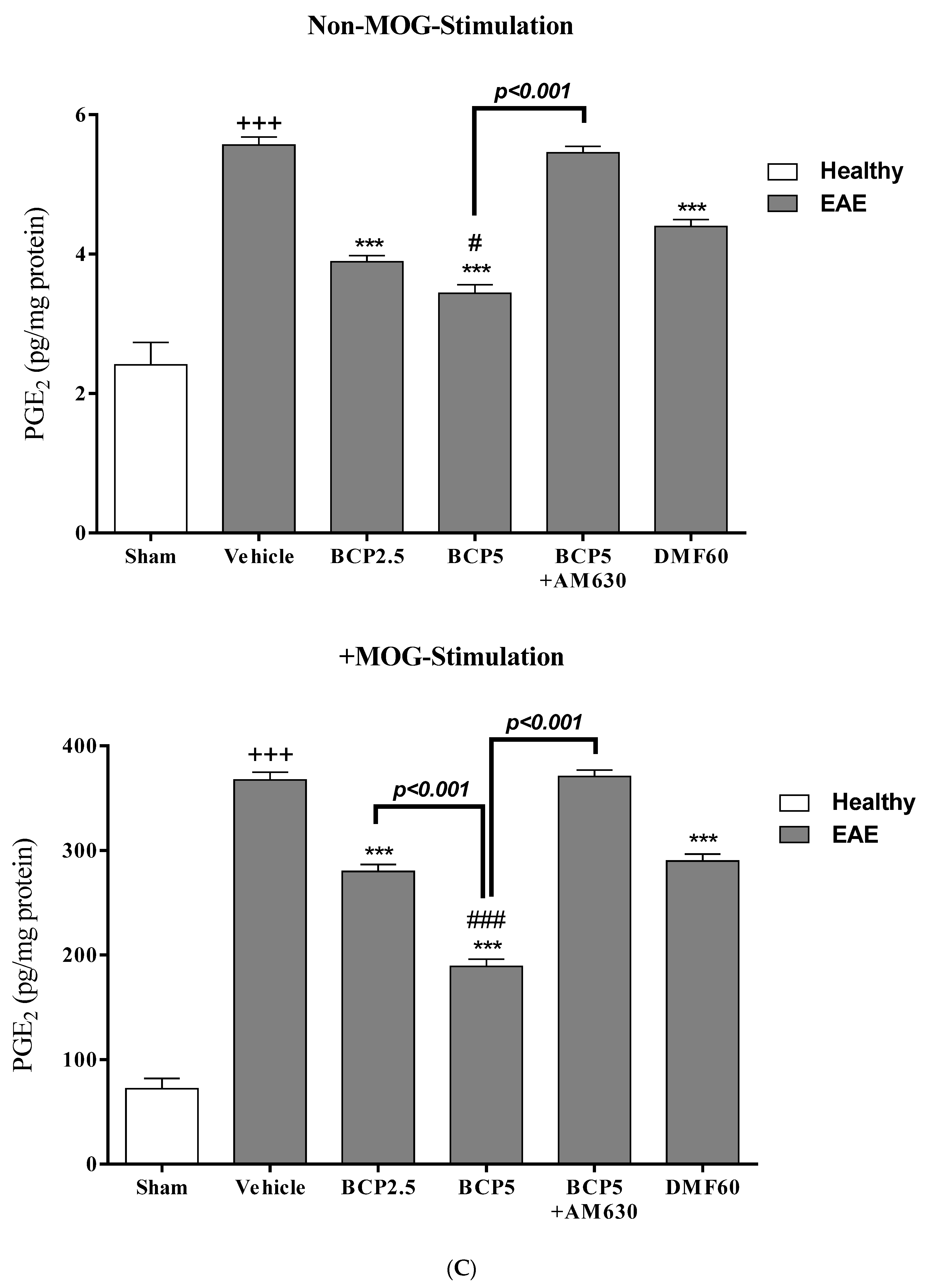
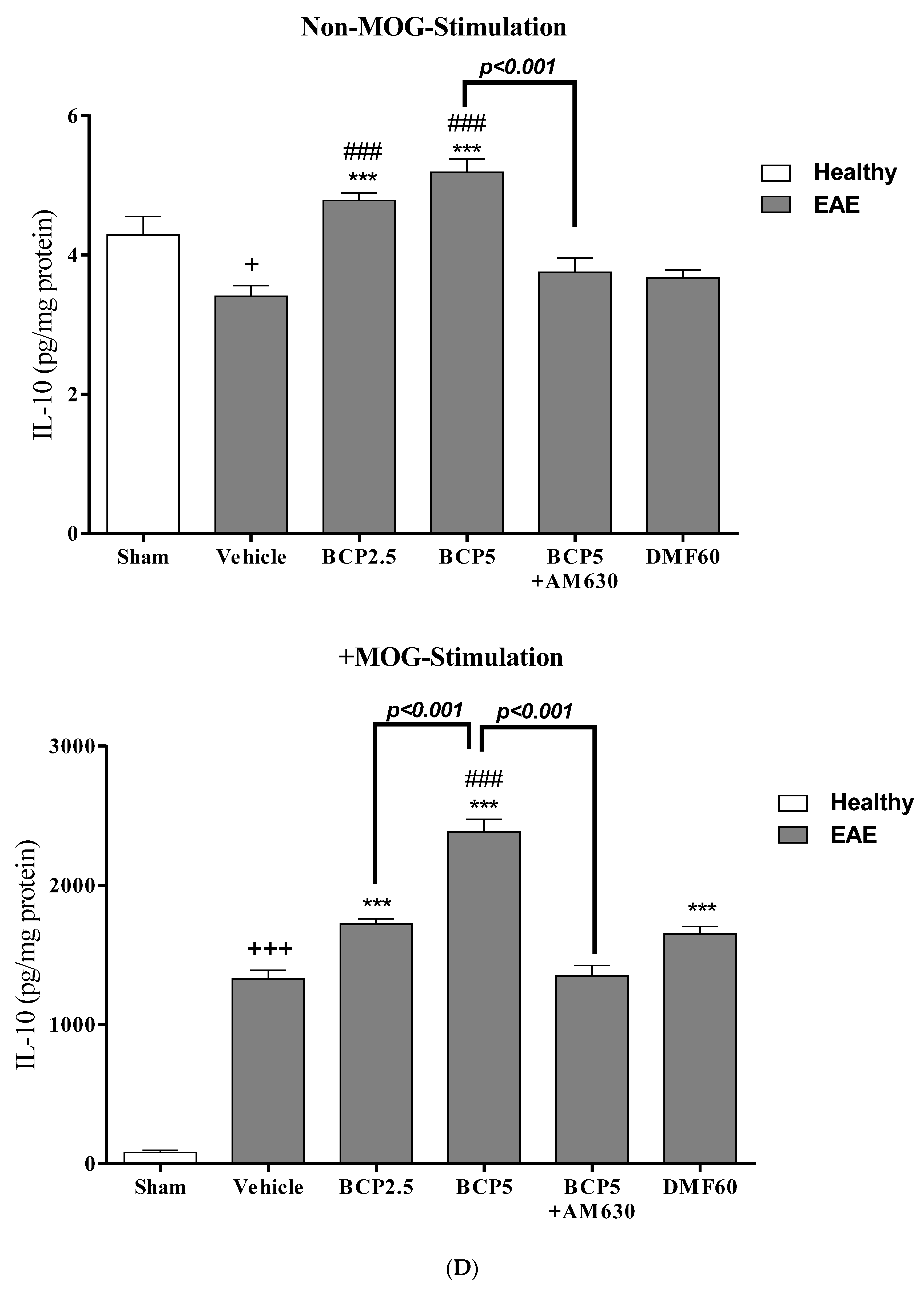
3.4. The Effects of BCP on Cell Proliferation and Cytokines Levels of Spleen Lymphocytes of EAE Mice with and without MOG Stimulation
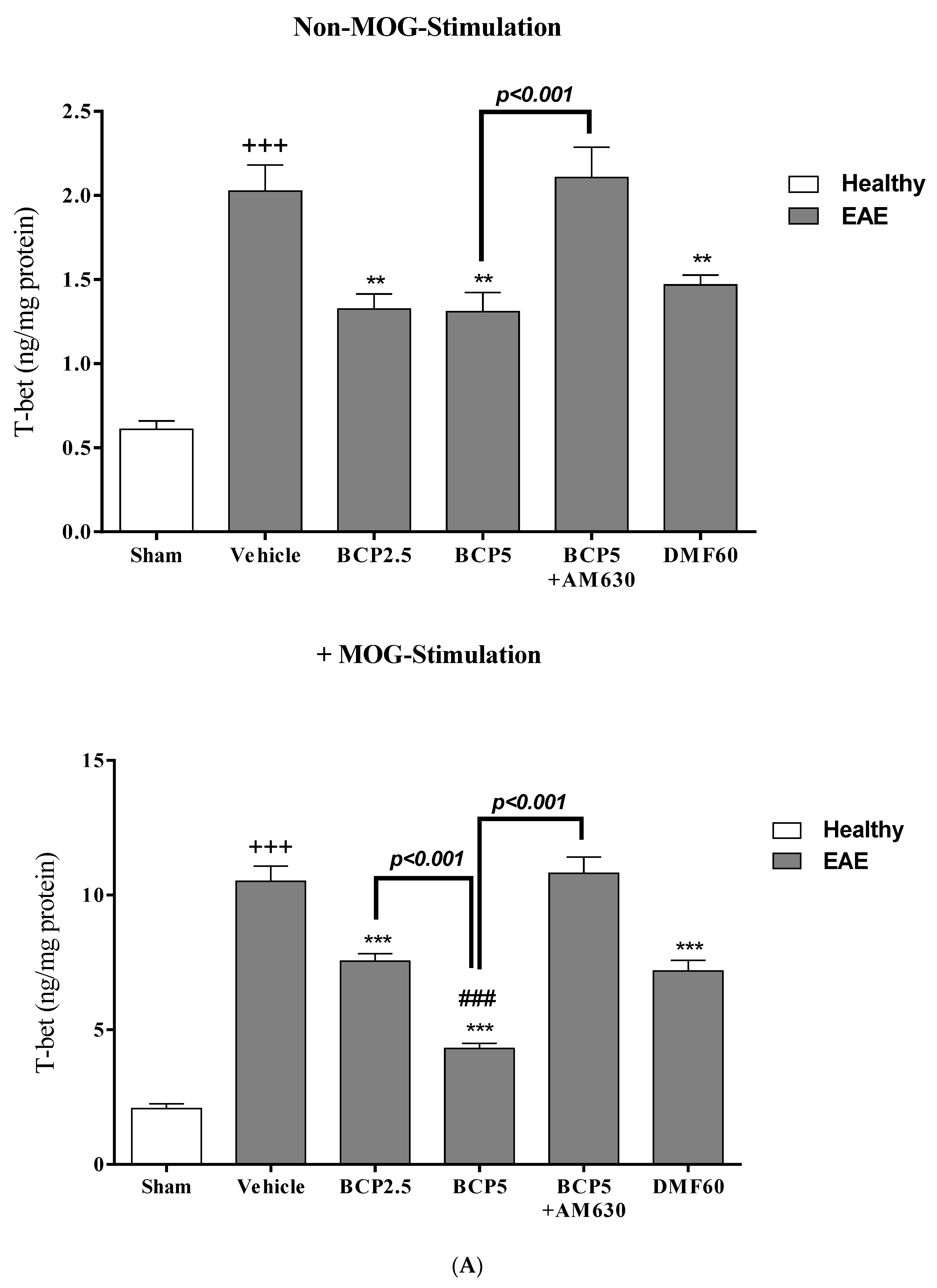
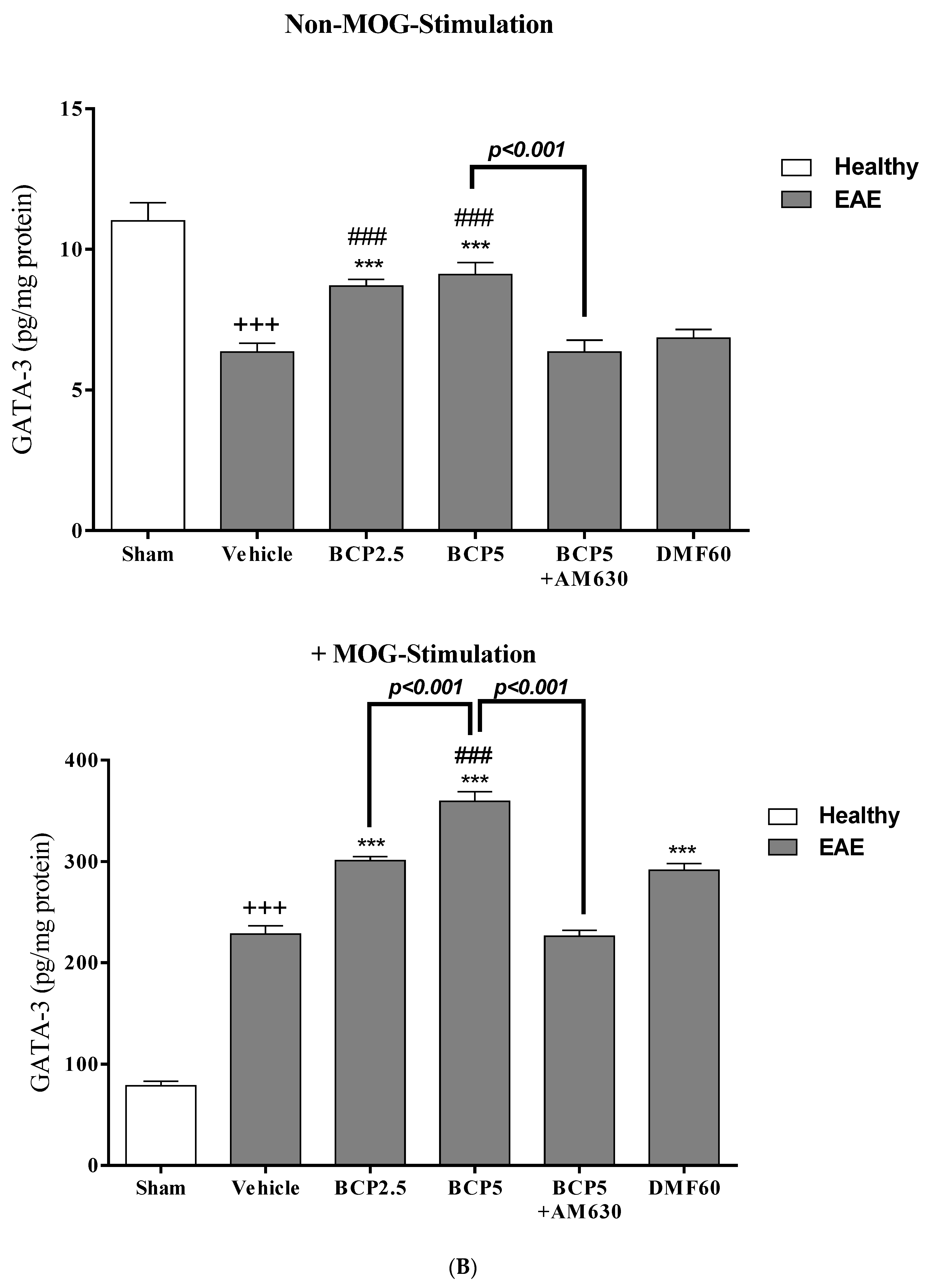
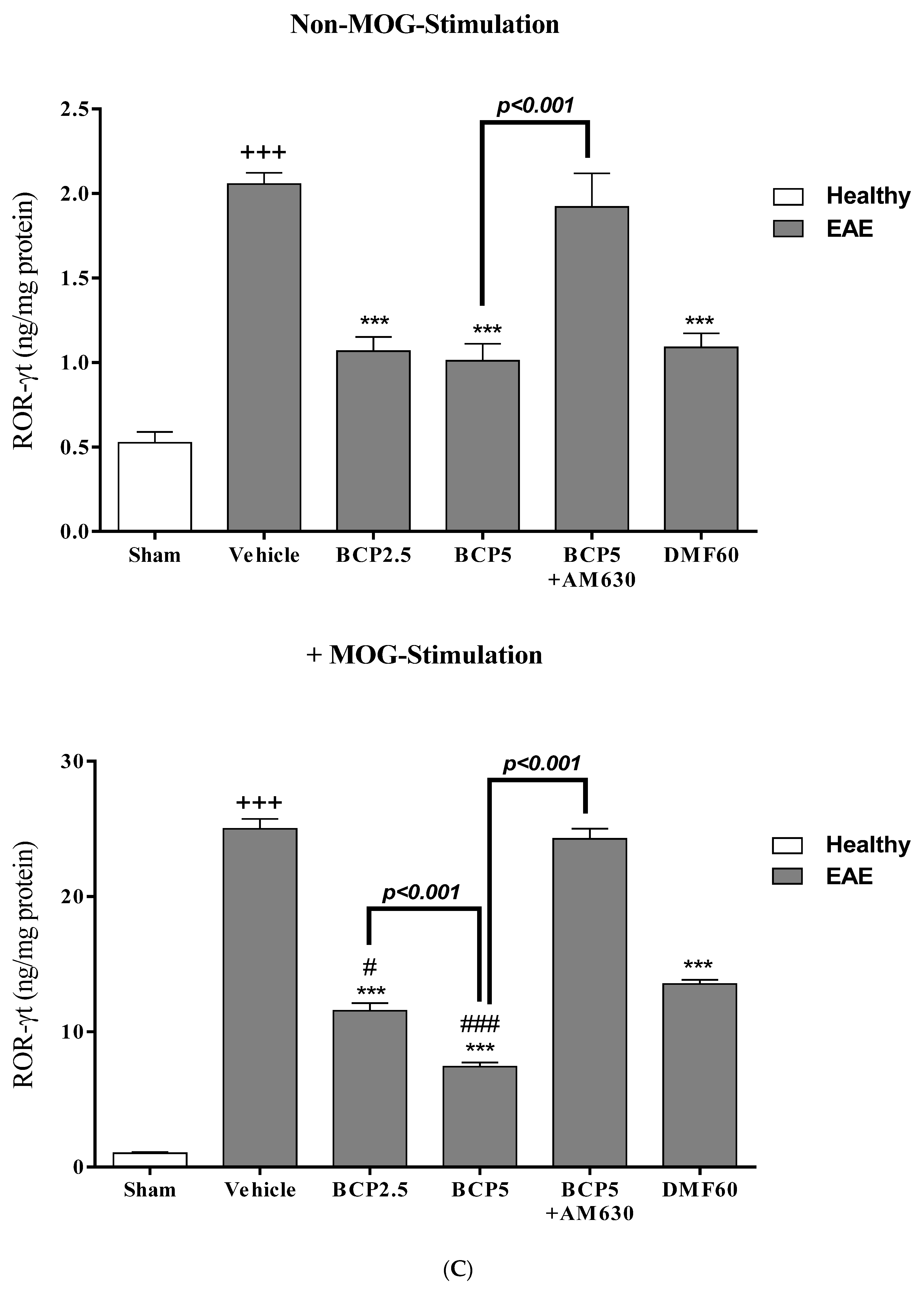
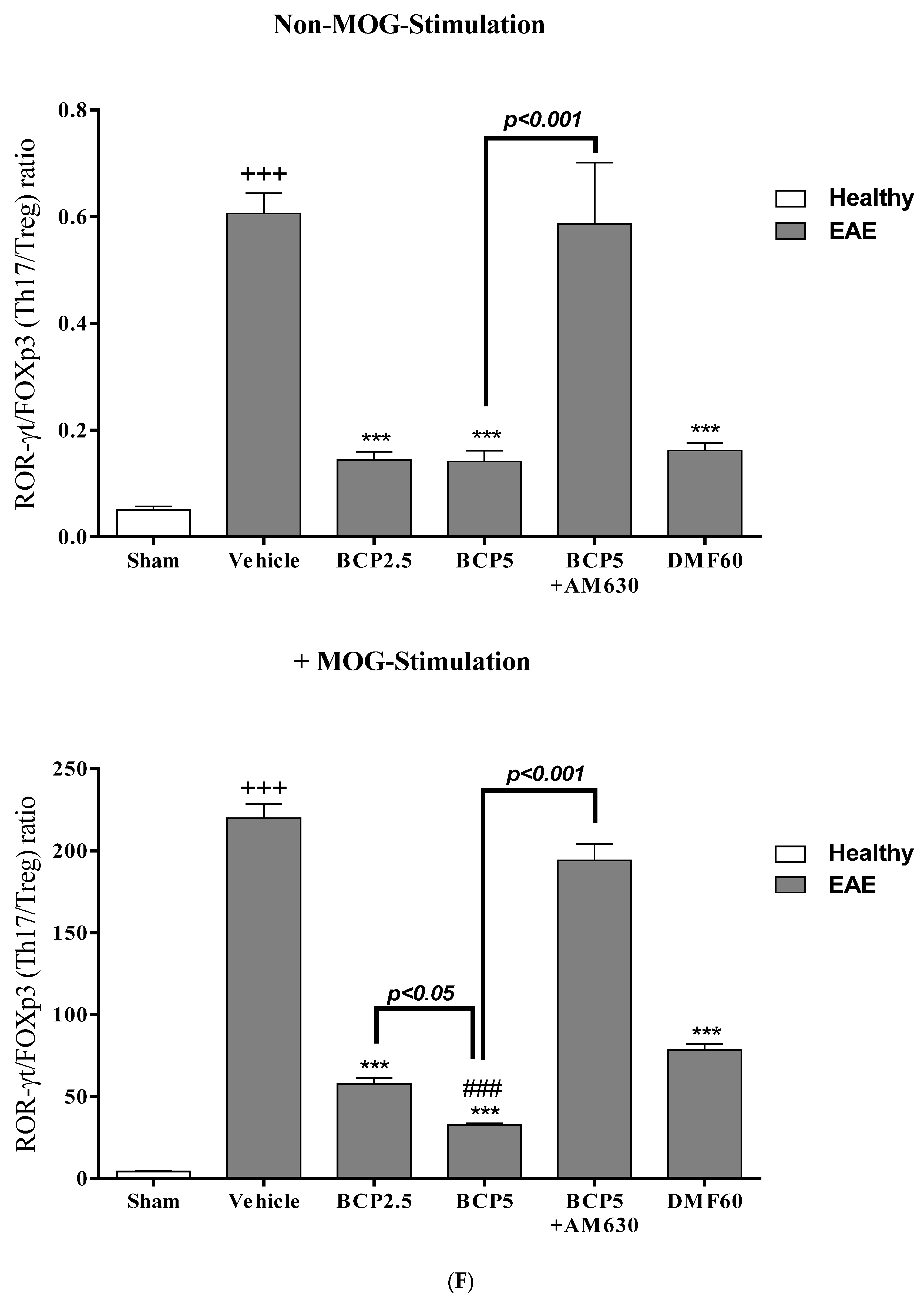
3.5. The Effects of BCP on the Levels of Transcription Factors T-Bet (Th1), ROR-γt (Th17), Foxp3 (Treg), and GATA3 (Th2) of EAE Mice Lymphocytes with and without MOG Stimulation
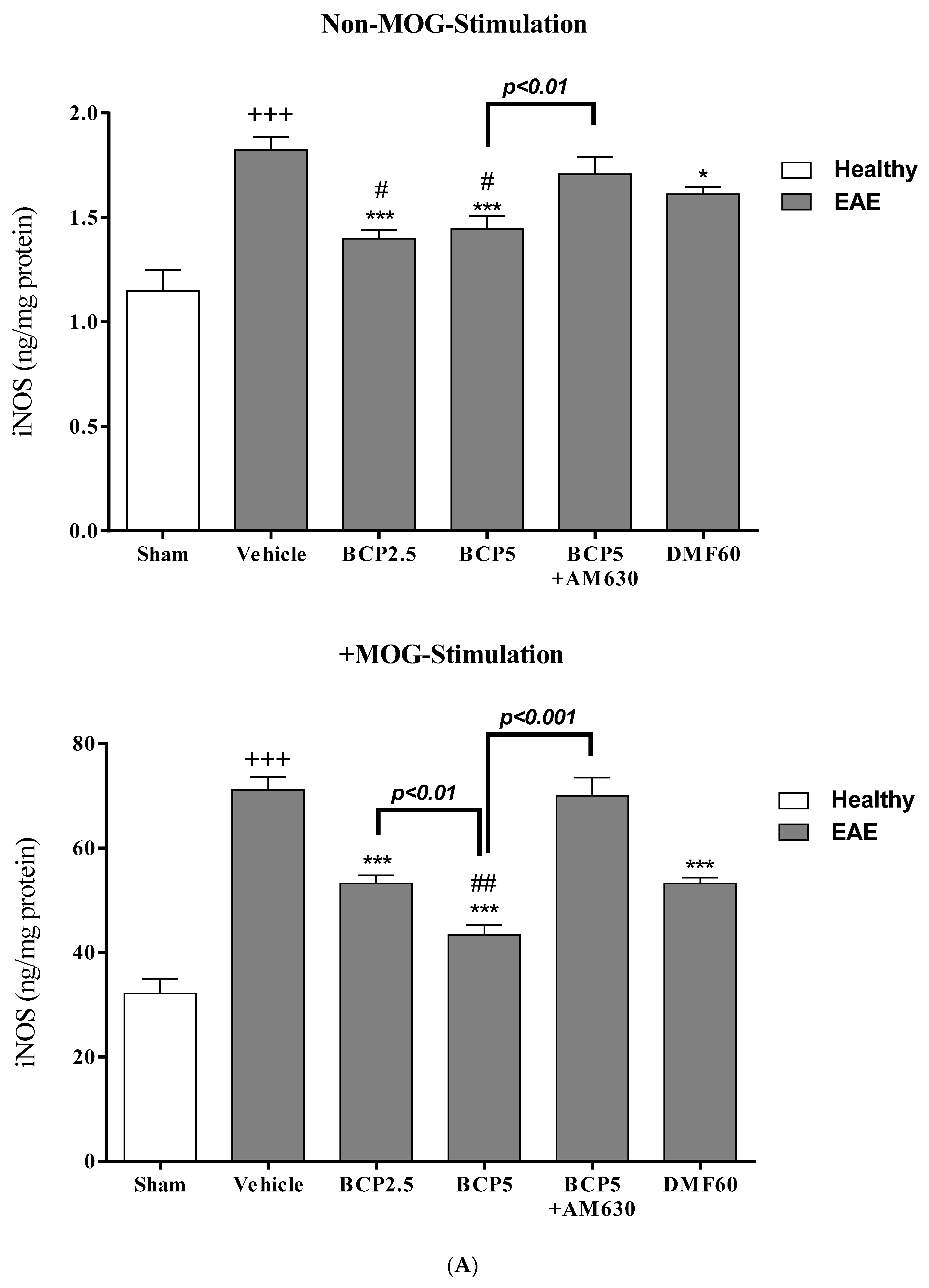
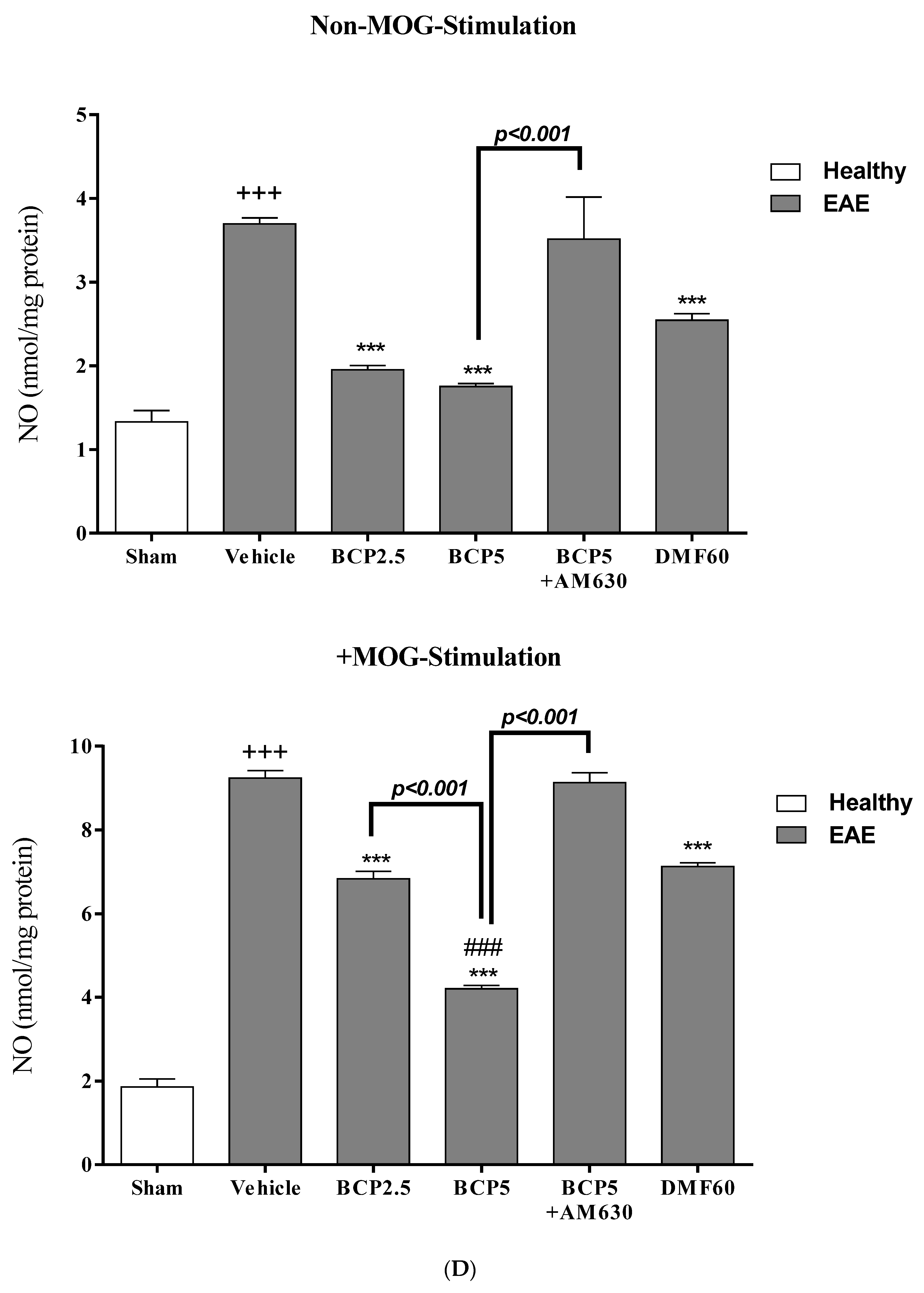
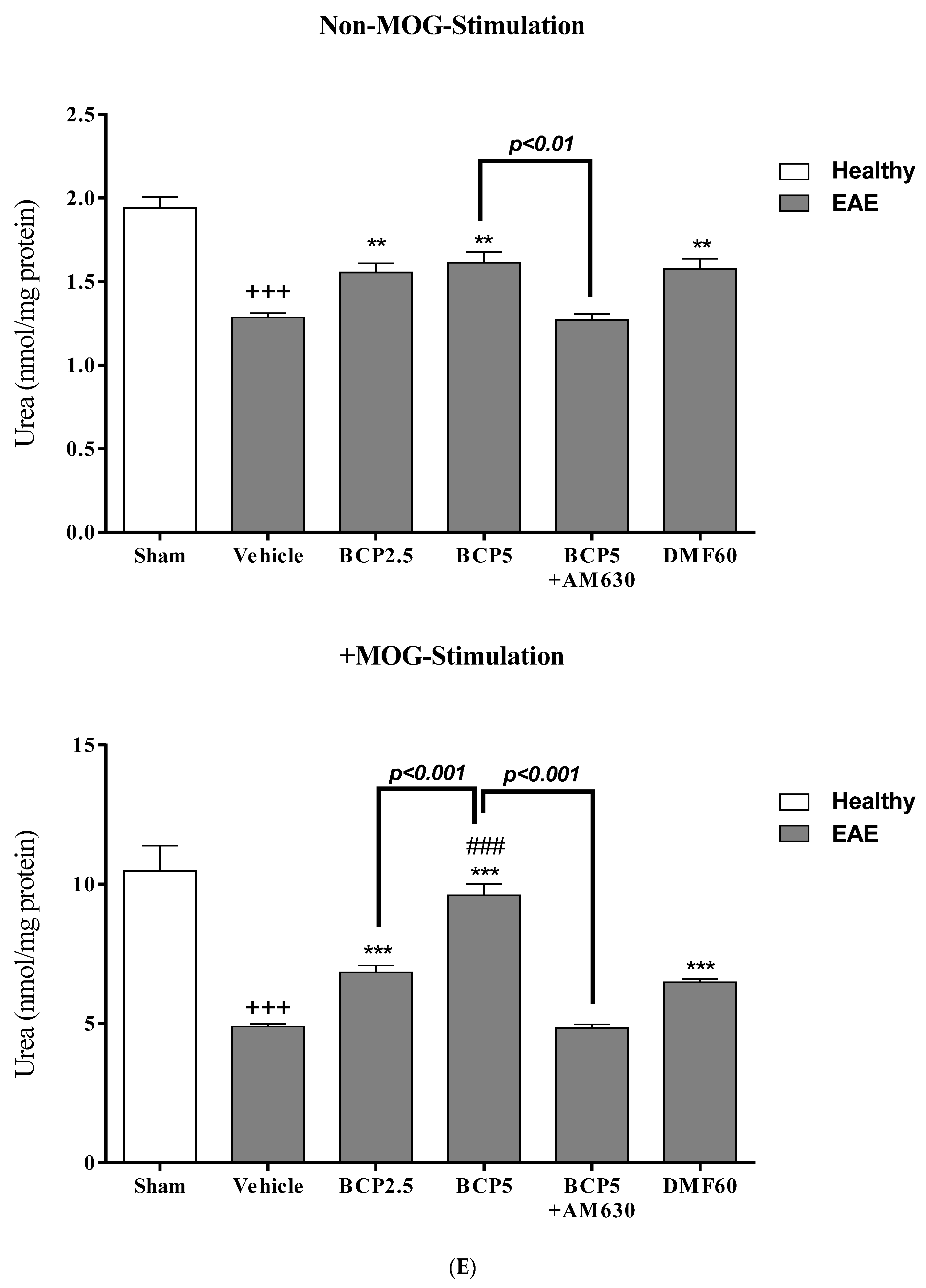
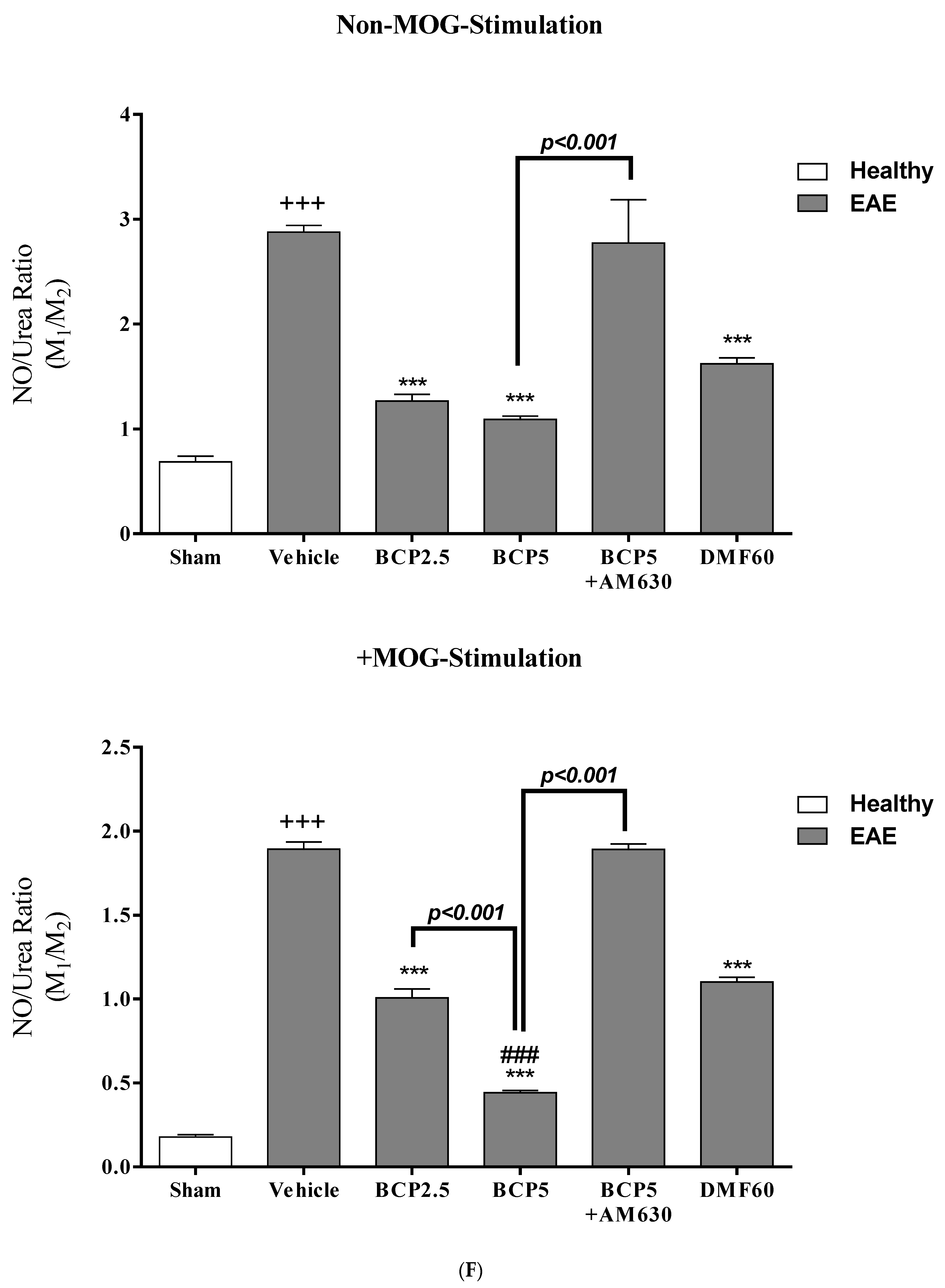
3.6. The BCP Effects on Cell Proliferation, Cytokines Profile, and Markers of Microglia of EAE Mice with and without MOG Stimulation
3.7. The Relative Effects of BCP on the CNS Immunity and Systemic Immunity Levels in EAE Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Dobson, R.; Giovannoni, G. Multiple sclerosis—A review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef]
- Goodin, D.S. Chapter 11—The epidemiology of multiple sclerosis: Insights to a causal cascade. In Handbook of Clinical Neurology; Aminoff, M.J., Boller, F., Swaab, D.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 138, pp. 173–206. [Google Scholar] [CrossRef]
- Greenfield, A.L.; Hauser, S.L. B-cell Therapy for Multiple Sclerosis: Entering an era. Ann. Neurol. 2018, 83, 13–26. [Google Scholar] [CrossRef]
- Grace, P.M.; Loram, L.C.; Christianson, J.P.; Strand, K.A.; Flyer-Adams, J.G.; Penzkover, K.R.; Forsayeth, J.R.; van Dam, A.-M.; Mahoney, M.J.; Maier, S.F.; et al. Behavioral assessment of neuropathic pain, fatigue, and anxiety in experimental autoimmune encephalomyelitis (EAE) and attenuation by interleukin-10 gene therapy. Brain Behav. Immun. 2017, 59, 49–54. [Google Scholar] [CrossRef]
- Constantinescu, C.S.; Farooqi, N.; O′Brien, K.; Gran, B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br. J. Pharmacol. 2011, 164, 1079–1106. [Google Scholar] [CrossRef]
- Álvarez-Sánchez, N.; Cruz-Chamorro, I.; López-González, A.; Utrilla, J.C.; Fernández-Santos, J.M.; Martínez-López, A.; Lardone, P.J.; Guerrero, J.M.; Carrillo-Vico, A. Melatonin controls experimental autoimmune encephalomyelitis by altering the T effector/regulatory balance. Brain Behav. Immun. 2015, 50, 101–114. [Google Scholar] [CrossRef]
- Askari, V.R.; Baradaran Rahimi, V.; Rezaee, S.A.; Boskabady, M.H. Auraptene regulates Th1/Th2/TReg balances, NF-κB nuclear localization and nitric oxide production in normal and Th2 provoked situations in human isolated lymphocytes. Phytomedicine 2018, 43, 1–10. [Google Scholar] [CrossRef]
- Fernández-Ruiz, J.; Moreno-Martet, M.; Rodríguez-Cueto, C.; Palomo-Garo, C.; Gómez-Cañas, M.; Valdeolivas, S.; Guaza, C.; Romero, J.; Guzmán, M.; Mechoulam, R.; et al. Prospects for cannabinoid therapies in basal ganglia disorders. Br. J. Pharmacol. 2011, 163, 1365–1378. [Google Scholar] [CrossRef]
- Lisboa, S.F.; Gomes, F.V.; Guimaraes, F.S.; Campos, A.C. Microglial Cells as a Link between Cannabinoids and the Immune Hypothesis of Psychiatric Disorders. Front. Neurol. 2016, 7, 5. [Google Scholar] [CrossRef]
- Barrie, N.; Manolios, N. The endocannabinoid system in pain and inflammation: Its relevance to rheumatic disease. Eur. J. Rheumatol. 2017, 4, 210–218. [Google Scholar] [CrossRef]
- Guida, F.; Luongo, L.; Boccella, S.; Giordano, M.E.; Romano, R.; Bellini, G.; Manzo, I.; Furiano, A.; Rizzo, A.; Imperatore, R.; et al. Palmitoylethanolamide induces microglia changes associated with increased migration and phagocytic activity: Involvement of the CB2 receptor. Sci. Rep. 2017, 7, 375. [Google Scholar] [CrossRef]
- Ladak, N.; Beishon, L.; Thompson, J.P.; Lambert, D.G. Cannabinoids and sepsis. Trends Anaesth. Crit. Care 2011, 1, 191–198. [Google Scholar] [CrossRef]
- Dhopeshwarkar, A.; Mackie, K. CB(2) Cannabinoid Receptors as a Therapeutic Target—What Does the Future Hold? Mol. Pharmacol. 2014, 86, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Askari, V.R.; Shafiee-Nick, R. The protective effects of β-caryophyllene on LPS-induced primary microglia M1/M2 imbalance: A mechanistic evaluation. Life Sci. 2019, 219, 40–73. [Google Scholar] [CrossRef]
- Askari, V.R.; Shafiee-Nick, R. Promising neuroprotective effects of β-caryophyllene against LPS-induced oligodendrocyte toxicity: A mechanistic study. Biochem. Pharmacol. 2019, 159, 154–171. [Google Scholar] [CrossRef]
- Cianchi, F.; Papucci, L.; Schiavone, N.; Lulli, M.; Magnelli, L.; Vinci, M.C.; Messerini, L.; Manera, C.; Ronconi, E.; Romagnani, P.; et al. Cannabinoid receptor activation induces apoptosis through tumor necrosis factor alpha-mediated ceramide de novo synthesis in colon cancer cells. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008, 14, 7691–7700. [Google Scholar] [CrossRef] [PubMed]
- Rivera, I.-G.; Ordoñez, M.; Presa, N.; Gomez-Larrauri, A.; Simón, J.; Trueba, M.; Gomez-Muñoz, A. Sphingomyelinase D/Ceramide 1-Phosphate in Cell Survival and Inflammation. Toxins 2015, 7, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Mercado, N.; Kizawa, Y.; Ueda, K.; Xiong, Y.; Kimura, G.; Moses, A.; Curtis, J.M.; Ito, K.; Barnes, P.J. Activation of transcription factor Nrf2 signalling by the sphingosine kinase inhibitor SKI-II is mediated by the formation of Keap1 dimers. PLoS ONE 2014, 9, e88168. [Google Scholar] [CrossRef]
- Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.Z.; Xie, X.Q.; Altmann, K.H.; Karsak, M.; Zimmer, A. Beta-caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef]
- Baradaran Rahimi, V.; Askari, V.R. A mechanistic review on immunomodulatory effects of selective type two cannabinoid receptor beta-caryophyllene. Biofactors 2022, 48, 857–882. [Google Scholar] [CrossRef]
- Wang, G.; Ma, W.; Du, J. beta-Caryophyllene (BCP) ameliorates MPP+ induced cytotoxicity. Biomed. Pharmacother. 2018, 103, 1086–1091. [Google Scholar] [CrossRef]
- Hu, Y.; Zeng, Z.; Wang, B.; Guo, S. Trans-caryophyllene inhibits amyloid β (Aβ) oligomer-induced neuroinflammation in BV-2 microglial cells. Int. Immunopharmacol. 2017, 51, 91–98. [Google Scholar] [CrossRef]
- Shan, J.; Chen, L.; Lu, K. Protective effects of trans-caryophyllene on maintaining osteoblast function. IUBMB Life 2017, 69, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Assis, L.C.; Straliotto, M.R.; Engel, D.; Hort, M.A.; Dutra, R.C.; de Bem, A.F. beta-Caryophyllene protects the C6 glioma cells against glutamate-induced excitotoxicity through the Nrf2 pathway. Neuroscience 2014, 279, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Alberti, T.B.; Barbosa, W.L.R.; Vieira, J.L.F.; Raposo, N.R.B.; Dutra, R.C. (−)-β-Caryophyllene, a CB2 Receptor-Selective Phytocannabinoid, Suppresses Motor Paralysis and Neuroinflammation in a Murine Model of Multiple Sclerosis. Int. J. Mol. Sci. 2017, 18, 691. [Google Scholar] [CrossRef] [PubMed]
- Fontes, L.B.A.; Dias, D.D.S.; Aarestrup, B.J.V.; Aarestrup, F.M.; Da Silva Filho, A.A.; Correa, J. beta-Caryophyllene ameliorates the development of experimental autoimmune encephalomyelitis in C57BL/6 mice. Biomed. Pharmacother. 2017, 91, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Bento, A.F.; Marcon, R.; Dutra, R.C.; Claudino, R.F.; Cola, M.; Leite, D.F.; Calixto, J.B. beta-Caryophyllene inhibits dextran sulfate sodium-induced colitis in mice through CB2 receptor activation and PPARgamma pathway. Am. J. Pathol. 2011, 178, 1153–1166. [Google Scholar] [CrossRef] [PubMed]
- Bittner, S.; Afzali, A.M.; Wiendl, H.; Meuth, S.G. Myelin oligodendrocyte glycoprotein (MOG35-55) induced experimental autoimmune encephalomyelitis (EAE) in C57BL/6 mice. J. Vis. Exp. JoVE 2014, 86, e51275. [Google Scholar] [CrossRef]
- Pitarokoili, K.; Ambrosius, B.; Meyer, D.; Schrewe, L.; Gold, R. Dimethyl Fumarate Ameliorates Lewis Rat Experimental Autoimmune Neuritis and Mediates Axonal Protection. PLoS ONE 2015, 10, e0143416. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, P.; Yellanki, S.; Medishetti, R.; Sriram, D.; Saxena, U.; Yogeeswari, P. Novel Zebrafish EAE model: A quick in vivo screen for multiple sclerosis. Mult. Scler Relat. Disord. 2017, 11, 32–39. [Google Scholar] [CrossRef]
- Javed, H.; Azimullah, S.; Haque, M.E.; Ojha, S.K. Cannabinoid Type 2 (CB2) Receptors Activation Protects against Oxidative Stress and Neuroinflammation Associated Dopaminergic Neurodegeneration in Rotenone Model of Parkinson’s Disease. Front. Neurosci. 2016, 10, 321. [Google Scholar] [CrossRef]
- Youssef, D.A.; El-Fayoumi, H.M.; Mahmoud, M.F. Beta-caryophyllene alleviates diet-induced neurobehavioral changes in rats: The role of CB2 and PPAR-γ receptors. Biomed. Pharmacother. 2019, 110, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Al Mansouri, S.; Ojha, S.; Al Maamari, E.; Al Ameri, M.; Nurulain, S.M.; Bahi, A. The cannabinoid receptor 2 agonist, beta-caryophyllene, reduced voluntary alcohol intake and attenuated ethanol-induced place preference and sensitivity in mice. Pharmacol. Biochem. Behav. 2014, 124, 260–268. [Google Scholar] [CrossRef]
- Askari, V.R.; Baradaran Rahimi, V.; Assaran, A.; Iranshahi, M.; Boskabady, M.H. Evaluation of the anti-oxidant and anti-inflammatory effects of the methanolic extract of Ferula szowitsiana root on PHA-induced inflammation in human lymphocytes. Drug Chem. Toxicol. 2020, 43, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Roohbakhsh, Y.; Baradaran Rahimi, V.; Silakhori, S.; Rajabi, H.; Rahmanian-Devin, P.; Samzadeh-Kermani, A.; Rakhshandeh, H.; Hasanpour, M.; Iranshahi, M.; Mousavi, S.H.; et al. Evaluation of the Effects of Peritoneal Lavage with Rosmarinus officinalis Extract against the Prevention of Postsurgical-Induced Peritoneal Adhesion. Planta Med. 2020, 86, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Datler, H.; Vogel, A.; Kerndl, M.; Baumgartinger, C.; Musiejovsky, L.; Makivic, N.; Frech, S.; Niederreiter, B.; Haider, T.; Pühringer, M.; et al. PI3K activity in dendritic cells exerts paradoxical effects during autoimmune inflammation. Mol. Immunol. 2019, 111, 32–42. [Google Scholar] [CrossRef]
- Ernst, O.; Zor, T. Linearization of the bradford protein assay. J. Vis. Exp. JoVE 2010, 1918. [Google Scholar] [CrossRef]
- Hegde, V.L.; Hegde, S.; Cravatt, B.F.; Hofseth, L.J.; Nagarkatti, M.; Nagarkatti, P.S. Attenuation of experimental autoimmune hepatitis by exogenous and endogenous cannabinoids: Involvement of regulatory T cells. Mol. Pharmacol. 2008, 74, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Tansey, M.G. Microglia isolation from adult mouse brain. Methods Mol. Biol. 2013, 1041, 17–23. [Google Scholar] [CrossRef]
- Askari, V.R.; Baradaran Rahimi, V.; Tabatabaee, S.A.; Shafiee-Nick, R. Combination of Imipramine, a sphingomyelinase inhibitor, and beta-caryophyllene improve their therapeutic effects on experimental autoimmune encephalomyelitis (EAE). Int. Immunopharmacol. 2019, 77, 105923. [Google Scholar] [CrossRef]
- Shafiee-Nick, R.; Afshari, A.R.; Mousavi, S.H.; Rafighdoust, A.; Askari, V.R.; Mollazadeh, H.; Fanoudi, S.; Mohtashami, E.; Rahimi, V.B.; Mohebbi, M.; et al. A comprehensive review on the potential therapeutic benefits of phosphodiesterase inhibitors on cardiovascular diseases. Biomed. Pharmacother. 2017, 94, 541–556. [Google Scholar] [CrossRef]
- Ramirez, F.; Fowell, D.J.; Puklavec, M.; Simmonds, S.; Mason, D. Glucocorticoids promote a TH2 cytokine response by CD4+ T cells in vitro. J. Immunol. 1996, 156, 2406–2412. [Google Scholar] [CrossRef]
- Askari, V.R.; Rahimi, V.B.; Zargarani, R.; Ghodsi, R.; Boskabady, M.; Boskabady, M.H. Anti-oxidant and anti-inflammatory effects of auraptene on phytohemagglutinin (PHA)-induced inflammation in human lymphocytes. Pharmacol. Rep. 2021, 73, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Baradaran Rahimi, V.; Mousavi, S.H.; Haghighi, S.; Soheili-Far, S.; Askari, V.R. Cytotoxicity and apoptogenic properties of the standardized extract of Portulaca oleracea on glioblastoma multiforme cancer cell line (U-87): A mechanistic study. EXCLI J. 2019, 18, 165–186. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.J.; Bond, R.A.; Spina, D.; Ahluwalia, A.; Alexander, S.P.; Giembycz, M.A.; Gilchrist, A.; Hoyer, D.; Insel, P.A.; Izzo, A.A.; et al. Experimental design and analysis and their reporting: New guidance for publication in BJP. Br. J. Pharmacol. 2015, 172, 3461–3471. [Google Scholar] [CrossRef] [PubMed]
- George, C.H.; Stanford, S.C.; Alexander, S.; Cirino, G.; Docherty, J.R.; Giembycz, M.A.; Hoyer, D.; Insel, P.A.; Izzo, A.A.; Ji, Y.; et al. Updating the guidelines for data transparency in the British Journal of Pharmacology—Data sharing and the use of scatter plots instead of bar charts. Br. J. Pharmacol. 2017, 174, 2801–2804. [Google Scholar] [CrossRef]
- Alexander, S.P.H.; Roberts, R.E.; Broughton, B.R.S.; Sobey, C.G.; George, C.H.; Stanford, S.C.; Cirino, G.; Docherty, J.R.; Giembycz, M.A.; Hoyer, D.; et al. Goals and practicalities of immunoblotting and immunohistochemistry: A guide for submission to the British Journal of Pharmacology. Br. J. Pharmacol. 2018, 175, 407–411. [Google Scholar] [CrossRef]
- Lassmann, H.; Bradl, M. Multiple sclerosis: Experimental models and reality. Acta Neuropathol. 2017, 133, 223–244. [Google Scholar] [CrossRef]
- Van Kaer, L.; Postoak, J.L.; Wang, C.; Yang, G.; Wu, L. Innate, innate-like and adaptive lymphocytes in the pathogenesis of MS and EAE. Cell. Mol. Immunol. 2019, 16, 531–539. [Google Scholar] [CrossRef]
- Lee, S.U.; Li, C.F.; Mortales, C.L.; Pawling, J.; Dennis, J.W.; Grigorian, A.; Demetriou, M. Increasing cell permeability of N-acetylglucosamine via 6-acetylation enhances capacity to suppress T-helper 1 (TH1)/TH17 responses and autoimmunity. PLoS ONE 2019, 14, e0214253. [Google Scholar] [CrossRef]
- Robinson, A.P.; Harp, C.T.; Noronha, A.; Miller, S.D. The experimental autoimmune encephalomyelitis (EAE) model of MS: Utility for understanding disease pathophysiology and treatment. Handb. Clin. Neurol. 2014, 122, 173–189. [Google Scholar] [CrossRef]
- Beurel, E.; Kaidanovich-Beilin, O.; Yeh, W.-I.; Song, L.; Palomo, V.; Michalek, S.M.; Woodgett, J.R.; Harrington, L.E.; Eldar-Finkelman, H.; Martinez, A.; et al. Regulation of Th1 Cells and Experimental Autoimmune Encephalomyelitis by Glycogen Synthase Kinase-3. J. Immunol. 2013, 190, 5000. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Wang, J.; Yang, B.; Weng, Q.; He, Q. Targeting Microglia and Macrophages: A Potential Treatment Strategy for Multiple Sclerosis. Front. Pharmacol. 2019, 10, 286. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Yu, J.; Feng, L.; Hou, S.; Liu, Y.; Guo, M.; Xie, Y.; Meng, J.; Zhang, H.; et al. Targeting the shift from M1 to M2 macrophages in experimental autoimmune encephalomyelitis mice treated with fasudil. PLoS ONE 2013, 8, e54841. [Google Scholar] [CrossRef]
- Schimrigk, S.; Brune, N.; Hellwig, K.; Lukas, C.; Bellenberg, B.; Rieks, M.; Hoffmann, V.; Pohlau, D.; Przuntek, H. Oral fumaric acid esters for the treatment of active multiple sclerosis: An open-label, baseline-controlled pilot study. Eur. J. Neurol. 2006, 13, 604–610. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, Q.; Mao, G. Dimethyl Fumarate Selectively Reduces Memory T Cells and Shifts the Balance between Th1/Th17 and Th2 in Multiple Sclerosis Patients. J. Immunol. 2017, 198, 3069–3080. [Google Scholar] [CrossRef]
- de Jong, R.; Bezemer, A.C.; Zomerdijk, T.P.; van de Pouw-Kraan, T.; Ottenhoff, T.H.; Nibbering, P.H. Selective stimulation of T helper 2 cytokine responses by the anti-psoriasis agent monomethylfumarate. Eur. J. Immunol. 1996, 26, 2067–2074. [Google Scholar] [CrossRef]
- Montes Diaz, G.; Fraussen, J.; Van Wijmeersch, B.; Hupperts, R.; Somers, V. Dimethyl fumarate induces a persistent change in the composition of the innate and adaptive immune system in multiple sclerosis patients. Sci. Rep. 2018, 8, 8194. [Google Scholar] [CrossRef]
- Paraiso, H.C.; Kuo, P.C.; Curfman, E.T.; Moon, H.J.; Sweazey, R.D.; Yen, J.H.; Chang, F.L.; Yu, I.C. Dimethyl fumarate attenuates reactive microglia and long-term memory deficits following systemic immune challenge. J. Neuroinflamm. 2018, 15, 100. [Google Scholar] [CrossRef]
- Peng, H.; Li, H.; Sheehy, A.; Cullen, P.; Allaire, N.; Scannevin, R.H. Dimethyl fumarate alters microglia phenotype and protects neurons against pro-inflammatory toxic microenvironments. J. Neuroimmunol. 2016, 299, 35–44. [Google Scholar] [CrossRef]
- Lively, S.; Schlichter, L.C. Microglia Responses to Pro-inflammatory Stimuli (LPS, IFNγ+TNFα) and Reprogramming by Resolving Cytokines (IL-4, IL-10). Front. Cell. Neurosci. 2018, 12, 215. [Google Scholar] [CrossRef]
- Klauke, A.L.; Racz, I.; Pradier, B.; Markert, A.; Zimmer, A.M.; Gertsch, J.; Zimmer, A. The cannabinoid CB2 receptor-selective phytocannabinoid beta-caryophyllene exerts analgesic effects in mouse models of inflammatory and neuropathic pain. Eur. Neuropsychopharmacol. 2014, 24, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.V.; Matyas, C.; Erdelyi, K.; Cinar, R.; Nieri, D.; Chicca, A.; Nemeth, B.T.; Paloczi, J.; Lajtos, T.; Corey, L.; et al. beta-Caryophyllene protects against alcoholic steatohepatitis by attenuating inflammation and metabolic dysregulation in mice. Br. J. Pharmacol. 2018, 175, 320–334. [Google Scholar] [CrossRef] [PubMed]
- Katsuyama, S.; Mizoguchi, H.; Kuwahata, H.; Komatsu, T.; Nagaoka, K.; Nakamura, H.; Bagetta, G.; Sakurada, T.; Sakurada, S. Involvement of peripheral cannabinoid and opioid receptors in beta-caryophyllene-induced antinociception. Eur. J. Pain 2013, 17, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, C.; Dai, X.; Ao, Y.; Li, Y. Inhibitory effect of trans-caryophyllene (TC) on leukocyte-endothelial attachment. Toxicol. Appl. Pharmacol. 2017, 329, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Ribeiro, R.; Tanaka, M.; Zhang, Y. Activation of CB2 receptor is required for the therapeutic effect of ABHD6 inhibition in experimental autoimmune encephalomyelitis. Neuropharmacology 2015, 99, 196–209. [Google Scholar] [CrossRef]
- Shi, Y.; Duan, Y.H.; Ji, Y.Y.; Wang, Z.L.; Wu, Y.R.; Gunosewoyo, H.; Xie, X.Y.; Chen, J.Z. Amidoalkylindoles as Potent and Selective Cannabinoid Type 2 Receptor Agonists with in Vivo Efficacy in a Mouse Model of Multiple Sclerosis. J. Med. Chem. 2017, 60, 7067–7083. [Google Scholar] [CrossRef]
- Kong, W.; Li, H.; Tuma, R.F.; Ganea, D. Selective CB2 receptor activation ameliorates EAE by reducing Th17 differentiation and immune cell accumulation in the CNS. Cell. Immunol. 2014, 287, 1–17. [Google Scholar] [CrossRef]
- Zhang, M.; Martin, B.R.; Adler, M.W.; Razdan, R.J.; Kong, W.; Ganea, D.; Tuma, R.F. Modulation of cannabinoid receptor activation as a neuroprotective strategy for EAE and stroke. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2009, 4, 249–259. [Google Scholar] [CrossRef]
- Zarruk, J.G.; Fernandez-Lopez, D.; Garcia-Yebenes, I.; Garcia-Gutierrez, M.S.; Vivancos, J.; Nombela, F.; Torres, M.; Burguete, M.C.; Manzanares, J.; Lizasoain, I.; et al. Cannabinoid type 2 receptor activation downregulates stroke-induced classic and alternative brain macrophage/microglial activation concomitant to neuroprotection. Stroke 2012, 43, 211–219. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Wang, P.; Bjorling, D.E. Activation of cannabinoid receptor 2 inhibits experimental cystitis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R846–R853. [Google Scholar] [CrossRef]
- Herrera, B.; Carracedo, A.; Diez-Zaera, M.; Gomez del Pulgar, T.; Guzman, M.; Velasco, G. The CB2 cannabinoid receptor signals apoptosis via ceramide-dependent activation of the mitochondrial intrinsic pathway. Exp. Cell Res. 2006, 312, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Olea-Herrero, N.; Vara, D.; Malagarie-Cazenave, S.; Diaz-Laviada, I. Inhibition of human tumour prostate PC-3 cell growth by cannabinoids R(+)-Methanandamide and JWH-015: Involvement of CB2. Br. J. Cancer 2009, 101, 940–950. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Dong, Z.; Liu, S. beta-Caryophyllene ameliorates the Alzheimer-like phenotype in APP/PS1 Mice through CB2 receptor activation and the PPARgamma pathway. Pharmacology 2014, 94, 1–12. [Google Scholar] [CrossRef] [PubMed]
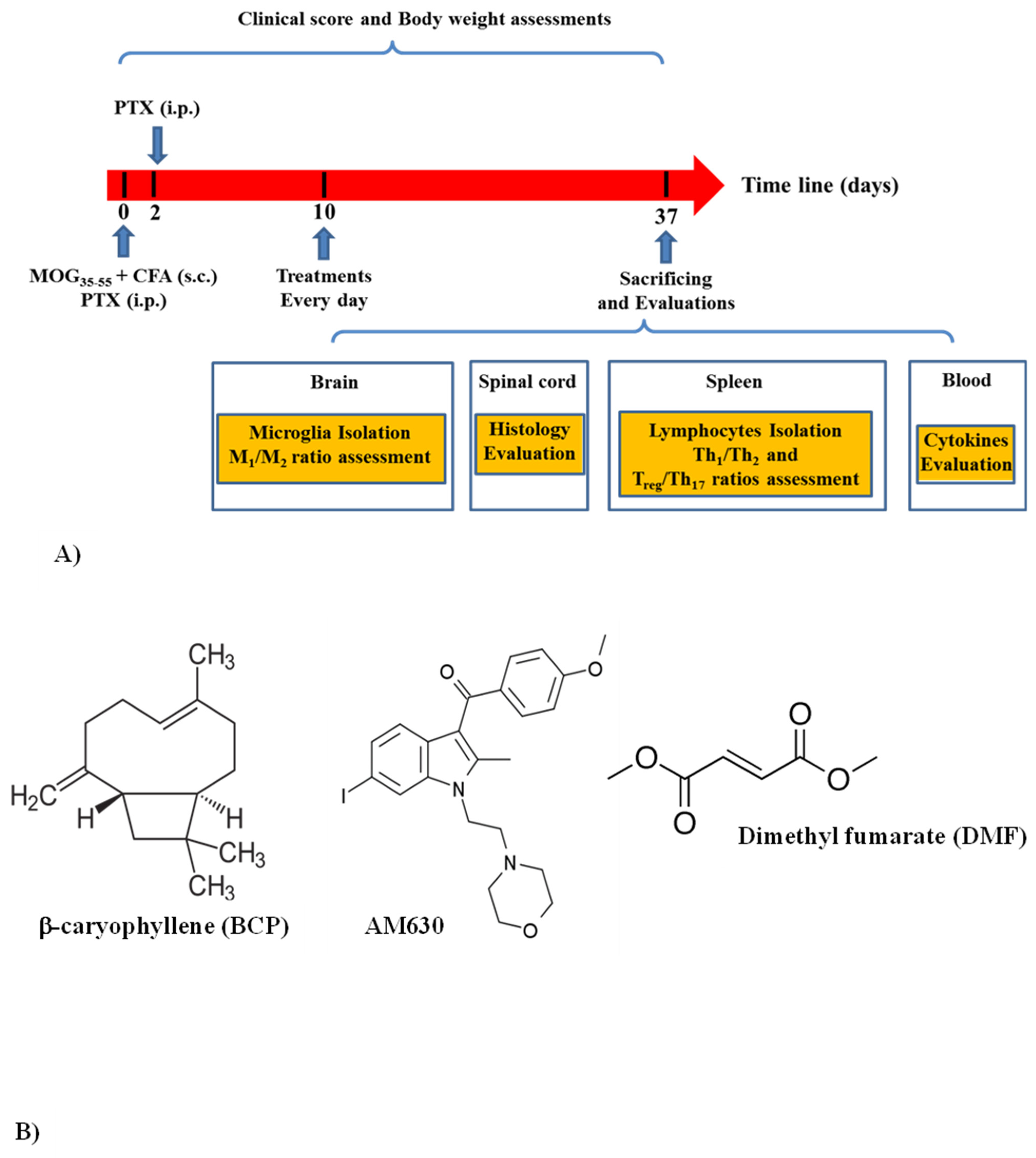
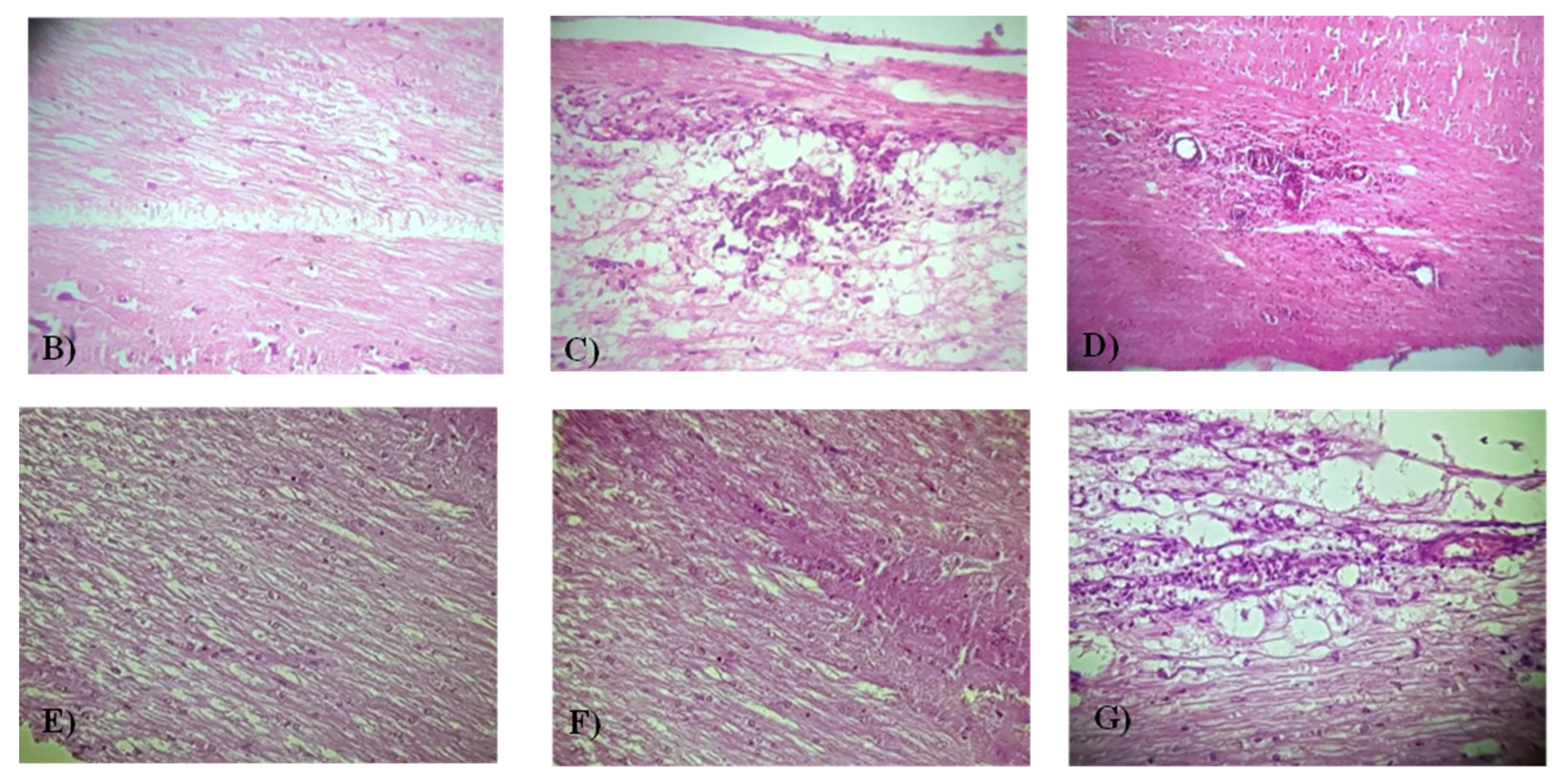
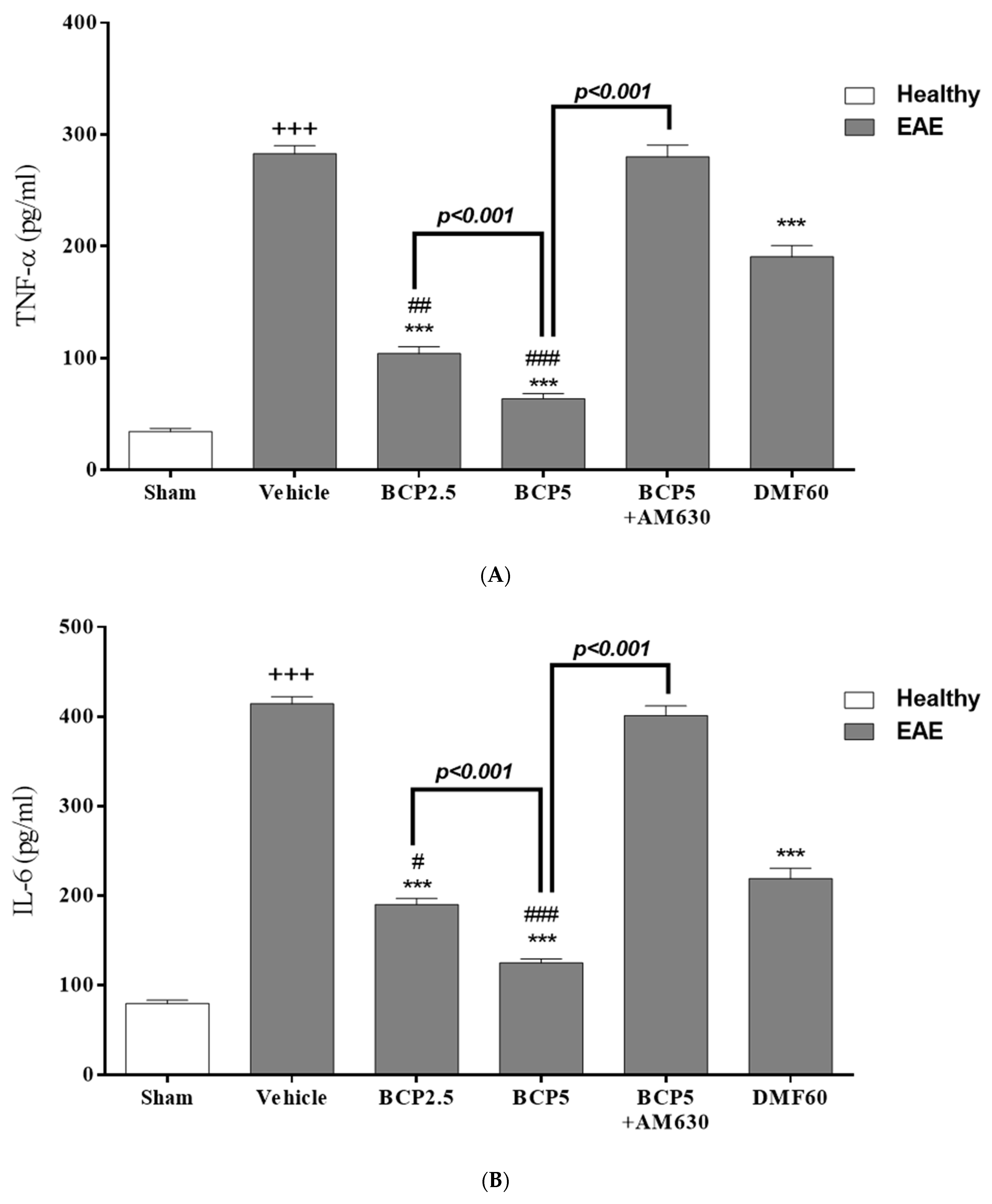
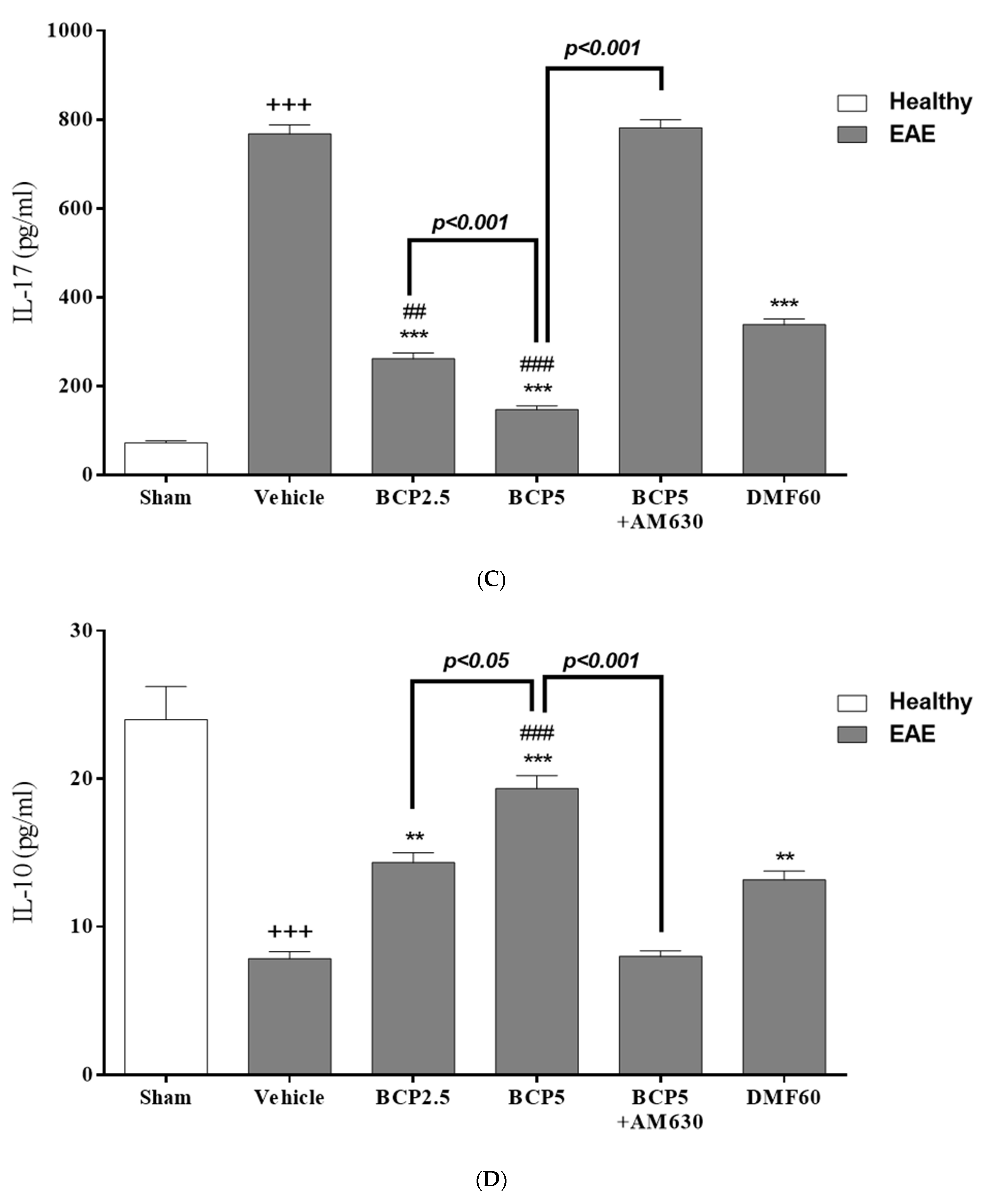
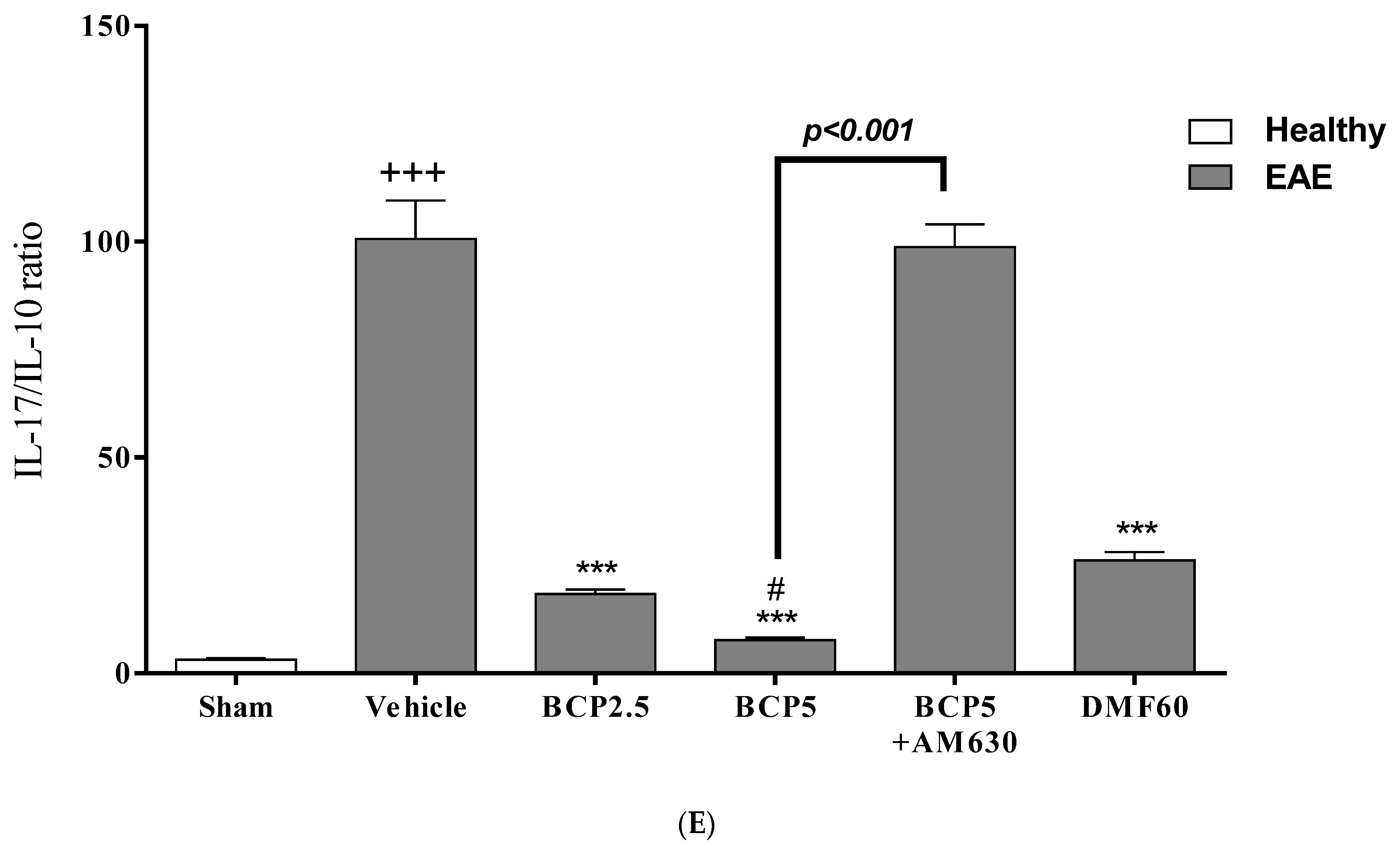












| Score | Clinical Manifestations (Significant Comment) |
|---|---|
| 0 | Without clinical symptoms |
| 1 | Partly limp tail (a tip of the tail droops, normal gait) |
| 2 | Paralyzed tail (tail droops, normal gait) |
| 3 | Uncoordinated movement, hind limb paresis (uncoordinated gait, hind limbs responding to pinching, tail limps) |
| 4 | One paralyzed hind limb (uncoordinated gait with dragging one hind limb, one hind limb not responding to pinch, tail limps) |
| 5 | Two paralyzed hind limbs (uncoordinated gait with both hind limbs dragging, two hind limbs not responding to pinch, tail limps) |
| 6 | Hind limbs paralyzed, weak forelimbs (uncoordinated gait with forelimbs struggling to pull body, tail limps, forelimbs reflex following pinching) |
| 7 | Hind limbs paralyzed, one paralyzed forelimb (no movement of the mouse, one forelimb responding to a toe pinch, tail limps) |
| 8 | Hind limbs paralyzed, both forelimbs paralyzed (no movement of the mouse, both forelimbs do not respond to a toe pinch, tail limps) |
| 9 | Moribund (worsened breathing, no movement) |
| 10 | Death |
| Protocol | Groups | EAE | From Day 10 to 37 (Every Day) | |
|---|---|---|---|---|
| 1 (n = 8/each group) | Sham | - | - | |
| Control | ✓ | Vehicle | ||
| BCP (2.5 mg/kg) | ✓ | BCP 2.5 mg/kg/day; p.o. | ||
| BCP (5 mg/kg) | ✓ | BCP 5 mg/kg/day; p.o. | ||
| DMF (60 mg/kg) | ✓ | DMF 30 mg/kg two times per day; p.o. | ||
| 2 (n = 8/each group) | 30 min later | |||
| Sham | - | - | - | |
| Control | ✓ | AM630 vehicle; i.p. | +BCP vehicle; p.o. | |
| BCP (5 mg/kg) | ✓ | AM630 vehicle; i.p. | +BCP 5 mg/kg/day; p.o. | |
| AM630 (1 mg/kg) + BCP (5 mg/kg) | ✓ | AM630 1 mg/kg/day; i.p. | +BCP 5 mg/kg/day; p.o. | |
| Groups | Incidence (Sick/Total) | MOD a | MMCS a | MCS at Peak EAE (Day 17) a | CDI a,b | Day Presents Significant Changes c |
|---|---|---|---|---|---|---|
| Sham | 0/16 (0%) | - | 0 ± 0 | 0 ± 0 | 0 | 0 |
| Vehicle | 16/16 (100%) +++ | 6.72 ± 0.42 +++ | 7.75 ± 0.47 +++ | 7.75 ± 0.47 +++ | 29.20 ± 1.45 +++ | 9 d |
| BCP 2.5 | 7/8 (87.5%) | 7.22 ± 0.25 | 4.00 ± 0.22 *** | 3.75 ± 0.18 *** | 15.68 ± 1.11 *** | 15 e |
| BCP 5 | 9/16 (56.25%) ***, xxx | 6.82 ± 0.52 | 3.42 ± 0.18 ***, xxx | 2.75 ± 0.43 ***, xxx | 10.70 ± 0.83 ***, xxx | 15 e |
| BCP 5 + AM630 | 8/8 (100%) ∆∆∆ | 7.11 ± 0.68 | 7.55 ± 0.48 ∆∆∆ | 7.35 ± 0.69 ∆∆∆ | 28.38 ± 2.12 ∆∆∆ | 9 d |
| DMF 60 | 7/8 (87.5%) | 6.52 ± 0.55 | 4.62 ± 0.21 *** | 3.43 ± 0.31 *** | 15.81 ± 1.52 *** | 15 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Askari, V.R.; Baradaran Rahimi, V.; Shafiee-Nick, R. Low Doses of β-Caryophyllene Reduced Clinical and Paraclinical Parameters of an Autoimmune Animal Model of Multiple Sclerosis: Investigating the Role of CB2 Receptors in Inflammation by Lymphocytes and Microglial. Brain Sci. 2023, 13, 1092. https://doi.org/10.3390/brainsci13071092
Askari VR, Baradaran Rahimi V, Shafiee-Nick R. Low Doses of β-Caryophyllene Reduced Clinical and Paraclinical Parameters of an Autoimmune Animal Model of Multiple Sclerosis: Investigating the Role of CB2 Receptors in Inflammation by Lymphocytes and Microglial. Brain Sciences. 2023; 13(7):1092. https://doi.org/10.3390/brainsci13071092
Chicago/Turabian StyleAskari, Vahid Reza, Vafa Baradaran Rahimi, and Reza Shafiee-Nick. 2023. "Low Doses of β-Caryophyllene Reduced Clinical and Paraclinical Parameters of an Autoimmune Animal Model of Multiple Sclerosis: Investigating the Role of CB2 Receptors in Inflammation by Lymphocytes and Microglial" Brain Sciences 13, no. 7: 1092. https://doi.org/10.3390/brainsci13071092
APA StyleAskari, V. R., Baradaran Rahimi, V., & Shafiee-Nick, R. (2023). Low Doses of β-Caryophyllene Reduced Clinical and Paraclinical Parameters of an Autoimmune Animal Model of Multiple Sclerosis: Investigating the Role of CB2 Receptors in Inflammation by Lymphocytes and Microglial. Brain Sciences, 13(7), 1092. https://doi.org/10.3390/brainsci13071092