Listening Effort in Tinnitus: A Pilot Study Employing a Light EEG Headset and Skin Conductance Assessment during the Listening to a Continuous Speech Stimulus under Different SNR Conditions
Abstract
1. Introduction
- The investigation of listening effort neurophysiological indices in normal hearing tinnitus patients in comparison to normal hearing controls during a continuous speech stimulus.
- The assessment of the influence of the different background noise levels on listening effort indices and the difficulty and pleasantness perception of the stimulus by tinnitus participants in comparison to healthy controls.
2. Materials and Methods
2.1. Participants
2.2. Self-Report Audiological Questionnaires
2.3. Experimental Protocol
2.4. EEG Signal Acquisition and Processing
2.5. Autonomic Activity Signal Acquisition and Processing
2.6. Statistical Analysis
3. Results
3.1. Behavioural Results
3.2. EEG Results
3.2.1. Parietal Alpha
3.2.2. Frontal Alpha
3.3. EDA Results
4. Discussion
5. Conclusions
- 1.
- The investigation of listening effort neurophysiological indices in normal hearing tinnitus patients in comparison to normal hearing controls during a continuous speech stimulus.
- Despite a general lack of difference between the TIN and CTRL groups concerning performances and perceived pleasantness or difficulty, possibly explained by the normal hearing condition shared by both groups, neurophysiological patterns and correlations retrieved in the TIN group support the hypothesis of a relation between listening effort underpinnings and tinnitus symptoms. The relevance of employing continuous speech stimuli presents a step forward in identifying neural patterns mirroring the daily communication conditions experienced by tinnitus patients.
- 2.
- The assessment of the influence of the different background noise levels on listening effort indices and the difficulty and pleasantness perception of the stimulus by tinnitus participants in comparison to healthy controls.
- In the present study, the level of SNR appears to not influence the listening effort experienced by the TIN group, probably due to the normal hearing condition chosen for patients, a condition purposefully selected to avoid biases due to the hearing-impaired condition effect on listening effort. Such a result should be further investigated on an enlarged sample.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McGarrigle, R.; Munro, K.J.; Dawes, P.; Stewart, A.J.; Moore, D.R.; Barry, J.G.; Amitay, S. Listening effort and fatigue: What exactly are we measuring? A British Society of Audiology Cognition in Hearing Special Interest Group ‘white paper’. Int. J. Audiol. 2014, 53, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Pichora-Fuller, M.K.; Kramer, S.E.; Eckert, M.A.; Edwards, B.; Hornsby, B.W.Y.; Humes, L.E.; Lemke, U.; Lunner, T.; Matthen, M.; Mackersie, C.L.; et al. Hearing Impairment and Cognitive Energy: The Framework for Understanding Effortful Listening (FUEL). Ear Hear. 2016, 37, 5S–27S. [Google Scholar] [CrossRef] [PubMed]
- Weisz, N.; Hartmann, T.; Müller, N.; Lorenz, I.; Obleser, J. Alpha rhythms in audition: Cognitive and clinical perspectives. Front. Psychol. 2011, 2, 73. [Google Scholar] [CrossRef] [PubMed]
- Obleser, J.; Wöstmann, M.; Hellbernd, N.; Wilsch, A.; Maess, B. Adverse Listening Conditions and Memory Load Drive a Common Alpha Oscillatory Network. J. Neurosci. 2012, 32, 12376–12383. [Google Scholar] [CrossRef] [PubMed]
- Vaden, K.I.; Kuchinsky, S.E.; Cute, S.L.; Ahlstrom, J.B.; Dubno, J.R.; Eckert, M.A. The cingulo-opercular network provides word-recognition benefit. J. Neurosci. 2013, 33, 18979–18986. [Google Scholar] [CrossRef]
- Shatzer, H.E.; Russo, F.A. Brightening the Study of Listening Effort with Functional Near-Infrared Spectroscopy: A Scoping Review. Semin. Hear. 2023, 44, 188–210. [Google Scholar] [CrossRef]
- White, B.E.; Langdon, C. The cortical organization of listening effort: New insight from functional near-infrared spectroscopy. Neuroimage 2021, 240, 118324. [Google Scholar] [CrossRef]
- Zekveld, A.A.; Kramer, S.E.; Festen, J.M. Pupil Response as an Indication of Effortful Listening: The Influence of Sentence Intelligibility. Ear Hear. 2010, 31, 480–490. [Google Scholar] [CrossRef]
- Ohlenforst, B.; Zekveld, A.A.; Lunner, T.; Wendt, D.; Naylor, G.; Wang, Y.; Versfeld, N.J.; Kramer, S.E. Impact of stimulus-related factors and hearing impairment on listening effort as indicated by pupil dilation. Hear. Res. 2017, 351, 68–79. [Google Scholar] [CrossRef]
- Mackersie, C.L.; MacPhee, I.X.; Heldt, E.W. Effects of Hearing Loss on Heart Rate Variability and Skin Conductance Measured During Sentence Recognition in Noise. Ear Hear. 2015, 36, 145. [Google Scholar] [CrossRef]
- Mackersie, C.L.; Cones, H. Subjective and psychophysiological indices of listening effort in a competing-talker task. J. Am. Acad. Audiol. 2011, 22, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Gatehouse, S.; Noble, W. The Speech, Spatial and Qualities of Hearing Scale (SSQ). Int. J. Audiol. 2004, 43, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Van Esch, T.E.M.; Kollmeier, B.; Vormann, M.; Lyzenga, J.; Houtgast, T.; Hällgren, M.; Larsby, B.; Athalye, S.P.; Lutman, M.E.; Dreschler, W.A. Evaluation of the preliminary auditory profile test battery in an international multi-centre study. Int. J. Audiol. 2013, 52, 305–321. [Google Scholar] [CrossRef]
- Fraser, S.; Gagné, J.-P.; Alepins, M.; Dubois, P. Evaluating the effort expended to understand speech in noise using a dual-task paradigm: The effects of providing visual speech cues. J. Speech Lang. Hear. Res. 2010, 53, 18–33. [Google Scholar] [CrossRef]
- Miles, K.; McMahon, C.; Boisvert, I.; Ibrahim, R.; de Lissa, P.; Graham, P.; Lyxell, B. Objective Assessment of Listening Effort: Coregistration of Pupillometry and EEG. Trends Hear. 2017, 21, 2331216517706396. [Google Scholar] [CrossRef]
- Seifi Ala, T.; Graversen, C.; Wendt, D.; Alickovic, E.; Whitmer, W.M.; Lunner, T. An exploratory Study of EEG Alpha Oscillation and Pupil Dilation in Hearing-Aid Users During Effortful listening to Continuous Speech. PLoS ONE 2020, 15, e0235782. [Google Scholar] [CrossRef]
- Klimesch, W.; Sauseng, P.; Hanslmayr, S. EEG alpha oscillations: The inhibition–timing hypothesis. Brain Res. Rev. 2007, 53, 63–88. [Google Scholar] [CrossRef]
- Jensen, O.; Mazaheri, A. Shaping Functional Architecture by Oscillatory Alpha Activity: Gating by Inhibition. Front. Hum. Neurosci. 2010, 4, 186. [Google Scholar] [CrossRef]
- Dimitrijevic, A.; Smith, M.L.; Kadis, D.S.; Moore, D.R. Cortical Alpha Oscillations Predict Speech Intelligibility. Front. Hum. Neurosci. 2017, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Cartocci, G.; Maglione, A.G.; Vecchiato, G.; Flumeri, G.D.; Colosimo, A.; Scorpecci, A.; Marsella, P.; Giannantonio, S.; Malerba, P.; Borghini, G.; et al. Mental workload estimations in unilateral deafened children. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 1654–1657. [Google Scholar]
- Cartocci, G.; Maglione, A.G.; Rossi, D.; Modica, E.; Borghini, G.; Malerba, P.; Piccioni, L.O.; Babiloni, F. Alpha and Theta EEG Variations as Indices of Listening Effort to Be Implemented in Neurofeedback Among Cochlear Implant Users. In Symbiotic Interaction; Ham, J., Spagnolli, A., Blankertz, B., Gamberini, L., Jacucci, G., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 30–41. [Google Scholar]
- Marsella, P.; Scorpecci, A.; Cartocci, G.; Giannantonio, S.; Maglione, A.G.; Venuti, I.; Brizi, A.; Babiloni, F. EEG activity as an objective measure of cognitive load during effortful listening: A study on pediatric subjects with bilateral, asymmetric sensorineural hearing loss. Int. J. Pediatr. Otorhinolaryngol. 2017, 99, 1–7. [Google Scholar] [CrossRef]
- Wöstmann, M.; Herrmann, B.; Wilsch, A.; Obleser, J. Neural Alpha Dynamics in Younger and Older Listeners Reflect Acoustic Challenges and Predictive Benefits. J. Neurosci. 2015, 35, 1458–1467. [Google Scholar] [CrossRef]
- Obleser, J.; Weisz, N. Suppressed Alpha Oscillations Predict Intelligibility of Speech and its Acoustic Details. Cereb. Cortex 2012, 22, 2466–2477. [Google Scholar] [CrossRef] [PubMed]
- Foxe, J.J.; Snyder, A.C. The Role of Alpha-Band Brain Oscillations as a Sensory Suppression Mechanism during Selective Attention. Front. Psychol. 2011, 2, 154. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, L.; Seifi Ala, T.; Graversen, C.; Alickovic, E.; Lunner, T.; Wendt, D. Hearing Aid Noise Reduction Lowers the Sustained Listening Effort during Continuous Speech in Noise-A Combined Pupillometry and EEG Study. Ear Hear. 2021, 42, 1590–1601. [Google Scholar] [CrossRef]
- Petersen, E.B.; Wöstmann, M.; Obleser, J.; Stenfelt, S.; Lunner, T. Hearing loss impacts neural alpha oscillations under adverse listening conditions. Front. Psychol. 2015, 6, 177. [Google Scholar] [CrossRef]
- Wisniewski, M.G.; Thompson, E.R.; Iyer, N. Theta- and alpha-power enhancements in the electroencephalogram as an auditory delayed match-to-sample task becomes impossibly difficult. Psychophysiology 2017, 54, 1916–1928. [Google Scholar] [CrossRef] [PubMed]
- Cartocci, G.; Scorpecci, A.; Borghini, G.; Maglione, A.G.; Inguscio, B.M.S.; Giannantonio, S.; Giorgi, A.; Malerba, P.; Rossi, D.; Modica, E.; et al. EEG rhythms lateralization patterns in children with unilateral hearing loss are different from the patterns of normal hearing controls during speech-in-noise listening. Hear. Res. 2019, 379, 31–42. [Google Scholar] [CrossRef]
- Piccioni, L.O.; Cartocci, G.; Maglione, A.G.; Modica, E.; Rossi, D.; Mancini, M.; Babiloni, F. EEG variations as estimators of listening effort during recognition of words in noise in unilateral and bilateral sequential adult cochlear implant users. J. Hear. Sci. 2018, 8, 116. [Google Scholar]
- Cartocci, G.; Maglione, A.G.; Rossi, D.; Modica, E.; Malerba, P.; Borghini, G.; Flumeri, G.D.; Aricò, P.; Babiloni, F. Applications in cochlear implants and avionic: Examples of how neurometric measurements of the human perception could help the choice of appropriate human-machine interaction solutions beyond behavioral data. PsychNol. J. 2016, 14, 67–84. [Google Scholar]
- Cartocci, G. The influence of different cochlear implant features use on the mental workload index during a word in noise recognition task. Int. J. Bioelectromagn. 2016, 18, 60–66. [Google Scholar]
- Quaranta, N.; Zinfollino, M.; Casulli, M.; Ardito, A.; Bartoli, R.; Cartocci, G.; Maglione, A.G.; Modica, E.; Rossi, D.; Mancini, M.; et al. Listening effort during speech in noise recognition: A neurophysiologic evaluation of consecutive sound processors. J. Hear. Sci. 2018, 8, 116. [Google Scholar]
- Esmaili, A.A.; Renton, J. A review of tinnitus. Aust. J. Gen. Pract. 2018, 47, 205–208. [Google Scholar] [CrossRef]
- Molnár, A.; Mavrogeni, P.; Tamás, L.; Maihoub, S. Correlation Between Tinnitus Handicap and Depression and Anxiety Scores. Ear. Nose Throat J. 2022, 01455613221139211. [Google Scholar] [CrossRef] [PubMed]
- Noreña, A.J.; Lacher-Fougère, S.; Fraysse, M.-J.; Bizaguet, E.; Grevin, P.; Thai-Van, H.; Moati, L.; Le Pajolec, C.; Fournier, P.; Ohresser, M. Chapter 21—A contribution to the debate on tinnitus definition. In Progress in Brain Research; Langguth, B., Kleinjung, T., De Ridder, D., Schlee, W., Vanneste, S., Eds.; Tinnitus—An Interdisciplinary Approach towards Individualized Treatment: Towards Understanding the Complexity of Tinnitus; Elsevier: Amsterdam, The Netherlands, 2021; Volume 262, pp. 469–485. [Google Scholar]
- Møller, A.R. Tinnitus: Presence and future. In Progress in Brain Research; Langguth, B., Hajak, G., Kleinjung, T., Cacace, A., Møller, A.R., Eds.; Tinnitus: Pathophysiology and Treatment; Elsevier: Amsterdam, The Netherlands, 2007; Volume 166, pp. 3–16. [Google Scholar]
- Møller, A.R. Pathophysiology of tinnitus. Otolaryngol. Clin. N. Am. 2003, 36, 249–266. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, J.; Kong, L.; Zhao, Y.; Diao, T.; Ma, X. Subjective tinnitus patients with normal pure-tone hearing still suffer more informational masking in the noisy environment. Front. Neurosci. 2022, 16, 983427. [Google Scholar] [CrossRef]
- Makar, S.K.; Biswas, A.; Shatapathy, P. The Impact of Tinnitus on Sufferers in Indian Population. Indian J. Otolaryngol. Head Neck Surg. 2014, 66, 37–51. [Google Scholar] [CrossRef]
- Mavrogeni, P.; Maihoub, S.; Tamás, L.; Molnár, A. Tinnitus characteristics and associated variables on Tinnitus Handicap Inventory among a Hungarian population. J. Otol. 2022, 17, 136–139. [Google Scholar] [CrossRef]
- Tunkel, D.E.; Bauer, C.A.; Sun, G.H.; Rosenfeld, R.M.; Chandrasekhar, S.S.; Cunningham, E.R.; Archer, S.M.; Blakley, B.W.; Carter, J.M.; Granieri, E.C.; et al. Clinical Practice Guideline: Tinnitus. Otolaryngol. Head Neck Surg 2014, 151, S1–S40. [Google Scholar] [CrossRef]
- Sendesen, E.; Kılıç, S.; Erbil, N.; Aydın, Ö.; Turkyilmaz, D. An Exploratory Study of the Effect of Tinnitus on Listening Effort Using EEG and Pupillometry. Otolaryngol. Head Neck Surg. 2023, 1–9. [Google Scholar] [CrossRef]
- Newman, C.W.; Jacobson, G.P.; Spitzer, J.B. Development of the Tinnitus Handicap Inventory. Arch. Otolaryngol. Head Neck Surg. 1996, 122, 143–148. [Google Scholar] [CrossRef]
- Monzani, D.; Genovese, E.; Marrara, A.; Gherpelli, C.; Pingani, L.; Forghieri, M.; Rigatelli, M.; Guadagnin, T.; Arslan, E. Validity of the Italian adaptation of the Tinnitus Handicap Inventory; focus on quality of life and psychological distress in tinnitus-sufferers. Acta Otorhinolaryngol. Ital. 2008, 28, 126–134. [Google Scholar] [PubMed]
- Moschen, R.; Fioretti, A.; Eibenstein, A.; Natalini, E.; Cuda, D.; Chiarella, G.; Rumpold, G.; Riedl, D. Validation of the Italian Tinnitus Questionnaire Short Form (TQ 12-I) as a Brief Test for the Assessment of Tinnitus-Related Distress: Results of a Cross-Sectional Multicenter-Study. Front. Psychol. 2018, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Mauss, I.B.; Robinson, M.D. Measures of emotion: A review. Cogn. Emot. 2009, 23, 209–237. [Google Scholar] [CrossRef] [PubMed]
- Chiarella, S.G.; Torromino, G.; Gagliardi, D.M.; Rossi, D.; Babiloni, F.; Cartocci, G. Investigating the negative bias towards artificial intelligence: Effects of prior assignment of AI-authorship on the aesthetic appreciation of abstract paintings. Comput. Hum. Behav. 2022, 137, 107406. [Google Scholar] [CrossRef]
- Francis, A.L.; MacPherson, M.K.; Chandrasekaran, B.; Alvar, A.M. Autonomic Nervous System Responses During Perception of Masked Speech may Reflect Constructs other than Subjective Listening Effort. Front. Psychol. 2016, 7, 263. [Google Scholar] [CrossRef] [PubMed]
- Cederroth, C.R.; Lugo, A.; Edvall, N.K.; Lazar, A.; Lopez-Escamez, J.-A.; Bulla, J.; Uhlen, I.; Hoare, D.J.; Baguley, D.M.; Canlon, B.; et al. Association between Hyperacusis and Tinnitus. J. Clin. Med. 2020, 9, 2412. [Google Scholar] [CrossRef]
- Ralli, M.; Salvi, R.J.; Greco, A.; Turchetta, R.; De Virgilio, A.; Altissimi, G.; Attanasio, G.; Cianfrone, G.; de Vincentiis, M. Characteristics of somatic tinnitus patients with and without hyperacusis. PLoS ONE 2017, 12, e0188255. [Google Scholar] [CrossRef]
- Khalfa, S.; Dubal, S.; Veuillet, E.; Perez-Diaz, F.; Jouvent, R.; Collet, L. Psychometric Normalization of a Hyperacusis Questionnaire. ORL 2002, 64, 436–442. [Google Scholar] [CrossRef]
- Asp, F.; Mäki-Torkko, E.; Karltorp, E.; Harder, H.; Hergils, L.; Eskilsson, G.; Stenfelt, S. A longitudinal study of the bilateral benefit in children with bilateral cochlear implants. Int. J. Audiol. 2015, 54, 77–88. [Google Scholar] [CrossRef]
- Healy, E.W.; Yoho, S.E. Difficulty understanding speech in noise by the hearing impaired: Underlying causes and technological solutions. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 89–92. [Google Scholar]
- Inguscio, B.M.S.; Mancini, P.; Greco, A.; Nicastri, M.; Giallini, I.; Leone, C.A.; Grassia, R.; Di Nardo, W.; Di Cesare, T.; Rossi, F.; et al. ‘Musical effort’ and ‘musical pleasantness’: A pilot study on the neurophysiological correlates of classical music listening in adults normal hearing and unilateral cochlear implant users. Hear. Balance Commun. 2022, 20, 79–88. [Google Scholar] [CrossRef]
- Strauß, A.; Kotz, S.A.; Scharinger, M.; Obleser, J. Alpha and theta brain oscillations index dissociable processes in spoken word recognition. Neuroimage 2014, 97, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Hannemann, R.; Obleser, J.; Eulitz, C. Top-down knowledge supports the retrieval of lexical information from degraded speech. Brain Res. 2007, 1153, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Eckert, M.A.; Teubner-Rhodes, S.; Vaden, K.I. Is Listening in Noise Worth It? The Neurobiology of Speech Recognition in Challenging Listening Conditions. Ear Hear. 2016, 37, 101S–110S. [Google Scholar] [CrossRef] [PubMed]
- McCombe, A.; Baguley, D.; Coles, R.; McKenna, L.; McKinney, C.; Windle-Taylor, P. Guidelines for the grading of tinnitus severity: The results of a working group commissioned by the British Association of Otolaryngologists, Head and Neck Surgeons, 1999. Clin. Otolaryngol. Allied Sci. 2001, 26, 388–393. [Google Scholar] [CrossRef]
- Mohsen, S.; Mahmoudian, S.; Talbian, S.; Pourbakht, A. Correlation Analysis of the Tinnitus Handicap Inventory and Distress Network in Chronic Tinnitus: An EEG Study. Basic Clin. Neurosci. 2019, 10, 499–514. [Google Scholar] [CrossRef]
- Fioretti, A.; Tortorella, F.; Masedu, F.; Valenti, M.; Fusetti, M.; Pavaci, S. Validity of the Italian version of Khalfa’s questionnaire on hyperacusis. Acta Otorhinolaryngol. Ital 2015, 35, 110–115. [Google Scholar]
- Vernon, J.A. Pathophysiology of tinnitus: A special case—Hyperacusis and a proposed treatment. Am. J. Otol. 1987, 8, 201–202. [Google Scholar]
- Hiller, W.; Goebel, G. Rapid assessment of tinnitus-related psychological distress using the Mini-TQ. Int. J. Audiol. 2004, 43, 600–604. [Google Scholar] [CrossRef]
- Russo, A.G.; De Martino, M.; Mancuso, A.; Iaconetta, G.; Manara, R.; Elia, A.; Laudanna, A.; Di Salle, F.; Esposito, F. Semantics-weighted lexical surprisal modeling of naturalistic functional MRI time-series during spoken narrative listening. Neuroimage 2020, 222, 117281. [Google Scholar] [CrossRef]
- Inguscio, B.M.S.; Cartocci, G.; Sciaraffa, N.; Nicastri, M.; Giallini, I.; Greco, A.; Babiloni, F.; Mancini, P. Gamma-Band Modulation in Parietal Area as the Electroencephalographic Signature for Performance in Auditory-Verbal Working Memory: An Exploratory Pilot Study in Hearing and Unilateral Cochlear Implant Children. Brain Sci. 2022, 12, 1291. [Google Scholar] [CrossRef]
- Cartocci, G.; Giorgi, A.; Inguscio, B.M.S.; Scorpecci, A.; Giannantonio, S.; De Lucia, A.; Garofalo, S.; Grassia, R.; Leone, C.A.; Longo, P.; et al. Higher Right Hemisphere Gamma Band Lateralization and Suggestion of a Sensitive Period for Vocal Auditory Emotional Stimuli Recognition in Unilateral Cochlear Implant Children: An EEG Study. Front. Neurosci. 2021, 15, 608156. [Google Scholar] [CrossRef] [PubMed]
- Turrini, M.; Cutugno, F.; Maturi, P.; Prosser, S.; Leoni, F.A.; Arslan, E. Bisyllabic words for speech audiometry: A new italian material. Acta Otorhinolaryngol. Ital. 1993, 13, 63–77. [Google Scholar] [PubMed]
- Lovallo, W.R. Stress and Health: Biological and Psychological Interactions; SAGE Publications: Thousand Oaks, CA, USA, 2015; ISBN 978-1-4833-4743-1. [Google Scholar]
- Attanasio, G.; Cartocci, G.; Covelli, E.; Ambrosetti, E.; Martinelli, V.; Zaccone, M.; Ponzanetti, A.; Gueli, N.; Filipo, R.; Cacciafesta, M. The Mozart effect in patients suffering from tinnitus. Acta Otolaryngol. 2012, 132, 1172–1177. [Google Scholar] [CrossRef] [PubMed]
- Sciaraffa, N.; Di Flumeri, G.; Germano, D.; Giorgi, A.; Di Florio, A.; Borghini, G.; Vozzi, A.; Ronca, V.; Varga, R.; van Gasteren, M.; et al. Validation of a Light EEG-Based Measure for Real-Time Stress Monitoring during Realistic Driving. Brain Sci. 2022, 12, 304. [Google Scholar] [CrossRef] [PubMed]
- Oostenveld, R.; Praamstra, P. The five percent electrode system for high-resolution EEG and ERP measurements. Clin. Neurophysiol. 2001, 112, 713–719. [Google Scholar] [CrossRef]
- Di Flumeri, G.; Aricó, P.; Borghini, G.; Colosimo, A.; Babiloni, F. A new regression-based method for the eye blinks artifacts correction in the EEG signal, without using any EOG channel. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 3187–3190. [Google Scholar]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Vozzi, A.; Ronca, V.; Aricò, P.; Borghini, G.; Sciaraffa, N.; Cherubino, P.; Trettel, A.; Babiloni, F.; Di Flumeri, G. The Sample Size Matters: To What Extent the Participant Reduction Affects the Outcomes of a Neuroscientific Research. A Case-Study in Neuromarketing Field. Sensors 2021, 21, 6088. [Google Scholar] [CrossRef]
- Klimesch, W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res. Rev. 1999, 29, 169–195. [Google Scholar] [CrossRef]
- Boucsein, W.; Fowles, D.C.; Grimnes, S.; Ben-Shakhar, G.; Roth, W.T.; Dawson, M.E.; Filion, D.L. Society for Psychophysiological Research Ad Hoc Committee on Electrodermal Measures Publication recommendations for electrodermal measurements. Psychophysiology 2012, 49, 1017–1034. [Google Scholar] [CrossRef]
- Benedek, M.; Kaernbach, C. A continuous measure of phasic electrodermal activity. J. Neurosci. Methods 2010, 190, 80–91. [Google Scholar] [CrossRef]
- Ronca, V.; Martinez-Levy, A.; Vozzi, A.; Giorgi, A.; Aricò, P.; Capotorto, R.; Borghini, G.; Flumeri, G.D.; Babiloni, F. Wearable Technologies for Electrodermal and Cardiac Activity measurements: A Comparison between Fitbit Sense, Empatica E4 and Shimmer GSR3+ 2023. Sensors 2023, 23, 5847. [Google Scholar] [CrossRef]
- Siegel, S. Nonparametric Statistics. Am. Stat. 1957, 11, 13–19. [Google Scholar] [CrossRef]
- Weber, M.; Sawilowsky, S. Comparative Power Of The Independent t, Permutation t, and WilcoxonTests. J. Mod. Appl. Stat. Methods 2009, 8, 10–15. [Google Scholar] [CrossRef]
- Mohamad, N.; Hoare, D.J.; Hall, D.A. The consequences of tinnitus and tinnitus severity on cognition: A review of the behavioural evidence. Hear. Res. 2016, 332, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Alhanbali, S.; Dawes, P.; Millman, R.E.; Munro, K.J. Measures of Listening Effort Are Multidimensional. Ear Hear. 2019, 40, 1084–1097. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Li, D.-S.; Tsai, M.-H.; Chen, C.-H.; Cheng, Y.-F. The Impact of Acute Tinnitus on Listening Effort: A Study Based on Clinical Observations of Sudden Sensorineural Hearing Loss Patients. Int. J. Environ. Res. Public Health 2022, 19, 3661. [Google Scholar] [CrossRef]
- Hennig, T.R.; Costa, M.J.; Urnau, D.; Becker, K.T.; Schuster, L.C. Recognition of Speech of Normal-hearing Individuals with Tinnitus and Hyperacusis. Int. Arch. Otorhinolaryngol. 2011, 15, 21–28. [Google Scholar]
- Colagrosso, E.M.G.; Fournier, P.; Fitzpatrick, E.M.; Hébert, S. A Qualitative Study on Factors Modulating Tinnitus Experience. Ear Hear. 2019, 40, 636–644. [Google Scholar] [CrossRef]
- Cartocci, G.; Attanasio, G.; Fattapposta, F.; Locuratolo, N.; Mannarelli, D.; Filipo, R. An electrophysiological approach to tinnitus interpretation. Int. Tinnitus J. 2012, 17, 152–157. [Google Scholar] [CrossRef]


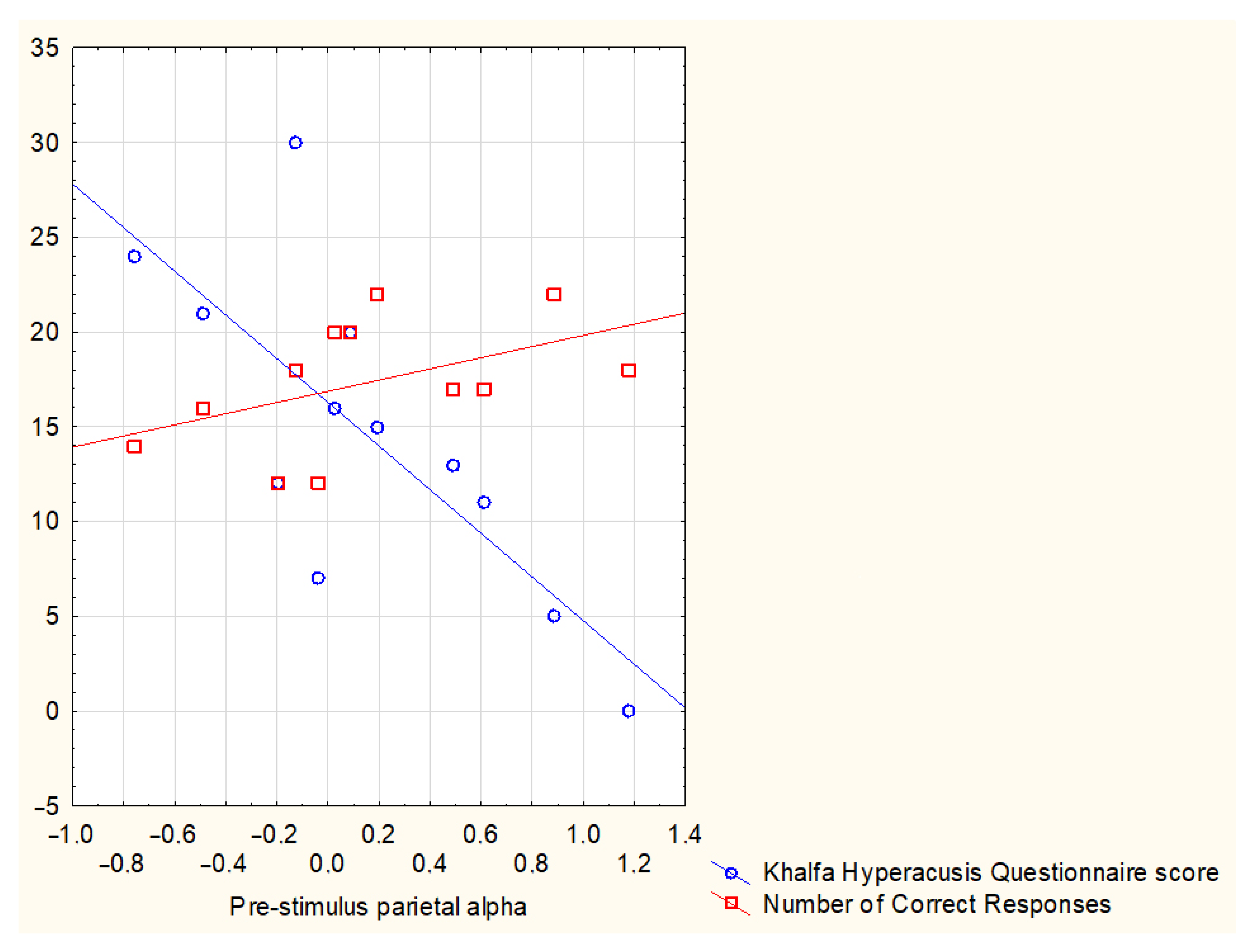
 and negative
and negative  correlations between Parietal alpha levels, Tinnitus Questionnaire 12-item short form (TQ12-I) scores, and electrodermal activity (EDA) levels in the chronic tinnitus (TIN) group.
correlations between Parietal alpha levels, Tinnitus Questionnaire 12-item short form (TQ12-I) scores, and electrodermal activity (EDA) levels in the chronic tinnitus (TIN) group.
 and negative
and negative  correlations between Parietal alpha levels, Tinnitus Questionnaire 12-item short form (TQ12-I) scores, and electrodermal activity (EDA) levels in the chronic tinnitus (TIN) group.
correlations between Parietal alpha levels, Tinnitus Questionnaire 12-item short form (TQ12-I) scores, and electrodermal activity (EDA) levels in the chronic tinnitus (TIN) group.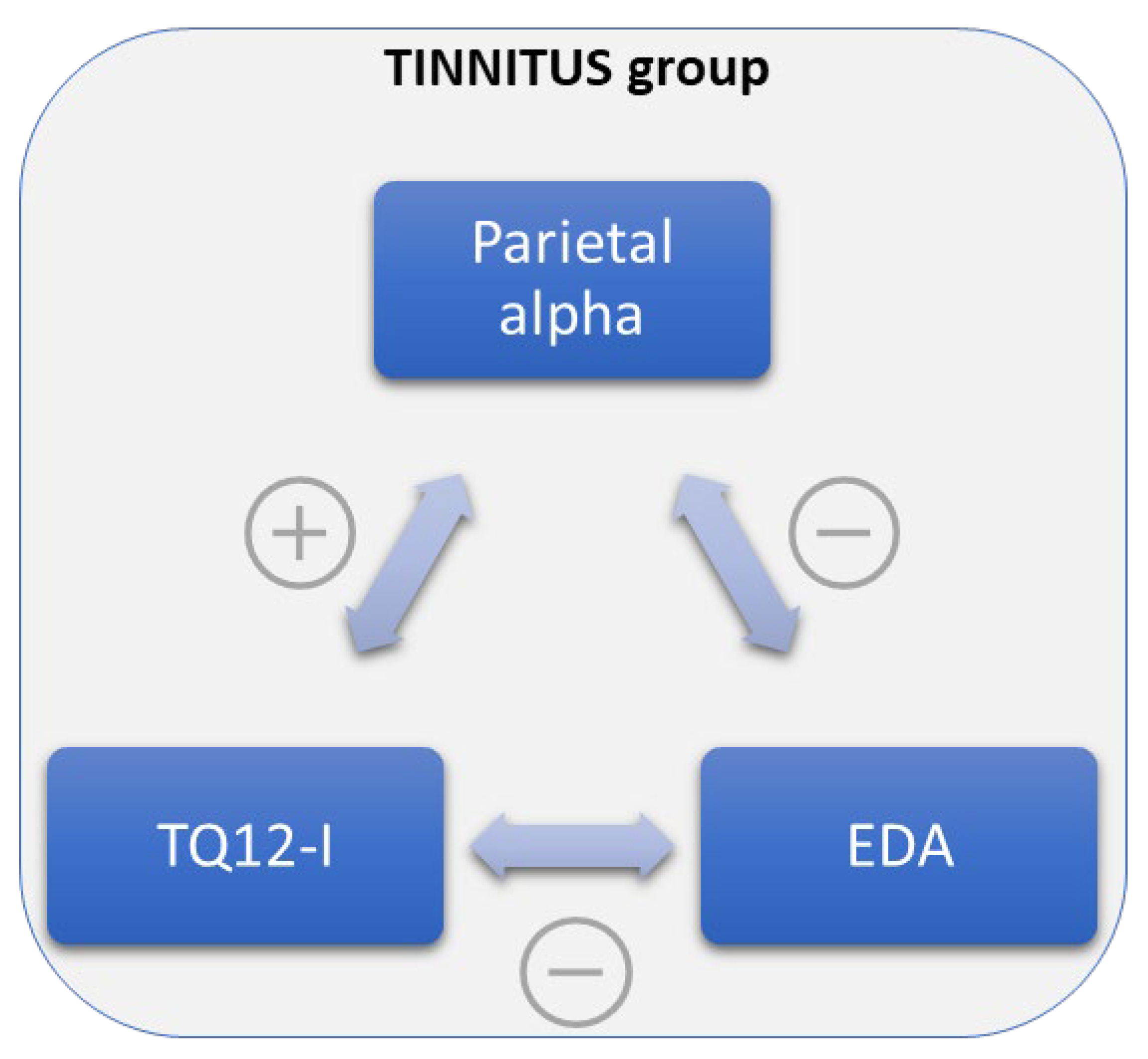


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cartocci, G.; Inguscio, B.M.S.; Giliberto, G.; Vozzi, A.; Giorgi, A.; Greco, A.; Babiloni, F.; Attanasio, G. Listening Effort in Tinnitus: A Pilot Study Employing a Light EEG Headset and Skin Conductance Assessment during the Listening to a Continuous Speech Stimulus under Different SNR Conditions. Brain Sci. 2023, 13, 1084. https://doi.org/10.3390/brainsci13071084
Cartocci G, Inguscio BMS, Giliberto G, Vozzi A, Giorgi A, Greco A, Babiloni F, Attanasio G. Listening Effort in Tinnitus: A Pilot Study Employing a Light EEG Headset and Skin Conductance Assessment during the Listening to a Continuous Speech Stimulus under Different SNR Conditions. Brain Sciences. 2023; 13(7):1084. https://doi.org/10.3390/brainsci13071084
Chicago/Turabian StyleCartocci, Giulia, Bianca Maria Serena Inguscio, Giovanna Giliberto, Alessia Vozzi, Andrea Giorgi, Antonio Greco, Fabio Babiloni, and Giuseppe Attanasio. 2023. "Listening Effort in Tinnitus: A Pilot Study Employing a Light EEG Headset and Skin Conductance Assessment during the Listening to a Continuous Speech Stimulus under Different SNR Conditions" Brain Sciences 13, no. 7: 1084. https://doi.org/10.3390/brainsci13071084
APA StyleCartocci, G., Inguscio, B. M. S., Giliberto, G., Vozzi, A., Giorgi, A., Greco, A., Babiloni, F., & Attanasio, G. (2023). Listening Effort in Tinnitus: A Pilot Study Employing a Light EEG Headset and Skin Conductance Assessment during the Listening to a Continuous Speech Stimulus under Different SNR Conditions. Brain Sciences, 13(7), 1084. https://doi.org/10.3390/brainsci13071084










