Effect of Berberine against Cognitive Deficits in Rat Model of Thioacetamide-Induced Liver Cirrhosis and Hepatic Encephalopathy (Behavioral, Biochemical, Molecular and Histological Evaluations)
Abstract
1. Introduction
2. Materials and Methods
2.1. Agents
2.2. Animals
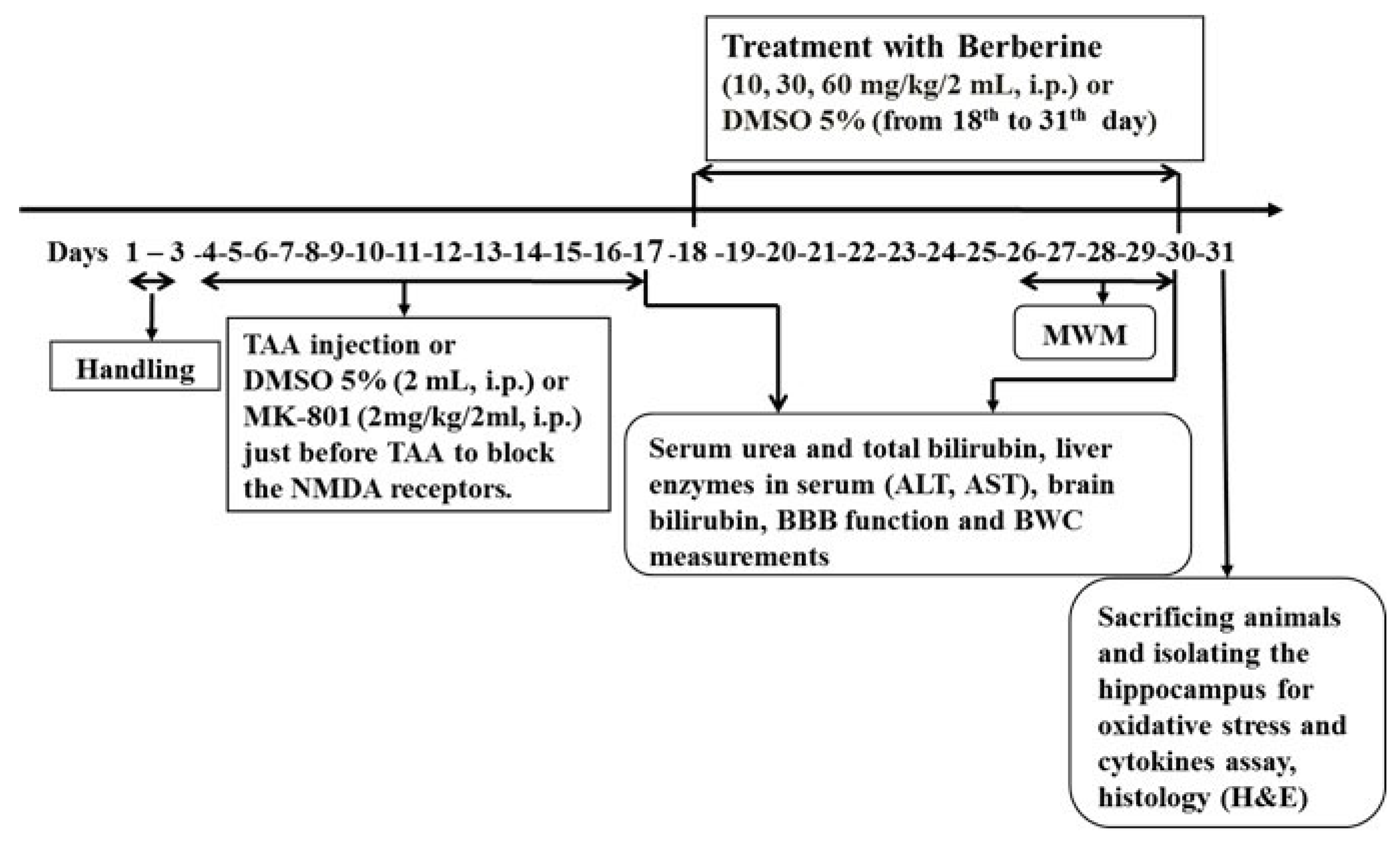
2.3. Experimental Protocols
- 1.
- Spatial memory evaluation in Morris water maze (MWM) and then brain histological evaluation (n = 8).
- 2.
- BBB function measurement (n = 5). Serum biomarkers such as urea, total bilirubin concentration, liver enzyme levels, total bilirubin concentration in brain tissue.
- 3.
- Brain water content measurement (n = 5).
- 4.
- Levels of MDA, GPx, tumor necrosis factor alpha (TNF-α) and interleukin-10 (IL-10) as cytokines in hippocampal tissue (n = 8). The timeline and experimental protocols are shown in Figure 1.
2.4. Induction of Experimental Hepatic Encephalopathy (HE)
2.5. Spatial Learning and Memory Evaluation
2.6. Serum Biochemical Factor Assay
2.7. BBB Permeability Measurement
2.8. Brain Water Content Measurement
2.9. Inflammatory Cytokine Assay
2.10. Oxidative Stress Assay
2.11. Histopathological Study of the Hippocampus
2.12. Statistical Analysis
3. Results
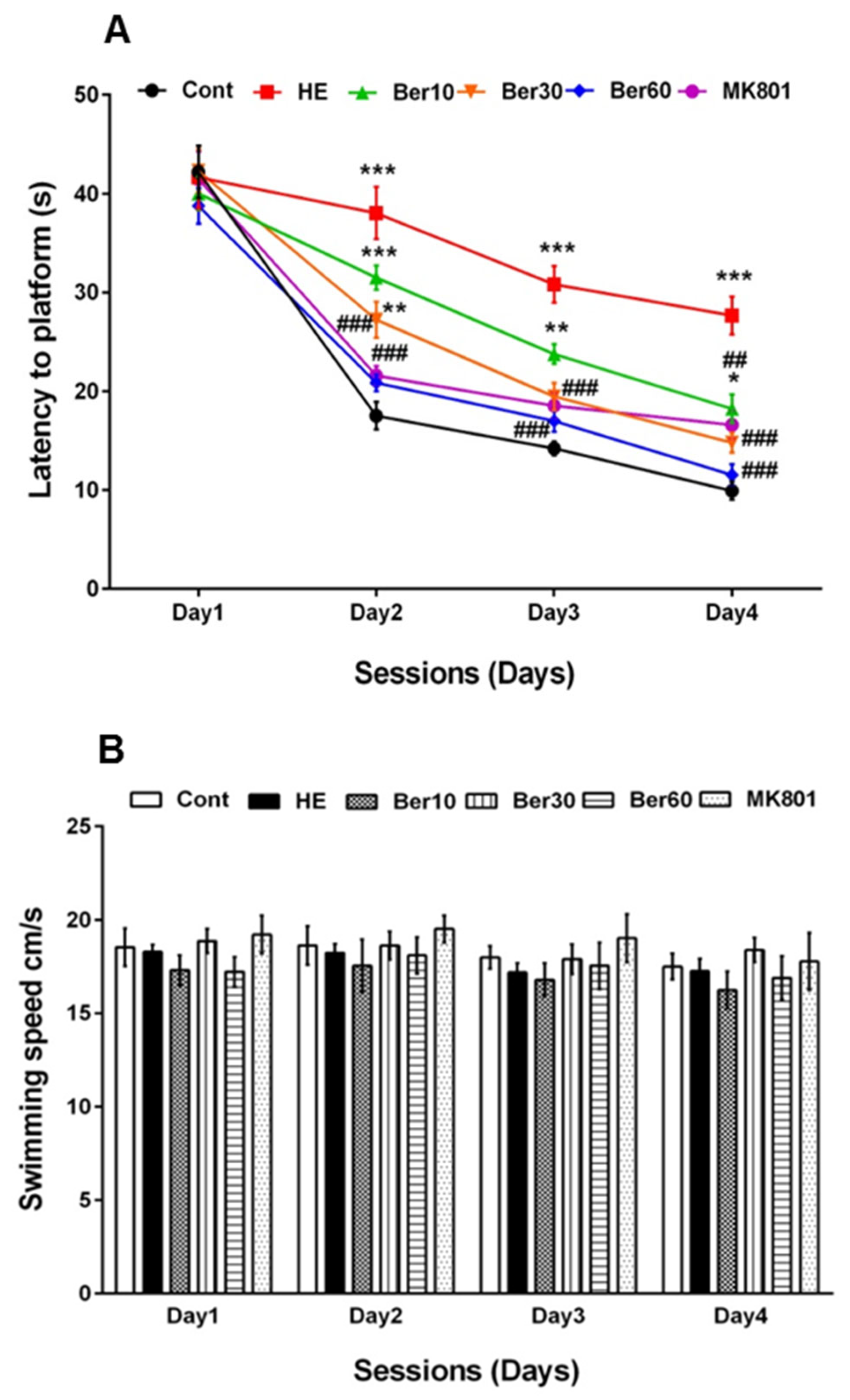
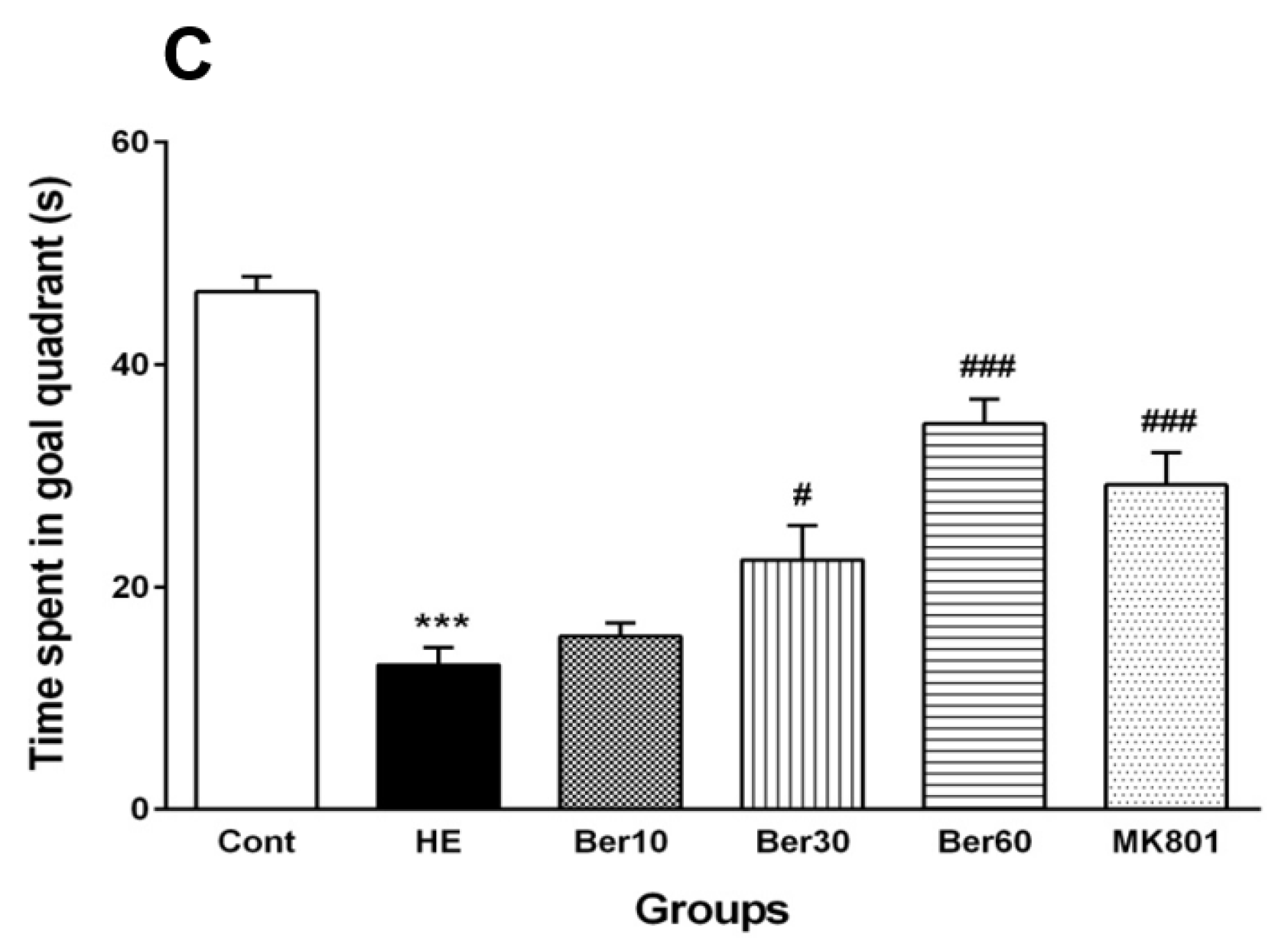
3.1. Spatial Learning and Memory
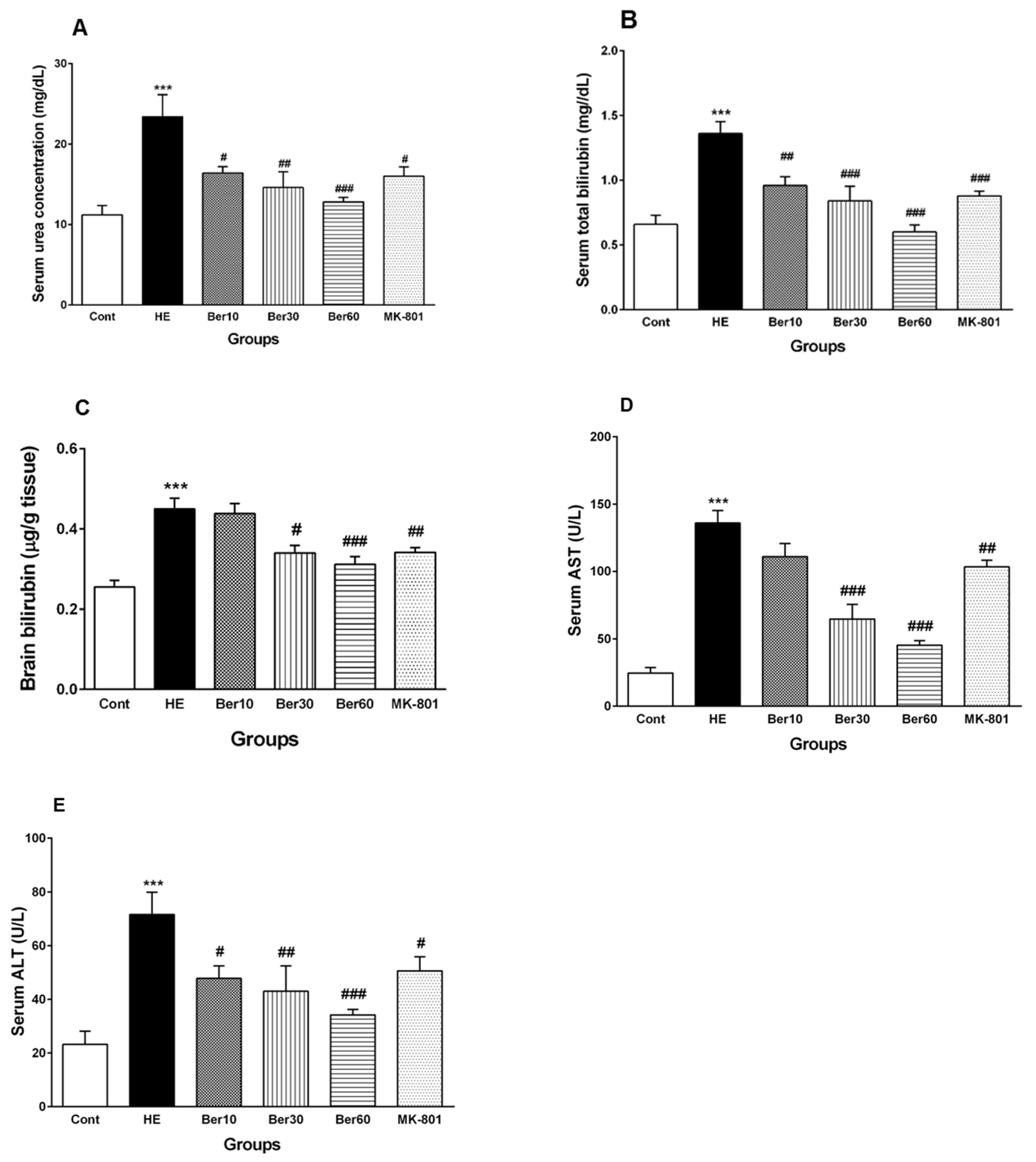
3.2. Serum Biochemical Biomarkers
3.3. Blood–Brain Barrier Permeability
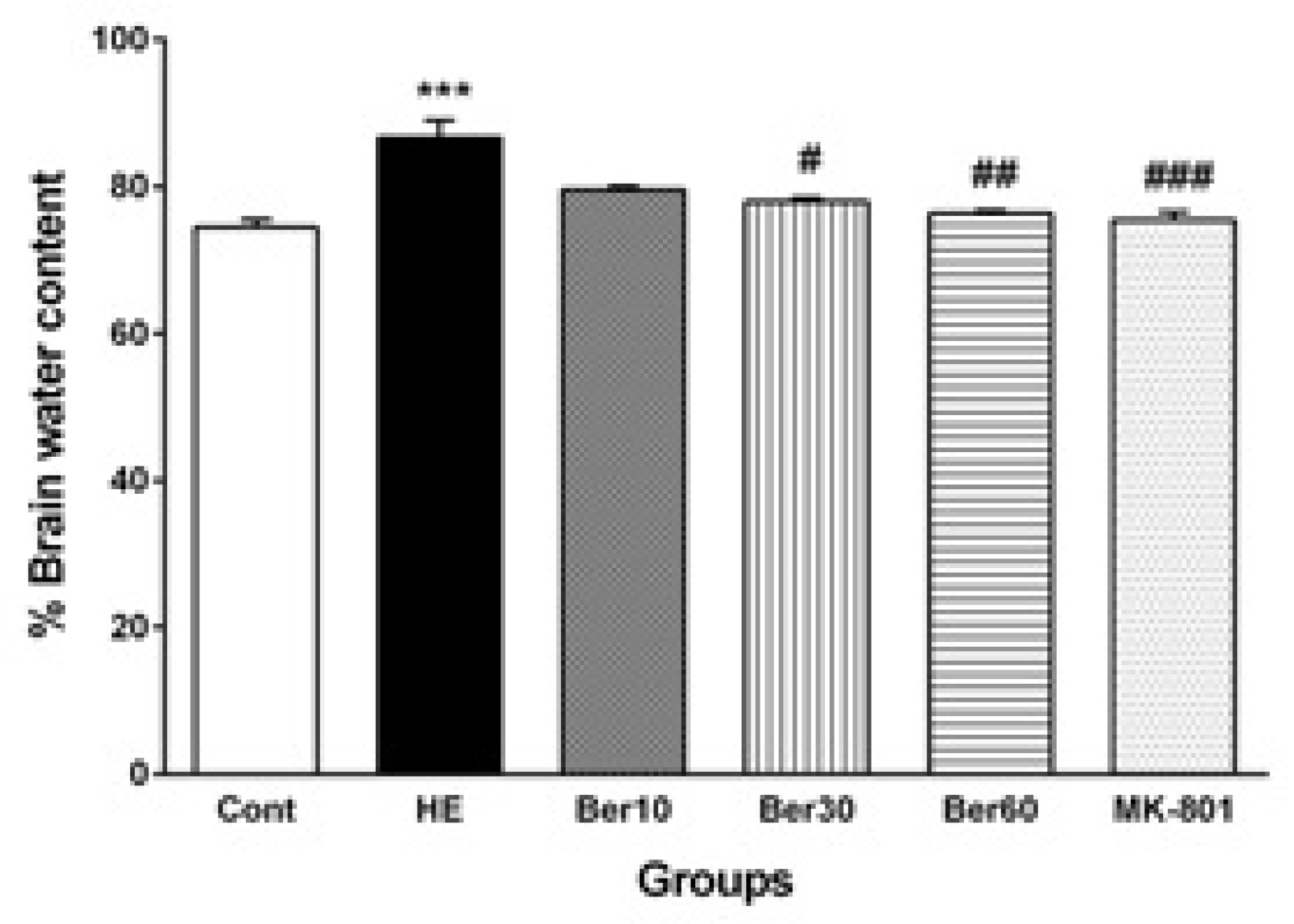
3.4. Brain Water Content
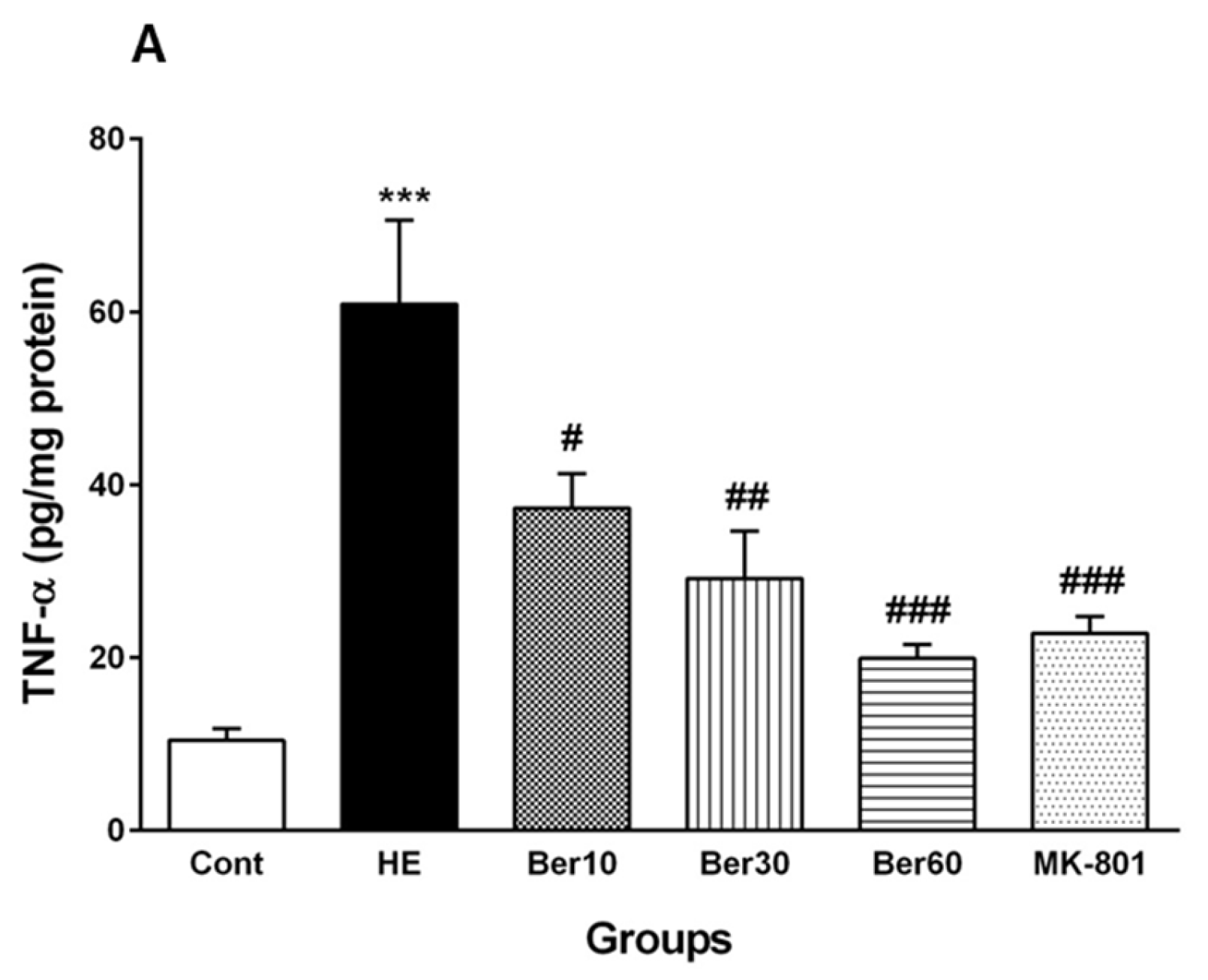
3.5. Inflammatory Cytokines in Hippocampal Tissue
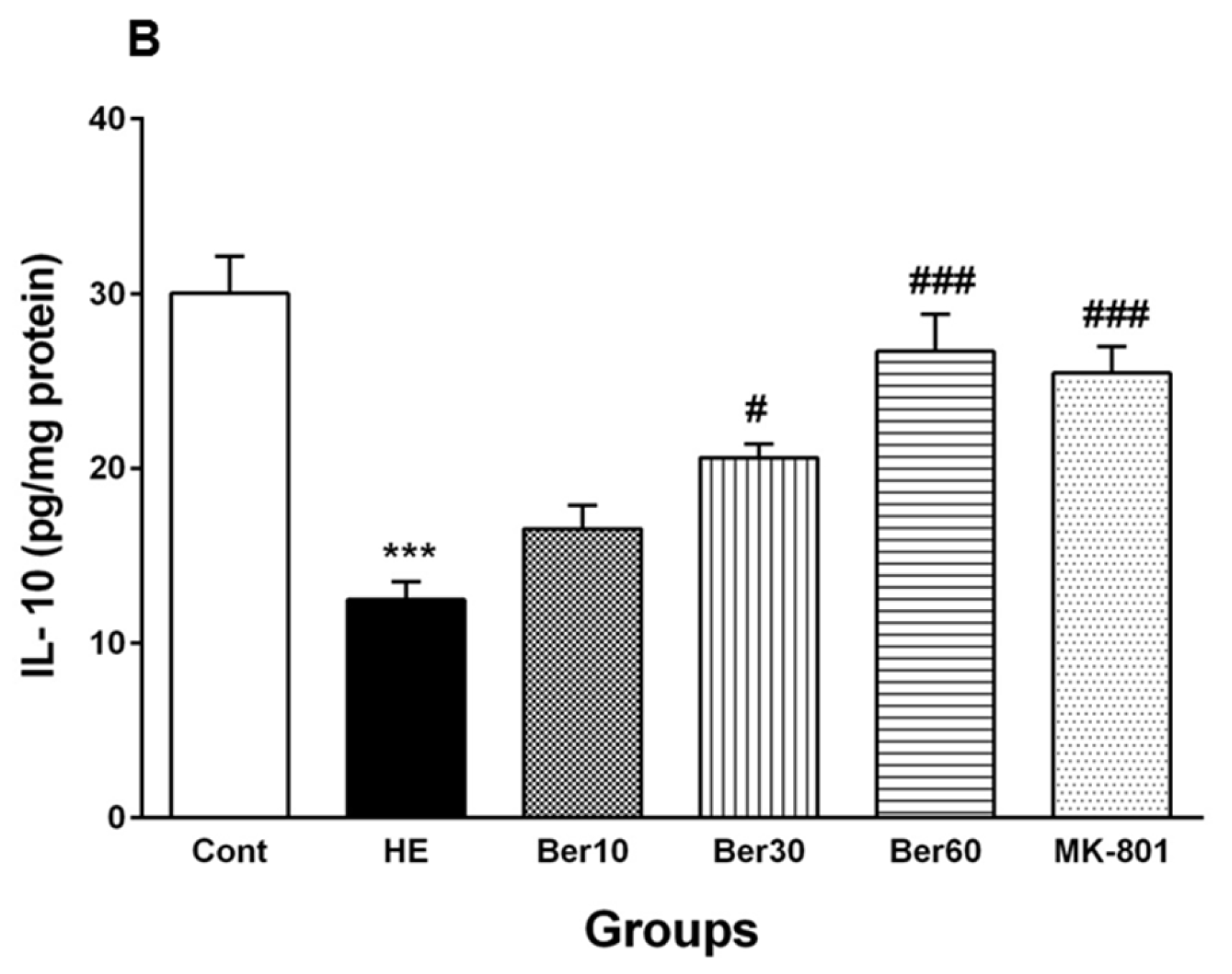
3.6. Oxidative Stress in Hippocampal Tissue
3.7. Histopathology of Hippocampus
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Abul, H.; Mathew, T.C.; Dashti, H.M.; Al-Bader, A. Level of superoxide dismutase, glutathione peroxidase and uric acid in thioacetamide-induced cirrhotic rats. Anat. Histol. Embryol. 2002, 31, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Chilakapati, J.; Korrapati, M.C.; Hill, R.A.; Warbritton, A.; Latendresse, J.R.; Mehendale, H.M. Toxicokinetics and toxicity of thioacetamide sulfoxide: A metabolite of thioacetamide. Toxicology 2007, 230, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Palacios, R.S.; Roderfeld, M.; Hemmann, S.; Rath, T.; Atanasova, S.; Tschuschner, A. Activation of hepatic stellate cells is associated with cytokine expression in thioacetamide-induced hepatic fibrosis in mice. Lab. Investig. 2008, 88, 192–203. [Google Scholar] [CrossRef]
- McMillin, M.; Grant, S.; Frampton, G.; Petrescu, A.D.; Williams, E.; Jefferson, B.; Thomas, A.; Brahmaroutu, A.; DeMorrow, S. Elevated circulating TGFbeta1 during acute liver failure activates TGFbetaR2 on cortical neurons and exacerbates neuroinflammation and hepatic encephalopathy in mice. J. Neuroinflamm. 2019, 16, 69. [Google Scholar] [CrossRef]
- McMillin, M.; Frampton, G.; Grant, S.; Khan, S.; Diocares, J.; Petrescu, A.; Wyatt, A.; Kain, J.; Jefferson, B.; DeMorrow, S. Bile Acid-Mediated Sphingosine-1-Phosphate Receptor 2 Signaling Promotes Neuroinflammation during Hepatic Encephalopathy in Mice. Front Cell Neurosci. 2017, 11, 191. [Google Scholar] [CrossRef]
- McMillin, M.; Frampton, G.; Tobin, R.; Dusio, G.; Smith, J.; Shin, H.; Newell-Rogers, K.; Grant, S.; DeMorrow, S. TGR5 signaling reduces neuroinflammation during hepatic encephalopathy. J. Neurochem. 2015, 135, 565–576. [Google Scholar] [CrossRef]
- Zhang, L.J.; Zhong, J.; Lu, G.M. Multimodality MR imaging findings of low-grade brain edema in hepatic encephalopathy. AJNR Am. J. Neuroradiol. 2013, 34, 707–715. [Google Scholar] [CrossRef]
- Avraham, Y.; Israeli, E.; Gabbay, E.; Okun, A.; Zolotarev, O.; Silberman, I.; Ganzburg, V.; Dagon, Y.; Magen, I.; Vorobia, L.; et al. Endocannabinoids affect neurological and cognitive function in thioacetamide-induced hepatic encephalopathy in mice. Neurobiol. Dis. 2006, 21, 237–245. [Google Scholar] [CrossRef]
- Butterworth, R.F. Pathogenesis of hepatic encephalopathy and brain edema in acute liver failure. J. Clin. Exp. Hepatol. 2015, 5 (Suppl. S1), S96–S103. [Google Scholar] [CrossRef]
- Chieli, E.; Malvaldi, G. Role of the microsomal FAD-containing monooxygenase in the liver toxicity of thioacetamide S-oxide. Toxicology 1984, 31, 41–52. [Google Scholar] [CrossRef]
- Porter, W.R.; Neal, R.A. Metabolism of thioacetamide and thioacetamide S-oxide by rat liver microsomes. Drug Metab. Dispos. 1978, 6, 379–388. [Google Scholar] [PubMed]
- Bruck, R.; Aeed, H.; Shirin, H.; Matas, Z.; Zaidel, L.; Avni, Y.; Halpern, Z. The hydroxyl radical scavengers dimethylsulfoxide and dimethylthiourea protect rats against thioacetamide-induced fulminant hepatic failure. J. Hepatol. 1999, 31, 27–38. [Google Scholar] [CrossRef] [PubMed]
- . Butterworth, R.F. Glutamate transporters in hyperammonemia. Neurochem. Int. 2002, 41, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.V.B.; Murthy, C.R.; Reddanna, P. Fulminant hepatic failure induced oxidative stress in nonsynaptic mitochondria of cerebral cortex in rats. Neurosci. Lett. 2004, 368, 15–20. [Google Scholar] [CrossRef]
- Bruck, R.; Aeed, H.; Avni, Y.; Shirin, H.; Matas, Z.; Shahmurov, M.; Avinoach, I.; Zozulya, G.; Weizman, N.; Hochman, A. Melatonin inhibits nuclear factor kappa B activation and oxidative stress and protects against thioacetamide induced liver damage in rats. J. Hepatol. 2004, 40, 86–93. [Google Scholar] [CrossRef]
- McMillin, M.; Grant, S.; Frampton, G.; Andry, S.; Brown, A.; DeMorrow, S. Fractalkine suppression during hepatic encephalopathy promotes neuroinflammation in mice. J. Neuroinflamm. 2016, 13, 198. [Google Scholar] [CrossRef]
- Chastre, A.; Belanger, M.; Nguyen, B.N.; Butterworth, R.F. Lipopolysaccharide precipitates hepatic encephalopathy and increases blood-brain barrier permeability in mice with acute liver failure. Liver Int. 2014, 34, 353–361. [Google Scholar] [CrossRef]
- Zarros, A.; Theocharis, S.; Skandali, N.; Tsakiris, S. Effects of fulminant hepatic encephalopathy on the adult rat brain antioxidant status and the activities of acetylcholinesterase, (Na+,K+)- and Mg2+)-ATPase: Comparison of the enzymes’ response to in vitro treatment with ammonia. Metab. Brain Dis. 2008, 23, 255–264. [Google Scholar] [CrossRef]
- Vaquero, J.; Polson, J.; Chung, C.; Helenowski, I.; Schiodt, F.V.; Reisch, J.; Lee, W.M.; Blei, A.T. Infection and the progression of hepatic encephalopathy in acute liver failure. Gastroenterology 2003, 125, 755–764. [Google Scholar] [CrossRef]
- Ding, S.; Liu, L.; Jing, H.; Xie, J.; Wang, X.; Mao, J.; Chen, B.; Zhuge, Q. Dopamine from cirrhotic liver contributes to the impaired learning and memory ability of hippocampus in minimal hepatic encephalopathy. Hepatol. Int. 2013, 7, 923–936. [Google Scholar] [CrossRef]
- Celik, T.; Uzbay, T.; Cinar, K.; Bozkaya, H.; Uzunalimoglu, O.; Yurdaydin, C. Combination treatment of hepatic encephalopathy due to thioacetamide-induced fulminant hepatic failure in the rat with benzodiazepine and opioid receptor antagonists. J. Hepatol. 1999, 31, 880–886. [Google Scholar] [CrossRef]
- Ye, M.; Fu, S.; Pi, R.; He, F. Neuropharmacological and pharmacokinetic properties of berberine: A review of recent research. J. Pharm. Pharmacol. 2009, 61, 831–837. [Google Scholar] [CrossRef]
- Alemardan, A.; Asadi, W.; Rezaei, M.; Tabrizi, L.; Mohammadi, S. Cultivation of Iranian seedless barberry (Berberis integerrima ‘Bidaneh’): A medicinal shrub. Ind. Crops Prod. 2013, 50, 276–287. [Google Scholar] [CrossRef]
- Neag, M.A.; Mocan, A.; Echeverria, J.; Pop, R.M.; Bocsan, C.I.; Crisan, G.; Buzoianu, A.D. Berberine: Botanical Occurrence, Traditional Uses, Extraction Methods, and Relevance in Cardiovascular, Metabolic, Hepatic, and Renal Disorders. Front. Pharmacol. 2018, 9, 557. [Google Scholar] [CrossRef]
- Tang, J.; Feng, Y.; Tsao, S.; Wang, N.; Curtain, R.; Wang, Y. Berberine and Coptidis rhizoma as novel antineoplastic agents: A review of traditional use and biomedical investigations. J. Ethnopharmacol. 2009, 126, 5–17. [Google Scholar] [CrossRef]
- Javad-Mousavi, S.A.; Hemmati, A.A.; Mehrzadi, S.; Hosseinzadeh, A.; Houshmand, G.; Nooshabadi, M.R.R.; Mehrabani, M.; Goudarzi, M. Protective effect of Berberis vulgaris fruit extract against Paraquat-induced pulmonary fibrosis in rats. Biomed. Pharmacother. 2016, 81, 329–336. [Google Scholar] [CrossRef]
- Rad, S.Z.K.; Rameshrad, M.; Hosseinzadeh, H. Toxicology effects of Berberis vulgaris (barberry) and its active constituent, berberine: A review. Iran. J. Basic Med. Sci. 2017, 20, 516–529. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Tawari, S.; Mundhada, D.; Nadeem, S. Protective effect of berberine, an isoquinoline alkaloid ameliorates ethanol-induced oxidative stress and memory dysfunction in rats. Pharmacol. Biochem. Behav. 2015, 136, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Y.; Zhu, Z.; Liu, H.; Guo, H.; Xiong, C.; Xie, K.; Zhang, X.; Su, S. Protective effect of berberine on doxorubicin-induced acute hepatorenal toxicity in rats. Mol. Med. Rep. 2016, 13, 3953–3960. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Sheng, M.; Weng, Y.; Xu, R.; Lu, N.; Du, H.; Yu, W. Berberine protects against ischemia/reperfusion injury after orthotopic liver transplantation via activating Sirt1/FoxO3α induced autophagy. Biochem. Biophys. Res. Commun. 2017, 483, 885–891. [Google Scholar] [CrossRef]
- Feng, Y.; Siu, K.-Y.; Ye, X.; Wang, N.; Yuen, M.-F.; Leung, C.-H.; Tong, Y.; Kobayashi, S. Hepatoprotective effects of berberine on carbon tetrachloride-induced acute hepatotoxicity in rats. Chin. Med. 2010, 5, 33. [Google Scholar] [CrossRef]
- McClure, L.A.; Loop, M.S.; Crosson, W.; Kleindorfer, D.; Kissela, B.; Al-Hamdan, M. Fine Particulate Matter (PM2.5) and the Risk of Stroke in the REGARDS Cohort. J. Stroke Cerebrovasc. Dis. 2017, 26, 1739–1744. [Google Scholar] [CrossRef]
- Adil, M.; Kandhare, A.D.; Dalvi, G.; Ghosh, P.; Venkata, S.; Raygude, K.S.; Bodhankar, S.L. Ameliorative effect of berberine against gentamicin-induced nephrotoxicity in rats via attenuation of oxidative stress, inflammation, apoptosis and mitochondrial dysfunction. Ren. Fail. 2016, 38, 996–1006. [Google Scholar] [CrossRef]
- Grant, S.; McMillin, M.; Frampton, G.; Petrescu, A.D.; Williams, E.; Jaeger, V.; Kain, J.; DeMorrow, S. Direct Comparison of the Thioacetamide and Azoxymethane Models of Type A Hepatic Encephalopathy in Mice. Gene Expr. 2018, 18, 171–185. [Google Scholar] [CrossRef]
- Chu, C.J.; Hsiao, C.C.; Wang, T.F.; Chan, C.Y.; Lee, F.Y.; Chang, F.Y.; Chen, Y.C.; Huang, H.C.; Wang, S.S.; Lee, S.D. Prostacyclin inhibition by indomethacin aggravates hepatic damage and encephalopathy in rats with thioacetamide-induced fulminant hepatic failure. World J. Gastroenterol. 2005, 11, 232–236. [Google Scholar] [CrossRef]
- Zhang, S.; Huo, X.; Zhang, Y.; Huang, Y.; Zheng, X.; Xu, X. Ambient fine particulate matter inhibits innate airway antimicrobial activity in preschool children in e-waste areas. Environ. Int. 2019, 123, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Karimi, S.A.; Salehi, I.; Shykhi, T.; Zare, S.; Komaki, A. Effects of exposure to extremely low-frequency electromagnetic fields on spatial and passive avoidance learning and memory, anxiety-like behavior and oxidative stress in male rats. Behav. Brain Res. 2019, 359, 630–638. [Google Scholar] [CrossRef]
- Sekaran, H.; Gan, C.-Y.; Latiff, A.A.; Harvey, T.M.; Nazri, L.M.; Hanapi, N.A.; Azizi, J.; Yusof, S.R. Changes in blood-brain barrier permeability and ultrastructure, and protein expression in a rat model of cerebral hypoperfusion. Brain Res. Bull. 2019, 152, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Saraswat, D.; Nehra, S.; Chaudhary, K.; CVS, S.P. Novel vascular endothelial growth factor blocker improves cellular viability and reduces hypobaric hypoxia-induced vascular leakage and oedema in rat brain. Clin. Exp. Pharmacol. Physiol. 2015, 42, 475–484. [Google Scholar] [CrossRef] [PubMed]
- He, X.L.; Wang, Y.H.; Bi, M.G.; Du, G.H. Chrysin improves cognitive deficits and brain damage induced by chronic cerebral hypoperfusion in rats. Eur. J. Pharmacol. 2012, 680, 41–48. [Google Scholar] [CrossRef]
- Tirumanyam, M.; Nadella, R.; Kondammagari, S.; Borelli, D.P.R.; Nannepaga, J.S. Bacopa phospholipid complex retrieves aluminum maltolate complex-induced oxidative stress and apoptotic alterations in the brain regions of albino rat. Environ. Sci. Pollut. Res. Int. 2019, 26, 12071–12079. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wei, K.; Li, X.; Hu, R.; Qiu, J.; Zhang, Y.; Yao, W.; Zhang, C.; Zhu, C. Upregulation of Cdh1 signaling in the hippocampus attenuates brain damage after transient global cerebral ischemia in rats. Neurochem. Int. 2018, 112, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Hossen, M.J.; Yang, W.S.; Kim, D.; Aravinthan, A.; Kim, J.-H.; Cho, J.Y. Thymoquinone: An IRAK1 inhibitor with in vivo and in vitro anti-inflammatory activities. Sci. Rep. 2017, 7, 42995. [Google Scholar] [CrossRef] [PubMed]
- Hassani-Bafrani, H.; Najaran, H.; Razi, M.; Rashtbari, H. Berberine ameliorates experimental varicocele-induced damages at testis and sperm levels; evidences for oxidative stress and inflammation. Andrologia 2019, 51, e13179. [Google Scholar] [CrossRef]
- Wang, L.; Li, S.; Wang, H.Y.; Zeng, J.; Zhang, Z.Z.; Lv, D.Y.; Kuang, W.H. In a Rat Model of Acute Liver Failure, Icaritin Improved the Therapeutic Effect of Mesenchymal Stem Cells by Activation of the Hepatocyte Growth Factor/c-Met Pathway. Evid.-Based Complement. Altern. Med. 2019, 2019, 1–13. [Google Scholar] [CrossRef]
- Aguilar, M.; Miñarro, J.; Felipo, V. Chronic moderate hyperammonemia impairs active and passive avoidance behavior and conditional discrimination learning in rats. Exp. Neurol. 2000, 161, 704–713. [Google Scholar] [CrossRef]
- Erceg, S.; Monfort, P.; Hernández-Viadel, M.; Rodrigo, R.; Montoliu, C.; Felipo, V. Oral administration of sildenafil restores learning ability in rats with hyperammonemia and with portacaval shunts. Hepatology 2005, 41, 299–306. [Google Scholar] [CrossRef]
- Mohammadian, F.; Firouzjaei, M.A.; Haghani, M.; Shabani, M.; Moosavi, S.M.S.; Mohammadi, F. Inhibition of inflammation is not enough for recovery of cognitive impairment in hepatic encephalopathy: Effects of minocycline and ibuprofen. Brain Res. Bull. 2019, 149, 96–105. [Google Scholar] [CrossRef]
- Monfort, P.; Erceg, S.; Piedrafita, B.; Llansola, M.; Felipo, V. Chronic liver failure in rats impairs glutamatergic synaptic transmission and long-term potentiation in hippocampus and learning ability. Eur. J. Neurosci. 2007, 25, 2103–2111. [Google Scholar] [CrossRef]
- Hernández-Rabaza, V.; Cabrera-Pastor, A.; Taoro-González, L.; Malaguarnera, M.; Agustí, A.; Llansola, M.; Felipo, V. Hyperammonemia induces glial activation, neuroinflammation and alters neurotransmitter receptors in hippocampus, impairing spatial learning: Reversal by sulforaphane. J. Neuroinflamm. 2016, 13, 41. [Google Scholar] [CrossRef]
- Cauli, O.; Rodrigo, R.; Boix, J.; Piedrafita, B.; Agusti, A.; Felipo, V. Acute liver failure-induced death of rats is delayed or prevented by blocking NMDA receptors in brain. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G503–G511. [Google Scholar] [CrossRef]
- Coltart, I.; Tranah, T.H.; Shawcross, D.L. Inflammation and hepatic encephalopathy. Arch. Biochem. Biophys. 2013, 536, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Montoliu, C.; Llansola, M.; Felipo, V. Neuroinflammation and neurological alterations in chronic liver diseases. Neuroimmunol. Neuroinflamm.. 2015, 2, 138–144. [Google Scholar]
- Hajipour, S.; Farbood, Y.; Dianat, M.; Rashno, M.; Khorsandi, L.S.; Sarkaki, A. Thymoquinone improves cognitive and hippocampal long-term potentiation deficits due to hepatic encephalopathy in rats. Iran. J. Basic Med. Sci. 2021, 24, 881. [Google Scholar]
- Wang, J.; Zhang, Y. Neuroprotective effect of berberine agonist against impairment of learning and memory skills in severe traumatic brain injury via Sirt1/p38 MAPK expression. Mol. Med. Rep. 2018, 17, 6881–6886. [Google Scholar] [CrossRef]
- Pires, E.N.S.; Frozza, R.L.; Hoppe, J.B.; de Melo Menezes, B.; Salbego, C.G. Berberine was neuroprotective against an in vitro model of brain ischemia: Survival and apoptosis pathways involved. Brain Res. 2014, 1557, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Kalalian-Moghaddam, H.; Baluchnejadmojarad, T.; Roghani, M.; Goshadrou, F.; Ronaghi, A. Hippocampal synaptic plasticity restoration and anti-apoptotic effect underlie berberine improvement of learning and memory in streptozotocin-diabetic rats. Eur. J. Pharmacol. 2013, 698, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Hussien, H.M.; Abd-Elmegied, A.; Ghareeb, D.A.; Hafez, H.S.; Ahmed, H.E.; Abd El-moneam, N. Neuroprotective effect of berberine against environmental heavy metals-induced neurotoxicity and Alzheimer’s-like disease in rats. Food Chem. Toxicol. 2018, 111, 432–444. [Google Scholar] [CrossRef]
- Huang, S.-X.; Qiu, G.; Cheng, F.-R.; Pei, Z.; Yang, Z.; Deng, X.-H.; Zhu, J.-H.; Chen, L.; Chen, C.-C.; Lin, W.-F. Berberine protects secondary injury in mice with traumatic brain injury through anti-oxidative and anti-inflammatory modulation. Neurochem. Res. 2018, 43, 1814–1825. [Google Scholar] [CrossRef]
- Méndez, M.; Méndez-López, M.; López, L.; Aller, M.A.; Arias, J.; Arias, J.L. Working memory impairment and reduced hippocampal and prefrontal cortex c-Fos expression in a rat model of cirrhosis. Physiol. Behav. 2008, 95, 302–307. [Google Scholar] [CrossRef]
- Méndez, M.; Méndez-López, M.; López, L.; Aller, M.Á.; Arias, J.; Arias, J.L. Basal and learning task-related brain oxidative metabolism in cirrhotic rats. Brain Res. Bull. 2009, 78, 195–201. [Google Scholar] [CrossRef]
- Singh, S.; Trigun, S. Low grade cirrhosis induces cognitive impairment and motor dysfunction in rats: Could be a model for minimal hepatic encephalopathy. Neurosci. Lett. 2014, 559, 136–140. [Google Scholar] [CrossRef]
- Kocerha, J.; Faghihi, M.A.; Lopez-Toledano, M.A.; Huang, J.; Ramsey, A.J.; Caron, M.G.; Sales, N.; Willoughby, D.; Elmen, J.; Hansen, H.F. MicroRNA-219 modulates NMDA receptor-mediated neurobehavioral dysfunction. Proc. Natl. Acad. Sci. USA 2009, 106, 3507–3512. [Google Scholar] [CrossRef]
- Lau, C.G.; Zukin, R.S. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat. Rev. Neurosci. 2007, 8, 413–426. [Google Scholar] [CrossRef]
- Riedel, G.; Platt, B.; Micheau, J. Glutamate receptor function in learning and memory. Behav. Brain Res. 2003, 140, 1–47. [Google Scholar] [CrossRef]
- Hernández-Frausto, M.; López-Rubalcava, C.; Galván, E.J. Progressive Alterations in Synaptic Transmission and Plasticity of Area CA1 Precede the Cognitive Impairment Associated with Neonatal Administration of MK-801. Neuroscience 2019, 404, 205–217. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Song, T.; Hu, C.; Tang, Y.; Zhang, X.; Zhao, J. Contribution of K+–Cl− cotransporter 2 in MK-801-induced impairment of long term potentiation. Behav. Brain Res. 2009, 201, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, R.; Cauli, O.; Boix, J.; ElMlili, N.; Agusti, A.; Felipo, V. Role of NMDA receptors in acute liver failure and ammonia toxicity: Therapeutical implications. Neurochem. Int. 2009, 55, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Sadraie, S.; Kiasalari, Z.; Razavian, M.; Azimi, S.; Sedighnejad, L.; Afshin-Majd, S.; Baluchnejadmojarad, T.; Roghani, M. Berberine ameliorates lipopolysaccharide-induced learning and memory deficit in the rat: Insights into underlying molecular mechanisms. Metab. Brain Dis. 2019, 34, 245–255. [Google Scholar] [CrossRef]
- Cui, H.-S.; Matsumoto, K.; Murakami, Y.; Hori, H.; Zhao, Q.; Obi, R. Berberine exerts neuroprotective actions against in vitro ischemia-induced neuronal cell damage in organotypic hippocampal slice cultures: Involvement of B-cell lymphoma 2 phosphorylation suppression. Biol. Pharm. Bull. 2009, 32, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.-Y.; Hwang, I.K.; Lim, B.O.; Kang, T.-C.; Kim, D.-W.; Kim, S.M.; Lee, H.Y.; Dai Kim, J.; Won, M.H. Berberry extract reduces neuronal damage and N-Methyl-D-aspartate receptor 1 immunoreactivity in the gerbil hippocampus after transient forebrain ischemia. Biol. Pharm. Bull. 2006, 29, 623–628. [Google Scholar] [CrossRef]
- Imanshahidi, M.; Hosseinzadeh, H. Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, berberine. Phytother. Res. 2008, 22, 999–1012. [Google Scholar] [CrossRef]
- Masago, K.; Kihara, Y.; Yanagida, K.; Hamano, F.; Nakagawa, S.; Niwa, M.; Shimizu, T. Lysophosphatidic acid receptor, LPA6, regulates endothelial blood-brain barrier function: Implication for hepatic encephalopathy. Biochem. Biophys. Res. Commun. 2018, 501, 1048–1054. [Google Scholar] [CrossRef]
- Wang, L.-Q.; Zhou, H.-J.; Pan, C.-F.; Zhu, S.-M.; Xu, L.-M. Expression of IL-1β, IL-6 and TNF-α in rats with thioacetamide-induced acute liver failure and encephalopathy: Correlation with brain edema. Asian Biomed. 2011, 5, 205–215. [Google Scholar] [CrossRef]
- Cauli, O.; López–Larrubia, P.; Rodrigo, R.; Agusti, A.; Boix, J.; Nieto–Charques, L.; Cerdán, S.; Felipo, V. Brain region-selective mechanisms contribute to the progression of cerebral alterations in acute liver failure in rats. Gastroenterology 2011, 140, 638–645. [Google Scholar] [CrossRef]
- Maleki, S.N.; Aboutaleb, N.; Souri, F. Berberine confers neuroprotection in coping with focal cerebral ischemia by targeting inflammatory cytokines. J. Chem. Neuroanat. 2018, 87, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, X.; Wang, C.; Li, Y.; Dong, L.; Cui, L.; Wang, L.; Liu, Z.; Qiao, H.; Zhu, C. Neuroprotection of early and short-time applying berberine in the acute phase of cerebral ischemia: Up-regulated pAkt, pGSK and pCREB, down-regulated NF-κB expression, ameliorated BBB permeability. Brain Res. 2012, 1459, 61–70. [Google Scholar] [CrossRef]
- Chen, C.-C.; Hung, T.-H.; Lee, C.Y.; Wang, L.-F.; Wu, C.-H.; Ke, C.-H.; Chen, S.-F. Berberine protects against neuronal damage via suppression of glia-mediated inflammation in traumatic brain injury. PLoS ONE 2014, 9, e115694. [Google Scholar] [CrossRef] [PubMed]
- Gressner, O.A.; Weiskirchen, R.; Gressner, A.M. Biomarkers of liver fibrosis: Clinical translation of molecular pathogenesis or based on liver-dependent malfunction tests. Clin. Chim. Acta 2007, 381, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Hasanein, P.; Ghafari-Vahed, M.; Khodadadi, I. Effects of isoquinoline alkaloid berberine on lipid peroxidation, antioxidant defense system, and liver damage induced by lead acetate in rats. Redox Rep. 2017, 22, 42–50. [Google Scholar] [CrossRef]
- Domitrović, R.; Jakovac, H.; Blagojević, G. Hepatoprotective activity of berberine is mediated by inhibition of TNF-α, COX-2, and iNOS expression in CCl4-intoxicated mice. Toxicology 2011, 280, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Prakash, A.; Pahwa, D. Galantamine potentiates the protective effect of rofecoxib and caffeic acid against intrahippocampal Kainic acid-induced cognitive dysfunction in rat. Brain Res. Bull. 2011, 85, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Lou, T.; Zhang, Z.; Xi, Z.; Liu, K.; Li, L.; Liu, B.; Huang, F. Berberine inhibits inflammatory response and ameliorates insulin resistance in hepatocytes. Inflammation 2011, 34, 659–667. [Google Scholar] [CrossRef]
- Mohammadzadeh, N.; Mehri, S. Hosseinzadeh H. Berberis vulgaris and its constituent berberine as antidotes and protective agents against natural or chemical toxicities. Iran. J. Basic Med. Sci. 2017, 20, 538–551. [Google Scholar] [PubMed]
- Saxena, N.; Bhatia, M.; Joshi, Y.K.; Garg, P.K.; Dwivedi, S.N.; Tandon, R.K. Electrophysiological and neuropsychological tests for the diagnosis of subclinical hepatic encephalopathy and prediction of overt encephalopathy. Liver 2002, 22, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Tahamtan, M.; Aghaei, I.; Pooladvand, V.; Sheibani, V.; Khaksari, M.; Shabani, M. Characterization of the CA1 pyramidal neurons in rat model of hepatic cirrhosis: Insights into their electrophysiological properties. Metab. Brain Dis. 2017, 32, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, H.N.; El Awdan, S.A.; Hegazy, G.A. Protective role of antioxidants on thioacetamide-induced acute hepatic encephalopathy: Biochemical and ultrastructural study. Tissue Cell 2013, 45, 350–362. [Google Scholar] [CrossRef]
- Saleh, A.A.S.; Ahmed, H.H.; Saeed, R.; Mohamed, Y.S. Towards Understanding the Mechanisms of Action of L-carnitine and Alpha Lipoic Acid in Counteracting Hepatic Encephalopathy. Der. Pharma. Chem. 2017, 9, 48–61. [Google Scholar]
- Kosenko, E.; Kaminsky, M.; Kaminsky, A.; Valencia, M.; Lee, L.; Hermenegildo, C.; Felipo, V. Superoxide production and antioxidant enzymes in ammonia intoxication in rats. Free. Radic. Res. 1997, 27, 637–644. [Google Scholar] [CrossRef]
- Fadillioglu, E.; Gursul, C.; Iraz, M. Effects of caffeic acid phenethyl ester on thioacetamide-induced hepatic encephalopathy in rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 1440–1445. [Google Scholar] [CrossRef]
- Irimia, R.; Ciobica, A.; Stanciu, C.; Trifan, A. The relevance of oxidative stress in cirrhotic patients with different forms of hepatic encephalopathy. Arch. Biol. Sci. 2013, 65, 1245–1252. [Google Scholar] [CrossRef]
- Alzoubi, K.H.; Khabour, O.F.; Salah, H.A.; Hasan, Z. Vitamin E prevents high-fat high-carbohydrates diet-induced memory impairment: The role of oxidative stress. Physiol. Behav. 2013, 119, 72–78. [Google Scholar] [CrossRef]
- Serrano, F.; Klann, E. Reactive oxygen species and synaptic plasticity in the aging hippocampus. Ageing Res. Rev. 2004, 3, 431–443. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Hozayen, W.G.; Ramadan, S.M. Berberine ameliorates methotrexate-induced liver injury by activating Nrf2/HO-1 pathway and PPARγ, and suppressing oxidative stress and apoptosis in rats. Biomed. Pharmacother. 2017, 94, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Othman, M.S.; Safwat, G.; Aboulkhair, M.; Moneim, A.E.A. The potential effect of berberine in mercury-induced hepatorenal toxicity in albino rats. Food Chem. Toxicol. 2014, 69, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Laamech, J.; El-Hilaly, J.; Fetoui, H.; Chtourou, Y.; Gouitaa, H.; Tahraoui, A.; Lyoussi, B. Berberis vulgaris L. effects on oxidative stress and liver injury in lead-intoxicated mice. J. Complement. Integr. Med. 2017, 14. [Google Scholar] [CrossRef]
- Lao-ong, T.; Chatuphonprasert, W.; Nemoto, N.; Jarukamjorn, K. Alteration of hepatic glutathione peroxidase and superoxide dismutase expression in streptozotocin-induced diabetic mice by berberine. Pharm. Biol. 2012, 50, 1007–1012. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, J.-Q.; He, B.-C.; Zhou, Q.-X.; Yu, H.-R.; Tang, Y.; Liu, B.-Z. Berberine and total base from rhizoma coptis chinensis attenuate brain injury in an aluminum-induced rat model of neurodegenerative disease. Saudi. Med. J. 2009, 30, 760–766. [Google Scholar]
- Aski, M.L.; Rezvani, M.E.; Khaksari, M.; Hafizi, Z.; Pirmoradi, Z.; Niknazar, S.; Mehrjerdi, F.Z. Neuroprotective effect of berberine chloride on cognitive impairment and hippocampal damage in experimental model of vascular dementia. Iran. J. Basic Med. Sci. 2018, 21, 53–58. [Google Scholar] [CrossRef]
- Ho, C.E.; Goh, Y.L.; Zhang, C. From prejudice to evidence: The case of rhizoma coptidis in singapore. Evid. Based Complement Altern. Med. 2014, 2014, 871720. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Zamani Taghizadeh Rabe, S.; Balali-Mood, M.; Karimi, G.; Memar, B.; Rahnama, M.; Tabasi, N.; Khazaee, M.; Riahi-Zanjani, B. Immunotoxicity induced in mice by subacute exposure to berberine. J. Immunotoxicol. 2016, 13, 255–262. [Google Scholar] [CrossRef] [PubMed]

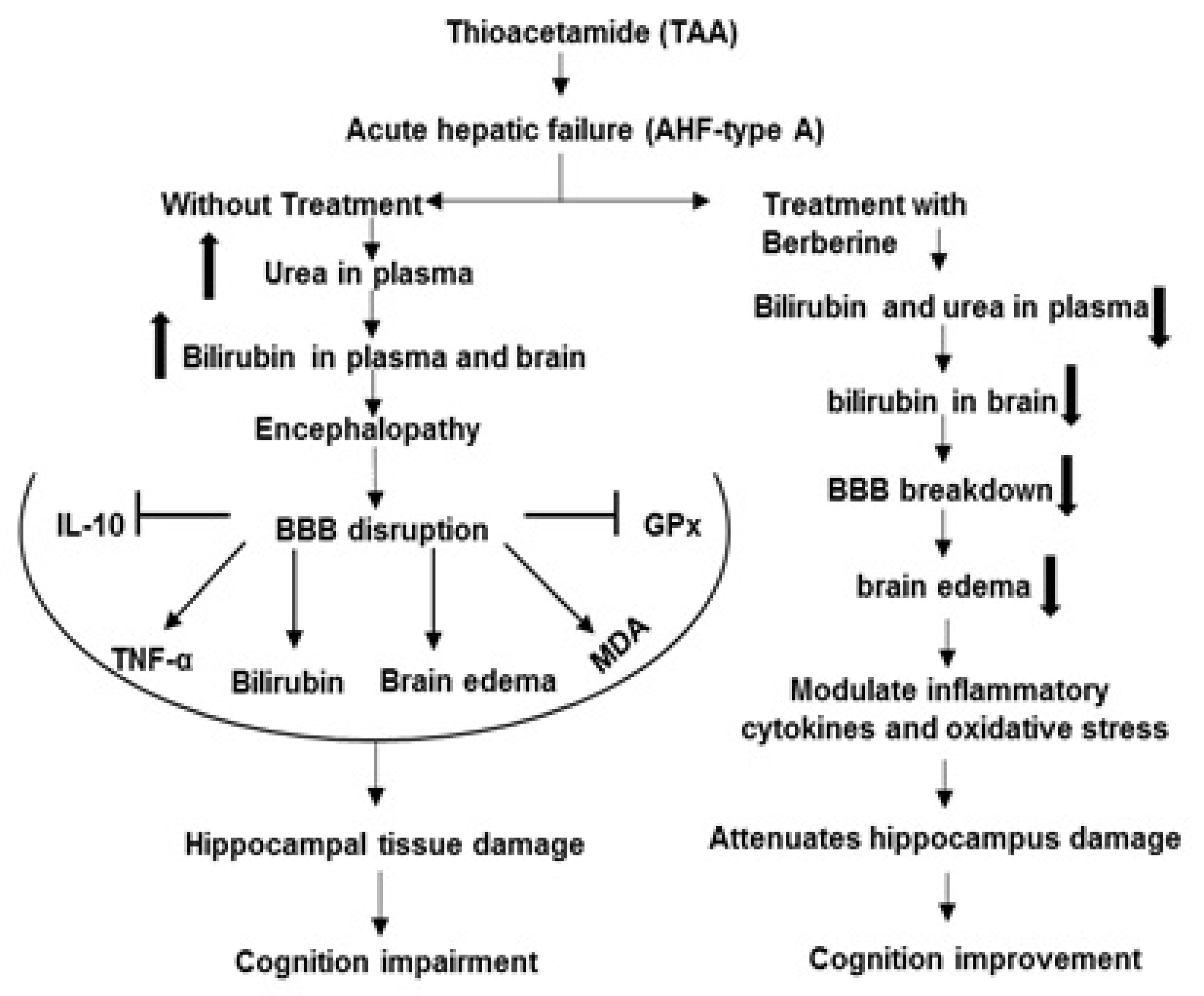
 , Stimulation
, Stimulation  , Increase
, Increase  , Decrease
, Decrease  .
.
 , Stimulation
, Stimulation  , Increase
, Increase  , Decrease
, Decrease  .
.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajipour, S.; Farbood, Y.; Dianat, M.; Nesari, A.; Sarkaki, A. Effect of Berberine against Cognitive Deficits in Rat Model of Thioacetamide-Induced Liver Cirrhosis and Hepatic Encephalopathy (Behavioral, Biochemical, Molecular and Histological Evaluations). Brain Sci. 2023, 13, 944. https://doi.org/10.3390/brainsci13060944
Hajipour S, Farbood Y, Dianat M, Nesari A, Sarkaki A. Effect of Berberine against Cognitive Deficits in Rat Model of Thioacetamide-Induced Liver Cirrhosis and Hepatic Encephalopathy (Behavioral, Biochemical, Molecular and Histological Evaluations). Brain Sciences. 2023; 13(6):944. https://doi.org/10.3390/brainsci13060944
Chicago/Turabian StyleHajipour, Somayeh, Yaghoob Farbood, Mahin Dianat, Ali Nesari, and Alireza Sarkaki. 2023. "Effect of Berberine against Cognitive Deficits in Rat Model of Thioacetamide-Induced Liver Cirrhosis and Hepatic Encephalopathy (Behavioral, Biochemical, Molecular and Histological Evaluations)" Brain Sciences 13, no. 6: 944. https://doi.org/10.3390/brainsci13060944
APA StyleHajipour, S., Farbood, Y., Dianat, M., Nesari, A., & Sarkaki, A. (2023). Effect of Berberine against Cognitive Deficits in Rat Model of Thioacetamide-Induced Liver Cirrhosis and Hepatic Encephalopathy (Behavioral, Biochemical, Molecular and Histological Evaluations). Brain Sciences, 13(6), 944. https://doi.org/10.3390/brainsci13060944




