Who Is Attending? The Role of Child Ethnicity and Maternal Demographics in Research Engagement and Early Identification of Autism
Abstract
1. Introduction
1.1. Socioeconomic Status
1.2. Australian Healthcare System
2. Materials and Methods
2.1. Study Design
2.2. Local Government Area Demographics
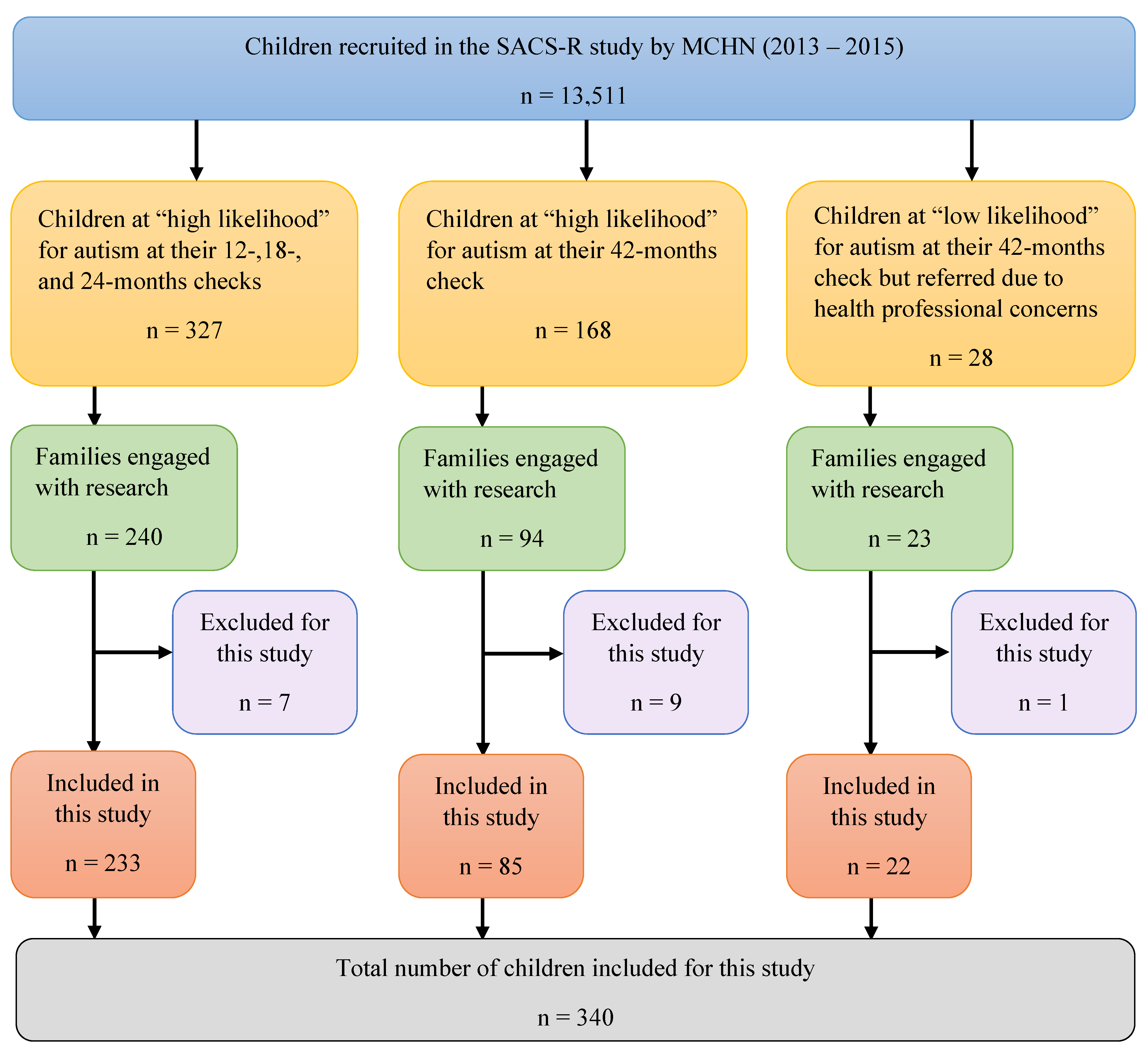
2.3. Study Recruitment
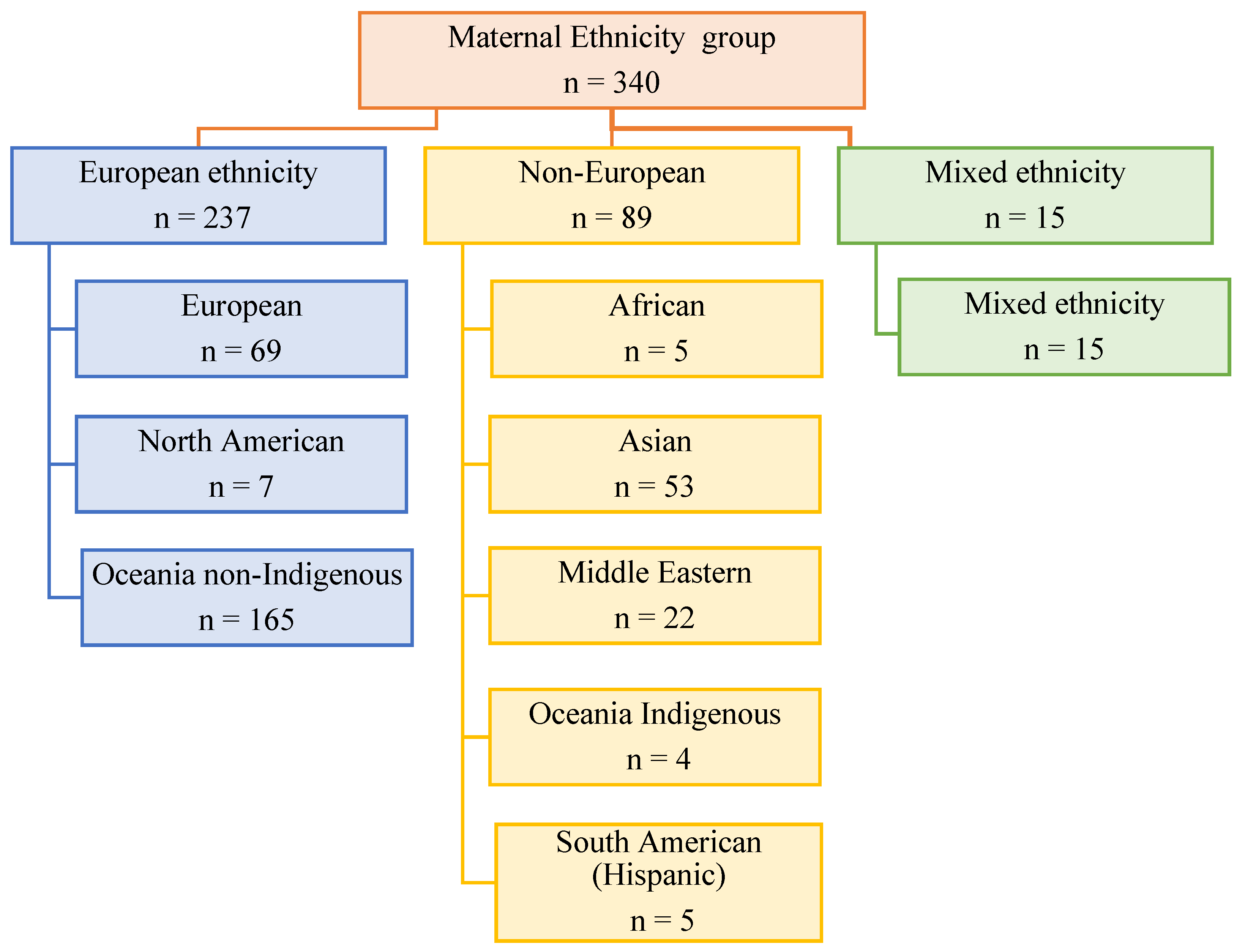
2.4. Maternal and Child Groups
2.5. Analysis
3. Results
3.1. Local Government Area Statistics
3.2. SACS-R+PR Recruitment Demographics
3.3. Maternal and Child Descriptive Statistics
3.4. Child Clinical Outcomes
4. Discussion
4.1. Prevalence
4.2. Maternal Employment and Language
4.3. Maternal Education and Annual Family Income
4.4. Ethnicity
4.5. Limitations and Future Research Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Psychiatric Association [APA]. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Centers for Disease Control and Prevention. Data & Statistics on Autism Spectrum Disorder. Available online: https://www.cdc.gov/ncbddd/autism/data.html (accessed on 10 August 2022).
- Autism and Developmental Disabilities Monitoring (Addm) Network. Community Report Autism 2023; Centers for Disease Control and Prevention (CDC): Altanta, GA, USA, 2023.
- Baron-Cohen, S.; Scott, J.; Allison, C.; Williams, J.; Bolton, P.; Matthews, E.; Brayne, C. Prevalence of autism-spectrum conditions: UK school-based population study. Br. J. Psychiatry 2009, 194, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Australian Bureau of Statistics [ABS]. Autism in Australia. Available online: https://www.abs.gov.au/AUSSTATS/abs@.nsf/Lookup/4430.0Main+Features102018 (accessed on 22 July 2020).
- Autism Spectrum Australia. Autism Prevalence Rate Up by an Estimated 40% to 1 in 70 People. Available online: https://www.autismspectrum.org.au/news/autism-prevalence-rate-up-by-an-estimated-40-to-1-in-70-people-11-07-2018 (accessed on 22 July 2020).
- Bent, C.; Barbaro, J.; Dissanayake, C. Change in autism diagnoses prior to and following the introduction of DSM-5. J. Autism Dev. Disord. 2017, 47, 163–171. [Google Scholar] [CrossRef]
- Barbaro, J.; Sadka, N.; Gilbert, M.; Beattie, E.; Li, X.; Ridgway, L.; Lawson, L.P.; Dissanayake, C. Diagnostic accuracy of the social attention and communication surveillance–Revised with preschool tool for early autism detection in very young children. JAMA Netw. Open 2022, 5, e2146415. [Google Scholar] [CrossRef] [PubMed]
- Baird, G.; Simonoff, E.; Pickles, A.; Changler, S.; Loucas, T.; Meldrum, D.; Charman, T. Prevalence of disorders of the autism spectrum in a population cohort of children in South Thames: The special needs and autism project (SNAP). Lancet 2006, 365, 210–215. [Google Scholar] [CrossRef]
- Boyle, C.A.; Boulet, S.; Schieve, L.A.; Cohen, R.A.; Blumberg, S.J.; Yeargin-Allsopp, M.; Visser, S.; Kogan, M.D. Trends in the prevalence of developmental disabilities in US children, 1997–2008. Pediatrics 2011, 127, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Durkin, M.S.; Maenner, M.J.; Meaney, F.J.; Levy, S.E.; DiGuiseppi, C.; Nicholas, J.S.; Kirby, R.S.; Pinto-Martin, J.A.; Schieve, L.A. Socioeconomic inequality in the prevalence of autism spectrum disorder: Evidence from a U.S. cross-sectional study. PLoS ONE 2010, 5, e11551. [Google Scholar] [CrossRef]
- Nassar, N.; Dixon, G.; Bourke, J.; Bower, C.; Glasson, E.; de Klerk, N.; Leonard, H. Autism spectrum disorders in young children: Effect of changes in diagnostic practices. Int. J. Epidemiol. 2009, 38, 1245–1254. [Google Scholar] [CrossRef]
- Nevison, C.; Blaxill, M.; Zahorodny, W. California autism prevalence trends from 1931 to 2014 and comparison to national ASD data from IDEA and ADDM. J. Autism Dev. Disord. 2018, 48, 4103–4117. [Google Scholar] [CrossRef]
- Maenner, M.; Durkin, M. Trends in the prevalence of autism on the basis of special education data. Pediatrics 2010, 126, e1018–e1025. [Google Scholar] [CrossRef]
- Lai, M.; Lombardo, M.; Baron-Cohen, S. Autism. Lancet 2014, 383, 896–910. [Google Scholar] [CrossRef]
- Baio, J.; Wiggins, L.; Christensen, D.; Maenner, M.; Daniels, J.; Warren, Z.; Kurzius-Spencer, M.; Zahorodny, W.; Robinson Rosenberg, C.; White, T.; et al. Prevalence of autism spectrum disorder among children aged 8 Years—Autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveill. Summ. 2018, 67, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Maenner, M.; Shaw, K.; Baio, J.; Washington, A.; Patrick, M.; DiRienzo, M.; Christensen, D.; Wiggins, L.; Pettygrove, S.; Andrews, J.; et al. Prevalence of autism spectrum disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 sites, United States, 2016. Surveill. Summ. 2020, 69, 1–12. [Google Scholar] [CrossRef]
- Abdullahi, I.; Leonard, H.; Cherian, S.; Mutch, R.; Glasson, E.; de Klerk, N.; Downs, J. The risk of neurodevelopmental disabilities in children of immigrant and refugee parents: Current knowledge and directions for future research. Rev. J. Autism Dev. Disord. 2018, 5, 29–42. [Google Scholar] [CrossRef]
- Abdullahi, I.; Wong, K.; Mutch, R.; Glasson, E.; de Klerk, N.; Cherian, S.; Downs, J.; Leonard, H. Risk of developmental disorders in children of immigrant mothers: A population-based data linkage evaluation. J. Pediatr. 2019, 204, 275–284. [Google Scholar] [CrossRef]
- Gao, X.; Zhao, Y.; Wang, N.; Yang, L. Migration modulates the prevalence of ASD and ADHD: A systematic review and meta-analysis. BMC Psychiatry 2022, 22, 395. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Geng, H.; Liu, W.; Zhang, G. Prenatal, perinatal, and postnatal factors associated with autism: A meta-analysis. Medicine 2017, 96, e6696. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Son, M.J.; Son, C.Y.; Radua, J.; Eisenhut, M.; Gressier, F.; Koyanagi, A.; Carvalho, A.F.; Stubbs, B.; Solmi, M. Environmental risk factors and biomarkers for autism spectrum disorder: An umbrella review of the evidence. Lancet Psychiatry 2019, 6, 590–600. [Google Scholar] [CrossRef]
- Daina, C. Migration and Autism Diagnosis. In Autism; Michael, F., Jane, Y., Eds.; IntechOpen: Rijeka, Croatia, 2017; Chapter 5. [Google Scholar]
- Fernell, E.; Bejerot, S.; Westerlund, J.; Miniscalco, C.; Simila, H.; Eyles, D.; Gillberg, C.; Humble, M.B. Autism spectrum disorder and low vitamin D at birth: A sibling control study. Mol. Autism 2015, 6, 3. [Google Scholar] [CrossRef]
- Keen, D.V.; Reid, F.D.; Arnone, D. Autism, ethnicity and maternal immigration. Br. J. Psychiatry 2010, 196, 274–281. [Google Scholar] [CrossRef]
- Magnusson, C.; Rai, D.; Goodman, A.; Lundberg, M.; Idring, S.; Svensson, A.; Koupil, I.; Serlachius, E.; Dalman, C. Migration and autism spectrum disorder: Population-based study. Br. J. Psychiatry 2012, 201, 109–115. [Google Scholar] [CrossRef]
- Williams, K.; Helmer, M.; Duncan, G.W.; Peat, J.K.; Mellis, C.M. Perinatal and maternal risk factors for autism spectrum disorders in New South Wales, Australia. Child Care Health Dev. 2008, 34, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Becerra, T.A.; von Ehrenstein, O.S.; Heck, J.E.; Olsen, J.; Arah, O.A.; Jeste, S.S.; Rodriguez, M.; Ritz, B. Autism spectrum disorders and race, ethnicity, and nativity: A population-based study. Pediatrics 2014, 134, e63–e71. [Google Scholar] [CrossRef]
- WHO. Social Determinants of Health. Available online: https://www.who.int/health-topics/social-determinants-of-health#tab=tab_1 (accessed on 12 October 2020).
- VicHealth. About Fair Foundations and Promoting Health Equity; Victorian Health Promotion Foundation: Melbourne, VIC, Australia, 2015; pp. 1–20. [Google Scholar]
- Bhasin, T.K.; Schendel, D. Sociodemographic Risk Factors for Autism in a US Metropolitan Area. J. Autism Dev. Disord. 2007, 37, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Delobel-Ayoub, M.; Ehlinger, V.; Klapouszczak, D.; Maffre, T.; Raynaud, J.; Delpierre, C.; Arnaud, C. Socioeconomic Disparities and Prevalence of Autism Spectrum Disorders and Intellectual Disability. PLoS ONE 2015, 5, e0141964. [Google Scholar] [CrossRef] [PubMed]
- Australian Bureau of Statistics [ABS]. 2071.0—Census of Population and Housing: Reflecting Australia—Stories from the Census. 2016. Available online: https://www.abs.gov.au/ausstats/abs@.nsf/Lookup/by%20Subject/2071.0~2016~Main%20Features~Socio-Economic%20Advantage%20and%20Disadvantage~123 (accessed on 30 March 2021).
- Ward, S.; Sullivan, K.; Gilmore, L. Practitioner Perceptions of the Assessment and Diagnosis of Autism in Australia. Aust. Psychol. 2016, 51, 272–279. [Google Scholar] [CrossRef]
- Bent, C.; Dissanayake, C.; Barbaro, J. Mapping the diagnosis of autism spectrum disorders in children aged under 7 years in Australia, 2010–2012. Med. J. Aust. 2015, 202, 317–320. [Google Scholar] [CrossRef]
- Van’t Hof, M.; Tisseur, C.; van Berckelear-Onnes, I.; van Nieuwenhuyzen, A.; Daniels, A.M.; Deen, M.; Hoek, H.W.; Ester, W.A. Age at autism spectrum disorder diagnosis: A systematic review and meta-analysis from 2012 to 2019. Autism 2021, 25, 862–873. [Google Scholar] [CrossRef]
- Clark, M.L.E.; Vinen, Z.; Barbaro, J.; Dissanayake, C. School Age Outcomes of Children Diagnosed Early and Later with Autism Spectrum Disorder. J. Autism Dev. Disord. 2018, 48, 92–102. [Google Scholar] [CrossRef]
- Department of Health and Human Services. Maternal & Child Health Services Annual Report Statewide 2017–2018; Department of Health and Human Services: Melbourne, VIC, Australia, 2019.
- Australian Bureau of Statistics [ABS]. Australia 2016 Census Community Profiles. Available online: https://abs.gov.au/census/find-census-data/quickstats/2016/ (accessed on 12 August 2021).
- Lord, C.; Risi, S.; Lambrecht, L.; Cook, E.H.J.; Leventhal, B.L.; DiLavore, P.C.; Pickles, A.; Rutter, M. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. J. Autism Dev. Disord. 2000, 30, 205–223. [Google Scholar] [CrossRef]
- Luyster, R.; Gotham, K.; Guthrie, W.; Coffing, M.; Petrak, R.; Pierce, K.; Bishop, S.; Esler, A.; Hus, V.; Oti, R.; et al. The Autism Diagnostic Observation Schedule-toddler module: A new module of a standardized diagnostic measure for autism spectrum disorders. J. Autism Dev. Disord. 2009, 39, 1305–1320. [Google Scholar] [CrossRef]
- Shank, L. Mullen Scales of Early Learning. In Encyclopedia of Clinical Neuropsychology; Kreutzer, J.S., DeLuca, J., Caplan, B., Eds.; Springer: New York, NY, USA, 2011; pp. 1669–1671. [Google Scholar]
- Lord, C.; Rutter, M.; Le Couteur, A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 1994, 24, 659–685. [Google Scholar] [CrossRef]
- Australian Bureau of Statistics. Australian Standard Classification of Cultural and Ethnic Groups (ASCCEG) 2019. Available online: https://www.abs.gov.au/ausstats/abs@.nsf/7d12b0f6763c78caca257061001cc588/8e81298ff9bfd160ca257ff1001e661e!OpenDocument (accessed on 29 July 2020).
- StataCorp. Stata Statistical Software: Release 16; StataCorp LLC: College Station, TX, USA, 2019. [Google Scholar]
- Gentles, S.; Nicholas, D.; Jack, S.; Mckibbon, K.; Szatmari, P. Coming to understand the child has autism: A process illustrating parents’ evolving readiness for engaging in care. Autism 2020, 24, 470–483. [Google Scholar] [CrossRef]
- Leonard, H.; Glasson, E.; Nassar, N.; Whitehouse, A.; Bebbington, K.; Bourke, J.; Jacoby, P.; Dixon, G.; Malacova, E.; Bower, C.; et al. Autism and Intellectual Disability Are Differentially Related to Sociodemographic Background at Birth. PLoS ONE 2011, 6, e17875. [Google Scholar] [CrossRef] [PubMed]
- Durkin, M.S.; Maenner, M.J.; Baio, J.; Christensen, D.; Daniels, J.; Fitzgerald, R.; Imm, P.; Lee, L.-C.; Schieve, L.A.; Van Naarden Braun, K.; et al. Autism Spectrum Disorder Among US Children (2002–2010): Socioeconomic, Racial, and Ethnic Disparities. Am. J. Public Health 2017, 107, 1818–1826. [Google Scholar] [CrossRef] [PubMed]
- Dealberto, M.J. Prevalence of autism according to maternal immigrant status and ethnic origin. Acta Psychiatr. Scand. 2011, 123, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Zahorodny, W.; Peng, B.; Kim, S.; Jani, N.; Halperin, W.; Brimacombe, M. The association of autism diagnosis with socioeconomic status. Autism 2012, 16, 201–213. [Google Scholar] [CrossRef]
- Maenner, M.J.; Arneson, C.L.; Durkin, M.S. Socioeconomic disparity in the prevalence of autism spectrum disorder in Wisconsin. Wis. Med. J. 2009, 108, 37–39. [Google Scholar]
- Maenner, M.; Sha, K.; Bakia, A.; Bilder, D.; Durkin, M.; Esler, A.; Furnier, S.; Hallas, L.; Hall-Lande, J.; Hudson, A.; et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2018. Surveill. Summ. 2021, 70, 1–16. [Google Scholar] [CrossRef]
- Elsabbagh, M.; Divan, G.; Koh, Y.; Kim, Y.; Kauchali, S.; Marcin, C.; Montiel-Nava, C.; Patel, V.; Paula, C.; Wang, C.; et al. Global prevalence of autism and other pervasive developmental disorders. Autism Res. 2012, 5, 160–179. [Google Scholar] [CrossRef]
- Global Research on Developmental Disabilities Collaborators. Developmental disabilities among children younger than 5 years in 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Glob. Health 2018, 6, e1100–e1121. [Google Scholar] [CrossRef]
- Shaw, K.A.; McArthur, D.; Hughes, M.M.; Bakian, A.V.; Lee, L.-C.; Pettygrove, S.; Maenner, M.J. Progress and Disparities in Early Identification of Autism Spectrum Disorder: Autism and Developmental Disabilities Monitoring Network, 2002–2016. J. Am. Acad. Child Adolesc. Psychiatry 2022, 61, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Bernie, C.; Williams, K.; O’Connor, B.; Rogers, S.; May, T. Referral, Assessment and Use of Screening Measures Related to Autism Spectrum Disorder at a Tertiary Hospital Setting. J. Autism Dev. Disord. 2021, 51, 2673–2685. [Google Scholar] [CrossRef] [PubMed]
- Mozolic-Staunton, B.; Donelly, M.; Yoxall, J.; Barbaro, J. Early detection for better outcomes: Universal developmental surveillance for autism across health and early childhood education settings. Res. Autism Spectr. Disord. 2020, 71, 101496. [Google Scholar] [CrossRef]
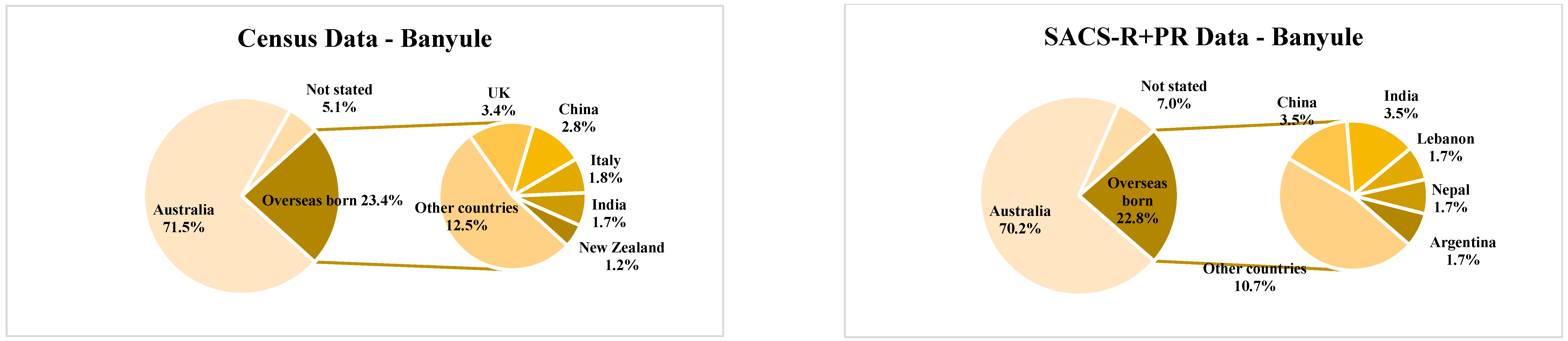
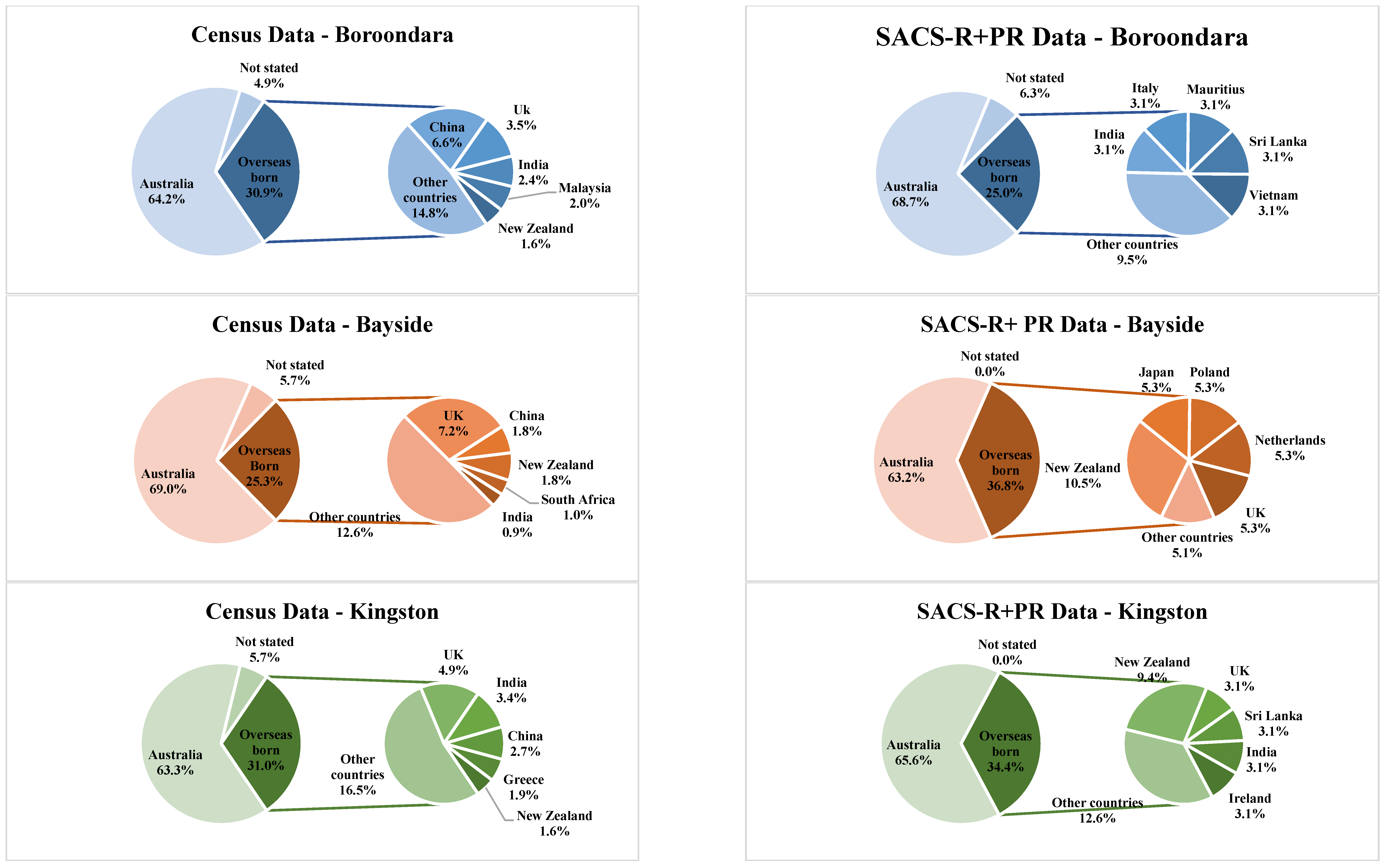
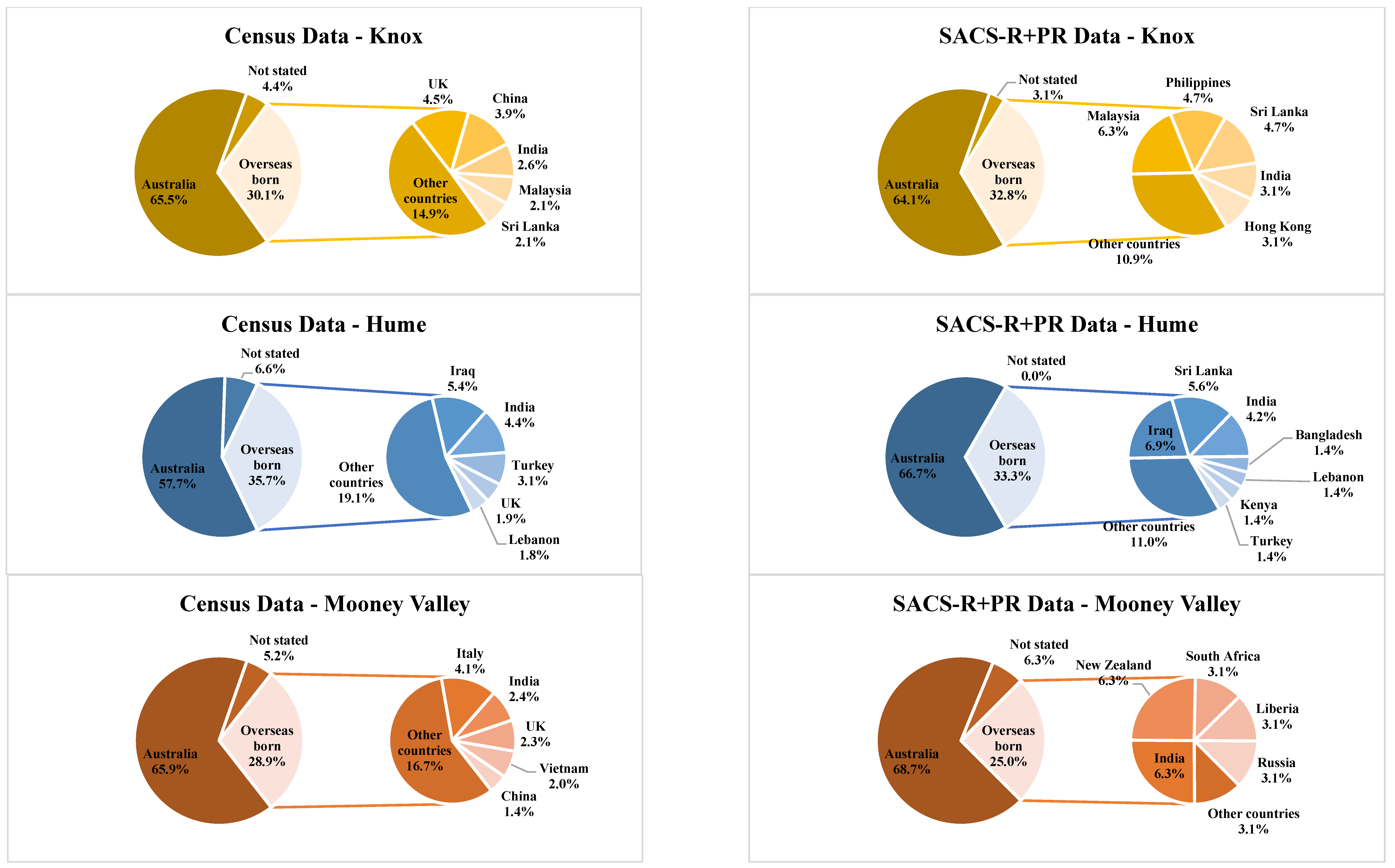

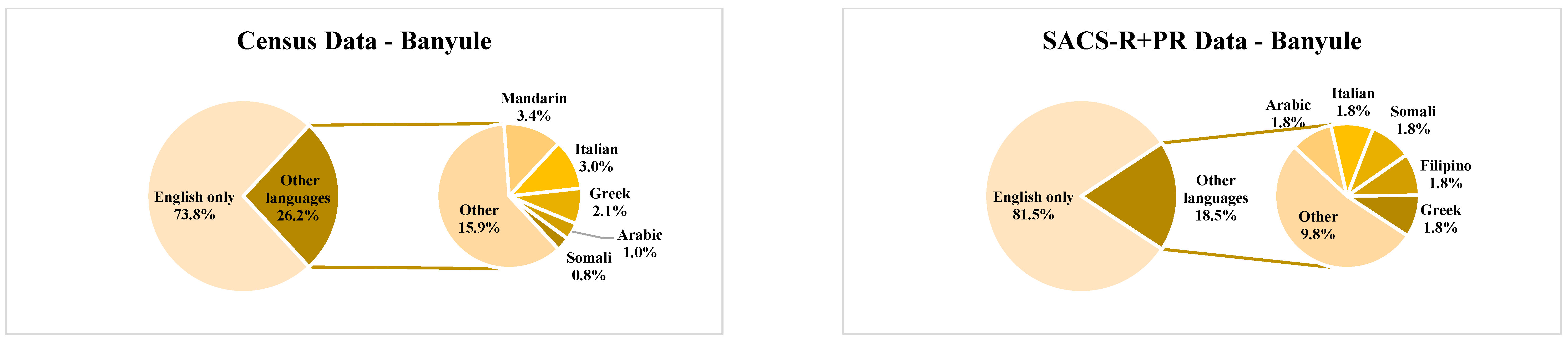
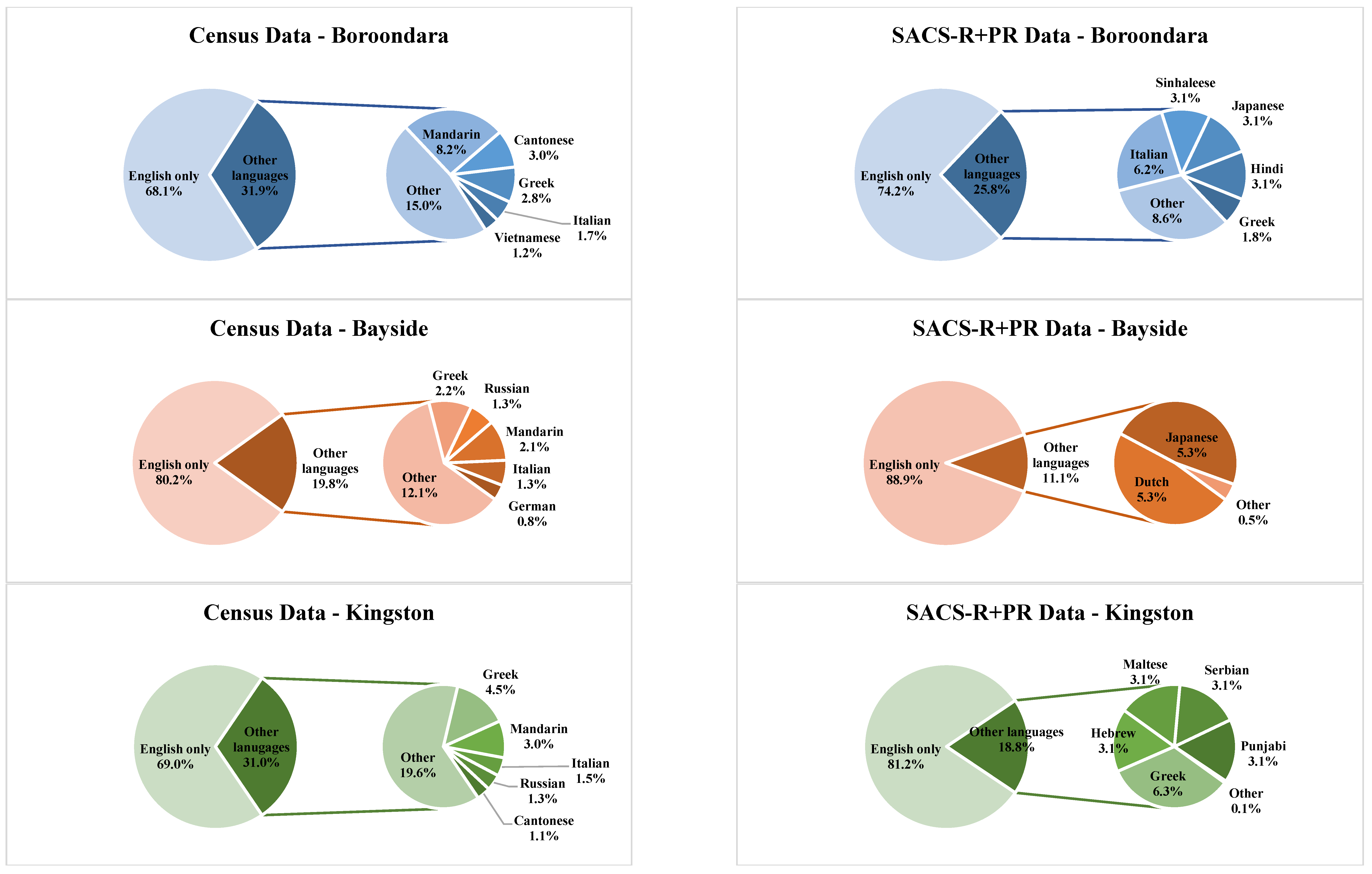
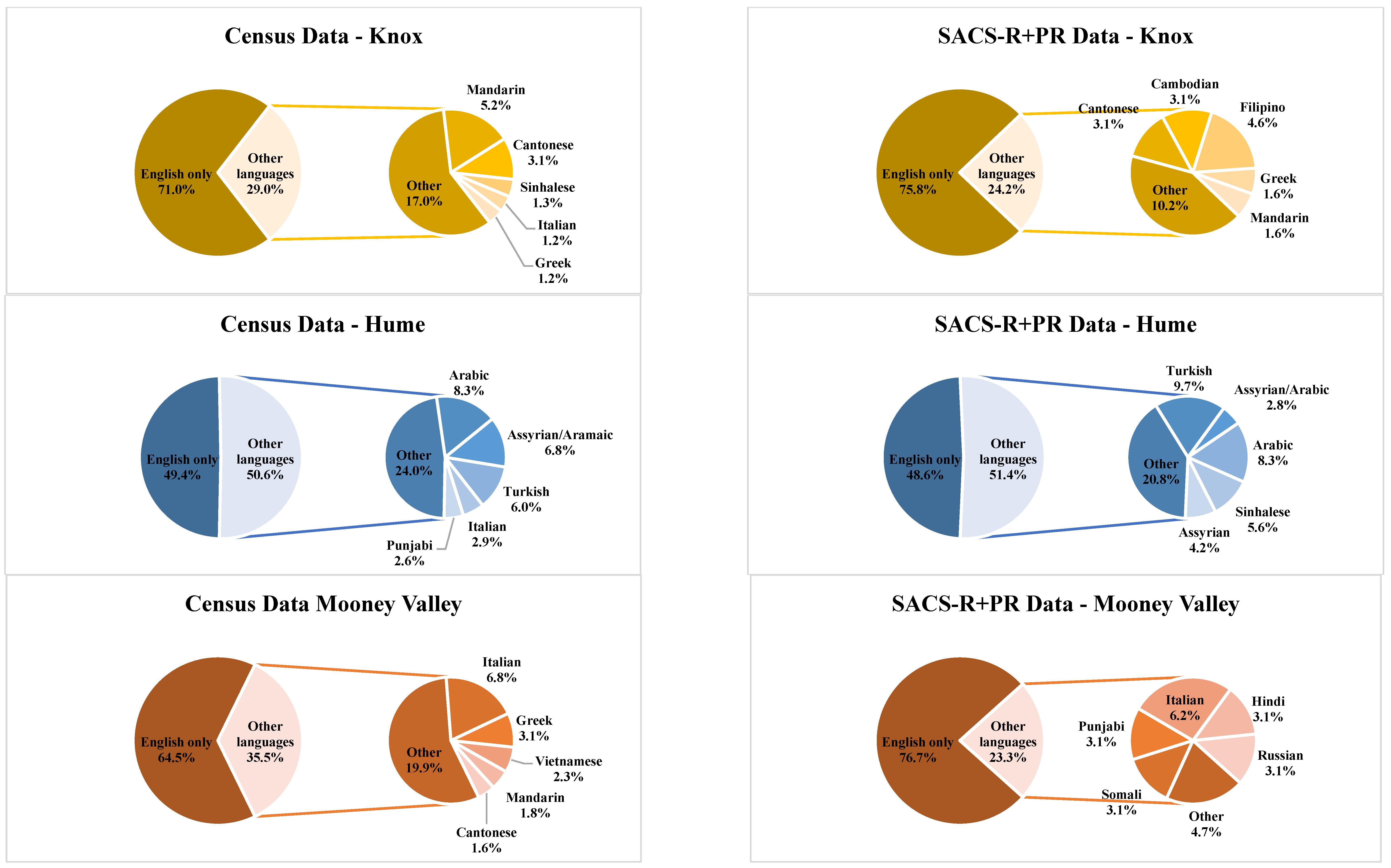

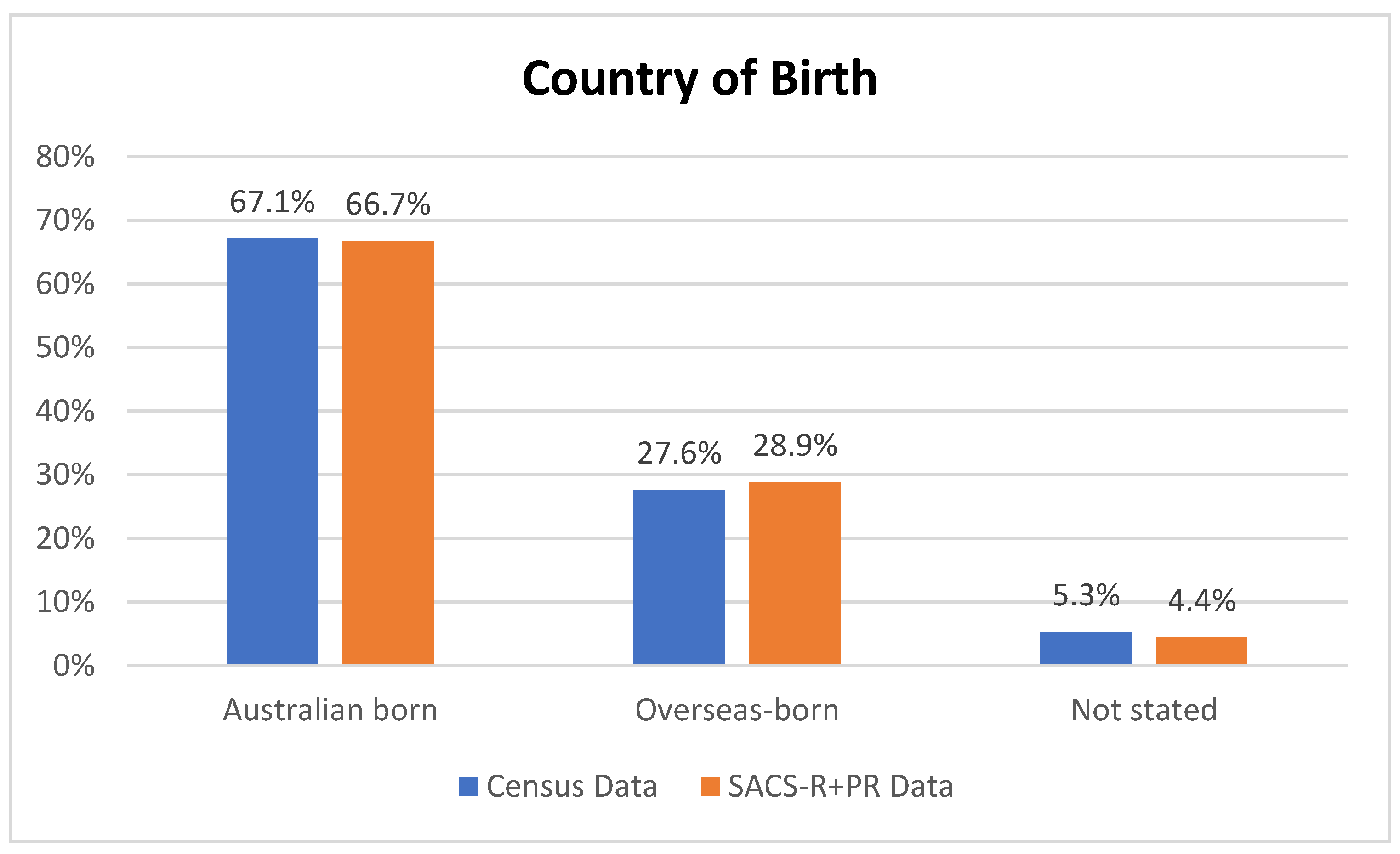
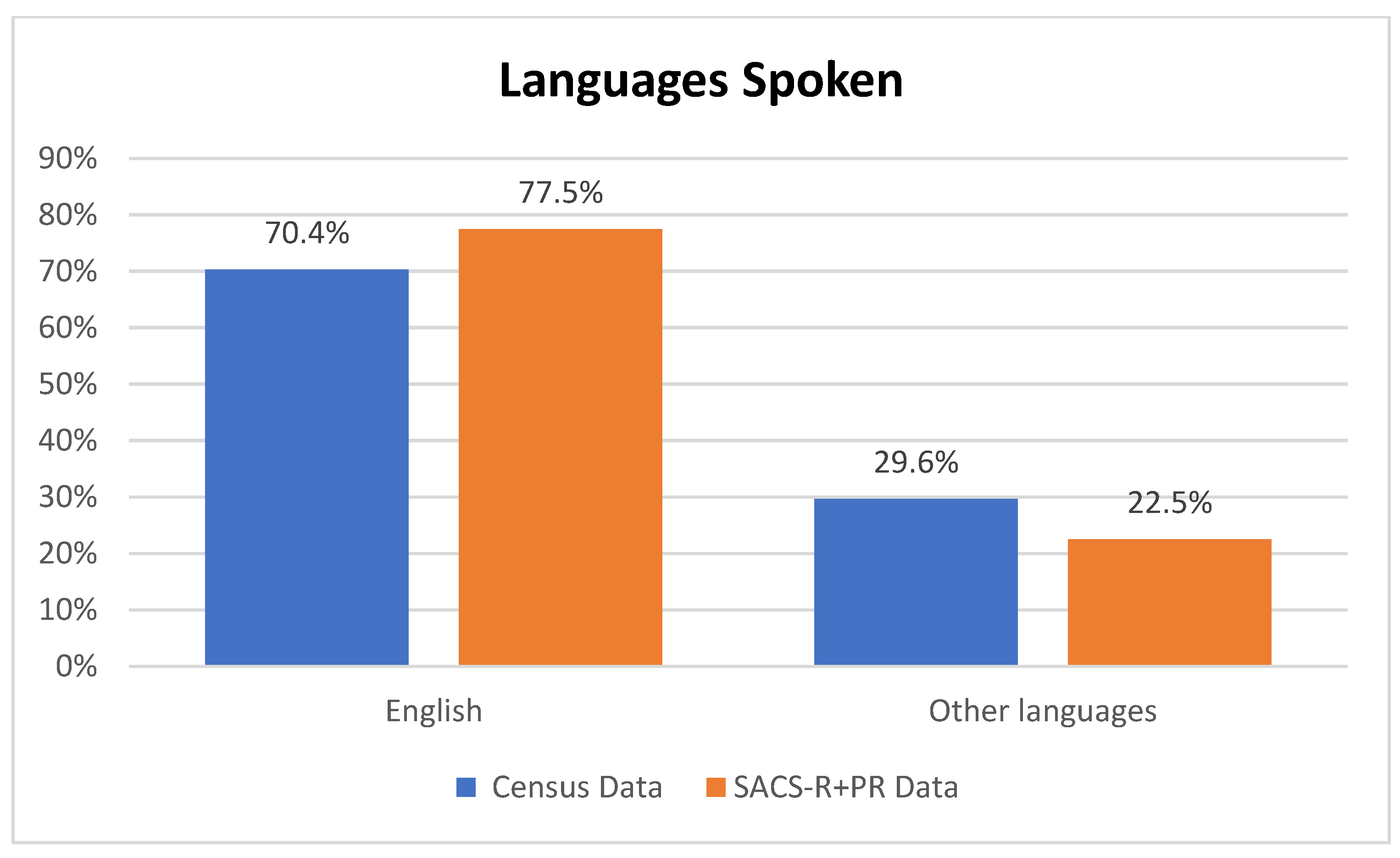
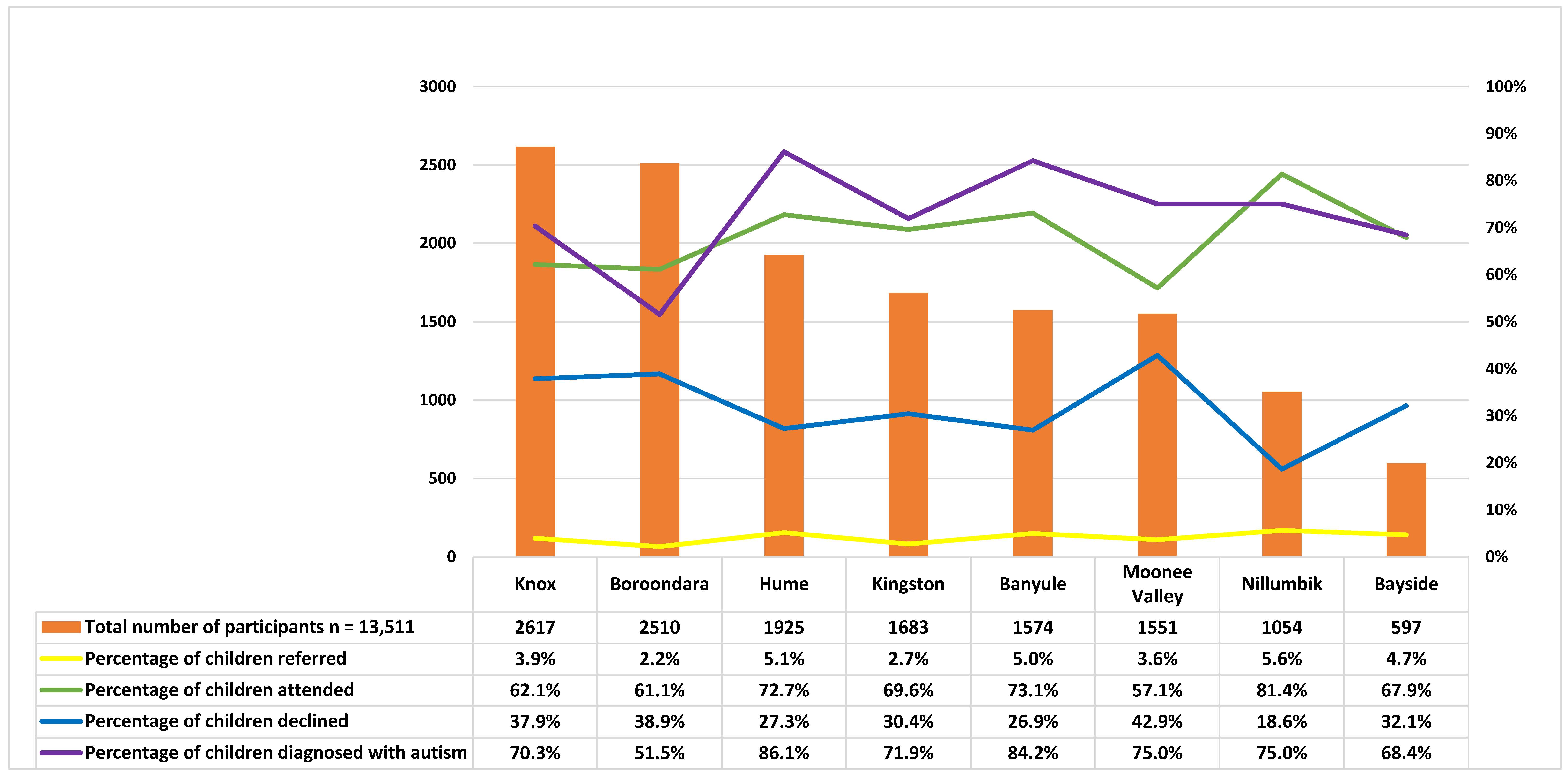
| Banyule | Bayside | Boroondara | Kingston | Knox | Hume | Moonee Valley | Nillumbik | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Location | North-Eastern | Southern | Eastern | South-Eastern | Eastern | North-Western | North-Western | Northern | ||
| Population | Census | 121,865 | 97,087 | 167,231 | 151,389 | 154,110 | 197,376 | 116,671 | 61,273 | |
| M: 48.6% | M: 47.6% | M: 47.9% | M: 48.6% | M: 48.9% | M: 49.8% | M: 48.3% | M: 49.4% | |||
| F: 51.4% | F: 52.4% | F: 52.1%. | F: 51.4% | F: 51.1% | F:50.2% | F: 51.7% | F: 50.6% | |||
| Study | 57 | 19 | 32 | 32 | 64 | 72 | 32 | 48 | ||
| M: 86.0% | M: 79.0% | M: 81.3% | M: 87.5% | M: 76.6% | M: 66.7% | M: 84.4% | M: 77.1% | |||
| F: 14.0% | F: 21.0% | F: 18.7% | F: 12.5% | F: 23.4% | F: 33.3% | F: 15.6% | F: 22.9% | |||
| Aboriginal and Torres Strait Islander peoples | Census | 0.6% | 0.2% | 0.2% | 0.4% | 0.5% | 0.7% | 0.4% | 0.4% | |
| Study | 0.0% | 0.0% | 0.0% | 0.0% | 3.2% | 0.0% | 6.3% | 0.0% | ||
| Household Income in Australian dollars | Census | Low | 17.0% | 15.4% | 15.4% | 18.7% | 16.2% | 18.4% | 18.5% | 11.1% |
| High | 20.1% | 35.8% | 33.8% | 17.4% | 15.5% | 11.2% | 22.1% | 28.4% | ||
| Study | Low | 10.5% | 10.5% | 0.0% | 3.1% | 7.8% | 15.3% | 9.4% | 8.3% | |
| High | 10.5% | 31.2% | 37.5% | 15.6% | 4.7% | 5.6% | 6.3% | 20.8% | ||
| IRSAD | Census | 1055 | 1125 | 1128 | 1042 | 1032 | 947 | 1046 | 1093 | |
| Banyule | Bayside | Boroondara | Hume | Kingston | Knox | MooneeValley | Nillumbik | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Number of children monitored at each age bracket | ||||||||||||||||||
| 12 months | 814 | 51.72 | 212 | 35.51 | 1195 | 47.61 | 902 | 46.86 | 746 | 44.33 | 1253 | 47.88 | 812 | 52.35 | 524 | 49.72 | 6458 | 47.80 |
| 18 months | 439 | 27.89 | 213 | 35.68 | 730 | 29.08 | 576 | 29.92 | 489 | 29.06 | 742 | 28.35 | 417 | 26.89 | 284 | 26.94 | 3890 | 28.79 |
| 24 months | 321 | 20.39 | 172 | 28.81 | 585 | 23.31 | 447 | 23.22 | 448 | 26.62 | 622 | 23.77 | 322 | 20.76 | 246 | 23.34 | 3163 | 23.41 |
| Total | 1574 | 100.00 | 597 | 100.00 | 2510 | 100.00 | 1925 | 100.00 | 1683 | 100.00 | 2617 | 100.00 | 1551 | 100.00 | 1054 | 100.00 | 13,511 | 100.00 |
| Number of children with only a single engagement with the MCH nurse at each age bracket | ||||||||||||||||||
| 12 months | 167 | 47.99 | 48 | 40.00 | 117 | 32.68 | 291 | 42.42 | 309 | 52.46 | 122 | 31.12 | 86 | 39.81 | 42 | 42.00 | 1182 | 42.06 |
| 18 months | 94 | 27.01 | 29 | 24.17 | 95 | 26.54 | 201 | 29.30 | 138 | 23.43 | 125 | 31.89 | 53 | 24.54 | 23 | 23.00 | 759 | 27.01 |
| 24 months | 87 | 25.00 | 43 | 35.83 | 146 | 40.78 | 194 | 28.28 | 142 | 24.11 | 145 | 36.99 | 77 | 35.65 | 35 | 35.00 | 869 | 30.93 |
| Total | 348 | 100.00 | 120 | 100.00 | 358 | 100.00 | 686 | 100.00 | 589 | 100.00 | 392 | 100.00 | 216 | 100.00 | 100 | 100.00 | 2810 | 100.00 |
| Number of attendances at the MCH checks overall | ||||||||||||||||||
| Single | 348 | 22.11 | 120 | 20.10 | 358 | 14.26 | 686 | 35.64 | 589 | 35.00 | 392 | 14.98 | 216 | 13.93 | 101 | 9.58 | 2810 | 20.80 |
| Multiple | 1226 | 77.89 | 477 | 79.90 | 2152 | 85.74 | 1239 | 64.36 | 1094 | 65.00 | 2225 | 85.02 | 1335 | 86.07 | 953 | 90.42 | 10,701 | 79.20 |
| Total | 1574 | 100.00 | 597 | 100.00 | 2510 | 100.00 | 1925 | 100.00 | 1683 | 100.00 | 2617 | 100.00 | 1551 | 100.00 | 1054 | 100.00 | 13,511 | 100.00 |
| Number of assessments by the MCH nurse at each age bracket | ||||||||||||||||||
| 12 months | 814 | 21.87 | 212 | 15.33 | 1195 | 18.63 | 902 | 24.04 | 746 | 23.38 | 1253 | 19.26 | 812 | 20.39 | 524 | 19.01 | 6458 | 20.37 |
| 18 months | 939 | 25.23 | 321 | 23.21 | 1617 | 25.20 | 996 | 26.55 | 715 | 22.41 | 1578 | 24.26 | 985 | 24.74 | 679 | 24.63 | 7830 | 24.69 |
| 24 months | 1020 | 27.40 | 433 | 31.31 | 1933 | 30.13 | 1067 | 28.44 | 768 | 24.07 | 1818 | 27.95 | 1119 | 28.10 | 843 | 30.58 | 9001 | 28.39 |
| 42 months | 949 | 25.50 | 417 | 30.15 | 1671 | 26.04 | 787 | 20.98 | 962 | 30.15 | 1856 | 28.53 | 1066 | 26.77 | 711 | 25.79 | 8419 | 26.55 |
| Total | 3722 | 100.00 | 1383 | 100.00 | 6416 | 100.00 | 3752 | 100.00 | 3191 | 100.00 | 6505 | 100.00 | 3982 | 100.00 | 2757 | 100.00 | 31,708 | 100.00 |
| Children identified at high likelihood for autism by age bracket | ||||||||||||||||||
| 12 months | 4 | 5.19 | 2 | 7.14 | 10 | 19.23 | 18 | 18.18 | 11 | 23.91 | 7 | 6.86 | 7 | 12.50 | 3 | 5.17 | 62 | 11.97 |
| 18 months | 20 | 25.97 | 4 | 14.29 | 6 | 11.54 | 24 | 24.24 | 13 | 28.26 | 13 | 12.75 | 15 | 26.79 | 12 | 20.69 | 107 | 20.66 |
| 24 months | 27 | 35.06 | 12 | 42.86 | 10 | 19.23 | 43 | 43.43 | 10 | 21.74 | 32 | 31.37 | 16 | 28.57 | 8 | 13.79 | 158 | 30.50 |
| 42 months | 24 | 31.17 | 10 | 35.71 | 21 | 40.38 | 14 | 14.14 | 12 | 26.09 | 43 | 42.16 | 17 | 30.36 | 27 | 46.55 | 168 | 32.43 |
| 42 months * | 3 | 3.85 | 0 | 0.00 | 7 | 12.96 | 0 | 0.00 | 0 | 0.00 | 8 | 7.77 | 1 | 1.79 | 9 | 15.25 | 28 | 5.35 |
| Total | 78 | 100.00 | 28 | 100.00 | 54 | 100.00 | 99 | 100.00 | 46 | 100.00 | 103 | 100.00 | 56 | 100.00 | 59 | 100.00 | 523 | 100.00 |
| Percentage | 78 | 5.0 | 28 | 4.70 | 54 | 2.15 | 99 | 5.14 | 46 | 2.73 | 103 | 3.94 | 56 | 3.61 | 59 | 5.60 | 523 | 3.87 |
| Research engagement | ||||||||||||||||||
| Attended | 57 | 73.08 | 19 | 67.86 | 33 | 61.11 | 72 | 72.73 | 32 | 69.57 | 64 | 62.14 | 32 | 57.14 | 48 | 81.36 | 357 | 68.92 |
| Declined | 21 | 26.92 | 9 | 32.14 | 21 | 38.89 | 27 | 27.27 | 14 | 30.43 | 39 | 37.86 | 24 | 42.86 | 11 | 18.64 | 166 | 31.74 |
| EME (n = 236) | MME (n = 15) | N-EME (n = 89) | Missing Data (n = 17) | Total (n = 357) | Difference 1 | |
|---|---|---|---|---|---|---|
| Demographic variables | ||||||
| Sex, n (%) | ||||||
| Female | 54 (22.9) | 1 (6.7) | 20 (22.5) | 3 (17.7) | 78 (22.9) | Pearson chi2(3) = 2.3682 Pr = 0.500 |
| Male | 182(77.1) | 14 (93.3) | 69 (77.5) | 14 (82.3) | 279 (78.1) | |
| Total | 236 (100.0) | 15 (100.0) | 89 (100.0) | 17 (100.0) | 357 (100.0) | |
| Country of birth, n (%) | ||||||
| Australia | 228 (96.6) | 15 (100.0) | 78 (92.9) | 4 (23.5) | 326 (95.9) | Pearson chi2(9) = 220.5623 Pr < 0.001 |
| Other | 6 (2.5) | 0 (0.0) | 5 (6.0) | 0 (0.0) | 11 (3.2) | |
| Missing data | 2 (0.9) | 0 (0.0) | 1 (1.1) | 13 (76.5) | 3 (0.9) | |
| Total | 236 (100.0) | 15 (100.0) | 89 (100.0) | 17 (100.0) | 357 (100.0) | |
| Language spoken at home, n (%) | ||||||
| English only | 210 (89.0) | 13 (86.7) | 28 (31.5) | 2 (11.8) | 253 (70.9) | Pearson chi2(9) = 375.3395 Pr < 0.001 |
| Multilingual | 22 (9.3) | 2 (13.3) | 51 (57.3) | 1 (5.9) | 76 (21.3) | |
| Non-English only | 2 (0.8) | 0 (0.0) | 10 (11.2) | 0 (0.0) | 12 (3.4) | |
| Missing | 2 (0.9) | 0 (0.0) | 0 (0.0) | 14 (82.3) | 16 (4.5) | |
| Total | 236 (100.0) | 15 (100.0) | 89 (100.0) | 17 (100.) | 357 (100.0) | |
| Local government area, n (%) | ||||||
| Banyule | 39 (16.5) | 0 (0.0) | 13 (14.6) | 5 (29.4) | 57 (16.0) | Pearson chi2(24) = 65.7298 Pr < 0.001 |
| Bayside | 16 (6.8) | 1 (6.7) | 2 (2.3) | 0 (0.0) | 19 (5.3) | |
| Boroondara | 22 (9.3) | 3 (20.0) | 5 (5.6) | 2 (11.8) | 32 (8.9) | |
| Hume | 35 (14.8) | 4 (26.7) | 33 (37.1) | 0 (0.0) | 72 (20.2) | |
| Kingston | 27 (11.4) | 1 (6.7) | 4 (4.5) | 0 (0.0) | 32 (9.0) | |
| Knox | 41 (17.4) | 2 (13.3) | 19 (21.4) | 2 (11.8) | 64 (17.9) | |
| Moonee Valley | 20 (8.5) | 2 (13.3) | 8 (9.0) | 2 (11.8) | 32 (8.9) | |
| Nillumbik | 37 (15.4) | 2 (13.3) | 5 (5.6) | 5 (29.4) | 48 (13.5) | |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (5.9) | 1 (0.3) | |
| Total | 236 (100.0) | 15 (100.0) | 89 (100.0) | 17 (100.0) | 357 (100.0) | |
| SEIFA- IRSAD Decile, n (%) | ||||||
| Low | 35 (14.8) | 4 (26.7) | 33 (37.1) | 0 (0.0) | 72 (21.3) | Pearson chi2(6) = 273.1901 Pr < 0.001 |
| High | 199 (84.3) | 11 (73.3) | 56 (62.9) | 3 (17.7) | 266 (78.7) | |
| Missing | 2 (0.8) | 0 (0.0) | 0 (0.0) | 14 (82.3) | 16 (4.5) | |
| Total | 236 (100.0) | 15 (100.0) | 89 (100.0) | 17 (100.0) | 357 (100.0) | |
| Maternal education (highest level completed), n (%) | ||||||
| Secondary and primary education | 43 (18.2) | 3 (20.0) | 11 (12.4) | 0 (0.0) | 57 (15.9) | Pearson chi2(6) = 15.2969 Pr = 0.018 |
| Diploma and vocational education | 65 (27.5) | 2 (13.3) | 10 (11.2) | 1 (5.9) | 78 (21.9) | |
| University | 124 (52.5) | 10 (66.7) | 66 (72.6) | 2 (11.8) | 202 (56.6) | |
| Missing | 4 (1.7) | 0 (0.0) | 2 (2.3) | 14 (82.3) | 20 (5.6) | |
| Total | 236 (100.0) | 15 (100.0) | 89 (100.0) | 17 (100.0) | 357 (100.0) | |
| Current employment status, n (%) | ||||||
| Employed | 108 (45.7) | 6 (40.0) | 46 (51.7) | 0 (0.0) | 160 (47.1) | Pearson chi2(9) = 291.4910 Pr < 0.001 |
| Unemployed | 120 (50.9) | 8 (53.3) | 38 (42.7) | 0 (0.0) | 166 (48.8) | |
| Other | 8 (3.4) | 1 (6.7) | 1 (1.1) | 0 (0.0) | 10 (2.9) | |
| Missing | 0 (0.0) | 0 (0.0) | 4 (4.5) | 17 (100.0) | 21 (5.9) | |
| Total | 236 (100.0) | 15 (100.0) | 89 (100.0) | 17 (100.0) | 357 (100.0) | |
| Annual family income (in Australian dollars), n (%) | ||||||
| Low | 22 (9.3) | 1 (6.7) | 8 (9.0) | 1 (5.9) | 32 (9.0) | Pearson chi2(9) = 42.2220 Pr < 0.001 |
| Medium | 125 (53.0) | 8 (53.3) | 52 (58.4) | 1 (5.9) | 186 (52.1) | |
| High | 36 (15.3) | 4 (26.7) | 8 (9.0) | 0 (0.0) | 48 (13.4) | |
| Missing * | 53 (22.5) | 2 (13.3) | 21 (23.6) | 15 (88.2) | 91 (25.5) | |
| Total | 236 (100.0) | 15 (100.0) | 89 (100.0) | 17 (100.0) | 357 (100.0) | |
| Clinical characteristics | ||||||
| Overall diagnosis, n (%) | ||||||
| Autism | 177 (75.0) | 7 (46.7) | 73 (82.0) | 11 (64.7) | 268 (75.1) | Pearson chi2(3) = 9.6873 Pr = 0.021 |
| DD/LD | 59 (25.0) | 8 (53.3) | 16 (18.0) | 6 (35.3) | 89 (24.9) | |
| Total | 236 (100.0) | 15 (100.0) | 89 (100.0) | 17 (100.0) | 357 (100.0) | |
| Autism prevalence in each group | ||||||
| 75. 0% | 46.7% | 82.0% | 64.7% | 75.1% | Pearson chi2(3) = 9.7411 Pr = 0.021 | |
| Overall Diagnosis | EME (n = 241) | MME (n = 15) | N-EME (n = 84) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | cRRR | aRRR * | n | cRRR | aRRR * | n | cRRR | aRRR * | |
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | ||||
| p value | p value | p value | p value | p value | p value | ||||
| DD/LD | Baseline | ||||||||
| Autism | 241 | Ref | Ref | 15 | 0.29 | 0.26 | 84 | 1.52 | 1.33 |
| (0.10, 0.83) | (0.09, 0.78), | (0.81, 2.86) | (0.60, 2.92), | ||||||
| p = 0.022 | p = 0.017 | p = 0.189 | p = 0.480 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdullahi, I.; Sadka, N.; Gilbert, M.; Barbaro, J. Who Is Attending? The Role of Child Ethnicity and Maternal Demographics in Research Engagement and Early Identification of Autism. Brain Sci. 2023, 13, 903. https://doi.org/10.3390/brainsci13060903
Abdullahi I, Sadka N, Gilbert M, Barbaro J. Who Is Attending? The Role of Child Ethnicity and Maternal Demographics in Research Engagement and Early Identification of Autism. Brain Sciences. 2023; 13(6):903. https://doi.org/10.3390/brainsci13060903
Chicago/Turabian StyleAbdullahi, Ifrah, Nancy Sadka, Melissa Gilbert, and Josephine Barbaro. 2023. "Who Is Attending? The Role of Child Ethnicity and Maternal Demographics in Research Engagement and Early Identification of Autism" Brain Sciences 13, no. 6: 903. https://doi.org/10.3390/brainsci13060903
APA StyleAbdullahi, I., Sadka, N., Gilbert, M., & Barbaro, J. (2023). Who Is Attending? The Role of Child Ethnicity and Maternal Demographics in Research Engagement and Early Identification of Autism. Brain Sciences, 13(6), 903. https://doi.org/10.3390/brainsci13060903






