Investigating the Robustness of a Rodent “Double Hit” (Post-Weaning Social Isolation and NMDA Receptor Antagonist) Model as an Animal Model for Schizophrenia: A Systematic Review
Abstract
1. Introduction
1.1. Study Aim
1.2. Study Objectives
2. Methods
2.1. Search Strategy
2.2. Selection Process
2.3. Data Extraction
2.4. Risk of Bias and Quality Assessment
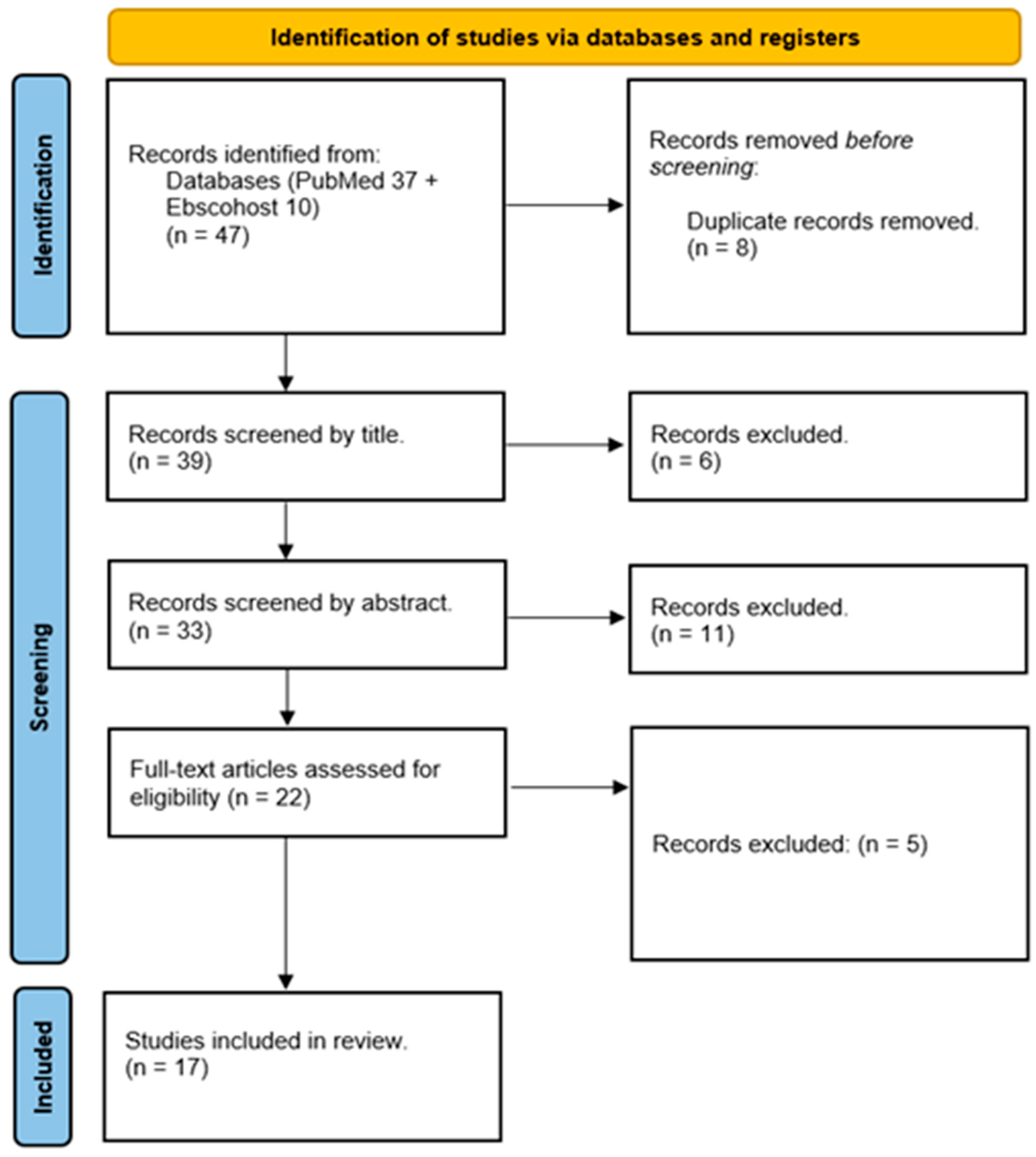
3. Results
3.1. Study Selection
3.2. Risk of Bias and Quality Assessment
3.3. Effectiveness and Robustness of the “Double Hit” (Post-Weaning Social Isolation and NMDA Receptor Antagonist) Model of Schizophrenia
3.4. The Effect of “Double Hit” (Post-Weaning Social Isolation and NMDA Receptor Antagonist) Model of Schizophrenia on Neurotransmitters
3.5. Use of “Double Hit” (Post-Weaning Social Isolation and NMDA Receptor Antagonist) Model as a Developmental Model of Schizophrenia
4. Discussion
4.1. “Double Hit” (Post-Weaning Social Isolation and NMDA Receptor Antagonist) Model on Neurotransmitters
4.2. “Double Hit” (Post-Weaning Social Isolation and NMDA Receptor Antagonist) Model on Positive Symptoms of Schizophrenia
4.3. “Double Hit” (Post-Weaning Social Isolation and NMDA Receptor Antagonist) Model on Cognitive Symptoms of Schizophrenia
4.4. Response of “Double Hit” (Post-Weaning Social Isolation and NMDA Receptor Antagonist) Model on Drugs Used to Reversed Symptoms of Schizophrenia
5. Conclusions, Limitations, and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McGrath, J.; Saha, S.; Chant, D.; Welham, J. Schizophrenia: A Concise Overview of Incidence, Prevalence, and Mortality. Epidemiol. Rev. 2008, 30, 67–76. [Google Scholar] [CrossRef]
- Crow, T.J. Molecular pathology of schizophrenia: More than one disease process? BMJ 1980, 280, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, N. Symptoms, signs, and diagnosis of schizophrenia. Lancet 1995, 346, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Bowie, C.R.; Harvey, P.D. Cognition in Schizophrenia: Impairments, Determinants, and Functional Importance. Psychiatr. Clin. N. Am. 2005, 28, 613–633. [Google Scholar] [CrossRef] [PubMed]
- Pratt, J.; Winchester, C.; Dawson, N.; Morris, B. Advancing schizophrenia drug discovery: Optimizing rodent models to bridge the translational gap. Nat. Rev. Drug Discov. 2012, 11, 560–579. [Google Scholar] [CrossRef]
- Jones, C.A.; Watson, D.J.G.; Fone, K. Animal models of schizophrenia. Br. J. Pharmacol. 2011, 164, 1162–1194. [Google Scholar] [CrossRef]
- McGrath, J.J. Variations in the Incidence of Schizophrenia: Data Versus Dogma. Schizophr. Bull. 2005, 32, 195–197. [Google Scholar] [CrossRef]
- Kahn, R.S.; Sommer, I.E. The neurobiology and treatment of first-episode schizophrenia. Mol. Psychiatry 2014, 20, 84–97. [Google Scholar] [CrossRef]
- Lin, A.; Wood, S.; Nelson, B.; Brewer, W.; Spiliotacopoulos, D.; Bruxner, A.; Broussard, C.; Pantelis, C.; Yung, A. Neurocognitive predictors of functional outcome two to 13years after identification as ultra-high risk for psychosis. Schizophr. Res. 2011, 132, 1–7. [Google Scholar] [CrossRef]
- Kahn, R.S.; Fleischhacker, W.W.; Boter, H.; Davidson, M.; Vergouwe, Y.; Keet, I.P.; Gheorghe, M.D.; Rybakowski, J.K.; Galderisi, S.; Libiger, J.; et al. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: An open randomised clinical trial. Lancet 2008, 371, 1085–1097. [Google Scholar] [CrossRef]
- Robinson, D. First-Episode Schizophrenia. CNS Spectr. 2010, 15, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Singh, V. Meta-Analysis of the Efficacy of Adjunctive NMDA Receptor Modulators in Chronic Schizophrenia. CNS Drugs 2011, 25, 859–885. [Google Scholar] [CrossRef] [PubMed]
- Heresco-Levy, U.; Javitt, D.C. Comparative effects of glycine and d-cycloserine on persistent negative symptoms in schizophrenia: A retrospective analysis. Schizophr. Res. 2004, 66, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.J.; Grunberg, N.E. Effects of housing on male and female rats: Crowding stresses males but calms females. Physiol. Behav. 1995, 58, 1085–1089. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, H. Age dependent effects of space limitation and social tension on open-field behavior in male rats. Physiol. Behav. 2005, 84, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Lapiz, M.D.; Fulford, A.; Muchimapura, S.; Mason, R.; Parker, T.; Marsden, C.A. Influence of postweaning social isolation in the rat on brain development, conditioned behaviour and neurotransmission. Ross. Fiziol. Zh. Im. I M Sechenova 2001, 87, 730–751. [Google Scholar]
- Fone, K.C.F.; Porkess, M.V. Behavioural and neurochemical effects of post-weaning social isolation in rodents—Relevance to developmental neuropsychiatric disorders. Neurosci. Biobehav. Rev. 2008, 32, 1087–1102. [Google Scholar] [CrossRef] [PubMed]
- Valzelli, L. The “isolation syndrome” in mice. Psychopharmacology 1973, 31, 305–320. [Google Scholar] [CrossRef]
- Einon, D.F.; Morgan, M.J. A critical period for social isolation in the rat. Dev. Psychobiol. 1977, 10, 123–132. [Google Scholar] [CrossRef]
- Heidbreder, C.; Weiss, I.; Domeney, A.; Pryce, C.; Homberg, J.; Hedou, G.; Feldon, J.; Moran, M.; Nelson, P. Behavioral, neurochemical and endocrinological characterization of the early social isolation syndrome. Neuroscience 2000, 100, 749–768. [Google Scholar] [CrossRef]
- Weiss, I.C.; Pryce, C.R.; Jongen-Rêlo, A.L.; Nanz-Bahr, N.I.; Feldon, J. Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav. Brain Res. 2004, 152, 279–295. [Google Scholar] [CrossRef]
- Marsden, C.A.; King, M.V.; Fone, K.C. Influence of social isolation in the rat on serotonergic function and memory—Relevance to models of schizophrenia and the role of 5-HT6 receptors. Neuropharmacology 2011, 61, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Silva-Gómez, A.B.; Rojas, D.; Juárez, I.; Flores, G. Decreased dendritic spine density on prefrontal cortical and hippocampal pyramidal neurons in postweaning social isolation rats. Brain Res. 2003, 983, 128–136. [Google Scholar] [CrossRef]
- Del Arco, A.; Zhu, S.; Terasmaa, A.; Mohammed, A.H.; Fuxe, K. Hyperactivity to novelty induced by social isolation is not correlated with changes in D2 receptor function and binding in striatum. Psychopharmacology 2003, 171, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Weiss, I.C.; Feldon, J.; Domeney, A.M. Isolation rearing-induced disruption of prepulse inhibition: Further evidence for fragility of the response. Behav. Pharmacol. 1999, 10, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Olney, J.W.; Farber, N.B. Glutamate Receptor Dysfunction and Schizophrenia. Arch. Gen. Psychiatry 1995, 52, 998–1007. [Google Scholar] [CrossRef]
- Tsai, G.; Coyle, J.T.; Carlsson, A.; Waters, N.; Holm-Waters, S.; Tedroff, J.; Nilsson, M.; Carlsson, M.L.; Lewis, D.A.; Levitt, P.; et al. Glutamatergic Mechanisms in Schizophrenia. Annu. Rev. Pharmacol. Toxicol. 2002, 42, 165–179. [Google Scholar] [CrossRef]
- Coyle, J.T.; Tsai, G.; Goff, D. Converging Evidence of NMDA Receptor Hypofunction in the Pathophysiology of Schizophrenia. Ann. N. Y. Acad. Sci. 2003, 1003, 318–327. [Google Scholar] [CrossRef]
- Martinez, Z.; Ellison, G.D.; Geyer, M.A.; Swerdlow, N.R. Effects of Sustained Phencyclidine Exposure on Sensorimotor Gating of Startle in Rats. Neuropsychopharmacology 1999, 21, 28–39. [Google Scholar] [CrossRef]
- Rung, J.P.; Carlsson, A.; Markinhuhta, K.R.; Carlsson, M.L. (+)-MK-801 induced social withdrawal in rats; a model for negative symptoms of schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2005, 29, 827–832. [Google Scholar] [CrossRef]
- Abdul-Monim, Z.; Neill, J.C.; Reynolds, G.P. Sub-chronic psychotomimetic phencyclidine induces deficits in reversal learning and alterations in parvalbumin-immunoreactive expression in the rat. J. Psychopharmacol. 2007, 21, 198–205. [Google Scholar] [CrossRef]
- Beninger, R.J.; Forsyth, J.K.; Van Adel, M.; Reynolds, J.N.; Boegman, R.J.; Jhamandas, K. Subchronic MK-801 behavioural deficits in rats: Partial reversal by the novel nitrate GT 1061. Pharmacol. Biochem. Behav. 2009, 91, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Gómez, E.; Pérez-Rando, M.; Bellés, M.; Gilabert-Juan, J.; Llorens, J.V.; Carceller, H.; Bueno-Fernández, C.; García-Mompó, C.; Ripoll-Martínez, B.; Curto, Y.; et al. Early Social Isolation Stress and Perinatal NMDA Receptor Antagonist Treatment Induce Changes in the Structure and Neurochemistry of Inhibitory Neurons of the Adult Amygdala and Prefrontal Cortex. Eneuro 2017, 4, ENEURO.0034-17.2017. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, X.; Hong, W.; Wang, D.; Chen, X. Establishment of a schizophrenic animal model through chronic administration of MK-801 in infancy and social isolation in childhood. Infant Behav. Dev. 2017, 46, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mompo, C.; Curto, Y.; Carceller, H.; Gilabert-Juan, J.; Rodriguez-Flores, E.; Guirado, R.; Nacher, J. Δ-9-Tetrahydrocannabinol treatment during adolescence and alterations in the inhibitory networks of the adult prefrontal cortex in mice subjected to perinatal NMDA receptor antagonist injection and to postweaning social isolation. Transl. Psychiatry 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s Risk of Bias Tool for Animal Studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Tuboly, G.; Benedek, G.; Horvath, G. Selective disturbance of pain sensitivity after social isolation. Physiol. Behav. 2009, 96, 18–22. [Google Scholar] [CrossRef]
- Ashby, D.M.; Habib, D.; Dringenberg, H.C.; Reynolds, J.N.; Beninger, R.J. Subchronic MK-801 treatment and post-weaning social isolation in rats: Differential effects on locomotor activity and hippocampal long-term potentiation. Behav. Brain Res. 2010, 212, 64–70. [Google Scholar] [CrossRef]
- Simpson, S.M.; Menard, J.L.; Reynolds, J.N.; Beninger, R.J. Post-weaning social isolation increases activity in a novel environment but decreases defensive burying and subchronic MK-801 enhances the activity but not the burying effect in rats. Pharmacol. Biochem. Behav. 2010, 95, 72–79. [Google Scholar] [CrossRef]
- Hickey, A.J.; Reynolds, J.N.; Beninger, R.J. Post-weaning social isolation and subchronic NMDA glutamate receptor blockade: Effects on locomotor activity and GABA signaling in the rat suggest independent mechanisms. Pharmacol. Biochem. Behav. 2012, 101, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.M.; Hickey, A.J.; Baker, G.B.; Reynolds, J.N.; Beninger, R.J. The antidepressant phenelzine enhances memory in the double Y-maze and increases GABA levels in the hippocampus and frontal cortex of rats. Pharmacol. Biochem. Behav. 2012, 102, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Hawken, E.R.; Delva, N.J.; Beninger, R.J. Increased Drinking following Social Isolation Rearing: Implications for Polydipsia Associated with Schizophrenia. PLoS ONE 2013, 8, e56105. [Google Scholar] [CrossRef]
- Inta, D.; Renz, P.; Lima-Ojeda, J.M.; Dormann, C.; Gass, P. Postweaning social isolation exacerbates neurotoxic effects of the NMDA receptor antagonist MK-801 in rats. J. Neural Transm. 2013, 120, 1605–1609. [Google Scholar] [CrossRef] [PubMed]
- Petrovszki, Z.; Adam, G.; Tuboly, G.; Kekesi, G.; Benedek, G.; Keri, S.; Horvath, G. Characterization of gene–environment interactions by behavioral profiling of selectively bred rats: The effect of NMDA receptor inhibition and social isolation. Behav. Brain Res. 2013, 240, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Gaskin, P.L.; Alexander, S.P.; Fone, K.C. Neonatal phencyclidine administration and post-weaning social isolation as a dual-hit model of ‘schizophrenia-like’ behaviour in the rat. Psychopharmacology 2014, 231, 2533–2545. [Google Scholar] [CrossRef]
- Gaskin, P.L.; Toledo-Rodriguez, M.; Alexander, S.P.; Fone, K.C. Down-Regulation of Hippocampal Genes Regulating Dopaminergic, GABAergic, and Glutamatergic Function Following Combined Neonatal Phencyclidine and Post-Weaning Social Isolation of Rats as a Neurodevelopmental Model for Schizophrenia. Int. J. Neuropsychopharmacol. 2016, 19, pyw062. [Google Scholar] [CrossRef]
- Wu, Z.-M.; Ding, Y.; Jia, H.-X.; Li, L. Different effects of isolation-rearing and neonatal MK-801 treatment on attentional modulations of prepulse inhibition of startle in rats. Psychopharmacology 2016, 233, 3089–3102. [Google Scholar] [CrossRef]
- Shortall, S.E.; Brown, A.M.; Newton-Mann, E.; Dawe-Lane, E.; Evans, C.; Fowler, M.; King, M.V. Calbindin Deficits May Underlie Dissociable Effects of 5-HT6 and mGlu7 Antagonists on Glutamate and Cognition in a Dual-Hit Neurodevelopmental Model for Schizophrenia. Mol. Neurobiol. 2020, 57, 3439–3457. [Google Scholar] [CrossRef]
- Hamieh, A.M.; Babin, D.; Sablé, E.; Hernier, A.M.; Castagné, V. Neonatal phencyclidine and social isolation in the rat: Effects of clozapine on locomotor activity, social recognition, prepulse inhibition, and executive functions deficits. Psychopharmacology 2020, 238, 517–528. [Google Scholar] [CrossRef]
- Klimczak, P.; Rizzo, A.; Castillo-Gómez, E.; Perez-Rando, M.; Gramuntell, Y.; Beltran, M.; Nacher, J. Parvalbumin Interneurons and Perineuronal Nets in the Hippocampus and Retrosplenial Cortex of Adult Male Mice After Early Social Isolation Stress and Perinatal NMDA Receptor Antagonist Treatment. Front. Synaptic Neurosci. 2021, 13, 733989. [Google Scholar] [CrossRef] [PubMed]
- Aleman, A.; Kahn, R.S.; Selten, J.-P. Sex Differences in the Risk of Schizophrenia: Evidence from meta-analysis. Arch. Gen. Psychiatry 2003, 60, 565–571. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.; Saha, S.; Welham, J.; El Saadi, O.; MacCauley, C.; Chant, D. A systematic review of the incidence of schizophrenia: The distribution of rates and the influence of sex, urbanicity, migrant status and methodology. BMC Med. 2004, 2, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Bryda, E.C. The Mighty Mouse: The impact of rodents on advances in biomedical research. Mo. Med. 2013, 110, 207–211. [Google Scholar]
- Rowland, L.M. Subanesthetic ketamine: How it alters physiology and behavior in humans. Aviat. Space Environ. Med. 2005, 76, C52–C58. [Google Scholar] [PubMed]
- Frohlich, J.; Van Horn, J.D. Reviewing the ketamine model for schizophrenia. J. Psychopharmacol. 2013, 28, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Powell, S.; Geyer, M.; Preece, M.; Pitcher, L.; Reynolds, G.; Swerdlow, N. Dopamine depletion of the nucleus accumbens reverses isolation-induced deficits in prepulse inhibition in rats. Neuroscience 2003, 119, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, L.; Massey, B.; Huang, M.; Oyamada, Y.; Meltzer, H. The Novel Object Recognition Test in Rodents in Relation to Cognitive Impairment in Schizophrenia. Curr. Pharm. Des. 2014, 20, 5104–5114. [Google Scholar] [CrossRef]
- Olney, J.W.; Labruyere, J.; Price, M.T. Pathological Changes Induced in Cerebrocortical Neurons by Phencyclidine and Related Drugs. Science 1989, 244, 1360–1362. [Google Scholar] [CrossRef]
- Leach, M.; Baxter, M.G.; Critchley, M.A.E. Neurochemical and Behavioral Aspects of Lamotrigine. Epilepsia 1991, 32, S4–S8. [Google Scholar] [CrossRef]
- Calabresi, P.; Centonze, D.; Marfia, G.A.; Pisani, A.; Bernardi, G. An in vitro electrophysiological study on the effects of phenytoin, lamotrigine and gabapentin on striatal neurons. Br. J. Pharmacol. 1999, 126, 689–696. [Google Scholar] [CrossRef] [PubMed]
| Studies | Selection Bias | Performance Bias | Detection Bias | Attrition Bias | Reporting Bias | Other | Judgement/Risk of Bias | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Was the Allocation Sequence Adequately Generated and Applied? | Were the Groups Similar at Baseline or Were They Adjusted for Confounders in the Analysis? | Was the Allocation Adequately Concealed? | Were the Animals Randomly Housed during the Experiment? | Were the Caregivers and/or Investigators Blinded from Knowledge of Which Intervention Each Animal Received during the Experiment? | Were Animals Selected at Random for Outcome Assessment? | Was the Outcome Assessor Blinded? | Were Incomplete Outcome Data Adequately Addressed? | Are Reports of the Study Free of Selective Outcome Reporting? | Was the Study Apparently Free of Other Problems That Could Result in High Risk of Bias? | ||
| [38] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| [39] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| [40] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| [41] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| [42] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| [43] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| [44] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| [45] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| [46] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| [47] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| [48] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Low |
| [33] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| [34] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| [35] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| [49] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| [50] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| [51] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Study | Location of the Study | Year of Publication | Species | Sex | NMDA Receptor Antagonist, Dosage, Period of Injection, Type of Injection | Commencement of Social Isolation, Period of Social Isolation | Research Groups per Study | Outcome of the “Double Hit” Model |
|---|---|---|---|---|---|---|---|---|
| [41] | Canada | 2012 | Sprague Dawley rat | Male | MK801, 0.5 mg/kg, (PND 56–62) 2 times daily for 7 days, i.p. injection | From PND21-PND78 | GH + Sal, GH + MK, SI + Sal, and SI + MK. | Combined post-weaning social isolation and subchronic MK801 treatment did not produce additive or synergistic effects on locomotor behaviour or Gaba signalling, but rather induce differential effects on GABAa receptor binding. |
| [44] | Germany | 2013 | Sprague Dawley rat | Male | MK801, (2 mg/kg) one injection, on PND64, i.p. injection | On PD21 (weaning age corresponding to pre-adolescence), rats were housed either individually or in groups of three in cages for a 6-week period from PND21 to PND63 | GH + Sal, GH + MK, SI + Sal, and SI + MK | Juvenile rats exposed to chronic isolation had increased MK801-triggered expression of heat shock protein 70, a marker of neuronal injury, in the retrosplenial cortex. This suggests an additive effect of juvenile stress and NMDA receptor blockade, with possible relevance for schizophrenia. |
| [42] | Canada | 2012 | Sprague Dawley rat | Male | MK801, 0.5 mg/kg, (PND56-PND62) was injected twice daily, i.p. injection. | On PND21-PND73 animals were socially isolated and rats remained in their assigned housing for the duration of the experiment. | GH + Sal + Sal, GH + MK + Sal, SI + Sal + Sal, SI + MK + Sal, GH + Sal + PLZ, GH + MK + PLZ, SI + Sal + PLZ, and SI + MK + PLZ | The combination of social isolation and subchronic MK801 did not produce greater behavioural changes than either treatment alone. |
| [47] | United Kingdom | 2016 | Lister-hooded rat | Male | PCP, 10 mg/kg on post-natal days (PND7, PND9, and PND11), s.c. injection | Started on PND 23 till the end of the study | V + GH + V, V + GH + L15, PCP + SI + V, PCP + SI + L10, and PCP + SI + L15 | Acute lamotrigine (10–15 mg/kg i.p.) reversed the hyperactivity and novel object recognition impairment induced by “double hit” model (PCP-SI) but had no effect on the prepulse inhibition deficit. |
| [35] | Spain | 2020 | GIN mice | Male | MK801, 1 mg/kg on PND 7 pups received one injection, i.p. injection | PND21 till the end of the study-PND133 | CTRL + V, CTRL + THC, (SI + MK) + V, and (SI + MK) + THC | We found that “double hit” had reductions in prepulse inhibition of the startle reflex (PPI), GAD67 expression and cingulate 1 cortex volume. |
| [45] | Hungary | 2013 | Wister rat | Both male and female | Ketamine, 30 mg/kg 5 times/week, 15 injections in total) from PND35-PND56 of age, i.p. injection | After weaning at 3 weeks of age (PND21–23 days), Rats were housed individually for 28 days (between 4 and 7 weeks of age) | NaNo, NaTr, SelNo, and SelTr | Selective breeding after juvenile isolation and ketamine treatment produces several signs which resemble those found in schizophrenia. |
| [39] | Canada | 2010 | Sprague Dawley rat | Male | MK801, 0.5 mg/kg, (PND56-PND62) 2 × day for seven days, i.p. injection. | Social isolation started at postnatal day PND21 until PND56 | GH + Sal, GH + MK, SI + Sal, and SI and MK | The lack of additive or synergistic effects in the “double hit model” suggests that combining isolation and subchronic MK801 treatment does not necessarily produce greater behavioural or physiological dysfunction than that seen with either treatment alone. |
| [50] | France | 2020 | Wister rat | Male | PCP (10 mg/kg) on (PND7, PND9, and PND11), s.c. injection | At the weaning day (PND 21), male rat pups were housed individually until the end of the study | V + GH + V, PCP + SI + V, and PCP + SI + Clo | The PCP-SI model presents with enduring and robust deficits (hyperactivity and social recognition impairment) associated with positive symptoms and cognitive/social deficits of schizophrenia, respectively. These deficits are normalized by chronic treatment with clozapine, thereby confirming the predictive validity of this animal model. |
| [38] | Hungary | 2009 | Wister rat | Male | Ketamine, 30 mg/kg, (PND28-PND42) one injection per day, i.p. injection | Wistar rats after weaning (PND21–PND23 days old) were either housed individually or grouped for 21 days. | GH + Sal, GH + Ket, SI + Sal, and SI + Ket | Since both social isolation and NMDA treatment are well-known animal models of schizophrenia, our results showed that juvenile isolation but not ketamine administration can stimulate hypoalgesia associated with this disease. |
| [34] | China | 2017 | Sprague Dawley rat | Male | MK801, 0.1, 0.3, and 0.5 mg/kg in PND7-PND21, s.c. injection | At PND21, rats were social isolated for four weeks (on PND49) | GH + Sal, SI + Sal, GH + MK0.1, SI + MK0.1, GH + MK0.3, SI + MK0.3, GH + MK0.5, and SI + MK0.5 | Administration of MK801 and social isolation are two independent factors on the neurodevelopmental defects. Combining social isolation and subchronic MK801 treatment does not necessarily produce greater behavioural or physiological dysfunction than that seen with either treatment alone. |
| [40] | Canada | 2010 | Sprague Dawley rat | Male | MK801, 0.5 mg/kg, injected twice per day for 7 days from PND56-PND62, i.p. injection. | Rats were obtained at weaning (PND21); they were socially isolated, or group housed according to their randomly assigned housing groups and remained in their assigned groups for the duration of the experiment. | GH + Sal, GH + MK, SI + Sal, and SI + MK | Locomotor activity was increased in social isolated rats. This activity was exacerbated in MK801-SI rats suggesting a possible decrease in hippocampal and/or prefrontal cortex GABA function. |
| [46] | United Kingdom | 2014 | Lister hooded rat | Male | PCP, 10 mg/kg, on post-natal day (PND7, PND9, and PND11), s.c. injection | Rats were socially isolated on PND23, and animals remain isolated for 6 weeks. | GH + CTRL, GH + PCP, SI + CTRL, and SI + PCP | Neonatal PCP and social isolation both produced behavioural deficits in adult rats resulting in severe cognitive impairment (visual recognition memory impairment). This provided a comprehensive preclinical model that can be used to determine the neurobiological aetiology of schizophrenia than either treatment alone. |
| [43] | Canada | 2013 | Sprague Dawley rat | Male | MK801 0.5 mg/kg, (PND62-PND68) twice daily for 7 days, i.p. injection | Rats reared in groups or in isolation beginning at PND21. | GH + Sal, GH + MK, SI + Sal, and SI + MK | Results showed that polydipsia is a schizophrenia-like behavioural effect caused by social isolation. The “double hit” model did not yield a more pronounced polydipsia effect than each treatment alone. |
| [49] | United Kingdom | 2020 | Lister hooded rat | Male | PCP-HCL, 10 mg/kg on (PND 7, PND9, and PND11), s.c. injection | Animals were socially isolated on PND21-PND63. | GH + V, GH + PCP, SI + V, and SI + PCP | Glutamate release was reduced in a “double hit” model; this reduced interneuron firing and caused impairment in the novel object discrimination task. |
| [48] | China | 2016 | Sprague Dawley rat | Male | MK801, 0.2 mg/kg, (PND7-PND10), i.p. injected | Animals were socially isolated on PND21 and remained isolated for 8 weeks. | GH + Sal, GH + MK, and SI + Sal | Both socially reared rats with neonatal exposure to the NMDA receptor antagonist MK-801 and isolation-reared rats exhibited augmented startle responses. |
| [33] | Spain | 2017 | Transgenic strain mice | Male | MK801, 1 mg/kg on PND7, once off/one injection, i.p. injection | Rats were socially isolated on PND21 and remained isolated for 10 weeks. | GH + Sal, GH + MK, SI + Sal, and SI + MK | The “double hit” model showed that the change in E/I balance in the key brain regions as one of the underlying causes of schizophrenia. |
| [51] | Spain | 2021 | FVB mice | Male | MK801, 1 mg/kg on PND7, once off/one injection, i.p. injection | Rats were socially isolated on PND21 and remained isolated for 10 weeks. | GH + Sal, GH + MK, SI + Sal, and SI + MK | The “double hit” model showed a significant decrease in the number of PV+ interneurons, perineuronal nets (PNNs), and PNNs+PV+ cells when compared to control grouped mice. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shangase, K.B.; Luvuno, M.; Mabandla, M.V. Investigating the Robustness of a Rodent “Double Hit” (Post-Weaning Social Isolation and NMDA Receptor Antagonist) Model as an Animal Model for Schizophrenia: A Systematic Review. Brain Sci. 2023, 13, 848. https://doi.org/10.3390/brainsci13060848
Shangase KB, Luvuno M, Mabandla MV. Investigating the Robustness of a Rodent “Double Hit” (Post-Weaning Social Isolation and NMDA Receptor Antagonist) Model as an Animal Model for Schizophrenia: A Systematic Review. Brain Sciences. 2023; 13(6):848. https://doi.org/10.3390/brainsci13060848
Chicago/Turabian StyleShangase, Khanyiso Bright, Mluleki Luvuno, and Musa V. Mabandla. 2023. "Investigating the Robustness of a Rodent “Double Hit” (Post-Weaning Social Isolation and NMDA Receptor Antagonist) Model as an Animal Model for Schizophrenia: A Systematic Review" Brain Sciences 13, no. 6: 848. https://doi.org/10.3390/brainsci13060848
APA StyleShangase, K. B., Luvuno, M., & Mabandla, M. V. (2023). Investigating the Robustness of a Rodent “Double Hit” (Post-Weaning Social Isolation and NMDA Receptor Antagonist) Model as an Animal Model for Schizophrenia: A Systematic Review. Brain Sciences, 13(6), 848. https://doi.org/10.3390/brainsci13060848




