Kullback–Leibler Divergence of Sleep-Wake Patterns Related with Depressive Severity in Patients with Epilepsy
Abstract
1. Introduction
2. Methods
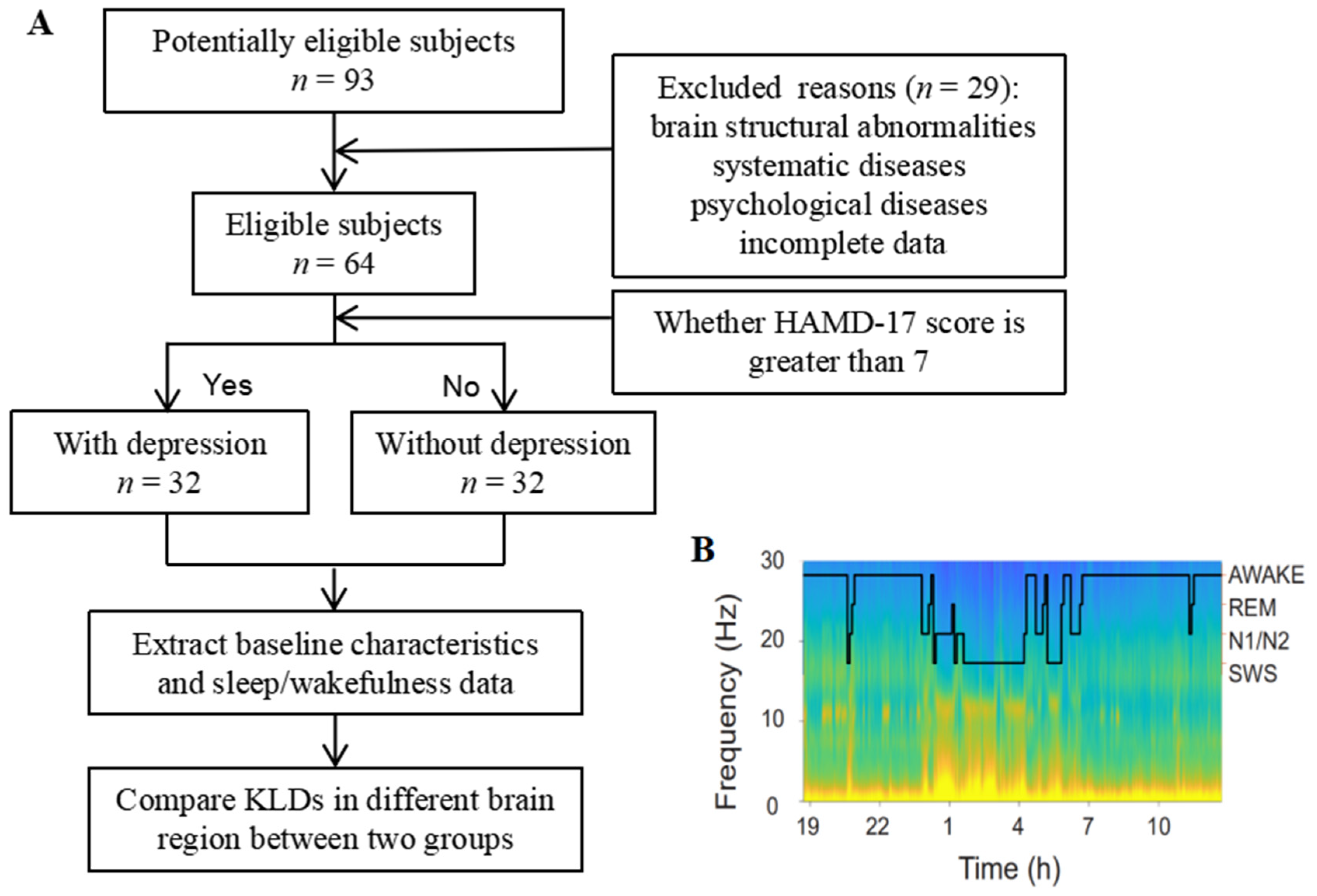
2.1. Study Participants
2.2. Data Acquisition
2.3. EEG Preprocessing
2.4. KLDs Calculation
2.5. Statistical Analyses
3. Results
3.1. Baseline Characteristics of the Participants
3.2. Comparison of Clinical Characteristics between Patients with and without Depression
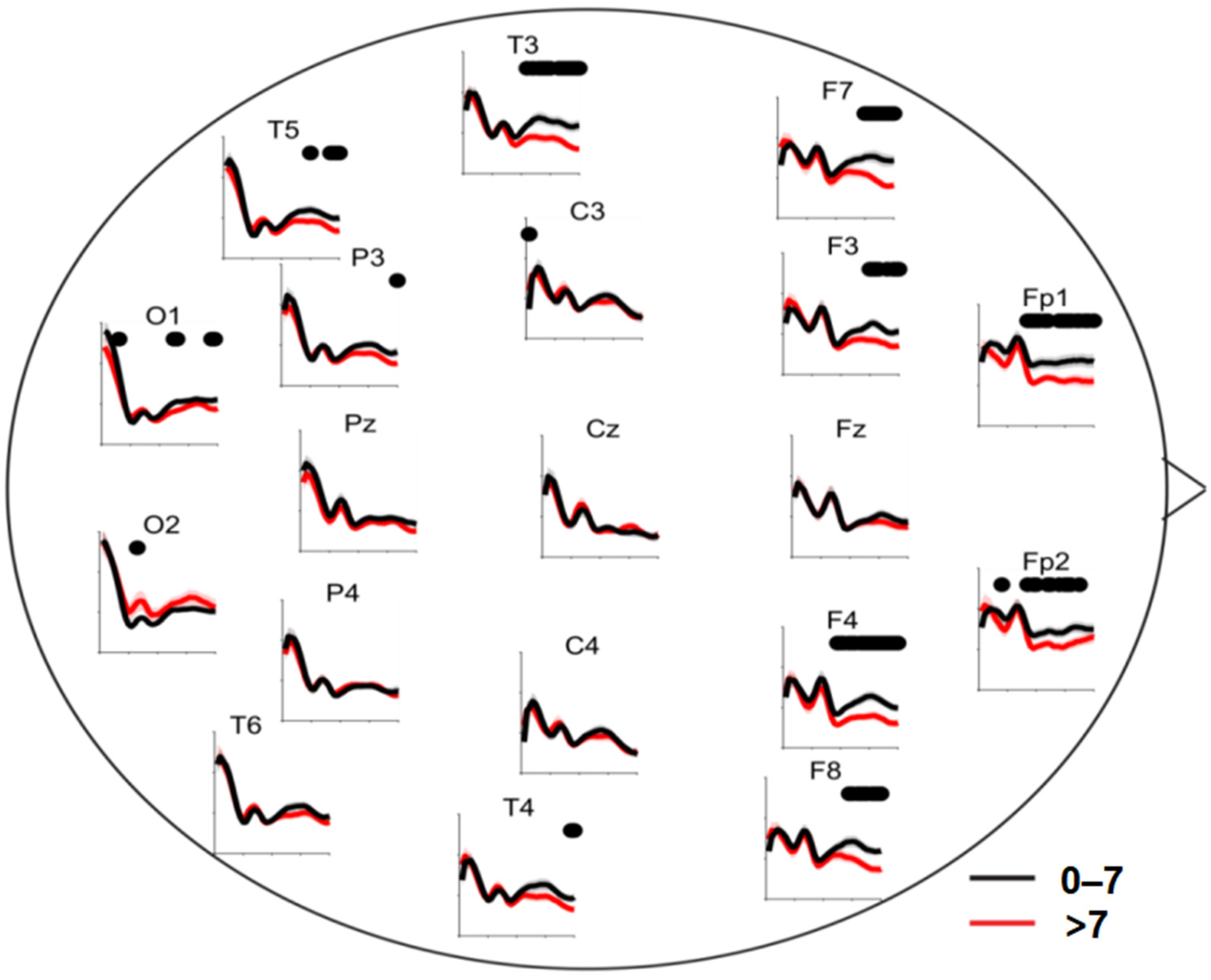
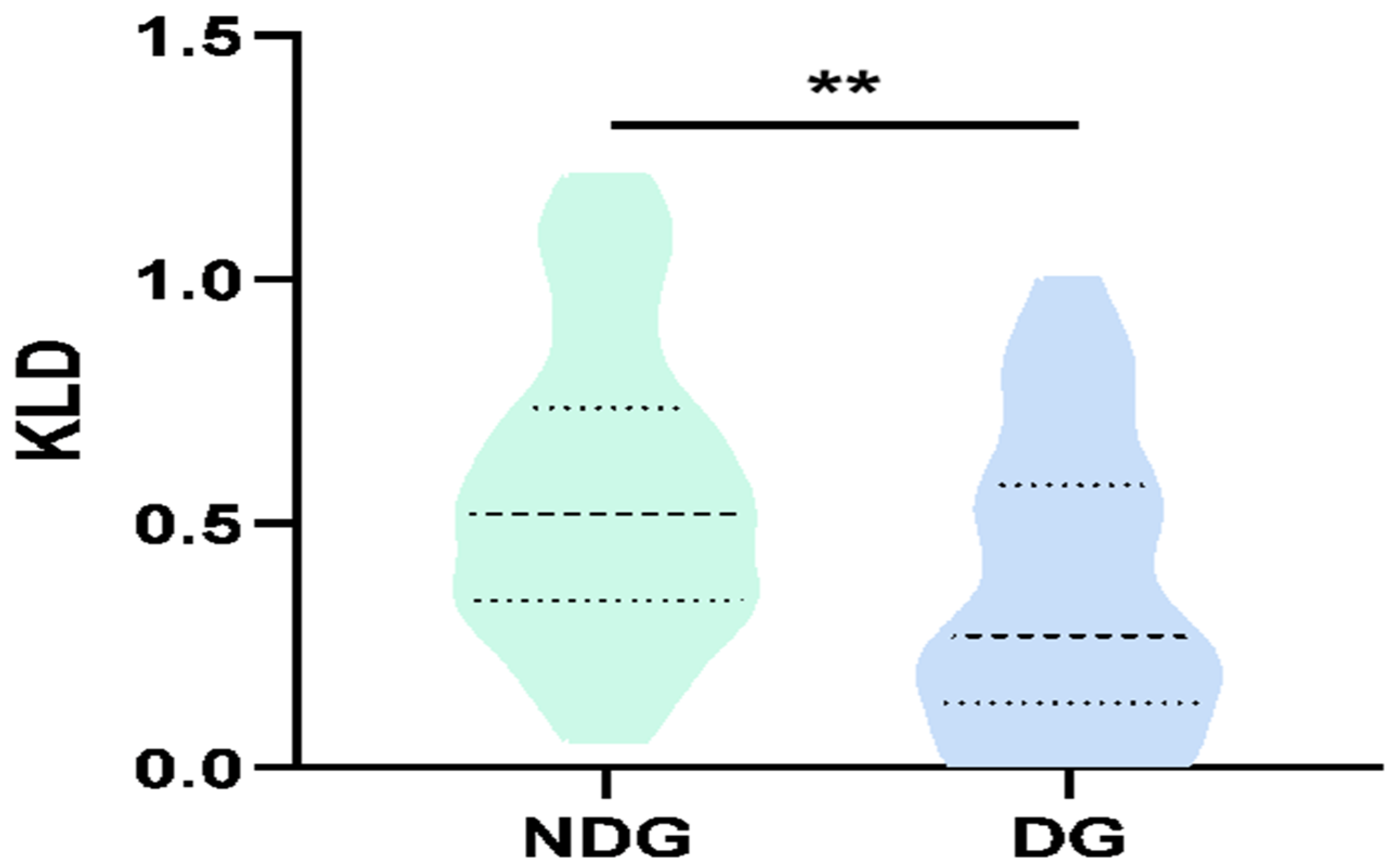
3.3. Global Analysis of KLDs between the Two Groups
3.4. Correlation Analysis between Regional-Specific KLDs and HAMD-17 Score
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moshé, S.L.; Perucca, E.; Ryvlin, P.; Tomson, T. Epilepsy: New advances. Lancet 2015, 385, 884–898. [Google Scholar] [CrossRef] [PubMed]
- Logan, R.W.; McClung, C.A. Rhythms of life: Circadian disruption and brain disorders across the lifespan. Nature reviews. Neuroscience 2019, 20, 49–65. [Google Scholar]
- Matos, G.; Andersen, M.L.; do Valle, A.C.; Tufifik, S. The relationship between sleep and epilepsy: Evidence from clinical trials and animal models. J. Neurol. Sci. 2010, 295, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mekky, J.F.; Elbhrawy, S.M.; Boraey, M.F.; Omar, H.M. Sleep architecture in patients with Juvenile Myoclonic Epilepsy. Sleep Med. 2017, 38, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jiang, J.H.; Yan, Z.Z.; Alberts, I.; Ge, J.J.; Zhang, H.W.; Zuo, C.T.; Yu, J.T.; Rominger, A.; Shi, K.Y. Individual brain metabolic connectome indicator based on Kullback-Leibler Divergence Similarity Estimation predicts progression from mild cognitive impairment to Alzheimer’s dementia. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2753–2764. [Google Scholar] [CrossRef] [PubMed]
- Butler, T.; Harvey, P.; Cardozo, L.; Zhu, Y.S.; Mosa, A.; Tanzi, E.; Pervez, F. Epilepsy, depression, and growth hormone. Epilepsy Behav. 2019, 94, 297–300. [Google Scholar] [CrossRef]
- Chang, P.P.; Ford, D.E.; Mead, L.A.; Cooper-Patrick, L.; Klag, M.J. Insomnia in young men and subsequent depression. Am. J. Epidemiol. 1997, 146, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Crouse, J.J.; Carpenter, J.S.; Song, Y.J.C.; Hockey, S.J.; Naismith, S.L.; Grunstein, R.R.; Scott, E.M.; Merikangas, K.R.; Scott, J.; Hickie, I.B. Circadian rhythm sleep-wake disturbances and depression in young people: Implications for prevention and early intervention. Lancet Psychiatry 2021, 8, 813–823. [Google Scholar] [CrossRef]
- Carpenter, J.S.; Crouse, J.J.; Scott, E.M.; Naismith, S.L.; Wilson, C.; Scott, J.; Merikangas, K.R.; Hickie, I.B. Circadian depression: A mood disorder phenotype. Neurosci. Biobehav. Rev. 2021, 126, 79–101. [Google Scholar] [CrossRef]
- Scheffer, I.E.; Berkovic, S.; Capovilla, G.; Connolly, M.B.; French, J.; Guilhoto, L.; Hirsch, E.; Jain, S.; Mathern, G.W.; Moshé, S.L.; et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 512–521. [Google Scholar] [CrossRef]
- Ren, Y.; Pan, L.; Du, X.; Li, X.; Hou, Y.; Bao, J.; Song, Y.J. Theta oscillation and functional connectivity alterations related to executive control in temporal lobe epilepsy with comorbid depression. Clin. Neurophysiol. 2020, 131, 1599–1609. [Google Scholar] [CrossRef]
- Jones-Gotman, M.; Smith, M.L.; Risse, G.L.; Westerveld, M.; Swanson, S.J.; Giovagnoli, A.R.; Lee, T.; Mader-Joaquim, M.J.; Piazzini, A. The contribution of neuropsychology to diagnostic assessment in epilepsy. Epilepsy Behav. 2010, 18, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, K.L.; Zhao, Q.H.; Li, F.; Guo, Q.H. Short-term delayed recall of auditory verbal learning test provides equivalent value to long-term delayed recall in predicting MCI clinical outcomes: A longitudinal follow-up study. Appl. Neuropsychol. Adult 2020, 27, 73–81. [Google Scholar] [CrossRef]
- Teplan, M. Fundamental of EEG Measurement. Meas. Sci. Rev. 2002, 2, 1–11. [Google Scholar]
- Staresina, B.P.; Bergmann, T.O.; Bonnefond, M.; van der Meij, R.; Jensen, O.; Deuker, L.; Elger, C.E.; Axmacher, N.; Fell, J. Hierarchical nesting of slow oscillations, spindles and ripples in the human hippocampus during sleep. Nat. Neurosci. 2015, 18, 1679–1686. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for Scoring Respiratory Events in Sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef] [PubMed]
- Hartoyo, A.; Cadusch, P.J.; Liley, D.T.J.; Hicks, D.G. Parameter estimation and identifiabi- lity in a neural population model for electro-cortical activity. PLoS Comput. Biol. 2019, 15, e1006694. [Google Scholar] [CrossRef]
- Zhong, J.; Liu, R.; Chen, P. Identifying critical state of complex diseases by single-sample Kullback-Leibler divergence. BMC Genom. 2020, 21, 87. [Google Scholar] [CrossRef]
- Fiest, K.M.; Patten, S.B.; Wiebe, S.; Bulloch, A.G.; Maxwell, C.J.; Jetté, N. Validating screening tools for depression in epilepsy. Epilepsia 2014, 55, 1642–1650. [Google Scholar] [CrossRef]
- Feldman, L.; Lapin, B.; Busch, R.M.; Bautista, J.F. Evaluating subjective cognitive impairment in the adult epilepsy clinic: Effects of depression, number of antiepileptic medications, and seizure frequency. Epilepsy Behav. 2018, 81, 18–24. [Google Scholar] [CrossRef]
- Gallinat, J.; Mulert, C.; Leicht, G. Significance of clinical electroencephalogram in psychiatry. Der Nervenarzt 2016, 87, 323–337. [Google Scholar] [CrossRef] [PubMed]
- de Aguiar Neto, F.S.; Rosa, J.L.G. Depression biomarkers using non-invasive EEG: A review. Neurosci. Biobehav. Rev. 2019, 105, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Pizzagalli, D.A.; Sherwood, R.J.; Henriques, J.B.; Davidson, R.J. Frontal brain asymmetry and reward responsiveness: A source-localization study. Psychol. Sci. 2005, 16, 805–813. [Google Scholar] [CrossRef]
- Fitzgerald, P.J.; Watson, B.O. Gamma oscillations as a biomarker for major depression: An emerging topic. Transl. Psychiatry 2018, 8, 177. [Google Scholar] [CrossRef]
- Downey, D.; Brigadoi, S.; Trevithick, L.; Elliott, R.; Elwell, C.; McAllist-Williams, R.H.; Anderson, I.M. Frontal haemodynamic responses in depression and the effect of electroconvulsive therapy. J. Psychopharmacol. 2019, 33, 1003–1014. [Google Scholar] [CrossRef] [PubMed]
- Olbrich, S.; Tränkner, A.; Chittka, T.; Hegerl, U.; Schönknecht, P. Functional connectivity in major depression: Increased phase synchronization between frontal cortical EEG-source estimates. Psychiatry Res. 2014, 222, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Lemogne, C.; Delaveau, P.; Freton, M.; Guionnet, S.; Fossati, P. Medial prefrontal cortex and the self in major depression. J. Affect. Disord. 2012, 136, e1–e11. [Google Scholar] [CrossRef]
- Goodwin, G.M. Neuropsychological and neuroimaging evidence for the involvement of the frontal lobes in depression. J. Psychopharmacol. 1997, 11, 115–122. [Google Scholar] [CrossRef]
- Wager, T.D.; Davidson, M.L.; Hughes, B.L.; Lindquist, M.A.; Ochsner, K.N. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron 2008, 59, 1037–1050. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, X.; Xu, S.; Hou, H.; Chen, X.; Zhang, Z.; Chen, W. Abnormal changes in functional connectivity between the amygdala and frontal regions are associated with depression in Alzheimer’s disease. Neuroradiology 2018, 60, 1315–1322. [Google Scholar] [CrossRef]
- Li, B.Z.; Cao, Y.; Zhang, Y.; Chen, Y.; Gao, Y.H.; Peng, J.X.; Shao, Y.C.; Zhang, X. Relation of Decreased Functional Connectivity Between Left Thalamus and Left Inferior Frontal Gyrus to Emotion Changes Following Acute Sleep Deprivation. Front. Neurol. 2021, 12, 642411. [Google Scholar] [CrossRef] [PubMed]
- Krause, A.J.; Simon, E.B.; Mander, B.A.; Greer, S.M.; Saletin, J.M.; Goldstein-Piekarski, A.N.; Walker, M.P. The sleep-deprived human brain. Nat. Rev. Neurosci. 2017, 18, 404–418. [Google Scholar] [CrossRef] [PubMed]
- Vallat, R.; Meunier, D.; Nicolas, A.; Ruby, P. Hard to wake up? The cerebral correlates of sleep inertia assessed using combined behavioral, EEG and fMRI measures. NeuroImage 2019, 184, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Javaheipour, N.; Shahdipour, N.; Noori, K.; Zarei, M.; Camilleri, J.; Laird, A.; Fox, P.T.; Eickhoff, S.B.; Eickhoff, C.L.; Rosenzweig, I.; et al. Functional brain alterations in acute sleep deprivation: An activation likelihood estimation meta-analysis. Sleep Med. Rev. 2019, 46, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.Y.; Chen, Y.S.; Su, T.P.; Hsieh, J.C.; Chen, L.F. Abnormal early gamma responses to emotional faces differentiate unipolar from bipolar disorder patients. Biomed. Res. Int. 2014, 2014, 906104. [Google Scholar] [CrossRef]
- Muthukumaraswamy, S.D.; Shaw, A.D.; Jackson, L.E.; Hall, J.; Moran, R.; Saxena, N. Evidence that Subanesthetic Doses of Ketamine Cause Sustained Disruptions of NMDA and AMPA-Mediated Frontoparietal Connectivity in Humans. J. Neurosci. 2015, 35, 11694–11706. [Google Scholar] [CrossRef]
- Hunt, M.J.; Raynaud, B.; Garcia, R. Ketamine dose-dependently induces high-frequency oscillations in the nucleus accumbens in freely moving rats. Biol. Psychiatry 2006, 60, 1206–1214. [Google Scholar] [CrossRef]
- Watson, B.O.; Ding, M.; Buzsáki, G. Temporal coupling of field potentials and action potentials in the neocortex. Eur. J. Neurosci. 2018, 48, 2482–2497. [Google Scholar] [CrossRef]
- Kim, M.; Kim, Y.S.; Kim, D.H.; Yang, T.W.; Kwon, O.Y. Major depressive disorder in epilepsy clinics: A meta-analysis. Epilepsy Behav. 2018, 84, 56–69. [Google Scholar] [CrossRef]
- Li, L.; Wu, C.; Gan, Y.; Qu, X.; Lu, Z. Insomnia and the risk of depression: A meta-analysis of prospective cohort studies. BMC Psychiatry 2016, 16, 375. [Google Scholar] [CrossRef]
- Gregory, A.M.; Rijsdijk, F.V.; Dahl, R.E.; McGuffin, P.; Eley, T.C. Associations between sleep problems, anxiety, and depression in twins at 8 years of age. Pediatrics 2006, 118, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Clemens, B.; Ménes, A.; Piros, P.; Bessenyei, M.; Altmann, A.; Jerney, J.; Kollár, K.; Rosdy, B.; Rózsavölgyi, M.; Steinecker, K.; et al. Quantitative EEG effects of carbamazepine, oxcarbazepine, valproate, lamotrigine, and possible clinical relevance of the findings. Epilepsy Res. 2006, 70, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Planas-Ballvé, A.; Grau-López, L.; Jiménez, M.; Ciurans, J.; Fumanal, A.; Becerra, J.L. Insomnia and poor sleep quality are associated with poor seizure control in patients with epilepsy. Neurologia (Engl. Ed.) 2022, 37, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.M.; Bruni, O.; Ferri, R.; Nunes, M.L. Sleep instability and cognitive status in drug-resistant epilepsies. Sleep Med. 2012, 13, 536–541. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | n (%) or Mean ± SD/M (P25, P75) |
|---|---|
| Sex | |
| Female | 36 (56.2) |
| Male | 28 (43.8) |
| Age | 36.3 ± 14.5 |
| Epilepsy duration | 7.5 (2.3, 15.8) |
| First diagnosis | |
| FD | 14 (21.9) |
| NFD | 50 (78.1) |
| Age at epilepsy diagnosis | 26.7 ± 14.5 |
| Epilepsy type | |
| Temporal lobe epilepsy | 41 (64.1) |
| Frontal lobe epilepsy | 2 (3.1) |
| Occipital lobe epilepsy | 1 (1.5) |
| Generalized epilepsy | 11 (17.2) |
| Unknown epilepsy | 9 (14.1) |
| ASM numbers | |
| ≤1 | 48 (75.0) |
| ≥2 | 16 (25.0) |
| HAMD-17 | 7.5 (1.0, 11.8) |
| MMSE | 29.0 (28.0, 30.0) |
| DS-forward | 8.0 (8.0, 9.0) |
| DS-backward | 5.2 ± 1.9 |
| VFT-animals | 16.2 ± 5.9 |
| SDMT | 48.4 ± 12.9 |
| AVLT immediate recall | 19.5 ± 5.4 |
| AVLT delayed recall | 8.0 ± 2.5 |
| AVLT recognition | 12.0 (11.0, 12.0) |
| Variable | Without Depression | With Depression | p-Value |
|---|---|---|---|
| (32) | (32) | ||
| Sex (male/female) | 17/15 | 11/21 | 0.13 |
| Age (x ± s) | 35.3 ± 13.8 | 37.0 ± 15.6 | 0.66 |
| Epilepsy duration M (P25, P75) | 6.5 (2.3, 18.3) | 9.5 (2.3, 15.0) | 0.76 |
| First diagnosis (FD/NFD) | 7/25 | 7/25 | 1.00 |
| Age at epilepsy diagnosis (x ± s) | 26.2 ± 14.0 | 27.6 ± 14.9 | 0.70 |
| Epilepsy type (n) | 0.15 | ||
| Temporal lobe epilepsy (n) | 25 | 16 | |
| Frontal lobe epilepsy (n) | 0 | 2 | |
| Occipital lobe epilepsy (n) | 0 | 1 | |
| Generalized epilepsy (n) | 4 | 7 | |
| Unknown epilepsy (n) | 3 | 6 | |
| ASM numbers (n) | 0.56 | ||
| ≤1 | 25 | 23 | |
| ≥2 | 7 | 9 | |
| ASM types (n) | |||
| CBZ | 7 | 6 | 0.76 |
| VA | 8 | 9 | 0.78 |
| OXC | 6 | 2 | 0.26 |
| LA | 7 | 4 | 0.32 |
| PB | 1 | 1 | 1.00 |
| TPM | 1 | 5 | 0.20 |
| LEV | 4 | 2 | 0.67 |
| PNT | 1 | 1 | 1.00 |
| CZP | 0 | 5 | 0.06 |
| HAMD-17 M (P25, P75) | 1.0 (0.0, 2.0) | 11.5 (9.0, 15.0) | 0.00 *** |
| MMSE M (P25, P75) | 28.0 (28.0, 29.0) | 29.0 (28.0, 30.0) | 0.02 * |
| DS-forward | 8.0 (8.0, 9.0) | 8.0 (7.0, 9.0) | 0.98 |
| DS-backward | 5.6 ± 1.6 | 4.8 ± 2.2 | 0.11 |
| VFT-animals | 16.9 ± 6.9 | 15.5 ± 4.9 | 0.37 |
| SDMT | 52.4 ± 6.8 | 44.6 ± 16.0 | 0.05 |
| AVLT immediate recall (x ± s) | 18.4 ± 6.2 | 20.4 ± 4.4 | 0.17 |
| AVLT delayed recall (x ± s) | 7.6 ± 3.1 | 8.5 ± 2.0 | 0.20 |
| AVLT recognition M (P25, P75) | 12.0 (10.5, 12.0) | 11.5 (11.0, 12.0) | 0.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Jiang, J.; Feng, Y.; Cai, Y.; Ding, J.; Wang, X. Kullback–Leibler Divergence of Sleep-Wake Patterns Related with Depressive Severity in Patients with Epilepsy. Brain Sci. 2023, 13, 823. https://doi.org/10.3390/brainsci13050823
Liu M, Jiang J, Feng Y, Cai Y, Ding J, Wang X. Kullback–Leibler Divergence of Sleep-Wake Patterns Related with Depressive Severity in Patients with Epilepsy. Brain Sciences. 2023; 13(5):823. https://doi.org/10.3390/brainsci13050823
Chicago/Turabian StyleLiu, Mingsu, Jian Jiang, Yu Feng, Yang Cai, Jing Ding, and Xin Wang. 2023. "Kullback–Leibler Divergence of Sleep-Wake Patterns Related with Depressive Severity in Patients with Epilepsy" Brain Sciences 13, no. 5: 823. https://doi.org/10.3390/brainsci13050823
APA StyleLiu, M., Jiang, J., Feng, Y., Cai, Y., Ding, J., & Wang, X. (2023). Kullback–Leibler Divergence of Sleep-Wake Patterns Related with Depressive Severity in Patients with Epilepsy. Brain Sciences, 13(5), 823. https://doi.org/10.3390/brainsci13050823






