Hippocampus Parcellation via Discriminative Embedded Clustering of fMRI Functional Connectivity
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Data Acquisition
2.2. Data Preprocessing
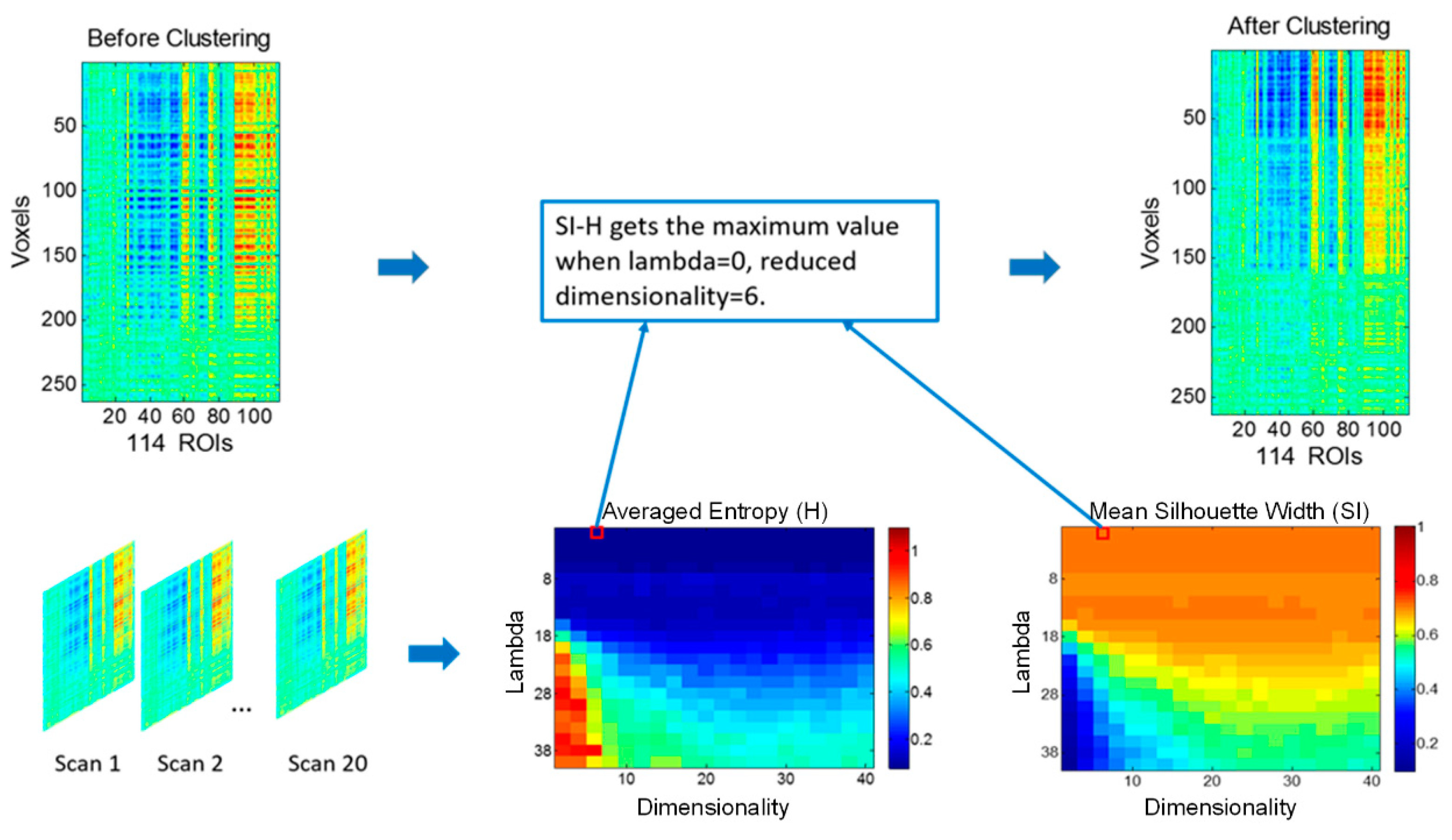
2.3. Discriminative Embedded Clustering
| Algorithm 1 DEC method |
| Input:, number of clusters , dimensionality of the embedding subspace , and balance parameter . Output: Transformation matrix , cluster centroid matrix and cluster indicator . |
| 1. Initialize by performing PCA, and initialize by conducting K-means on . 2. Optimize by fixing and . Similarly, optimize and by fixing . To avoid local optimization, initialize several times randomly and update according to relevant rules. 3. Repeat Step 2 until convergence. |
2.4. Functional Parcellation of the Hippocampus
- —the average distance between every pair of voxels assigned to the i-th cluster.
- —the average distance between within and out of the i-th cluster.
- —the number of clusters.
2.5. Comparison Analysis
3. Results
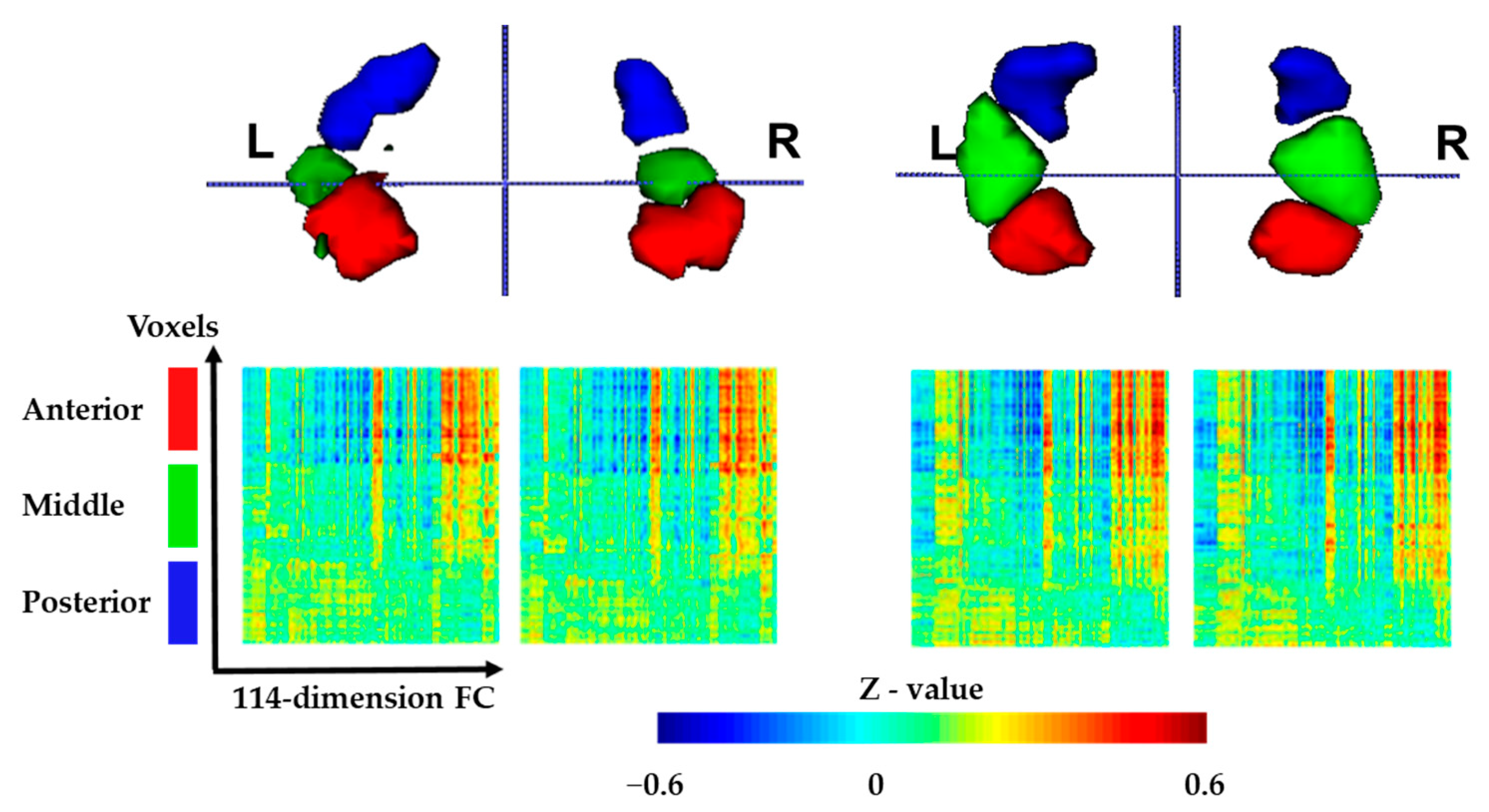
3.1. Functional Connectivity-Based Parcellation of the Hippocampus
3.2. Performance of DEC-Based Parcellation Framework Compared with Existing Methods
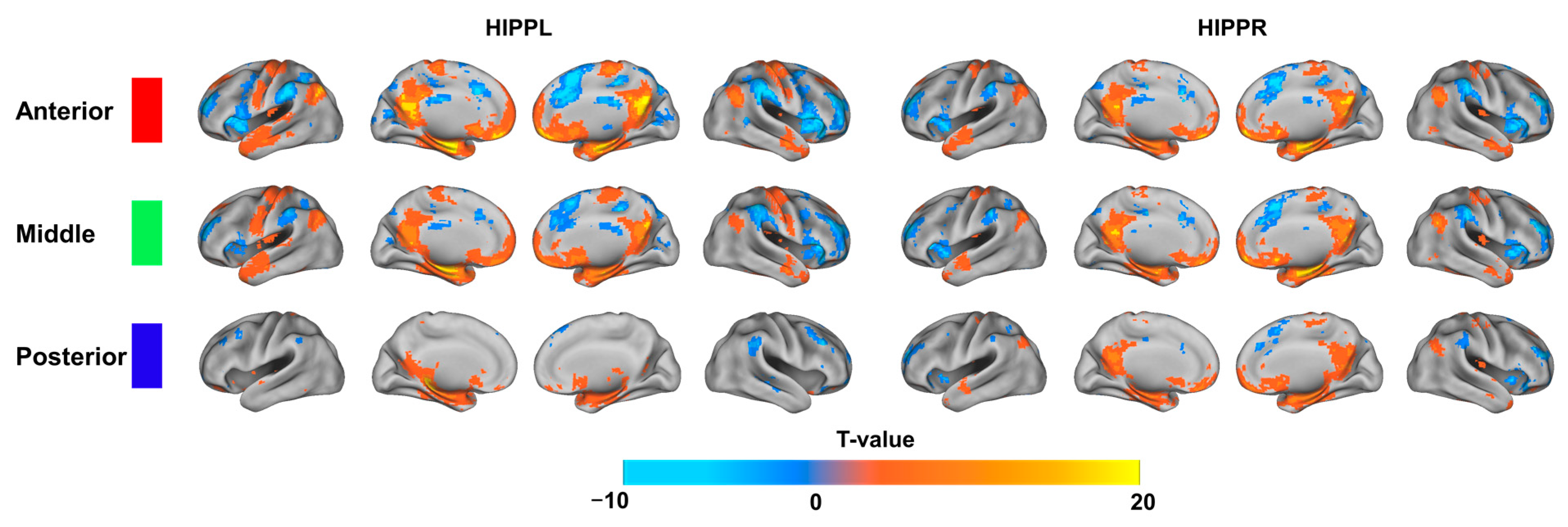
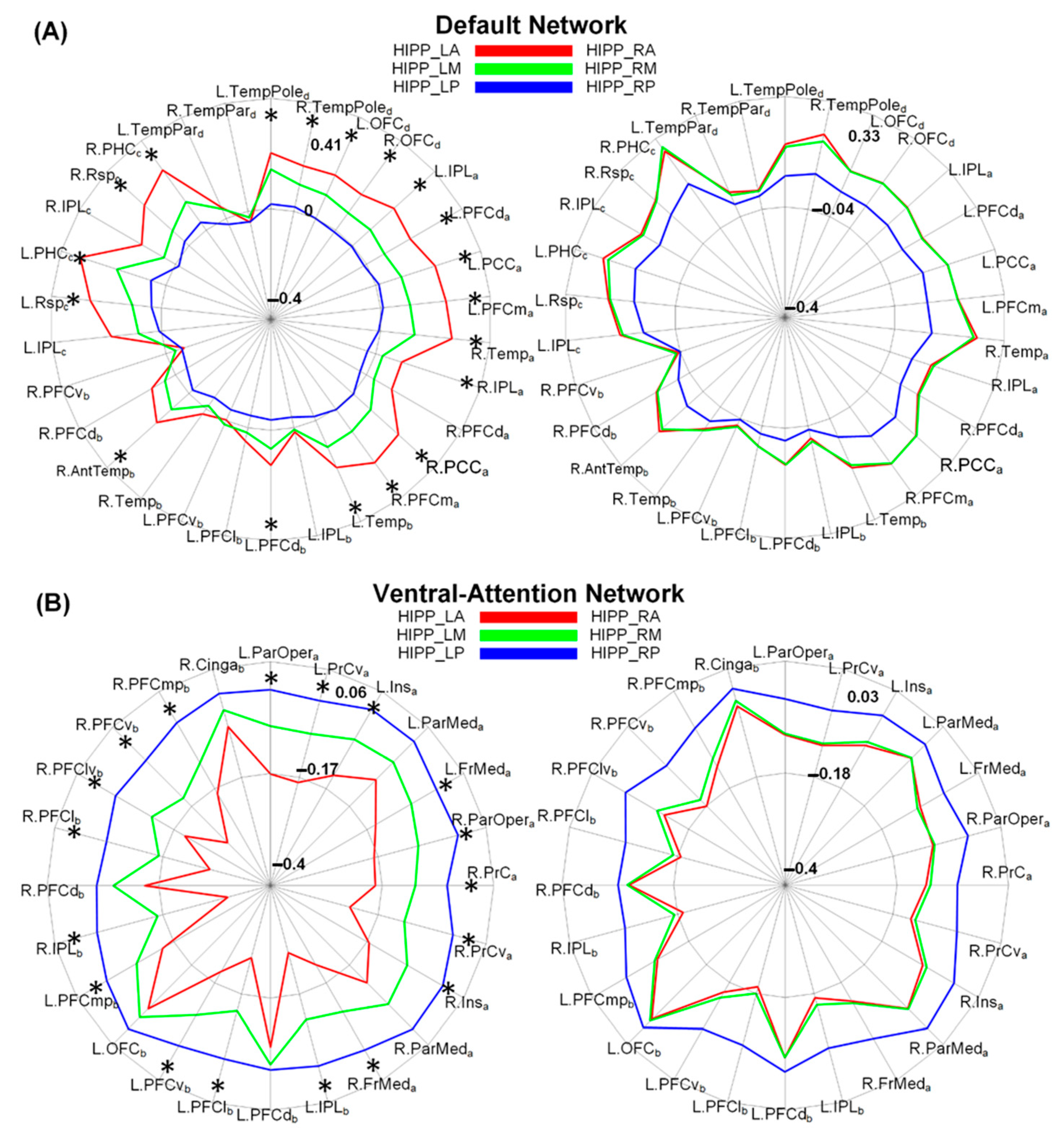
3.3. Discriminative Functional Patterns of the Hippocampal Subregions in the Taxi Drivers
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ke, Q.; Zhang, J.; Wozniak, M.; Wei, W. The Phase and Shift-Invariant Feature by Adaptive Independent Subspace Analysis for Cortical Complex Cells. Inf. Technol. Control 2019, 48, 58–70. [Google Scholar] [CrossRef]
- Ramya, J.; Maheswari, B.; Rajakumar, M.P.; Sonia, R. Alzheimer’s Disease Segmentation and Classification on MRI Brain Images Using Enhanced Expectation Maximization Adaptive Histogram (EEM-AH) and Machine Learning. Inf. Technol. Control 2022, 51, 786–800. [Google Scholar] [CrossRef]
- Ke, Q.; Zhang, J.; Wei, W.; Damaševičius, R.; Woźniak, M. Adaptive Independent Subspace Analysis of Brain Magnetic Resonance Imaging Data. IEEE Access 2019, 7, 12252–12261. [Google Scholar] [CrossRef]
- Sporns, O. Network attributes for segregation and integration in the human brain. Curr. Opin. Neurobiol. 2013, 23, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Eickhoff, S.B.; Yeo, B.T.T.; Genon, S. Imaging-based parcellations of the human brain. Nat. Rev. Neurosci. 2018, 19, 672–686. [Google Scholar] [CrossRef] [PubMed]
- Kong, R.; Li, J.; Orban, C.; Sabuncu, M.R.; Liu, H.; Schaefer, A.; Sun, N.; Zuo, X.-N.; Holmes, A.J.; Eickhoff, S.B.; et al. Spatial Topography of Individual-Specific Cortical Networks Predicts Human Cognition, Personality, and Emotion. Cereb. Cortex 2019, 29, 2533–2551. [Google Scholar] [CrossRef]
- Luo, Z.; Zeng, L.-L.; Qin, J.; Hou, C.; Shen, H.; Hu, D. Functional Parcellation of Human Brain Precuneus Using Density-Based Clustering. Cereb. Cortex 2019, 30, 269–282. [Google Scholar] [CrossRef]
- Kong, R.; Yang, Q.; Gordon, E.; Xue, A.; Yan, X.; Orban, C.; Zuo, X.-N.; Spreng, R.N.; Ge, T.; Holmes, A.; et al. Individual-Specific Areal-Level Parcellations Improve Functional Connectivity Prediction of Behavior. Cereb. Cortex 2021, 31, 4477–4500. [Google Scholar] [CrossRef]
- Peng, L.; Luo, Z.; Zeng, L.-L.; Hou, C.; Shen, H.; Zhou, Z.; Hu, D. Parcellating the human brain using resting-state dynamic functional connectivity. Cereb. Cortex 2022, 33, 3575–3590. [Google Scholar] [CrossRef]
- Su, J.; Shen, H.; Peng, L.; Hu, D. Few-shot domain-adaptive anomaly detection for cross-site brain images. IEEE Trans. Pattern Anal. Mach. Intell. 2021, 1. [Google Scholar] [CrossRef]
- Hwang, K.; Bertolero, M.; Liu, W.; Esposito, M. The human thalamus is an integrative hub for functional brain networks. J. Neurosci. 2017, 37, 5594–5607. [Google Scholar] [CrossRef]
- Hou, C.; Nie, F.; Yi, D.; Tao, D. Discriminative embedded clustering: A framework for grouping high-dimensional data. IEEE Trans. Neural Netw. Learn. Syst. 2015, 26, 1287–1299. [Google Scholar]
- Maguire, E.A.; Burgess, N.; Donnett, J.G.; Frackowiak, R.S.; Frith, C.D.; O’Keefe, J. Knowing where and getting there: A human navigation network. Science 1998, 280, 921. [Google Scholar] [CrossRef]
- Howard, L.R.; Javadi, A.H.; Yu, Y.; Mill, R.D.; Morrison, L.C.; Knight, R.; Loftus, M.M.; Staskute, L.; Spiers, H.J. The Hippocampus and Entorhinal Cortex Encode the Path and Euclidean Distances to Goals during Navigation. Curr. Biol. 2014, 24, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Morgan, L.K.; MacEvoy, S.P.; Aguirre, G.K.; Epstein, R.A. Distances between Real-World Locations Are Represented in the Human Hippocampus. J. Neurosci. 2011, 31, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Greicius, M.D.; Krasnow, B.; Boyettanderson, J.M.; Eliez, S.; Schatzberg, A.F.; Reiss, A.L.; Menon, V. Regional analysis of hippocampal activation during memory encoding and retrieval: fMRI study. Hippocampus 2003, 13, 164. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.; Beckmann, C.F.; Binnewijzend, M.A.A.; Schoonheim, M.M.; Oghabian, M.A.; Sanz-Arigita, E.J.; Scheltens, P.; Matthews, P.M.; Barkhof, F. Functional segmentation of the hippocampus in the healthy human brain and in Alzheimer’s disease. Neuroimage 2013, 66, 28–35. [Google Scholar] [CrossRef]
- Cheng, H.; Fan, Y. Functional parcellation of the hippocampus by clustering resting state fMRI signals. In Proceedings of the IEEE International Symposium on Biomedical Imaging, Beijing, China, 29 April–2 May 2014; pp. 5–8. [Google Scholar]
- Fanselow, M.S. Are the Dorsal and Ventral Hippocampus Functionally Distinct Structures? Neuron 2010, 65, 7–19. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, D.; Cui, H.; Zhao, T.; Chu, C.; Wang, J. Group-guided individual functional parcellation of the hippocampus and application to normal aging. Hum. Brain Mapp. 2021, 42, 5973–5984. [Google Scholar] [CrossRef]
- Cheng, H.; Zhu, H.; Zheng, Q.; Liu, J.; He, G. Functional parcellation of the hippocampus by semi-supervised clustering of resting state fMRI data. Sci. Rep. 2020, 10, 16402. [Google Scholar] [CrossRef]
- Cenquizca, L.A.; Swanson, L.W. Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Res. Rev. 2007, 56, 1–26. [Google Scholar] [CrossRef]
- Maguire, E.A.; Woollett, K.; Spiers, H.J. London taxi drivers and bus drivers: A structural MRI and neuropsychological analysis. Hippocampus 2006, 16, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Maguire, E.A.; Gadian, D.G.; Johnsrude, I.S.; Good, C.D.; Ashburner, J.; Frackowiak, R.S.J.; Frith, C.D. Navigation-related structural change in the hippocampi of taxi drivers. Proc. Natl. Acad. Sci. USA 2000, 97, 4398–4403. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Zeng, L.-L.; Liu, Q.; Wang, L.; Qin, J.; Xu, H.; Shen, H.; Li, H.; Hu, D. Functional connectivity changes in the entorhinal cortex of taxi drivers. Brain Behav. 2018, 8, e01022. [Google Scholar] [CrossRef] [PubMed]
- Glasser, M.F.; Sotiropoulos, S.N.; Wilson, J.A.; Coalson, T.S.; Fischl, B.; Andersson, J.L.; Xu, J.; Jbabdi, S.; Webster, M.; Polimeni, J.R.; et al. The minimal preprocessing pipelines for the Human Connectome Project. NeuroImage 2013, 80, 105–124. [Google Scholar] [CrossRef] [PubMed]
- Salimi-Khorshidi, G.; Douaud, G.; Beckmann, C.F.; Glasser, M.F.; Griffanti, L.; Smith, S.M. Automatic denoising of functional MRI data: Combining independent component analysis and hierarchical fusion of classifiers. NeuroImage 2014, 90, 449–468. [Google Scholar] [CrossRef]
- Zeng, L.L.; Shen, H.; Liu, L.; Hu, D. Unsupervised classification of major depression using functional connectivity MRI. Hum. Brain Mapp. 2014, 35, 1630–1641. [Google Scholar] [CrossRef]
- Zeng, L.L.; Wang, D.; Fox, M.D.; Sabuncu, M.; Hu, D.; Ge, M.; Buckner, R.L.; Liu, H. Neurobiological basis of head motion in brain imaging. Proc. Natl. Acad. Sci. USA 2014, 111, 6058. [Google Scholar] [CrossRef]
- Zeng, L.L.; Wang, H.; Hu, P.; Yang, B.; Pu, W.; Shen, H.; Chen, X.; Liu, Z.; Yin, H.; Tan, Q. Multi-Site Diagnostic Classification of Schizophrenia Using Discriminant Deep Learning with Functional Connectivity MRI. EBioMedicine 2018, 30, 74–85. [Google Scholar] [CrossRef]
- Yeo, B.T.T.; Krienen, F.M.; Sepulcre, J.; Sabuncu, M.R.; Lashkari, D.; Hollinshead, M.; Roffman, J.L.; Smoller, J.W.; Zöllei, L.; Polimeni, J.R. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011, 106, 1125–1165. [Google Scholar]
- Shen, X.; Papademetris, X.; Constable, R.T. Graph-Theory Based Parcellation of Functional Subunits in the Brain from Resting-State fMRI data. Neuroimage 2010, 50, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Craddock, R.C.; James, G.A.; Holtzheimer, P.E.; Hu, X.P.; Mayberg, H.S. A whole brain fMRI atlas generated via spatially constrained spectral clustering. Hum. Brain Mapp. 2012, 33, 1914–1928. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Zang, Y. DPARSF: A MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front. Syst. Neurosci. 2010, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Berthoz, A. Neural basis of spatial orientation and memory of routes: Topokinetic memory or topokinesthesic memory. Rev. Neurol. 2001, 157, 779–789. [Google Scholar] [PubMed]
- Burgess, N.; Maguire, E.A.; O’Keefe, J. The human hippocampus and spatial and episodic memory. Neuron 2002, 35, 625–641. [Google Scholar] [CrossRef]
- Bohbot, V.D.; Jech, R.; Růzicka, E.; Nadel, L.; Kalina, M.; Stepánková, K.; Bures, J. Rat spatial memory tasks adapted for humans: Characterization in subjects with intact brain and subjects with selective medial temporal lobe thermal lesions. Physiol. Res. 2002, 51 (Suppl. S1), S49. [Google Scholar]
- Buckner, R.L.; Andrewshanna, J.R.; Schacter, D.L. The brain’s default network: Anatomy, function, and relevance to disease. Ann. NY Acad. Sci. 2008, 1124, 1–38. [Google Scholar] [CrossRef]
- Ranganath, C.; Ritchey, M. Two cortical systems for memory-guided behaviour. Nat. Rev. Neurosci. 2012, 13, 713–726. [Google Scholar] [CrossRef]
- Raichle, M.E. The Brain’s Default Mode Network. Annu. Rev. Neurosci. 2015, 38, 433. [Google Scholar] [CrossRef]
- Poppenk, J.; Evensmoen, H.R.; Moscovitch, M.; Nadel, L. Long-axis specialization of the human hippocampus. Trends Cogn. Sci. 2013, 17, 230–240. [Google Scholar] [CrossRef]
- Aggleton, J.P.; Wright, N.F.; Vann, S.D.; Saunders, R.C. Medial Temporal Lobe Projections to the Retrosplenial Cortex of the Macaque Monkey. Hippocampus 2012, 22, 1883–1900. [Google Scholar] [CrossRef] [PubMed]
- Strange, B.A.; Witter, M.P.; Lein, E.S.; Moser, E.I. Functional organization of the hippocampal longitudinal axis. Nat. Rev. Neurosci. 2014, 15, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Knierim, J.J. Neural representations of location outside the hippocampus. Learn Mem. 2006, 13, 405–415. [Google Scholar] [CrossRef] [PubMed]
- van Strien, N.M.; Cappaert, N.L.; Witter, M.P. The anatomy of memory: An interactive overview of the parahippocampal-hippocampal network. Nat. Rev. Neurosci. 2009, 10, 272–282. [Google Scholar] [CrossRef]
- Javadi, A.H.; Emo, B.; Howard, L.R.; Zisch, F.E.; Yu, Y.; Knight, R.; Silva, J.P.; Spiers, H.J. Hippocampal and prefrontal processing of network topology to simulate the future. Nat. Commun. 2017, 8, 14652. [Google Scholar] [CrossRef]
- Rosenbaum, R.S.; Ziegler, M.; Winocur, G.; Grady, C.L.; Moscovitch, M. “I have often walked down this street before”: fMRI studies on the hippocampus and other structures during mental navigation of an old environment. Hippocampus 2010, 14, 826–835. [Google Scholar] [CrossRef]
- Patai, E.Z.; Javadi, A.-H.; Ozubko, J.D.; Ji, S.; Callaghan, A.; Robin, J.; Grady, C.; Winocur, G.; Rosenbaum, S.R.; Moscovitch, M.; et al. Long-term consolidation switches goal proximity coding from hippocampus to retrosplenial cortex. bioRxiv 2017, 25, 167882. [Google Scholar]
- Epstein, R.A.; Patai, E.Z.; Julian, J.B.; Spiers, H.J. The cognitive map in humans: Spatial navigation and beyond. Nat. Neurosci. 2017, 20, 1504–1513. [Google Scholar] [CrossRef]


| Variable | Drivers | Non-Drivers | p Value |
|---|---|---|---|
| Sample size | 20 | 20 | |
| Age (years) | 39.5 ± 5.8 | 41.1 ± 5.0 | 0.34 a |
| Sex (male/female) | 20/0 | 18/2 | 0.15 b |
| Education (years) | 9.5 ± 1.8 | 9.0 ± 1.4 | 0.37 a |
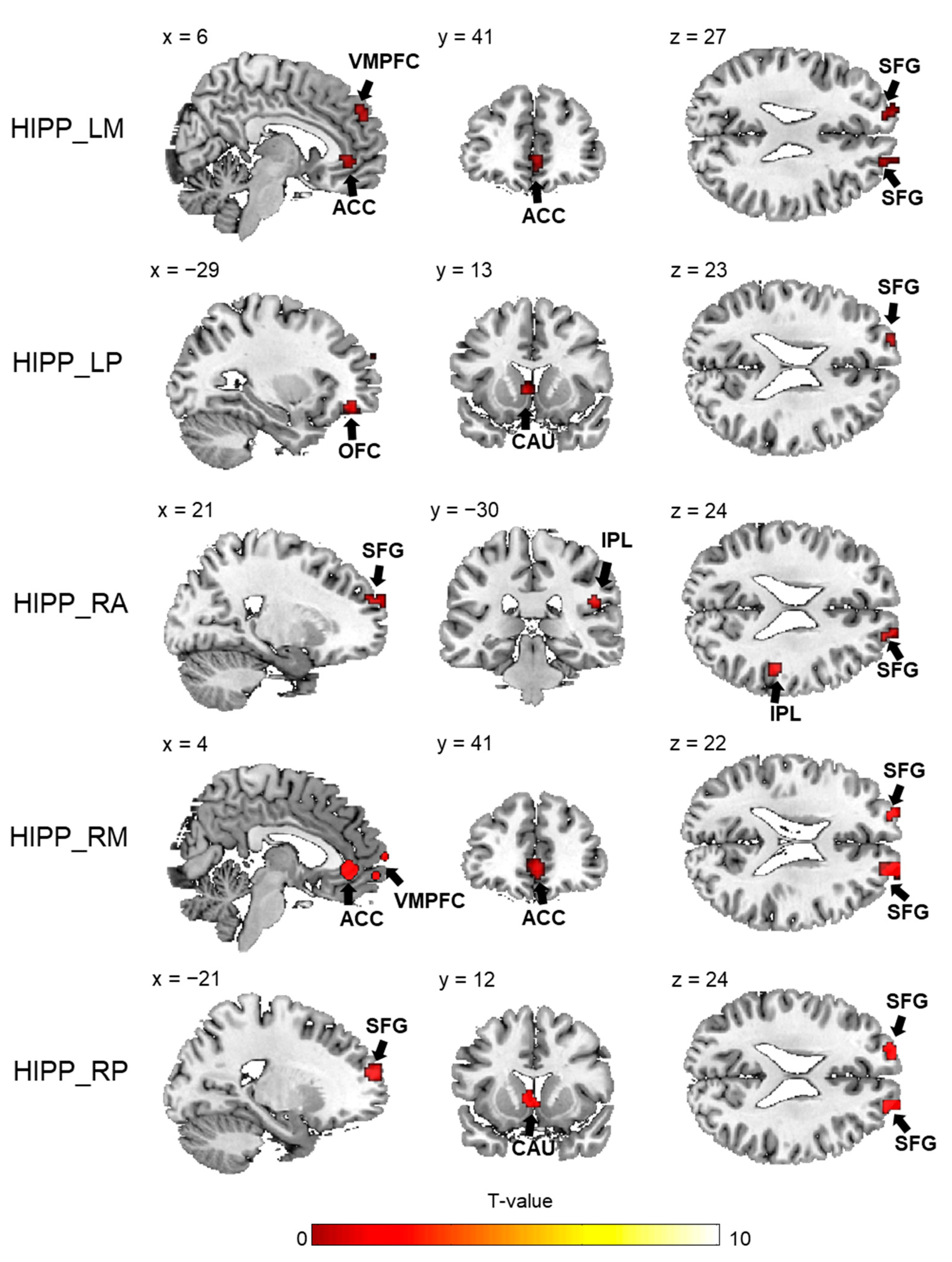
| Target Region | Side | BA | Cluster Size (Voxels) | MNI Coordinates | T-Value | |
|---|---|---|---|---|---|---|
| (x, y, z) | ||||||
| HIPP_LM | ||||||
| Anterior cingulate gyrus | R | 32 | 19 | 3, 42, −3 | −3.94 | |
| Superior frontal gyrus | R | 10 | 16 | 21, 57, 27 | −3.65 | |
| Superior frontal gyrus | L | 10 | 11 | −15, 60, 27 | −3.91 | |
| Superior frontal gyrus | R | 10 | 12 | 6, 51, 33 | −3.85 | |
| HIPP_LP | ||||||
| Orbitofrontal cortex | L | 11 | 17 | −27, 42, −12 | −4.96 | |
| Superior frontal gyrus | L | 10 | 11 | −21, 63, 24 | −4.04 | |
| Caudate nucleus | L | 13 | −3, 12, 0 | −4.61 | ||
| HIPP_RA | ||||||
| Superior frontal gyrus | R | 10 | 14 | 6, 66, 6 | −4.63 | |
| Inferior parietal lobule | R | 40 | 14 | 48, −27, 24 | −4.32 | |
| Superior frontal gyrus | R | 10 | 13 | 21, 66, 24 | −3.93 | |
| HIPP_RM | ||||||
| Superior temporal gyrus | L | 22 | 12 | −30, 6, −33 | −4.70 | |
| Anterior cingulate gyrus | R | 32 | 45 | 3, 39, −3 | −5.04 | |
| Superior frontal gyrus | R | 10 | 29 | 6, 66, 6 | −4.45 | |
| Superior frontal gyrus | L | 10 | 11 | −21, 63, 18 | −3.82 | |
| Superior frontal gyrus | R | 10 | 41 | 21, 66, 27 | −5.35 | |
| HIPP_RP | ||||||
| Superior frontal gyrus | L | 10 | 29 | −21, 57, 18 | −4.01 | |
| Middle frontal gyrus | R | 10 | 12 | 21, 66, 24 | −3.99 | |
| Caudate nucleus | L | 16 | −3, 12, 0 | −4.45 | ||
| HIPP_LA | ||||||
| None | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, L.; Hou, C.; Su, J.; Shen, H.; Wang, L.; Hu, D.; Zeng, L.-L. Hippocampus Parcellation via Discriminative Embedded Clustering of fMRI Functional Connectivity. Brain Sci. 2023, 13, 757. https://doi.org/10.3390/brainsci13050757
Peng L, Hou C, Su J, Shen H, Wang L, Hu D, Zeng L-L. Hippocampus Parcellation via Discriminative Embedded Clustering of fMRI Functional Connectivity. Brain Sciences. 2023; 13(5):757. https://doi.org/10.3390/brainsci13050757
Chicago/Turabian StylePeng, Limin, Chenping Hou, Jianpo Su, Hui Shen, Lubin Wang, Dewen Hu, and Ling-Li Zeng. 2023. "Hippocampus Parcellation via Discriminative Embedded Clustering of fMRI Functional Connectivity" Brain Sciences 13, no. 5: 757. https://doi.org/10.3390/brainsci13050757
APA StylePeng, L., Hou, C., Su, J., Shen, H., Wang, L., Hu, D., & Zeng, L.-L. (2023). Hippocampus Parcellation via Discriminative Embedded Clustering of fMRI Functional Connectivity. Brain Sciences, 13(5), 757. https://doi.org/10.3390/brainsci13050757






