Device-Aided Therapies in Parkinson’s Disease—Results from the German Care4PD Study
Abstract
1. Introduction
2. Materials and Methods
- (a)
- A medication frequency of >6 times per day (considering the intake of additional dopaminergic rescue medication);
- (b)
- An “off” time >25% during waking time (about 4 h in total of a 16 h waking time);
- (c)
- The existence of troublesome dyskinesia.
3. Results
3.1. Participants with Device-Aided Therapies
3.2. Participants without Device-Aided Therapies
3.2.1. Indications for aPD and the Possible Need for Device-Aided Therapies
- (a)
- Medication frequency (question #15): Oral medication was used in nearly all PwP (99%, n = 1107/1116) with good therapy adherence in 96% of PwP (n = 1070/1097), who reported taking their medication “always” or “often” sticking to their medication scheme. A total of 56% of PwP took their oral medication 4 to 6 times per day (n = 626/1113) and about 21% (n = 234/1113) even had a medication frequency of more than 6 times.
- (b)
- Amount of “off” time per day (question #9): Off phases, in general, were recognized by the majority of patients (n = 736/1089 = 68%, (remaining n = 231 “no off” or n = 122 “don’t know”)), of which 454/1089 patients (42%) suffered from >25% “off” time during waking hours, which is about 4 h per day when assuming about 16 h of waking time.
- (c)
- Troublesome dyskinesia (question #8, item “involuntary movements”) was reported in 15% of PwP (n = 170/1108).
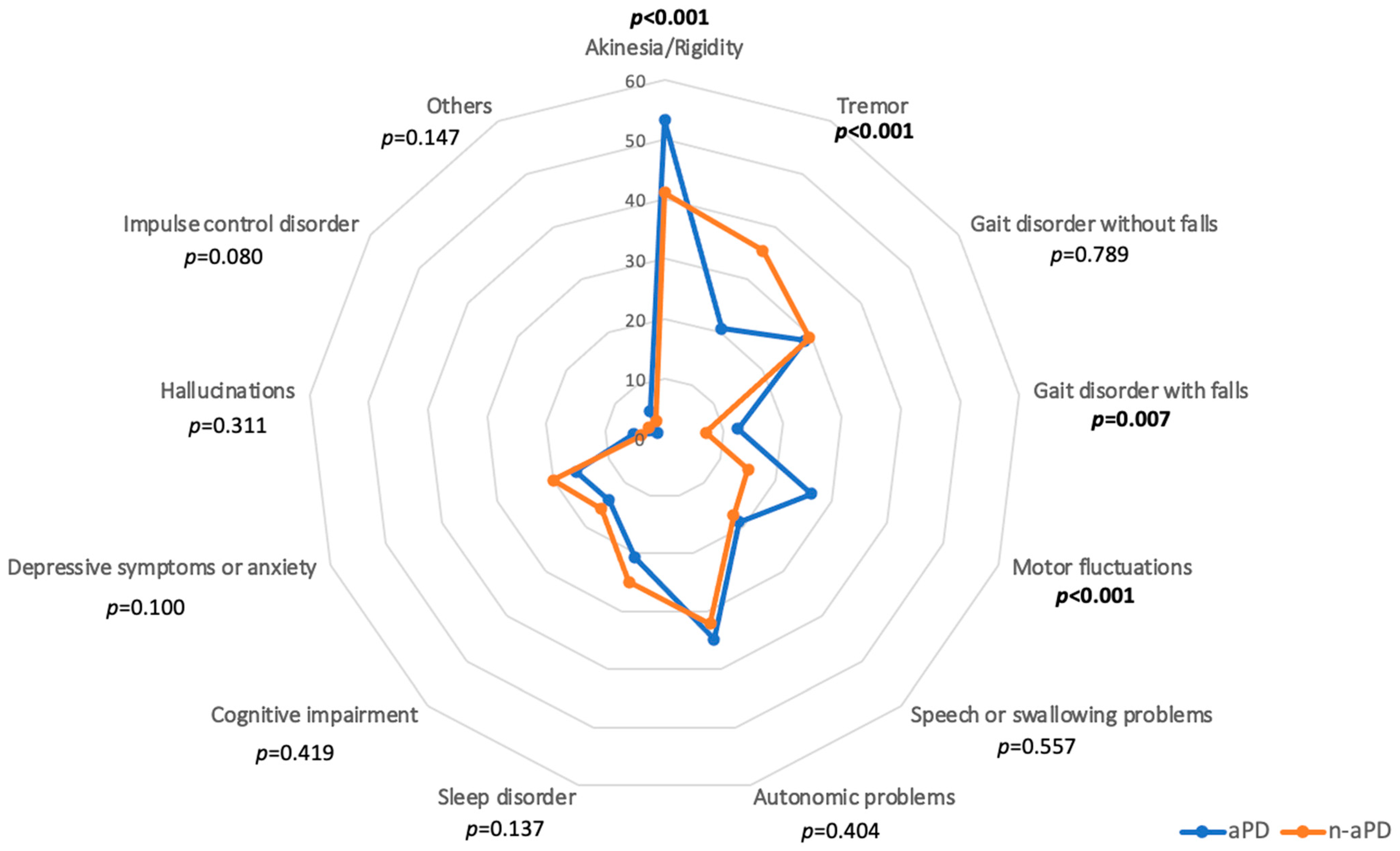
3.2.2. Most Bothersome Symptoms of PwP with and without Suspected aPD (Subitems of Question #8)
4. Discussion
- (1)
- About 12% of rather younger, more severely affected PD patients with earlier disease onset already receive DAT with preference for DBS.
- (2)
- Of the remaining patients, more than 50% show evidence for at least one of three aPD-suspect symptoms, indicating higher numbers of aPD patients as possible candidates for DAT.
- (3)
- The most bothersome symptom profiles of PwP with and without suspected aPD as well as their need for professional LTC vary between groups, possibly indicating different diagnostic and care demands.
4.1. Participants with Device-Aided Therapies
4.2. Participants without Device-Aided Therapies
4.2.1. Indications for aPD and the Possible Need for Device-Aided Therapies
4.2.2. Most Bothersome Symptoms of PwP with and without Suspected aPD
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fasano, A.; Fung, V.S.C.; Lopiano, L.; Elibol, B.; Smolentseva, I.G.; Seppi, K.; Takats, A.; Onuk, K.; Parra, J.C.; Bergmann, L.; et al. Characterizing advanced Parkinson’s disease: OBSERVE-PD observational study results of 2615 patients. BMC Neurol. 2019, 19, 50. [Google Scholar] [CrossRef]
- Antonini, A.; Stoessl, A.J.; Kleinman, L.S.; Skalicky, A.M.; Marshall, T.S.; Sail, K.R.; Onuk, K.; Odin, P.L.A. Developing consensus among movement disorder specialists on clinical indicators for identification and management of advanced Parkinson’s disease: A multi-country Delphi-panel approach. Curr. Med. Res. Opin. 2018, 34, 2063–2073. [Google Scholar] [CrossRef]
- Santos-Garcia, D.; de Deus Fonticoba, T.; Suarez Castro, E.; Aneiros Diaz, A.; McAfee, D. 5-2-1 Criteria: A Simple Screening Tool for Identifying Advanced PD Patients Who Need an Optimization of Parkinson’s Treatment. Park. Dis. 2020, 2020, 7537924. [Google Scholar] [CrossRef]
- Antonini, A.; Odin, P.; Schmidt, P.; Cubillos, F.; Standaert, D.G.; Henriksen, T.; Jimenez-Shahed, J.; Alobaidi, A.; Jalundhwala, Y.J.; Bao, Y.; et al. Validation and clinical value of the MANAGE-PD tool: A clinician-reported tool to identify Parkinson’s disease patients inadequately controlled on oral medications. Park. Relat. Disord. 2021, 92, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Deuschl, G.; Schade-Brittinger, C.; Krack, P.; Volkmann, J.; Schafer, H.; Botzel, K.; Daniels, C.; Deutschlander, A.; Dillmann, U.; Eisner, W.; et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N. Engl. J. Med. 2006, 355, 896–908. [Google Scholar] [CrossRef] [PubMed]
- Cernera, S.; Eisinger, R.S.; Wong, J.K.; Ho, K.W.D.; Lopes, J.L.; To, K.; Carbunaru, S.; Ramirez-Zamora, A.; Almeida, L.; Foote, K.D.; et al. Long-term Parkinson’s disease quality of life after staged DBS: STN vs GPi and first vs second lead. NPJ Park. Dis. 2020, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Valldeoriola, F.; Catalan, M.J.; Escamilla-Sevilla, F.; Freire, E.; Olivares, J.; Cubo, E.; Garcia, D.S.; Calopa, M.; Martinez-Martin, P.; Parra, J.C.; et al. Patient and caregiver outcomes with levodopa-carbidopa intestinal gel in advanced Parkinson’s disease. NPJ Park. Dis. 2021, 7, 108. [Google Scholar] [CrossRef]
- Antonini, A.; Odin, P.; Pahwa, R.; Aldred, J.; Alobaidi, A.; Jalundhwala, Y.J.; Kukreja, P.; Bergmann, L.; Inguva, S.; Bao, Y.; et al. The Long-Term Impact of Levodopa/Carbidopa Intestinal Gel on ‘Off’-time in Patients with Advanced Parkinson’s Disease: A Systematic Review. Adv. Ther. 2021, 38, 2854–2890. [Google Scholar] [CrossRef]
- Drapier, S.; Eusebio, A.; Degos, B.; Verin, M.; Durif, F.; Azulay, J.P.; Viallet, F.; Rouaud, T.; Moreau, C.; Defebvre, L.; et al. Quality of life in Parkinson’s disease improved by apomorphine pump: The OPTIPUMP cohort study. J. Neurol. 2016, 263, 1111–1119. [Google Scholar] [CrossRef]
- Meira, B.; Degos, B.; Corsetti, E.; Doulazmi, M.; Berthelot, E.; Virbel-Fleischman, C.; Dodet, P.; Meneret, A.; Mariani, L.L.; Delorme, C.; et al. Long-term effect of apomorphine infusion in advanced Parkinson’s disease: A real-life study. NPJ Park. Dis. 2021, 7, 50. [Google Scholar] [CrossRef]
- Smilowska, K.; van Wamelen, D.J.; Pietrzykowski, T.; Calvano, A.; Rodriguez-Blazquez, C.; Martinez-Martin, P.; Odin, P.; Chaudhuri, K.R. Cost-Effectiveness of Device-Aided Therapies in Parkinson’s Disease: A Structured Review. J. Park. Dis. 2021, 11, 475–489. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, D.J.; Gandor, F.; Jost, W.H.; Arlt, C.; Onuk, K.; Timmermann, L. Characterization of advanced Parkinson’s disease in Germany: Results of the non-interventional OBSERVE-PD study. Neurol. Res. Pract. 2022, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Fründt, O.; Hanff, A.M.; Mai, T.; Warnecke, T.; Wellach, I.; Eggers, C.; van Munster, M.; Dodel, R.; Kirchner, C.; Krüger, R.; et al. Scoping-Review zur stationären Langzeitpflege von Menschen mit idiopathischem Parkinson in Deutschland. DGNeurologie 2022, 5, 345–354. [Google Scholar] [CrossRef]
- Shih, T.M.; Sail, K.R.; Jalundhwala, Y.J.; Sullivan, J.; van Eijndhoven, E.; Zadikoff, C.; Marshall, T.S.; Lakdawalla, D.N. The effect of functional status impairment on nursing home admission risk among patients with advanced Parkinson’s disease. J. Med. Econ. 2020, 23, 297–307. [Google Scholar] [CrossRef]
- Stiftung, T. Thiemann Parkinson Care Research-Care4PD Study. Available online: https://thiemannstiftung.de/thiemann-parkinson-care-research/ (accessed on 5 March 2023).
- Thiemann Stiftung. Care4PD Study. Available online: https://thiemannstiftung.de/news/care4pd-studie/ (accessed on 5 March 2023).
- Fründt, O.; Hanff, A.-M.; Mai, T.; Kirchner, C.; Bouzanne des Mazery, E.; Amouzandeh, A.; Buhmann, C.; Krüger, R.; Südmeyer, M. Impact of COVID-19 Pandemic on (Health) Care Situation of People with Parkinson’s Disease in Germany (Care4PD). Brain Sci. 2022, 12, 62. [Google Scholar] [CrossRef]
- Green, J. Josephine Green. Available online: https://josephine-green.com/ (accessed on 23 April 2023).
- Edwards, P. Questionnaires in clinical trials: Guidelines for optimal design and administration. Trials 2010, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: Onset, progression and mortality. Neurology 1967, 17, 427–442. [Google Scholar] [CrossRef] [PubMed]
- Mischley, L.K.; Lau, R.C.; Weiss, N.S. Use of a self-rating scale of the nature and severity of symptoms in Parkinson’s Disease (PRO-PD): Correlation with quality of life and existing scales of disease severity. NPJ Park. Dis. 2017, 3, 20. [Google Scholar] [CrossRef] [PubMed]
- Health, U.S.N.I.O. Complementary & Alternative Medicine in Parkinson’s Disease (CAM Care in PD). Available online: https://clinicaltrials.gov/ct2/show/NCT02194816?term=mischley&rank=3 (accessed on 23 April 2023).
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef]
- Jenkinson, C.; Fitzpatrick, R.; Peto, V.; Greenhall, R.; Hyman, N. The PDQ-8: Development and validation of a short-form parkinson’s disease questionnaire. Psychol. Health 1997, 12, 805–814. [Google Scholar] [CrossRef]
- Katz, S. Assessing self-maintenance: Activities of daily living, mobility, and instrumental activities of daily living. J. Am. Geriatr. Soc. 1983, 31, 721–727. [Google Scholar] [CrossRef]
- OCR Systeme GmbH. FormPro Software Version 3.0, Leipzig, Germany. Available online: https://www.ocr-systeme.de/index/formpro/ (accessed on 5 March 2023).
- IBM. SPSS Statistics. Version 27, Armonk, North Castle, United States. Available online: https://www.ibm.com/de-de/analytics/spss-statistics-software (accessed on 5 March 2023).
- Timpka, J.; Nitu, B.; Datieva, V.; Odin, P.; Antonini, A. Device-Aided Treatment Strategies in Advanced Parkinson’s Disease. Int. Rev. Neurobiol. 2017, 132, 453–474. [Google Scholar] [CrossRef] [PubMed]
- Leta, V.; Dafsari, H.S.; Sauerbier, A.; Metta, V.; Titova, N.; Timmermann, L.; Ashkan, K.; Samuel, M.; Pekkonen, E.; Odin, P.; et al. Personalised Advanced Therapies in Parkinson’s Disease: The Role of Non-Motor Symptoms Profile. J. Pers. Med. 2021, 11, 773. [Google Scholar] [CrossRef] [PubMed]
- Boura, I.; Haliasos, N.; Giannopoulou Iota, A.; Karabetsos, D.; Spanaki, C. Combining Device-Aided Therapies in Parkinson’s Disease: A Case Series and a Literature Review. Mov. Disord. Clin. Pract. 2021, 8, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Richter, D.; Bartig, D.; Jost, W.; Jorges, C.; Stumpe, B.; Gold, R.; Krogias, C.; Tonges, L. Dynamics of device-based treatments for Parkinson’s disease in Germany from 2010 to 2017: Application of continuous subcutaneous apomorphine, levodopa-carbidopa intestinal gel, and deep brain stimulation. J. Neural Transm. 2019, 126, 879–888. [Google Scholar] [CrossRef]
- Wachter, T.; Minguez-Castellanos, A.; Valldeoriola, F.; Herzog, J.; Stoevelaar, H. A tool to improve pre-selection for deep brain stimulation in patients with Parkinson’s disease. J. Neurol. 2011, 258, 641–646. [Google Scholar] [CrossRef]
- Schuepbach, W.M.; Rau, J.; Knudsen, K.; Volkmann, J.; Krack, P.; Timmermann, L.; Halbig, T.D.; Hesekamp, H.; Navarro, S.M.; Meier, N.; et al. Neurostimulation for Parkinson’s disease with early motor complications. N. Engl. J. Med. 2013, 368, 610–622. [Google Scholar] [CrossRef]
- Dijk, J.M.; Espay, A.J.; Katzenschlager, R.; de Bie, R.M.A. The Choice Between Advanced Therapies for Parkinson’s Disease Patients: Why, What, and When? J. Park. Dis. 2020, 10, S65–S73. [Google Scholar] [CrossRef]
- Hanna, J.A.; Scullen, T.; Kahn, L.; Mathkour, M.; Gouveia, E.E.; Garces, J.; Evans, L.M.; Lea, G.; Houghton, D.J.; Biro, E.; et al. Comparison of elderly and young patient populations treated with deep brain stimulation for Parkinson’s disease: Long-term outcomes with up to 7 years of follow-up. J. Neurosurg. 2018, 131, 807–812. [Google Scholar] [CrossRef]
- Dafsari, H.S.; Martinez-Martin, P.; Rizos, A.; Trost, M.; Dos Santos Ghilardi, M.G.; Reddy, P.; Sauerbier, A.; Petry-Schmelzer, J.N.; Kramberger, M.; Borgemeester, R.W.K.; et al. EuroInf 2: Subthalamic stimulation, apomorphine, and levodopa infusion in Parkinson’s disease. Mov. Disord. 2019, 34, 353–365. [Google Scholar] [CrossRef]
- Carbone, F.; Djamshidian, A.; Seppi, K.; Poewe, W. Apomorphine for Parkinson’s Disease: Efficacy and Safety of Current and New Formulations. CNS Drugs 2019, 33, 905–918. [Google Scholar] [CrossRef]
- Marsili, L.; Bologna, M.; Miyasaki, J.M.; Colosimo, C. Parkinson’s disease advanced therapies—A systematic review: More unanswered questions than guidance. Park. Relat. Disord. 2021, 83, 132–139. [Google Scholar] [CrossRef]
- Henriksen, T.; Staines, H. Continuous Subcutaneous Apomorphine Infusion in Parkinson’s Disease: A Single-Center, Long-Term Follow-Up Study of the Causes for Discontinuation. J. Pers. Med. 2021, 11, 525. [Google Scholar] [CrossRef] [PubMed]
- Olivola, E.; Fasano, A.; Varanese, S.; Lena, F.; Santilli, M.; Femiano, C.; Centonze, D.; Modugno, N. Continuous subcutaneous apomorphine infusion in Parkinson’s disease: Causes of discontinuation and subsequent treatment strategies. Neurol. Sci. 2019, 40, 1917–1923. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, T.; Dalhoff, K.P.; Hansen, H.E.; Brenneche, A.W.; Lonberg, U.S.; Danielsen, E.H. Access and Use of Device-Aided Therapies for Parkinson’s Disease in Denmark. Mov. Disord. Clin. Pract. 2020, 7, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Nyholm, D.; Jost, W.H. Levodopa-entacapone-carbidopa intestinal gel infusion in advanced Parkinson’s disease: Real-world experience and practical guidance. Ther. Adv. Neurol. Disord. 2022, 15, 17562864221108018. [Google Scholar] [CrossRef]
- Senek, M.; Nielsen, E.I.; Nyholm, D. Levodopa-entacapone-carbidopa intestinal gel in Parkinson’s disease: A randomized crossover study. Mov. Disord. 2017, 32, 283–286. [Google Scholar] [CrossRef]
- Rosebraugh, M.; Stodtmann, S.; Liu, W.; Facheris, M.F. Foslevodopa/foscarbidopa subcutaneous infusion maintains equivalent levodopa exposure to levodopa-carbidopa intestinal gel delivered to the jejunum. Park. Relat. Disord. 2022, 97, 68–72. [Google Scholar] [CrossRef]
- Rosebraugh, M.; Voight, E.A.; Moussa, E.M.; Jameel, F.; Lou, X.; Zhang, G.G.Z.; Mayer, P.T.; Stolarik, D.; Carr, R.A.; Enright, B.P.; et al. Foslevodopa/Foscarbidopa: A New Subcutaneous Treatment for Parkinson’s Disease. Ann. Neurol. 2021, 90, 52–61. [Google Scholar] [CrossRef]
- Rosebraugh, M.; Liu, W.; Neenan, M.; Facheris, M.F. Foslevodopa/Foscarbidopa Is Well Tolerated and Maintains Stable Levodopa and Carbidopa Exposure Following Subcutaneous Infusion. J. Park. Dis. 2021, 11, 1695–1702. [Google Scholar] [CrossRef]
- Lee, K.S.; Clennell, B.; Steward, T.G.J.; Gialeli, A.; Cordero-Llana, O.; Whitcomb, D.J. Focused Ultrasound Stimulation as a Neuromodulatory Tool for Parkinson’s Disease: A Scoping Review. Brain Sci. 2022, 12, 289. [Google Scholar] [CrossRef]
- Lowin, J.; Sail, K.; Baj, R.; Jalundhwala, Y.J.; Marshall, T.S.; Konwea, H.; Chaudhuri, K.R. The cost-effectiveness of levodopa/carbidopa intestinal gel compared to standard care in advanced Parkinson’s disease. J. Med. Econ. 2017, 20, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Heald, A.H.; Livingston, M.; Stedman, M.; Wyrko, Z. Higher levels of apomorphine and rotigotine prescribing reduce overall secondary healthcare costs in Parkinson’s disease. Int. J. Clin. Pract. 2016, 70, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Odin, P.; Ray Chaudhuri, K.; Slevin, J.T.; Volkmann, J.; Dietrichs, E.; Martinez-Martin, P.; Krauss, J.K.; Henriksen, T.; Katzenschlager, R.; Antonini, A.; et al. Collective physician perspectives on non-oral medication approaches for the management of clinically relevant unresolved issues in Parkinson’s disease: Consensus from an international survey and discussion program. Park. Relat. Disord. 2015, 21, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Stangl, S.; Haas, K.; Eggers, C.; Reese, J.P.; Tonges, L.; Volkmann, J. Care of patients with Parkinson’s disease in Germany. Nervenarzt 2020, 91, 493–502. [Google Scholar] [CrossRef]
- Haas, K.; Stangl, S.; Steigerwald, F.; Matthies, C.; Gruber, D.; Kuhn, A.A.; Krauss, J.K.; Sixel-Doring, F.; von Eckardstein, K.; Deuschl, G.; et al. Development of evidence-based quality indicators for deep brain stimulation in patients with Parkinson’s disease and first year experience of implementation of a nation-wide registry. Park. Relat. Disord. 2019, 60, 3–9. [Google Scholar] [CrossRef]
- Sudmeyer, M.; Volkmann, J.; Wojtecki, L.; Deuschl, G.; Schnitzler, A.; Moller, B. Deep brain stimulation—Expectations and doubts. A nationwide questionnaire study of patients with Parkinson’s disease and their family members. Nervenarzt 2012, 83, 481–486. [Google Scholar] [CrossRef]
- Hamberg, K.; Hariz, G.M. The decision-making process leading to deep brain stimulation in men and women with parkinson’s disease—An interview study. BMC Neurol. 2014, 14, 89. [Google Scholar] [CrossRef]
- Katz, M.; Kilbane, C.; Rosengard, J.; Alterman, R.L.; Tagliati, M. Referring patients for deep brain stimulation: An improving practice. Arch. Neurol. 2011, 68, 1027–1032. [Google Scholar] [CrossRef]
- Bhidayasiri, R.; Boonpang, K.; Jitkritsadakul, O.; Calne, S.M.; Henriksen, T.; Trump, S.; Chaiwong, S.; Susang, P.; Boonrod, N.; Sringean, J.; et al. Understanding the role of the Parkinson’s disease nurse specialist in the delivery of apomorphine therpy. Park. Relat. Disord. 2016, 33, S49–S55. [Google Scholar] [CrossRef]
- Moro, E.; Allert, N.; Eleopra, R.; Houeto, J.L.; Phan, T.M.; Stoevelaar, H.; International Study Group on Referral Criteria for DBS. A decision tool to support appropriate referral for deep brain stimulation in Parkinson’s disease. J. Neurol. 2009, 256, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Online Pflegeschule Parkinson. Available online: https://www.online-pflegeschule.de/pflegeschule-parkinson/hauptseite (accessed on 5 March 2023).
- Chandra, V.; Hilliard, J.D.; Foote, K.D. Deep brain stimulation for the treatment of tremor. J. Neurol. Sci. 2022, 435, 120190. [Google Scholar] [CrossRef] [PubMed]
- Debu, B.; De Oliveira Godeiro, C.; Lino, J.C.; Moro, E. Managing Gait, Balance, and Posture in Parkinson’s Disease. Curr. Neurol. Neurosci. Rep. 2018, 18, 23. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, G.; Liu, J. Autonomic dysfunction in Parkinson’s disease: Implications for pathophysiology, diagnosis, and treatment. Neurobiol. Dis. 2020, 134, 104700. [Google Scholar] [CrossRef]
- Palma, J.A.; Kaufmann, H. Treatment of autonomic dysfunction in Parkinson disease and other synucleinopathies. Mov. Disord. 2018, 33, 372–390. [Google Scholar] [CrossRef]
- Dafsari, H.S.; Dos Santos Ghilardi, M.G.; Visser-Vandewalle, V.; Rizos, A.; Ashkan, K.; Silverdale, M.; Evans, J.; Martinez, R.C.R.; Cury, R.G.; Jost, S.T.; et al. Beneficial nonmotor effects of subthalamic and pallidal neurostimulation in Parkinson’s disease. Brain Stimul. 2020, 13, 1697–1705. [Google Scholar] [CrossRef]
- van der Heide, A.; Speckens, A.E.M.; Meinders, M.J.; Rosenthal, L.S.; Bloem, B.R.; Helmich, R.C. Stress and mindfulness in Parkinson’s disease—A survey in 5000 patients. NPJ Park. Dis. 2021, 7, 7. [Google Scholar] [CrossRef]
- Naisby, J.; Amjad, A.; Ratcliffe, N.; Yarnall, A.J.; Rochester, L.; Walker, R.; Baker, K. A Survey of People With Parkinson’s and Their Carers: The Management of Pain in Parkinson’s. J. Geriatr. Psychiatry Neurol. 2021, 35, 8919887211023592. [Google Scholar] [CrossRef]
- Yenilmez, F.; Frundt, O.; Hidding, U.; Buhmann, C. Cannabis in Parkinson’s Disease: The Patients’ View. J. Park. Dis. 2021, 11, 309–321. [Google Scholar] [CrossRef]
| Patients without Device-Aided Therapies (n = 1116) | |||||
|---|---|---|---|---|---|
| Parameter | aPD (n = 627) (Mean [Min-Max] ± SD) or n (%) | Available Data | n-aPD (n = 489) (Mean [Min-Max] ± SD) or n (%) | Available Data | Statistics p-Value |
| Clinical Characteristics | |||||
| Gender (#3) | ♂ 330 (53%) ♀ 290 (47%) | n = 620 | ♂ 262 (54%) ♀ 221 (46%) | n = 483 | p = 0.736 b |
| Age (years, #4) | 73.6 (45–95) ± SD 8.3 | n = 621 | 72.6 (43–91) ± SD 8.8 | n = 481 | p = 0.036 a |
| Age at diagnosis (years, #5) | 62.9 (26–85) ± SD 9.6 | n = 619 | 65.0 (22–87) ± SD 9.5 | n = 477 | p < 0.001 a |
| Hoehn and Yahr stage * (stages 1−5, #7) | 3.3 (1–5) ± SD 1.1 | n = 583 | 2.5 (1–5) ± SD 1.1 | n = 457 | p < 0.001 a |
| ADL (Katz index, total score 0−6, #13) | 4.2 (0–6) ± SD 1.9 | n = 556 | 5.3 (0–6) ± SD 1.2 | n = 457 | p < 0.001 a |
| Professional long-term care (LTC, #23) | LTC: 173 (27.6%) No LTC: 454 (72.4%) | n = 627 | LTC: 62 (12.7%) No LTC: 427 (87.3%) | n = 489 | p < 0.001 b |
| QoL (PDQ-8 total score, #10) | 39.5 (0–100) ± SD 17.3 | n = 515 | 25.6 (0–87.5) ± SD 16.8 | n = 409 | p < 0.001 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fründt, O.; Hanff, A.-M.; Möhl, A.; Mai, T.; Kirchner, C.; Amouzandeh, A.; Buhmann, C.; Krüger, R.; Südmeyer, M. Device-Aided Therapies in Parkinson’s Disease—Results from the German Care4PD Study. Brain Sci. 2023, 13, 736. https://doi.org/10.3390/brainsci13050736
Fründt O, Hanff A-M, Möhl A, Mai T, Kirchner C, Amouzandeh A, Buhmann C, Krüger R, Südmeyer M. Device-Aided Therapies in Parkinson’s Disease—Results from the German Care4PD Study. Brain Sciences. 2023; 13(5):736. https://doi.org/10.3390/brainsci13050736
Chicago/Turabian StyleFründt, Odette, Anne-Marie Hanff, Annika Möhl, Tobias Mai, Christiane Kirchner, Ali Amouzandeh, Carsten Buhmann, Rejko Krüger, and Martin Südmeyer. 2023. "Device-Aided Therapies in Parkinson’s Disease—Results from the German Care4PD Study" Brain Sciences 13, no. 5: 736. https://doi.org/10.3390/brainsci13050736
APA StyleFründt, O., Hanff, A.-M., Möhl, A., Mai, T., Kirchner, C., Amouzandeh, A., Buhmann, C., Krüger, R., & Südmeyer, M. (2023). Device-Aided Therapies in Parkinson’s Disease—Results from the German Care4PD Study. Brain Sciences, 13(5), 736. https://doi.org/10.3390/brainsci13050736






