Resting State Dynamic Reconfiguration of Spatial Attention Cortical Networks and Visuospatial Functioning in Non-Verbal Learning Disability (NVLD): A HD-EEG Investigation
Abstract
1. Introduction
1.1. Neural Correlates of NVLD
1.2. Visuospatial Working Memory and EEG Oscillations
1.3. Spatial Attention Networks
1.4. Aims
1.5. Hypotheses
2. Materials and Methods
2.1. Participants
2.2. Visuospatial Performance
2.3. EEG Resting-State Recording
2.4. EEG Preprocessing
2.5. EEG Source Modeling and Connectivity Analysis
2.6. Discrimination between NVLD and TD Groups: A Machine-Learning Approach
2.7. Behavioral Predictions from Functional Connectivity Matrices
2.8. Discrimination between NVLD and TD: A Graph Theory Approach
2.8.1. Graph Construction
2.8.2. Graph Measures
2.8.3. Global Measures
2.8.4. Nodal Measures
2.8.5. Statistical Analyses between NVLD and TD
3. Results
3.1. Visuospatial Performance Measures
3.2. Rs-Connectivity Differences in the DAN and in the VAN
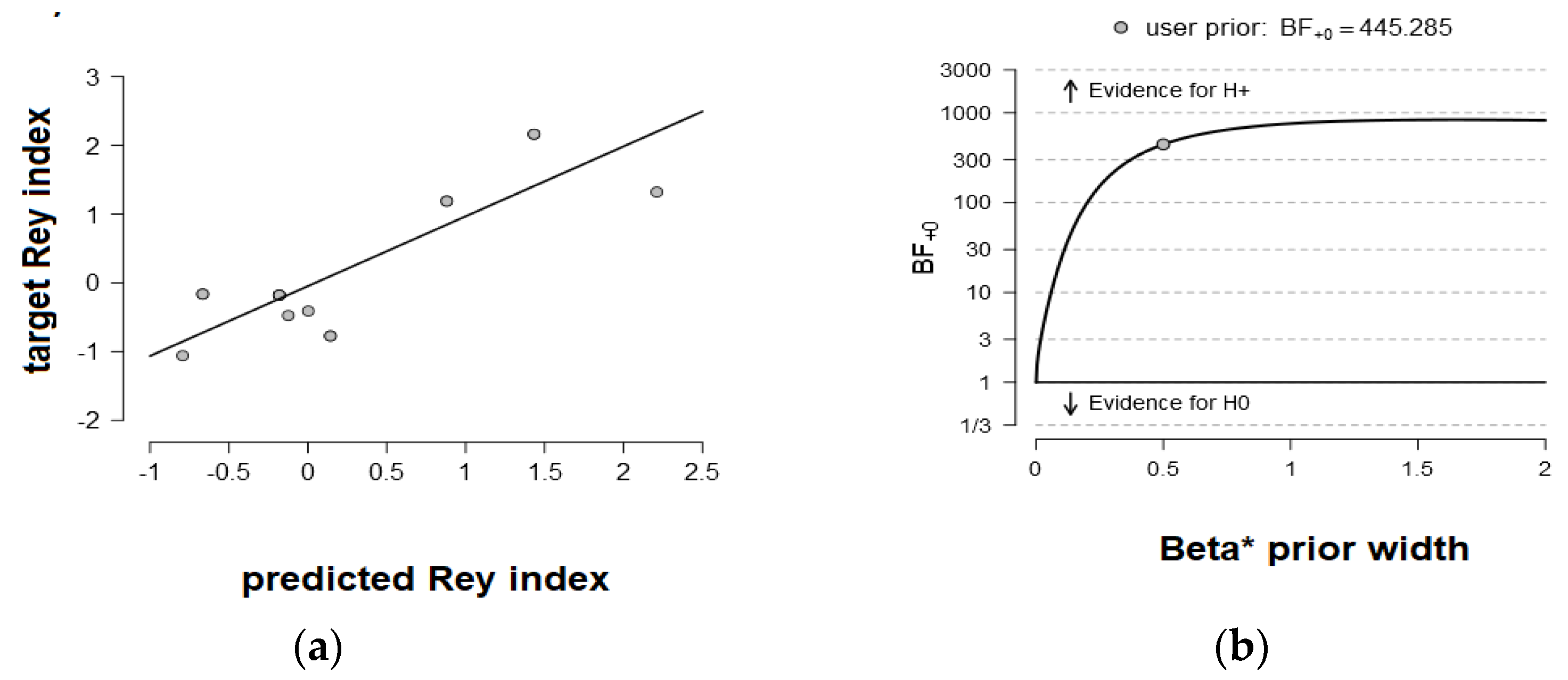
3.3. Behavior Prediction from Functional Connectivity Matrices
3.4. Discrimination between NVLD and TD: A Graph Theory Approach
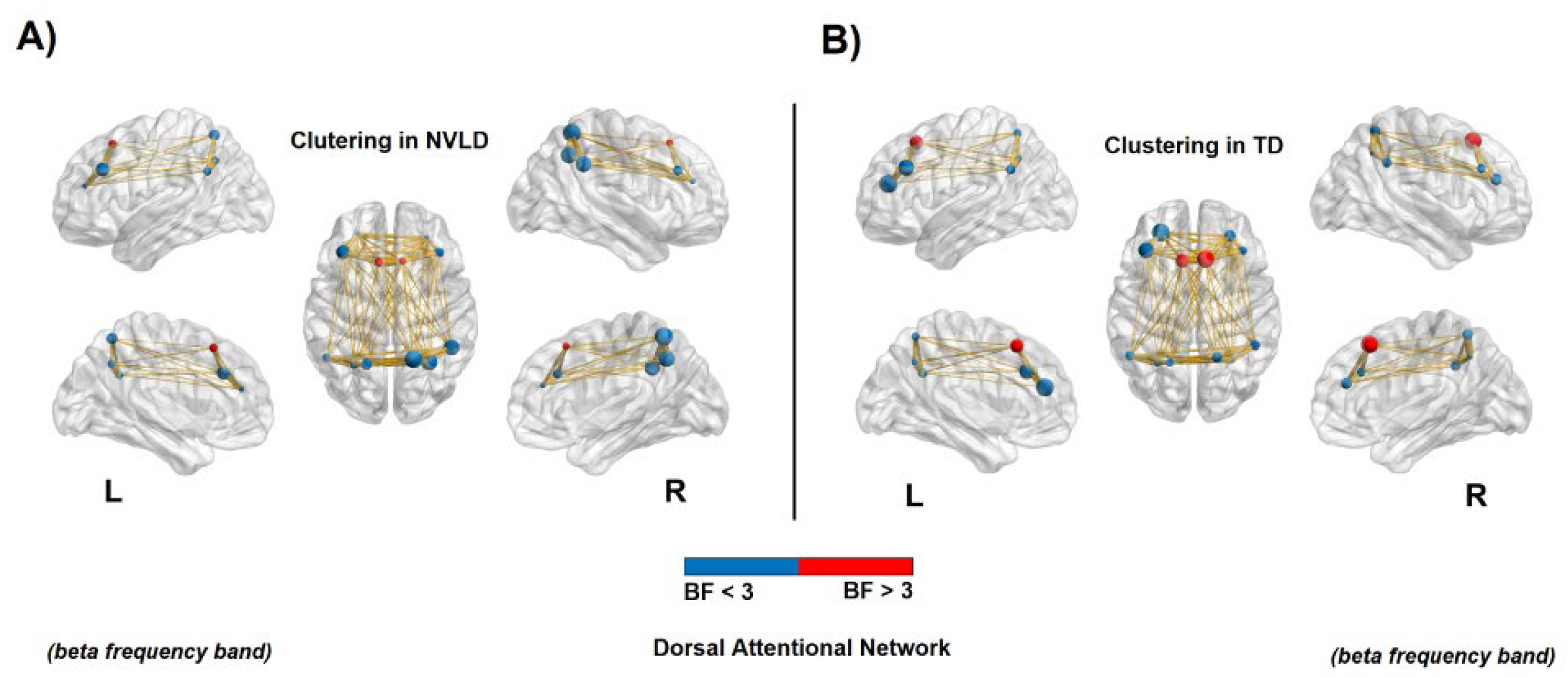
3.4.1. Global Measures
3.4.2. Nodal Measures: Dorsal Attention Network
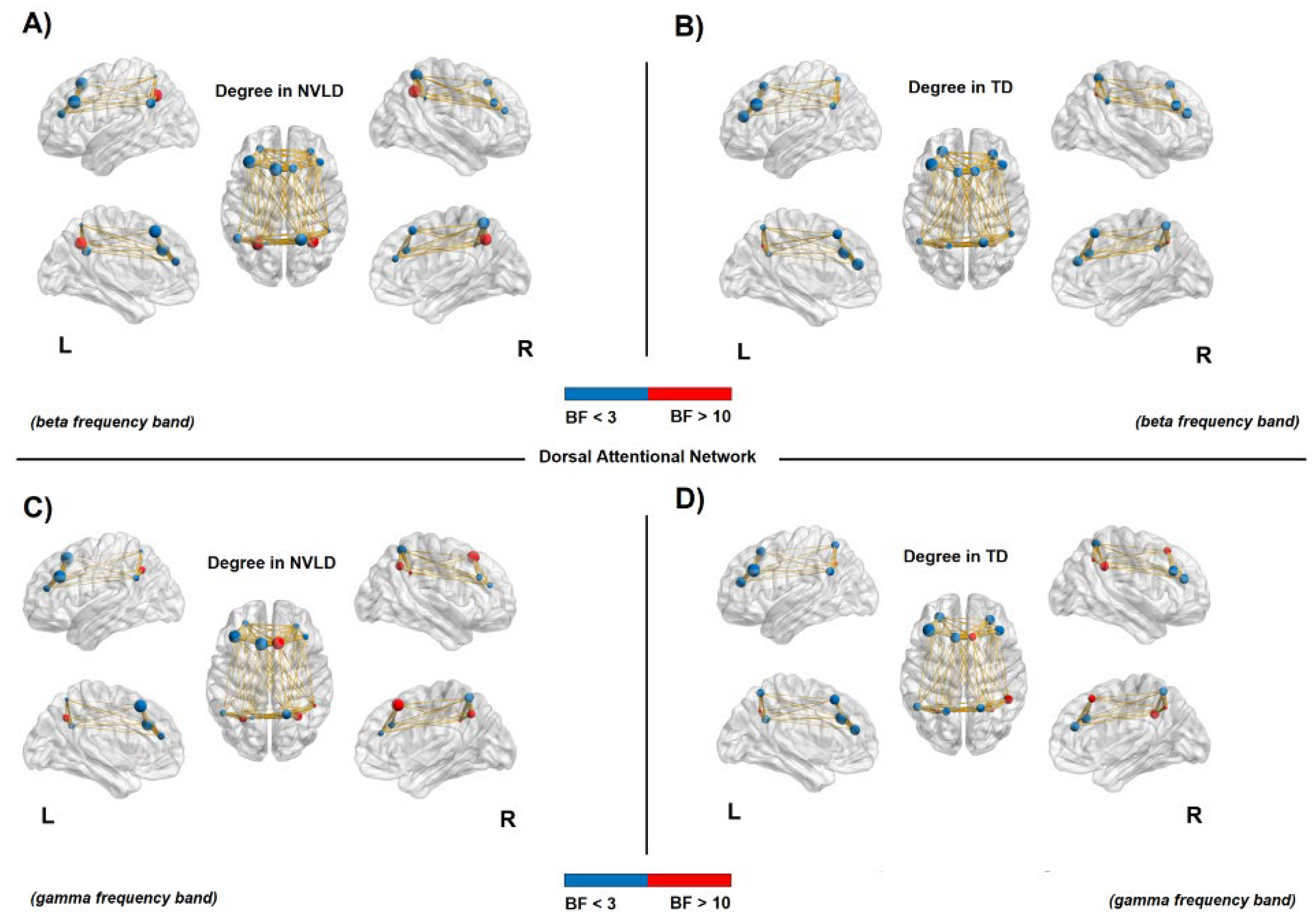
3.4.3. Nodal Measures: Ventral Attention Network
4. Discussion
4.1. Behavioral Measures
4.2. EEG Rs-Functional Connectivity: Discrimination between NVLD and TD
4.3. EEG Rs-Functional Connectivity: Behavior Prediction
4.4. EEG Rs-Functional Connectivity: Discrimination between NVLD and TD with a Graph Theory Approach
4.5. Reconfiguration of Rs-Functional Connectivity in NVLD
4.6. Hemispheric Differences
5. Limitations and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Beta Frequency Band | BF₁₀ | Group | n | Mean | SD | SE |
|---|---|---|---|---|---|---|
| L S_intrapariet_and_P_trans | 40.70 | NVLD | 16 | 1.438 | 1.031 | 0.258 |
| TD | 16 | 0.313 | 0.602 | 0.151 | ||
| R S_intrapariet_and_P_trans | 10.46 | NVLD | 16 | 1.438 | 0.892 | 0.223 |
| TD | 16 | 0.375 | 1.025 | 0.256 | ||
| Gamma frequncy band | BF₁₀ | Group | n | Mean | SD | SE |
| R G_front_sup | 3.521 | NVLD | 16 | 1.813 | 0.834 | 0.209 |
| TD | 16 | 1.000 | 0.966 | 0.242 | ||
| L S_intrapariet_and_P_trans | 218.118 | NVLD | 16 | 1.063 | 0.854 | 0.213 |
| TD | 16 | 0.063 | 0.250 | 0.063 | ||
| R S_intrapariet_and_P_trans | 20.932 | NVLD | 16 | 1.250 | 0.856 | 0.214 |
| TD | 16 | 0.250 | 0.775 | 0.194 | ||
| R S_interm_prim-Jensen | 8.123 | NVLD | 16 | 0.500 | 0.730 | 0.183 |
| TD | 16 | 1.250 | 0.683 | 0.171 |
| Beta Frequency band | BF₁₀ | Group | n | Mean | SD | SE |
|---|---|---|---|---|---|---|
| L S_intrapariet_and_P_trans | 35.45 | NVLD | 16 | 0.951 | 0.728 | 0.182 |
| TD | 16 | 0.178 | 0.407 | 0.102 | ||
| L S_front_middle | 31.10 | NVLD | 16 | 0.684 | 0.632 | 0.158 |
| TD | 16 | 1.399 | 0.464 | 0.116 | ||
| R S_intrapariet_and_P_trans | 39.96 | NVLD | 16 | 0.991 | 0.715 | 0.179 |
| TD | 16 | 0.163 | 0.512 | 0.128 | ||
| Gamma frequency band | BF₁₀ | Group | n | Mean | SD | SE |
| G_front_sup R | 1528.70 | NVLD | 16 | 1.362 | 0.534 | 0.133 |
| TD | 16 | 0.448 | 0.438 | 0.109 | ||
| S_intrapariet_and_P_trans L | 83.332 | NVLD | 16 | 0.696 | 0.660 | 0.165 |
| TD | 16 | 0.018 | 0.071 | 0.018 | ||
| S_front_middle L | 5.103 | NVLD | 16 | 0.709 | 0.612 | 0.153 |
| TD | 16 | 1.261 | 0.517 | 0.129 | ||
| G_front_sup L | 7.685 | NVLD | 16 | 1.391 | 0.696 | 0.174 |
| TD | 16 | 0.724 | 0.567 | 0.142 | ||
| S_intrapariet_and_P_trans R | 12.695 | NVLD | 16 | 0.830 | 0.659 | 0.165 |
| TD | 16 | 0.150 | 0.528 | 0.132 | ||
| G_front_middle R | 4.452 | NVLD | 16 | 0.608 | 0.651 | 0.163 |
| TD | 16 | 1.100 | 0.338 | 0.084 |
| Beta Frequency Band | BF₁₀ | Group | n | Mean | SD | SE |
|---|---|---|---|---|---|---|
| R G_front_sup | 3.716 | NVLD | 16 | 0.043 | 0.172 | 0.043 |
| TD | 16 | 0.281 | 0.326 | 0.082 | ||
| L S_front_middle | 17.602 | NVLD | 16 | 0.017 | 0.068 | 0.017 |
| TD | 16 | 0.295 | 0.322 | 0.080 |
| Beta Frequency Band | BF₁₀ | Group | n | Mean | SD | SE |
|---|---|---|---|---|---|---|
| L G_front_inf-Opercular | 4.386 | NVLD | 16 | 0.010 | 0.039 | 0.010 |
| TD | 16 | 0.213 | 0.302 | 0.075 | ||
| Gamma frequency band | BF₁₀ | Group | n | Mean | SD | SE |
| R G_temp_sup-G_T_transv | 7.256 | NVLD | 16 | 0.330 | 0.300 | 0.075 |
| TD | 16 | 0.069 | 0.189 | 0.047 | ||
| R G_temp_sup-Lateral | 11.286 | NVLD | 16 | 0.347 | 0.296 | 0.074 |
| TD | 16 | 0.069 | 0.189 | 0.047 |
References
- Rourke, B.P. Nonverbal Learning Disabilities: The Syndrome and the Model; Guilford Press: New York, NY, USA, 1989; ISBN 978-0-89862-378-9. [Google Scholar]
- Rourke, B.P. Syndrome of Nonverbal Learning Disabilities: Neurodevelopmental Manifestations; The Guilford Press: New York, NY, USA, 1995; p. 518. ISBN 978-0-89862-155-6. [Google Scholar]
- Mammarella, I.C.; Cornoldi, C. An Analysis of the Criteria Used to Diagnose Children with Nonverbal Learning Disability (NLD). Child Neuropsychol. 2014, 20, 255–280. [Google Scholar] [CrossRef] [PubMed]
- Nichelli, P.; Venneri, A. Right Hemisphere Developmental Learning Disability: A Case Study. Neurocase Case Stud. Neuropsychol. Neuropsychiatry Behav. Neurol. 1995, 1, 173–177. [Google Scholar] [CrossRef]
- Cornoldi, C.; Rigoni, F.; Tressoldi, P.E.; Vio, C. Imagery Deficits in Nonverbal Learning Disabilities. J. Learn. Disabil. 1999, 32, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Mammarella, I.C.; Cornoldi, C. Difficulties in the Control of Irrelevant Visuospatial Information in Children with Visuospatial Learning Disabilities. Acta Psychol. 2005, 118, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Mammarella, I.C.; Giofrè, D.; Ferrara, R.; Cornoldi, C. Intuitive Geometry and Visuospatial Working Memory in Children Showing Symptoms of Nonverbal Learning Disabilities. Child Neuropsychol. 2013, 19, 235–249. [Google Scholar] [CrossRef]
- Cardillo, R.; Vio, C.; Mammarella, I.C. A Comparison of Local-Global Visuospatial Processing in Autism Spectrum Disorder, Nonverbal Learning Disability, ADHD and Typical Development. Res. Dev. Disabil. 2020, 103, 103682. [Google Scholar] [CrossRef]
- Mammarella, I.C.; Cardillo, R.; Zoccante, L. Differences in Visuospatial Processing in Individuals with Nonverbal Learning Disability or Autism Spectrum Disorder without Intellectual Disability. Neuropsychology 2019, 33, 123–134. [Google Scholar] [CrossRef]
- Molenaar-Klumper, M. Non-Verbal Learning Disabilities: Characteristics, Diagnosis and Treatment within an Educational Setting; Jessica Kingsley Publishers: London, UK, 2002; ISBN 978-1-84642-347-5. [Google Scholar]
- Semrud-Clikeman, M.; Walkowiak, J.; Wilkinson, A.; Christopher, G. Neuropsychological Differences among Children with Asperger Syndrome, Nonverbal Learning Disabilities, Attention Deficit Disorder, and Controls. Dev. Neuropsychol. 2010, 35, 582–600. [Google Scholar] [CrossRef]
- Mammarella, I.C.; Meneghetti, C.; Pazzaglia, F.; Gitti, F.; Gomez, C.; Cornoldi, C. Representation of Survey and Route Spatial Descriptions in Children with Nonverbal (Visuospatial) Learning Disabilities. Brain Cogn. 2009, 71, 173–179. [Google Scholar] [CrossRef]
- Mammarella, I.C.; Meneghetti, C.; Pazzaglia, F.; Cornoldi, C. Memory and Comprehension Deficits in Spatial Descriptions of Children with Non-Verbal and Reading Disabilities. Front. Psychol. 2014, 5, 1534. [Google Scholar] [CrossRef]
- Schiff, R.; Bauminger, N.; Toledo, I. Analogical Problem Solving in Children with Verbal and Nonverbal Learning Disabilities. J. Learn. Disabil. 2009, 42, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Agaliotis, I.; Ismirlidou, E. Comparison of Students with Non-Verbal Learning Disabilities and Students with Asperger Syndrome in Solving Word Arithmetic Problems. Eur. J. Spec. Educ. Res. 2018, 3, 27–48. [Google Scholar] [CrossRef]
- Crollen, V.; Vanderclausen, C.; Allaire, F.; Pollaris, A.; Noël, M.-P. Spatial and Numerical Processing in Children with Non-Verbal Learning Disabilities. Res. Dev. Disabil. 2015, 47, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Mammarella, I.C.; Lucangeli, D.; Cornoldi, C. Spatial Working Memory and Arithmetic Deficits in Children with Nonverbal Learning Difficulties. J. Learn. Disabil. 2010, 43, 455–468. [Google Scholar] [CrossRef]
- Semrud-Clikeman, M.; Glass, K. Comprehension of Humor in Children with Nonverbal Learning Disabilities, Reading Disabilities, and without Learning Disabilities. Ann. Dyslexia 2008, 58, 163–180. [Google Scholar] [CrossRef]
- Semrud-Clikeman, M.; Walkowiak, J.; Wilkinson, A.; Minne, E.P. Direct and Indirect Measures of Social Perception, Behavior, and Emotional Functioning in Children with Asperger’s Disorder, Nonverbal Learning Disability, or ADHD. J. Abnorm. Child Psychol. 2010, 38, 509–519. [Google Scholar] [CrossRef]
- Weintraub, S.; Mesulam, M.-M. Developmental Learning Disabilities of the Right Hemisphere: Emotional, Interpersonal, and Cognitive Components. Arch. Neurol. 1983, 40, 463–468. [Google Scholar] [CrossRef]
- Voeller, K.K. Right-Hemisphere Deficit Syndrome in Children. Am. J. Psychiatry 1986, 143, 1004–1009. [Google Scholar] [CrossRef]
- Bogen, J.E.; Gazzaniga, M.S. Cerebral Commissurotomy in Man: Minor Hemisphere Dominance for Certain Visuospatial Functions. J. Neurosurg. 1965, 23, 394–399. [Google Scholar] [CrossRef]
- De Renzi, E. Disorders of Space Exploration and Cognition; John Wiley & Sons: Chichester, UK; New York, NY, USA, 1982; ISBN 978-0-47128-024-8. [Google Scholar]
- Kessels, R.P.C.; de Haan, E.H.F.; Kappelle, L.J.; Postma, A. Selective Impairments in Spatial Memory after Ischaemic Stroke. J. Clin. Exp. Neuropsychol. 2002, 24, 115–129. [Google Scholar] [CrossRef]
- Marshall, J.C.; Fink, G.R. Spatial Cognition: Where We Were and Where We Are. NeuroImage 2001, 14, S2–S7. [Google Scholar] [CrossRef] [PubMed]
- Njiokiktjien, C.; de Rijke, W.; Jonkman, E.J. Children with Nonverbal Learning Disabilities (NLD): Coherence Values in the Resting State May Reflect Hypofunctional Long Distance Connections in the Right Hemisphere. Hum. Physiol. 2001, 27, 523–528. [Google Scholar] [CrossRef]
- Thatcher, R.W.; Krause, P.J.; Hrybyk, M. Cortico-Cortical Associations and EEG Coherence: A Two-Compartmental Model. Electroencephalogr. Clin. Neurophysiol. 1986, 64, 123–143. [Google Scholar] [CrossRef] [PubMed]
- Tucker, D.M.; Roth, D.L.; Bair, T.B. Functional Connections among Cortical Regions: Topography of EEG Coherence. Electroencephalogr. Clin. Neurophysiol. 1986, 63, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Fine, J.G.; Musielak, K.A.; Semrud-Clikeman, M. Smaller Splenium in Children with Nonverbal Learning Disability Compared to Controls, High-Functioning Autism and ADHD. Child Neuropsychol. 2014, 20, 641–661. [Google Scholar] [CrossRef]
- Banker, S.M.; Ramphal, B.; Pagliaccio, D.; Thomas, L.; Rosen, E.; Sigel, A.N.; Zeffiro, T.; Marsh, R.; Margolis, A.E. Spatial Network Connectivity and Spatial Reasoning Ability in Children with Nonverbal Learning Disability. Sci. Rep. 2020, 10, 561. [Google Scholar] [CrossRef]
- Sempere-Ferrandez, A.; Andrés-Bayón, B.; Geijo-Barrientos, E. Callosal Responses in a Retrosplenial Column. Brain Struct. Funct. 2018, 223, 1051–1069. [Google Scholar] [CrossRef]
- Semrud-Clikeman, M.; Fine, J.G.; Bledsoe, J.; Zhu, D.C. Magnetic Resonance Imaging Volumetric Findings in Children with Asperger Syndrome, Nonverbal Learning Disability, or Healthy Controls. J. Clin. Exp. Neuropsychol. 2013, 35, 540–550. [Google Scholar] [CrossRef]
- Margolis, A.E.; Pagliaccio, D.; Thomas, L.; Banker, S.; Marsh, R. Salience Network Connectivity and Social Processing in Children with Nonverbal Learning Disability or Autism Spectrum Disorder. Neuropsychology 2019, 33, 135–143. [Google Scholar] [CrossRef]
- Bassett, D.S.; Wymbs, N.F.; Porter, M.A.; Mucha, P.J.; Carlson, J.M.; Grafton, S.T. Dynamic Reconfiguration of Human Brain Networks during Learning. Proc. Natl. Acad. Sci. USA 2011, 108, 7641–7646. [Google Scholar] [CrossRef]
- Muldoon, S.F.; Bassett, D.S. Why Network Neuroscience? Compelling Evidence and Current Frontiers. Comment on “Understanding Brain Networks and Brain Organization” by Luiz Pessoa. Phys. Life Rev. 2014, 11, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Braun, U.; Schäfer, A.; Walter, H.; Erk, S.; Romanczuk-Seiferth, N.; Haddad, L.; Schweiger, J.I.; Grimm, O.; Heinz, A.; Tost, H.; et al. Dynamic Reconfiguration of Frontal Brain Networks during Executive Cognition in Humans. Proc. Natl. Acad. Sci. USA 2015, 112, 11678–11683. [Google Scholar] [CrossRef] [PubMed]
- Maffei, A.; Sessa, P. Event-Related Network Changes Unfold the Dynamics of Cortical Integration during Face Processing. Psychophysiology 2021, 58, e13786. [Google Scholar] [CrossRef] [PubMed]
- Duma, G.M.; Danieli, A.; Mattar, M.G.; Baggio, M.; Vettorel, A.; Bonanni, P.; Mento, G. Resting State Network Dynamic Reconfiguration and Neuropsychological Functioning in Temporal Lobe Epilepsy: An HD-EEG Investigation. Cortex 2022, 157, 1–13. [Google Scholar] [CrossRef]
- Bhattacharya, J.; Petsche, H.; Feldmann, U.; Rescher, B. EEG Gamma-Band Phase Synchronization between Posterior and Frontal Cortex during Mental Rotation in Humans. Neurosci. Lett. 2001, 311, 29–32. [Google Scholar] [CrossRef]
- Gruber, T.; Müller, M.M.; Keil, A.; Elbert, T. Selective Visual-Spatial Attention Alters Induced Gamma Band Responses in the Human EEG. Clin. Neurophysiol. 1999, 110, 2074–2085. [Google Scholar] [CrossRef]
- Chen, H.; Guo, X.; Lv, Y.; Sun, J.; Tong, S. Mental Rotation Process for Mirrored and Identical Stimuli: A Beta-Band ERD Study. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 4948–4951. [Google Scholar]
- Tallon-Baudry, C. Oscillatory Synchrony and Human Visual Cognition. J. Physiol.-Paris 2003, 97, 355–363. [Google Scholar] [CrossRef]
- von Stein, A.; Sarnthein, J. Different Frequencies for Different Scales of Cortical Integration: From Local Gamma to Long Range Alpha/Theta Synchronization. Int. J. Psychophysiol. 2000, 38, 301–313. [Google Scholar] [CrossRef]
- Basso Garcia, R.; Mammarella, I.C.; Pancera, A.; Galera, C.; Cornoldi, C. Deficits in Visual Short-Term Memory Binding in Children at Risk of Non-Verbal Learning Disabilities. Res. Dev. Disabil. 2015, 45–46, 365–372. [Google Scholar] [CrossRef]
- Corbetta, M.; Shulman, G.L. Control of Goal-Directed and Stimulus-Driven Attention in the Brain. Nat. Rev. Neurosci. 2002, 3, 201–215. [Google Scholar] [CrossRef]
- Silver, M.A.; Kastner, S. Topographic Maps in Human Frontal and Parietal Cortex. Trends Cogn. Sci. 2009, 13, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Jerde, T.A.; Merriam, E.P.; Riggall, A.C.; Hedges, J.H.; Curtis, C.E. Prioritized Maps of Space in Human Frontoparietal Cortex. J. Neurosci. 2012, 32, 17382–17390. [Google Scholar] [CrossRef] [PubMed]
- Sprague, T.C.; Serences, J.T. Attention Modulates Spatial Priority Maps in the Human Occipital, Parietal and Frontal Cortices. Nat. Neurosci. 2013, 16, 1879–1887. [Google Scholar] [CrossRef]
- Sheremata, S.L.; Bettencourt, K.C.; Somers, D.C. Hemispheric Asymmetry in Visuotopic Posterior Parietal Cortex Emerges with Visual Short-Term Memory Load. J. Neurosci. 2010, 30, 12581–12588. [Google Scholar] [CrossRef] [PubMed]
- Szczepanski, S.M.; Konen, C.S.; Kastner, S. Mechanisms of Spatial Attention Control in Frontal and Parietal Cortex. J. Neurosci. 2010, 30, 148–160. [Google Scholar] [CrossRef]
- Corbetta, M.; Patel, G.; Shulman, G.L. The Reorienting System of the Human Brain: From Environment to Theory of Mind. Neuron 2008, 58, 306–324. [Google Scholar] [CrossRef]
- DiQuattro, N.E.; Geng, J.J. Contextual Knowledge Configures Attentional Control Networks. J. Neurosci. 2011, 31, 18026–18035. [Google Scholar] [CrossRef]
- Vossel, S.; Weidner, R.; Thiel, C.M.; Fink, G.R. What Is “Odd” in Posner’s Location-Cueing Paradigm? Neural Responses to Unexpected Location and Feature Changes Compared. J. Cogn. Neurosci. 2009, 21, 30–41. [Google Scholar] [CrossRef]
- Wechsler, D. Wechsler Intelligence Scale for Children—Fourth Edition; WISC-IV; The Psychological Corporation: San Antonio, TX, USA, 2003. [Google Scholar]
- Cornoldi, C.; Mammarella, I.C.; Fine, J.G. Nonverbal Learning Disabilities; Guilford Publications: New York, NY, USA, 2016; ISBN 978-1-46252-759-5. [Google Scholar]
- Beery, K. The Beery-Buktenica Development Test of Visual-Motor Integration: Beery VMI, Administration, Scoring, and Teaching Manual; NCS Pearson: Minneapolis, MN, USA, 2004. [Google Scholar]
- Rutter, M.; Le Couteur, A.; Lord, C. Autism Diagnostic Interview-Revised; Western Psychological Service: Los Angeles, CA, USA, 2003; Volume 29, p. 30. [Google Scholar]
- Rey, A. Épreuves Mnésiques et d’Apprentissage par André Rey: Bon Couverture Souple (1968)|BASEBOOKS. Available online: https://www.abebooks.fr/%C3%89preuves-Mn%C3%A9siques-dApprentissage-Andr%C3%A9-Rey-Delachaux/30610231778/bd (accessed on 9 March 2023).
- Destrieux, C.; Fischl, B.; Dale, A.; Halgren, E. Automatic Parcellation of Human Cortical Gyri and Sulci Using Standard Anatomical Nomenclature. Neuroimage 2010, 53, 1–15. [Google Scholar] [CrossRef]
- Duma, G.M.; Di Bono, M.G.; Mento, G. Grounding Adaptive Cognitive Control in the Intrinsic, Functional Brain Organization: An HD-EEG Resting State Investigation. Brain Sci. 2021, 11, 1513. [Google Scholar] [CrossRef]
- Yadav, S.; Shukla, S. Analysis of K-Fold Cross-Validation over Hold-Out Validation on Colossal Datasets for Quality Classification. In Proceedings of the 2016 IEEE 6th International Conference on Advanced Computing (IACC), Bhimavaram, India, 27–28 February 2016; pp. 78–83. [Google Scholar]
- Arlot, S.; Celisse, A. A Survey of Cross-Validation Procedures for Model Selection. Stat. Surv. 2010, 4, 40–79. [Google Scholar] [CrossRef]
- Di Bono, M.G.; Zorzi, M. Decoding Cognitive States from FMRI Data Using Support Vector Regression. PsychNology J. 2008, 6, 189–201. [Google Scholar]
- Langer, N.; Pedroni, A.; Jäncke, L. The Problem of Thresholding in Small-World Network Analysis. PLoS ONE 2013, 8, e53199. [Google Scholar] [CrossRef] [PubMed]
- Rey, A. L’examen Psychologique Dans Les Cas d’encéphalopathie Traumatique. (Les Problems.). Arch. Psychol. 1941, 28, 215–285. [Google Scholar]
- Cardillo, R.; Mammarella, I.C.; Garcia, R.B.; Cornoldi, C. Local and Global Processing in Block Design Tasks in Children with Dyslexia or Nonverbal Learning Disability. Res. Dev. Disabil. 2017, 64, 96–107. [Google Scholar] [CrossRef]
- Marosi, E.; Harmony, T.; Becker, J.; Reyes, A.; Bernal, J.; Fernández, T.; Rodríguez, M.; Silva, J.; Guerrero, V. Electroencephalographic Coherences Discriminate between Children with Different Pedagogical Evaluation. Int. J. Psychophysiol. 1995, 19, 23–32. [Google Scholar] [CrossRef]
- Jäncke, L.; Alahmadi, N. Resting State EEG in Children with Learning Disabilities: An Independent Component Analysis Approach. Clin. EEG Neurosci. 2016, 47, 24–36. [Google Scholar] [CrossRef]
- Roca-Stappung, M.; Fernández, T.; Bosch-Bayard, J.; Harmony, T.; Ricardo-Garcell, J. Electroencephalographic Characterization of Subgroups of Children with Learning Disorders. PLoS ONE 2017, 12, e0179556. [Google Scholar] [CrossRef]
- Harmony, T.; Hinojosa, G.; Marosi, E.; Becker, J.; Rodriguez, M.; Reyes, A.; Rocha, C. Correlation between EEG Spectral Parameters and an Educational Evaluation. Int. J. Neurosci. 1990, 54, 147–155. [Google Scholar] [CrossRef]
- Thatcher, R.W.; North, D.M.; Biver, C.J. Development of Cortical Connections as Measured by EEG Coherence and Phase Delays. Hum. Brain Mapp. 2008, 29, 1400–1415. [Google Scholar] [CrossRef]
- Scoville, W.B.; Milner, B. Loss of Recent Memory after Bilateral Hippocampal Lesions. J. Neurol. Neurosurg. Psychiatry 1957, 20, 11. [Google Scholar] [CrossRef] [PubMed]
- Ezzati, A.; Katz, M.J.; Zammit, A.R.; Lipton, M.L.; Zimmerman, M.E.; Sliwinski, M.J.; Lipton, R.B. Differential Association of Left and Right Hippocampal Volumes with Verbal Episodic and Spatial Memory in Older Adults. Neuropsychologia 2016, 93, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Cornoldi, C. I Disturbi dell’Apprendimento; il Mulino: Bologna, Italy, 2023. [Google Scholar]
- Smith, E.E.; Jonides, J. Storage and Executive Processes in the Frontal Lobes. Science 1999, 283, 1657–1661. [Google Scholar] [CrossRef]
- Majerus, S.; Cowan, N.; Péters, F.; Van Calster, L.; Phillips, C.; Schrouff, J. Cross-Modal Decoding of Neural Patterns Associated with Working Memory: Evidence for Attention-Based Accounts of Working Memory. Cereb. Cortex 2016, 26, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Todd, J.J.; Marois, R. Capacity Limit of Visual Short-Term Memory in Human Posterior Parietal Cortex. Nature 2004, 428, 751–754. [Google Scholar] [CrossRef]
- Majerus, S.; Attout, L.; D’Argembeau, A.; Degueldre, C.; Fias, W.; Maquet, P.; Martinez Perez, T.; Stawarczyk, D.; Salmon, E.; Van der Linden, M.; et al. Attention Supports Verbal Short-Term Memory via Competition between Dorsal and Ventral Attention Networks. Cereb. Cortex 2012, 22, 1086–1097. [Google Scholar] [CrossRef]
- Paulraj, S.R.; Schendel, K.; Curran, B.; Dronkers, N.F.; Baldo, J.V. Role of the Left Hemisphere in Visuospatial Working Memory. J. Neurolinguist. 2018, 48, 133–141. [Google Scholar] [CrossRef]
- Burgio, F.; Basso, A. Memory and Aphasia. Neuropsychologia 1997, 35, 759–766. [Google Scholar] [CrossRef]
- De Renzi, E.; Nichelli, P. Verbal and Non-Verbal Short-Term Memory Impairment Following Hemispheric Damage. Cortex 1975, 11, 341–354. [Google Scholar] [CrossRef]
- Kasselimis, D.S.; Simos, P.G.; Economou, A.; Peppas, C.; Evdokimidis, I.; Potagas, C. Are Memory Deficits Dependent on the Presence of Aphasia in Left Brain Damaged Patients? Neuropsychologia 2013, 51, 1773–1776. [Google Scholar] [CrossRef]
- Abu-Akel, A.; Shamay-Tsoory, S. Neuroanatomical and Neurochemical Bases of Theory of Mind. Neuropsychologia 2011, 49, 2971–2984. [Google Scholar] [CrossRef] [PubMed]
- Schurz, M.; Radua, J.; Tholen, M.G.; Maliske, L.; Margulies, D.S.; Mars, R.B.; Sallet, J.; Kanske, P. Toward a Hierarchical Model of Social Cognition: A Neuroimaging Meta-Analysis and Integrative Review of Empathy and Theory of Mind. Psychol. Bull. 2021, 147, 293–327. [Google Scholar] [CrossRef] [PubMed]
- Kamali, A.; Flanders, A.E.; Brody, J.; Hunter, J.V.; Hasan, K.M. Tracing Superior Longitudinal Fasciculus Connectivity in the Human Brain Using High Resolution Diffusion Tensor Tractography. Brain Struct. Funct. 2014, 219, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Janelle, F.; Iorio-Morin, C.; D’amour, S.; Fortin, D. Superior Longitudinal Fasciculus: A Review of the Anatomical Descriptions with Functional Correlates. Front. Neurol. 2022, 13, 794618. [Google Scholar] [CrossRef] [PubMed]




| Measures | NVLD (n = 16) Mean (SD) | TD (n = 16) Mean (SD) | Group Differences |
|---|---|---|---|
| Chronological age (months) | 157.19 (21.78) | 155.44 (23.09) | NS * |
| Gender (M:F) | 12:4 | 14:2 | NS |
| Vocabulary 1 | 11.56 (2.53) | 12.25 (1.98) | NS |
| VMI 2 | 77.92 (11.96) | 101.60 (11.86) | NVLD < TD |
| ADI-R 3: A (Reciprocal Social Interactions) | 6.31 (4.99) | 2.29 (2.28) | both groups < clinical cut-off |
| ADI-R 3: B (Language/ Communication) | 4.88 (3.65) | 2.12 (2.62) | both groups < clinical cut-off |
| ADI-R 3: C (Repetitive Behaviors/Interests) | 2.64 (2.37) | 0.41 (0.62) | both groups < clinical cut-off |
| Measures | NVLD (n = 16) Mean (SD) | TD (n = 16) Mean (SD) | BF10 | Group Significance |
|---|---|---|---|---|
| ROCFT copy trial 1 | −4.93 (2.69) | −0.88 (1.27) | 44,791.26 | NVLD < TD |
| ROCFT recall trial 1 | −2.52 (1.15) | −0.56 (1.21) | 357.90 | NVLD < TD |
| ROI | Freq. Bands | Group | BF10 |
|---|---|---|---|
| R-DAN | delta | NVLD | 445.285 |
| L-DAN | gamma | TD | 2343.641 |
| L-VAN | delta | TD | 5.645 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coccaro, A.; Di Bono, M.G.; Maffei, A.; Orefice, C.; Lievore, R.; Mammarella, I.; Liotti, M. Resting State Dynamic Reconfiguration of Spatial Attention Cortical Networks and Visuospatial Functioning in Non-Verbal Learning Disability (NVLD): A HD-EEG Investigation. Brain Sci. 2023, 13, 731. https://doi.org/10.3390/brainsci13050731
Coccaro A, Di Bono MG, Maffei A, Orefice C, Lievore R, Mammarella I, Liotti M. Resting State Dynamic Reconfiguration of Spatial Attention Cortical Networks and Visuospatial Functioning in Non-Verbal Learning Disability (NVLD): A HD-EEG Investigation. Brain Sciences. 2023; 13(5):731. https://doi.org/10.3390/brainsci13050731
Chicago/Turabian StyleCoccaro, Ambra, Maria Grazia Di Bono, Antonio Maffei, Camilla Orefice, Rachele Lievore, Irene Mammarella, and Mario Liotti. 2023. "Resting State Dynamic Reconfiguration of Spatial Attention Cortical Networks and Visuospatial Functioning in Non-Verbal Learning Disability (NVLD): A HD-EEG Investigation" Brain Sciences 13, no. 5: 731. https://doi.org/10.3390/brainsci13050731
APA StyleCoccaro, A., Di Bono, M. G., Maffei, A., Orefice, C., Lievore, R., Mammarella, I., & Liotti, M. (2023). Resting State Dynamic Reconfiguration of Spatial Attention Cortical Networks and Visuospatial Functioning in Non-Verbal Learning Disability (NVLD): A HD-EEG Investigation. Brain Sciences, 13(5), 731. https://doi.org/10.3390/brainsci13050731






