Sinomenine Protects against Early Brain Injury by Inhibiting Microglial Inflammatory Response via Nrf2-Dependent Pathway after Subarachnoid Hemorrhage
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Models of SAH
2.3. Drug Administration
2.4. Experimental Design
2.4.1. Brain Water Content
2.4.2. Neurological Evaluation
2.4.3. Nissl Staining
2.4.4. Western Blot Analysis
2.4.5. Immunohistochemical Staining
2.4.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.4.7. Immunofluorescence Analysis
2.4.8. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
3. Statistical Analyses
4. Results
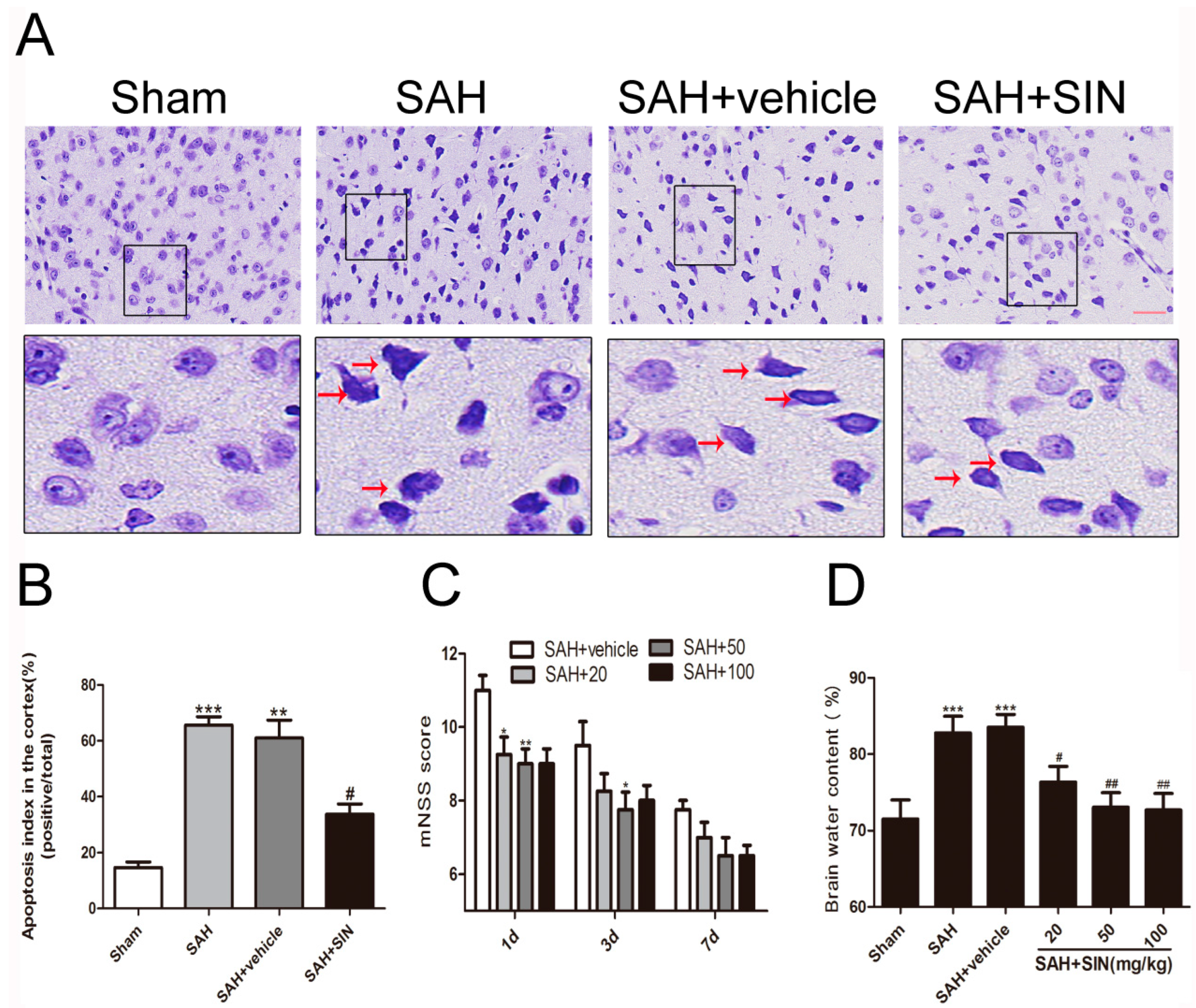
4.1. SIN Ameliorated EBI after SAH in Rats
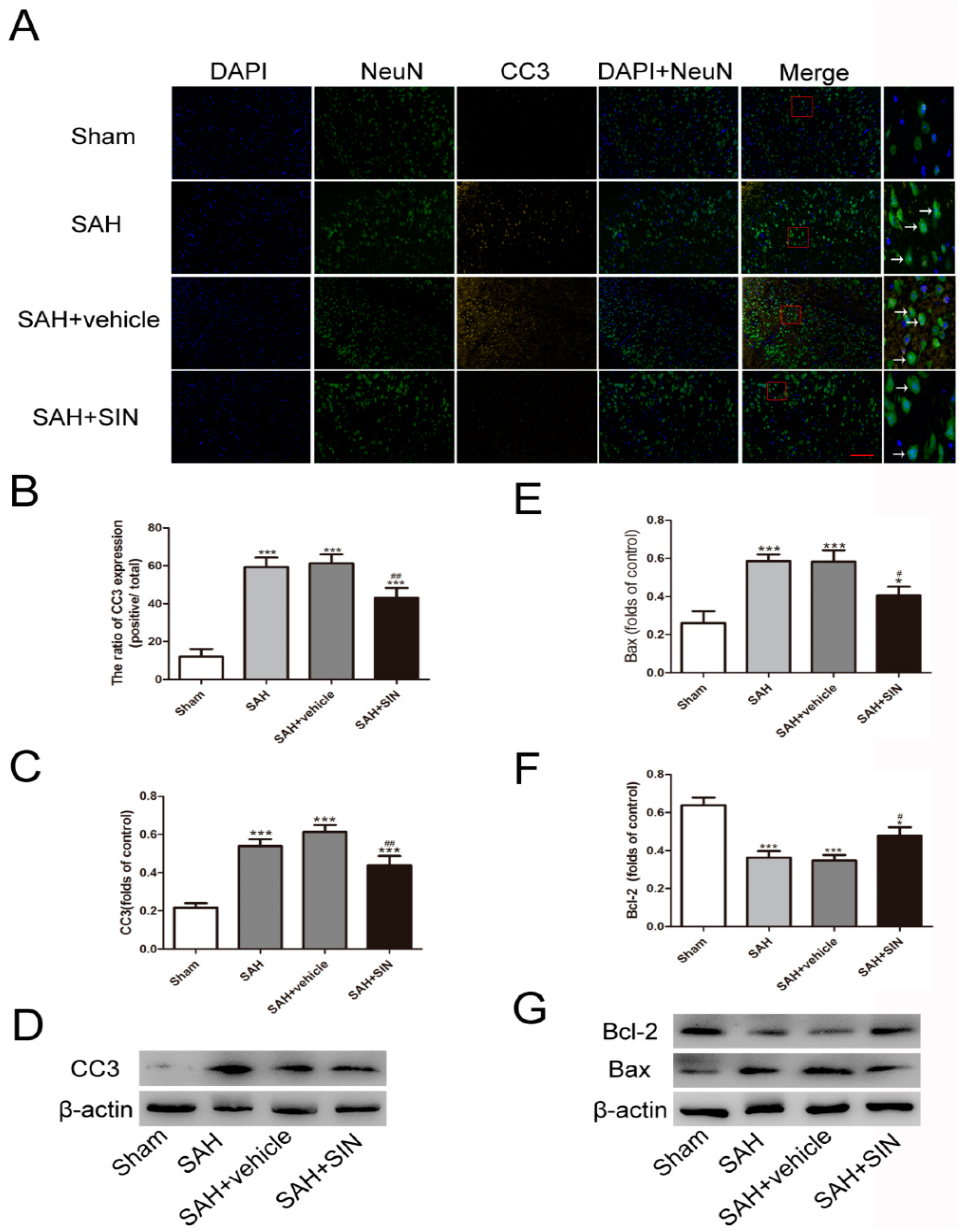
4.2. SIN Decreased Neuronal Degeneration
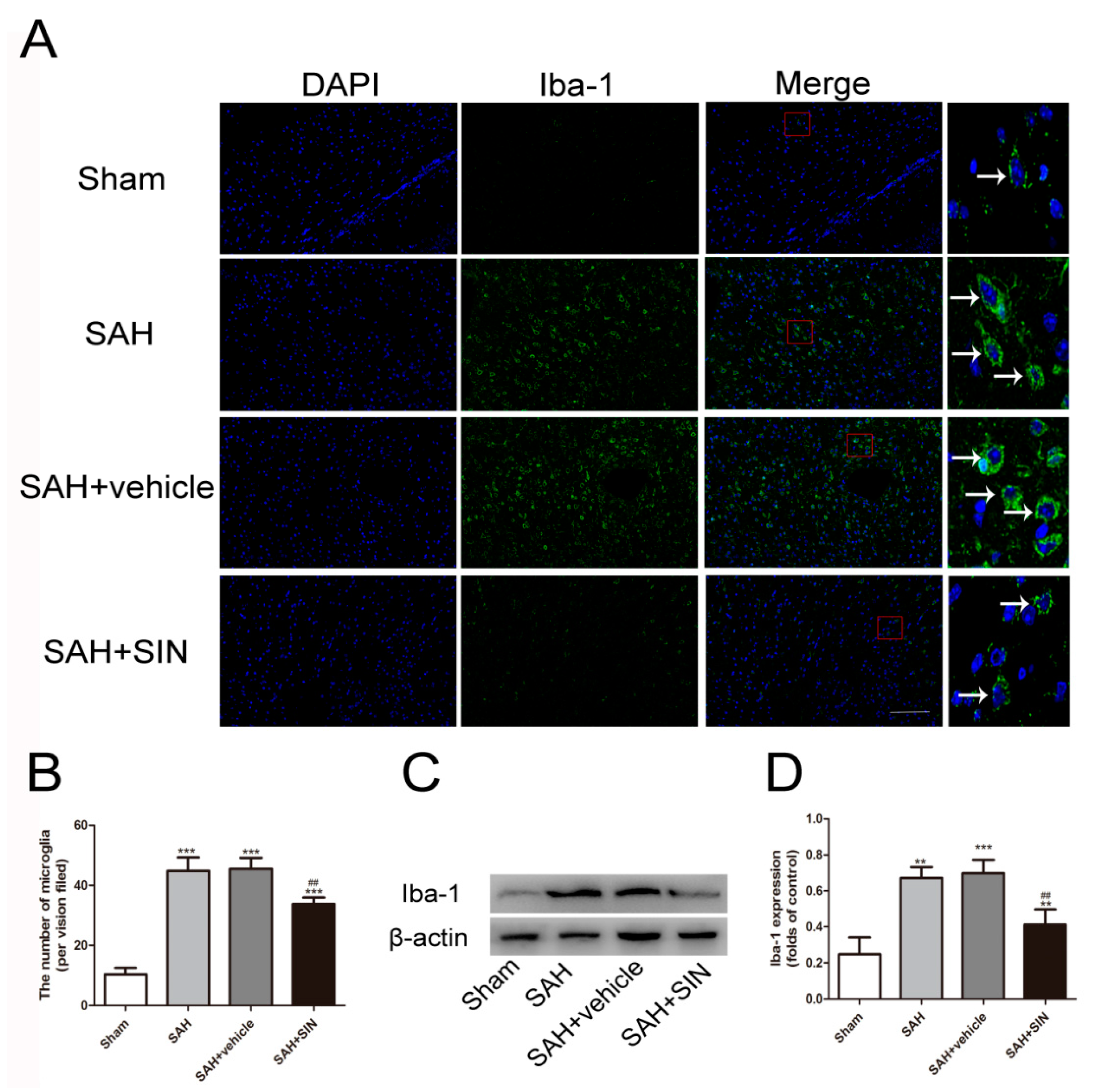
4.3. SIN Inhibits Microglial Activation and Microglia-Mediated Inflammatory Response after SAH
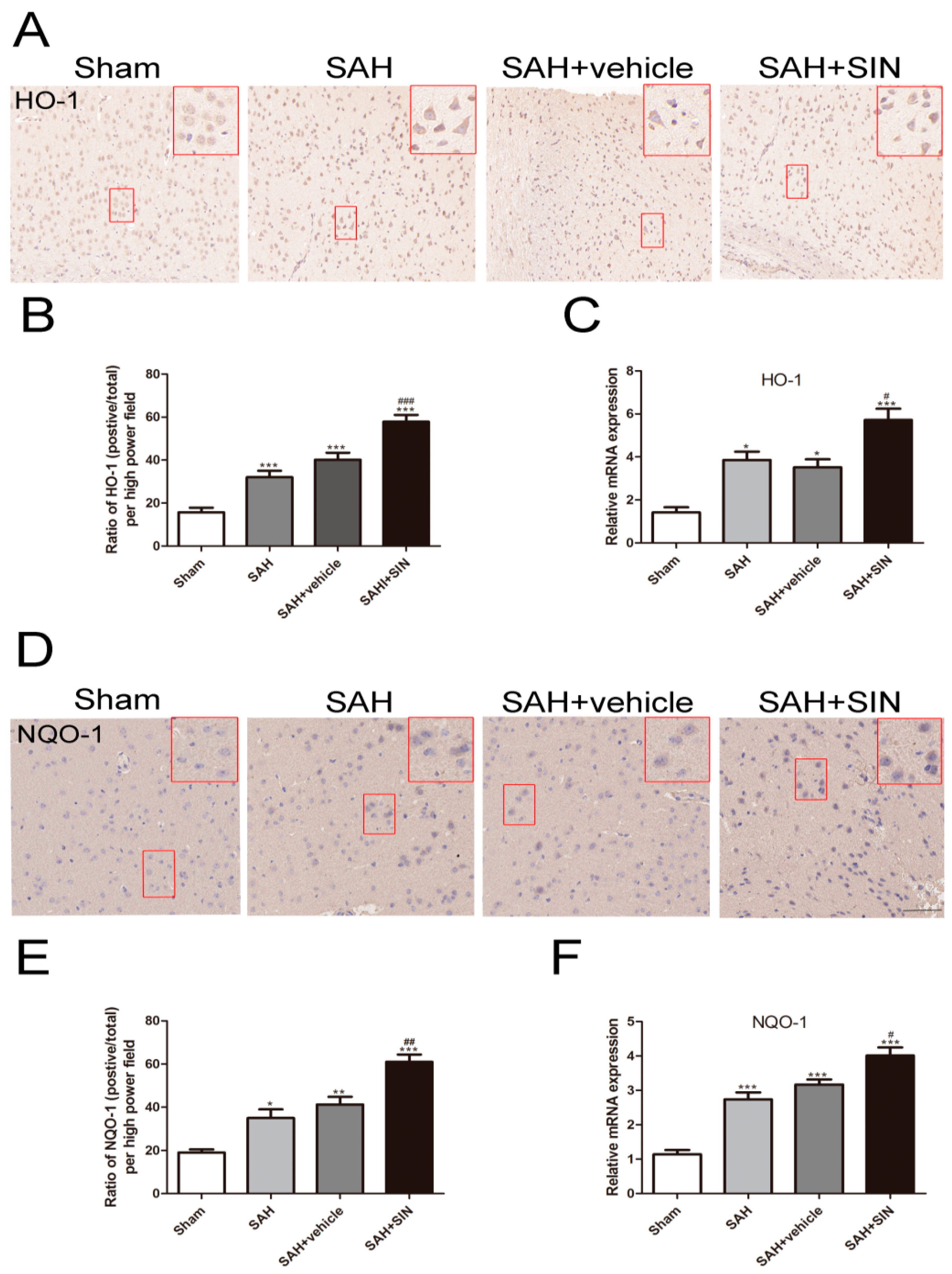
4.4. SIN Promoted Nrf2 Expression and Nuclear Translocation
4.5. SIN Accelerated the Expression of Nrf2 Downstream Factors
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ke, D.Q.; Chen, Z.Y.; Li, Z.L.; Huang, X.; Liang, H. Target inhibition of caspase-8 alleviates brain damage after subarachnoid hemorrhage. Neural Regen. Res. 2020, 15, 1283–1289. [Google Scholar]
- Zuo, Y.; Huang, L.; Enkhjargal, B.; Xu, W.; Umut, O.; Travis, Z.D.; Zhang, G.; Tang, J.; Liu, F.; Zhang, J.H. Activation of retinoid X receptor by bexarotene attenuates Neuroinflamm. via PPARγ/SIRT6/FoxO3a pathway after subarachnoid hemorrhage in rats. J. Neuroinflamm. 2019, 16, 47. [Google Scholar] [CrossRef]
- Xu, P.; Hong, Y.; Xie, Y.; Yuan, K.; Li, J.; Sun, R.; Zhang, X.; Shi, X.; Li, R.; Wu, J.; et al. TREM-1 Exacerbates Neuroinflammatory Injury via NLRP3 Inflammasome-Mediated Pyroptosis in Experimental Subarachnoid Hemorrhage. Transl. Stroke Res. 2020, 12, 643–659. [Google Scholar] [CrossRef]
- Xia, D.-Y.; Yuan, J.-L.; Jiang, X.-C.; Qi, M.; Lai, N.-S.; Wu, L.-Y.; Zhang, X.-S. SIRT1 Promotes M2 Microglia Polarization via Reducing ROS-Mediated NLRP3 Inflammasome Signaling After Subarachnoid Hemorrhage. Front. Immunol. 2021, 12, 770744. [Google Scholar] [CrossRef]
- Song, X.; Long, D. Nrf2 and Ferroptosis: A New Research Direction for Neurodegenerative Diseases. Front. Neurosci. 2020, 14, 267. [Google Scholar] [CrossRef]
- Chen, X.; Wei, G.; Li, D.; Fan, Y.; Zeng, Y.; Qian, Z.; Jia, Z.; Tang, Y.; Shi, Y.; Wu, H.; et al. Sirtuin 1 alleviates microglia-induced inflammation by modulating the PGC-1α/Nrf2 pathway after traumatic brain injury in male rats. Brain Res. Bull. 2022, 185, 28–38. [Google Scholar] [CrossRef]
- Osama, A.; Zhang, J.; Yao, J.; Yao, X.; Fang, J. Nrf2, a dark horse in Alzheimer’s disease treatment. Ageing Res. Rev. 2020, 64, 101206. [Google Scholar] [CrossRef]
- Ren, J.; Su, D.; Li, L.; Cai, H.; Zhang, M.; Zhai, J.; Li, M.; Wu, X.; Hu, K. Anti-inflammatory effects of Aureusidin in LPS-stimulated RAW264.7 macrophages via suppressing NF-κB and activating ROS- and MAPKs-dependent Nrf2/HO-1 signaling pathways. Toxicol. Appl. Pharmacol. 2020, 387, 114846. [Google Scholar] [CrossRef]
- Liu, Q.; Jin, Z.; Xu, Z.; Yang, H.; Li, L.; Li, G.; Li, F.; Gu, S.; Zong, S.; Zhou, J.; et al. Antioxidant effects of ginkgolides and bilobalide against cerebral ischemia injury by activating the Akt/Nrf2 pathway in vitro and in vivo. Cell Stress Chaperones 2019, 24, 441–452. [Google Scholar] [CrossRef]
- Kiasalari, Z.; Afshin-Majd, S.; Baluchnejadmojarad, T.; Azadi-Ahmadabadi, E.; Fakour, M.; Ghasemi-Tarie, R.; Jalalzade-Ogvar, S.; Khodashenas, V.; Tashakori-Miyanroudi, M.; Roghani, M. Sinomenine Alleviates Murine Experimental Autoimmune Encephalomyelitis Model of Multiple Sclerosis through Inhibiting NLRP3 Inflammasome. J. Mol. Neurosci. 2021, 71, 215–224. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, W.; Wang, Y.; Kou, F.; Lyu, C.; Wei, H. Metabolic mechanism and anti-inflammation effects of sinomenine and its major metabolites N-demethylsinomenine and sinomenine-N-oxide. Life Sci. 2020, 261, 118433. [Google Scholar] [CrossRef]
- Sharma, R.; Kambhampati, S.P.; Zhang, Z.; Sharma, A.; Chen, S.; Duh, E.I.; Kannan, S.; Tso, M.O.; Kannan, R.M. Dendrimer mediated targeted delivery of sinomenine for the treatment of acute Neuroinflamm. in traumatic brain injury. J. Control. Release 2020, 323, 361–375. [Google Scholar] [CrossRef]
- Shi, H.; Zheng, K.; Su, Z.; Su, H.; Zhong, M.; He, X.; Zhou, C.; Chen, H.; Xiong, Q.; Zhang, Y. Sinomenine enhances microglia M2 polarization and attenuates inflammatory injury in intracerebral hemorrhage. J. Neuroimmunol. 2016, 299, 28–34. [Google Scholar] [CrossRef]
- Singh, D.; Agrawal, A.; Singal, C.; Pandey, H.S.; Seth, P.; Sharma, S.K. Sinomenine inhibits amyloid beta-induced astrocyte activation and protects neurons against indirect toxicity. Mol. Brain. 2020, 13, 30. [Google Scholar] [CrossRef]
- Zeng, M.Y.; Tong, Q.Y. Anti-inflammation Effects of Sinomenine on Macrophages through Suppressing Activated TLR4/NF-κB Signaling Pathway. Curr. Med. Sci. 2020, 40, 130–137. [Google Scholar] [CrossRef]
- Zhang, X.S.; Wu, Q.; Wu, L.Y.; Ye, Z.N.; Jiang, T.W.; Li, W.; Zhuang, Z.; Zhou, M.L.; Zhang, X.; Huang, C.H. Sirtuin 1 activation protects against early brain injury after experimental subarachnoid hemorrhage in rats. Cell Death Dis. 2016, 7, e2416. [Google Scholar] [CrossRef]
- Gao, Y.; Li, J.; Wu, L.; Zhou, C.; Wang, Q.; Li, X.; Zhou, M.; Wang, H. Tetrahydrocurcumin provides neuroprotection in rats after traumatic brain injury: Autophagy and the PI3K/AKT pathways as a potential mechanism. J. Surg. Res. 2016, 206, 67–76. [Google Scholar] [CrossRef]
- Duan, H.; Li, L.; Shen, S.; Ma, Y.; Yin, X.; Liu, Z.; Yuan, C.; Wang, Y.; Zhang, J. Hydrogen Sulfide Reduces Cognitive Impairment in Rats After Subarachnoid Hemorrhage by Ameliorating Neuroinflamm. Mediated by the TLR4/NF-κB Pathway in Microglia. Front. Cell. Neurosci. 2020, 14, 210. [Google Scholar] [CrossRef]
- Tsai, T.-H.; Chang, C.-H.; Lin, S.-H.; Su, Y.-F.; Tsai, Y.-C.; Yang, S.-F.; Lin, C.-L. Therapeutic effect of and mechanisms underlying the effect of miR-195-5p on subarachnoid hemorrhage-induced vasospasm and brain injury in rats. PeerJ 2021, 9, e11395. [Google Scholar] [CrossRef]
- Wang, J.; Liang, J.; Deng, J.; Liang, X.; Wang, K.; Wang, H.; Qian, D.; Long, H.; Yang, K.; Qi, S. Emerging Role of Microglia-Mediated Neuroinflamm. in Epilepsy after Subarachnoid Hemorrhage. Mol. Neurobiol. 2021, 58, 2780–2791. [Google Scholar] [CrossRef]
- Qin, B.; Peng, Y.; Zhong, C.; Cai, Y.; Zhou, S.; Chen, H.; Zhuang, J.; Zeng, H.; Xu, C.; Xu, H.; et al. Mast Cells Mediate Inflammatory Injury and Aggravate Neurological Impairment in Experimental Subarachnoid Hemorrhage Through Microglial PAR-2 Pathway. Front. Cell. Neurosci. 2021, 15, 710481. [Google Scholar] [CrossRef]
- Zheng, Z.V.; Lyu, H.; Lam, S.; Lam, P.K.; Poon, W.S.; Wong, G. The Dynamics of Microglial Polarization Reveal the Resident Neuroinflammatory Responses After Subarachnoid Hemorrhage. Transl. Stroke Res. 2020, 11, 433–449. [Google Scholar] [CrossRef]
- Du, Y.; Lu, Z.; Yang, D.; Wang, D.; Jiang, L.; Shen, Y.; Du, Q.; Yu, W. MerTK inhibits the activation of the NLRP3 inflammasome after subarachnoid hemorrhage by inducing autophagy. Brain Res. 2021, 1766, 147525. [Google Scholar] [CrossRef]
- Shen, K.-H.; Hung, J.-H.; Liao, Y.-C.; Tsai, S.-T.; Wu, M.-J.; Chen, P.-S. Sinomenine Inhibits Migration and Invasion of Human Lung Cancer Cell through Downregulating Expression of miR-21 and MMPs. Int. J. Mol. Sci. 2020, 21, 3080. [Google Scholar] [CrossRef]
- Bi, F.; Zhang, Y.; Liu, W.; Xie, K. Sinomenine activation of Nrf2 signaling prevents inflammation and cerebral injury in a mouse model of ischemic stroke. Exp. Ther. Med. 2021, 21, 647. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, Y.; Yuan, F.; Li, Z.; Huang, S.; Shen, H.; Yuan, B. Sinomenine inhibits microglia activation and attenuates brain injury in intracerebral hemorrhage. Mol. Immunol. 2014, 60, 109–114. [Google Scholar] [CrossRef]
- Song, W.; Yang, X.; Wang, W.; Wang, Z.; Wu, J.; Huang, F. Sinomenine ameliorates septic acute lung injury in mice by modulating gut homeostasis via aryl hydrocarbon receptor/Nrf2 pathway. Eur. J. Pharmacol. 2021, 912, 174581. [Google Scholar] [CrossRef]
- Gou, Z.; Su, X.; Hu, X.; Zhou, Y.; Huang, L.; Fan, Y.; Lu, L. Melatonin improves hypoxic-ischemic brain damage through the Akt/Nrf2/Gpx4 signaling pathway. Brain Res Bull. 2020, 163, 40–48. [Google Scholar] [CrossRef]
- Zhang, X.S.; Lu, Y.; Li, W.; Tao, T.; Peng, L.; Wang, W.H.; Li, W. Astaxanthin ameliorates oxidative stress and neuronal apoptosis via SIRT1/NRF2/Prx2/ASK1/p38 after traumatic brain injury in mice. Br. J. Pharmacol. 2021, 178, 1114–1132. [Google Scholar] [CrossRef]
- Khan, H.; Tundis, R.; Ullah, H.; Aschner, M.; Belwal, T.; Mirzaei, H.; Akkol, E.K. Flavonoids targeting NRF2 in neurodegenerative disorders. Food Chem. Toxicol. 2020, 146, 111817. [Google Scholar] [CrossRef]
- Marchetti, B. Nrf2/Wnt resilience orchestrates rejuvenation of glia-neuron dialogue in Parkinson’s disease. Redox Biol. 2020, 36, 101664. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, X.-M.; Wu, L.-Y.; Liu, G.-J.; Xu, W.-D.; Zhang, X.-S.; Gao, Y.-Y.; Tao, T.; Zhou, Y.; Lu, Y.; et al. Aucubin alleviates oxidative stress and inflammation via Nrf2-mediated signaling activity in experimental traumatic brain injury. J. Neuroinflamm. 2020, 17, 188. [Google Scholar] [CrossRef]
- Kahroba, H.; Ramezani, B.; Maadi, H.; Sadeghi, M.R.; Jaberie, H.; Ramezani, F. The role of Nrf2 in neural stem/progenitors cells: From maintaining stemness and self-renewal to promoting differentiation capability and facilitating therapeutic application in neurodegenerative disease. Ageing Res. Rev. 2020, 65, 101211. [Google Scholar] [CrossRef]


| Motor Tests Points | |
|---|---|
| Raising rat by the tail | 3 |
| 1 Flexion of forelimb | |
| 1 Flexion of hindlimb | |
| 1 Head moved > 10° to vertical axis within 30 s | |
| Placing rat on the floor | 3 |
| 0 Normal walk | |
| 1 Inability to walk straight | |
| 2 Circling toward the paretic side | |
| 3 Fall down to the paretic side | |
| Sensory tests | 2 |
| 1 Placing test (visual and tactile test) | |
| 2 Proprioceptive test (deep sensation, pushing the paw against the table edge to stimulate limb muscles) | |
| Beam balance tests | 6 |
| 0 Balances with steady posture | |
| 1 Grasps side of beam | |
| 2 Hugs the beam and one limb fall down from the beam | |
| 3 Hugs the beam and two limbs fall down from the beam, or spins on beam (>60 s) | |
| 4 Attempts to balance on the beam but falls off (>40 s) | |
| 5 Attempts to balance on the beam but falls off (>20 s) | |
| 6 Falls off: No attempt to balance or hang on to the beam (<20 s) | |
| Reflexes absent and abnormal movements | 4 |
| 1 Pinna reflex (head shake when touching the auditory meatus) | |
| 1 Corneal reflex (eye blink when lightly touching the cornea with cotton) | |
| 1 Startle reflex (motor response to a brief noise from snapping a clipboard paper | |
| 1 Seizures, myoclonus, myodystony | |
| Maximum points | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, C.; Xin, H.; Qian, Z.; Li, X.; Gao, J.; Fan, Y.; Tang, Y.; Shi, Y.; Li, D.; Wu, H. Sinomenine Protects against Early Brain Injury by Inhibiting Microglial Inflammatory Response via Nrf2-Dependent Pathway after Subarachnoid Hemorrhage. Brain Sci. 2023, 13, 716. https://doi.org/10.3390/brainsci13050716
Fu C, Xin H, Qian Z, Li X, Gao J, Fan Y, Tang Y, Shi Y, Li D, Wu H. Sinomenine Protects against Early Brain Injury by Inhibiting Microglial Inflammatory Response via Nrf2-Dependent Pathway after Subarachnoid Hemorrhage. Brain Sciences. 2023; 13(5):716. https://doi.org/10.3390/brainsci13050716
Chicago/Turabian StyleFu, Chuanjing, Heng Xin, Zhengting Qian, Xiang Li, Juemin Gao, Youwu Fan, Yong Tang, Yan Shi, Ding Li, and Heming Wu. 2023. "Sinomenine Protects against Early Brain Injury by Inhibiting Microglial Inflammatory Response via Nrf2-Dependent Pathway after Subarachnoid Hemorrhage" Brain Sciences 13, no. 5: 716. https://doi.org/10.3390/brainsci13050716
APA StyleFu, C., Xin, H., Qian, Z., Li, X., Gao, J., Fan, Y., Tang, Y., Shi, Y., Li, D., & Wu, H. (2023). Sinomenine Protects against Early Brain Injury by Inhibiting Microglial Inflammatory Response via Nrf2-Dependent Pathway after Subarachnoid Hemorrhage. Brain Sciences, 13(5), 716. https://doi.org/10.3390/brainsci13050716





