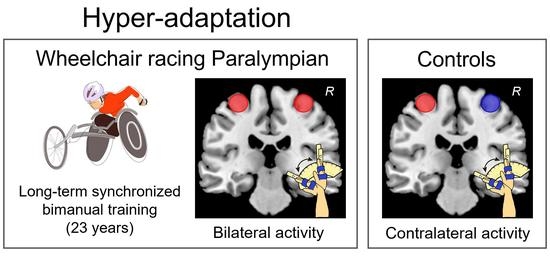
Functional and Structural Properties of Interhemispheric Interaction between Bilateral Precentral Hand Motor Regions in a Top Wheelchair Racing Paralympian
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. General Procedure
2.3. fMRI Experiment
2.4. Acquisition of MRI Data
2.4.1. Functional MRI
2.4.2. Diffusion MRI
2.5. fMRI Task Design
2.5.1. Right-Hand Task
2.5.2. Bimanual Task
2.6. fMRI Data Analysis
2.6.1. fMRI Data Preprocessing
2.6.2. Defining the Precentral Hand Regions
2.6.3. Single-Subject Analysis
2.6.4. Group Analysis
Brain Activation and Deactivation in the Left and Right ROIs in the Control Group
One-to-Many Two-Sample t-Test
Functional Connectivity Analysis
2.7. dMRI Data Analysis
2.7.1. dMRI Data Preprocessing
2.7.2. Tractography
2.7.3. Evaluating Tract Microstructural Properties and Statistical Analysis
3. Results
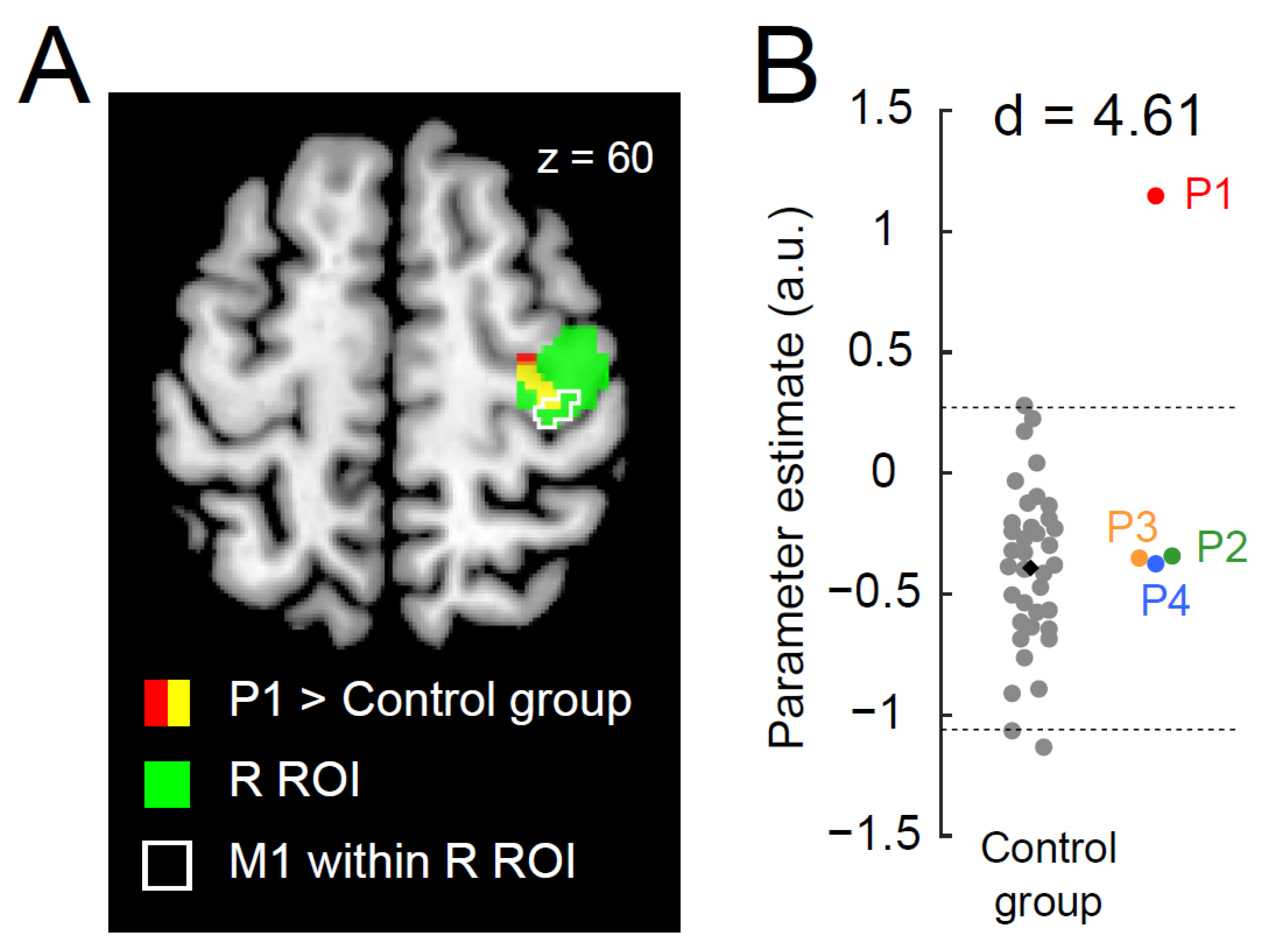
3.1. Ipsilateral Precentral Activation during the Right-Hand Task in P1
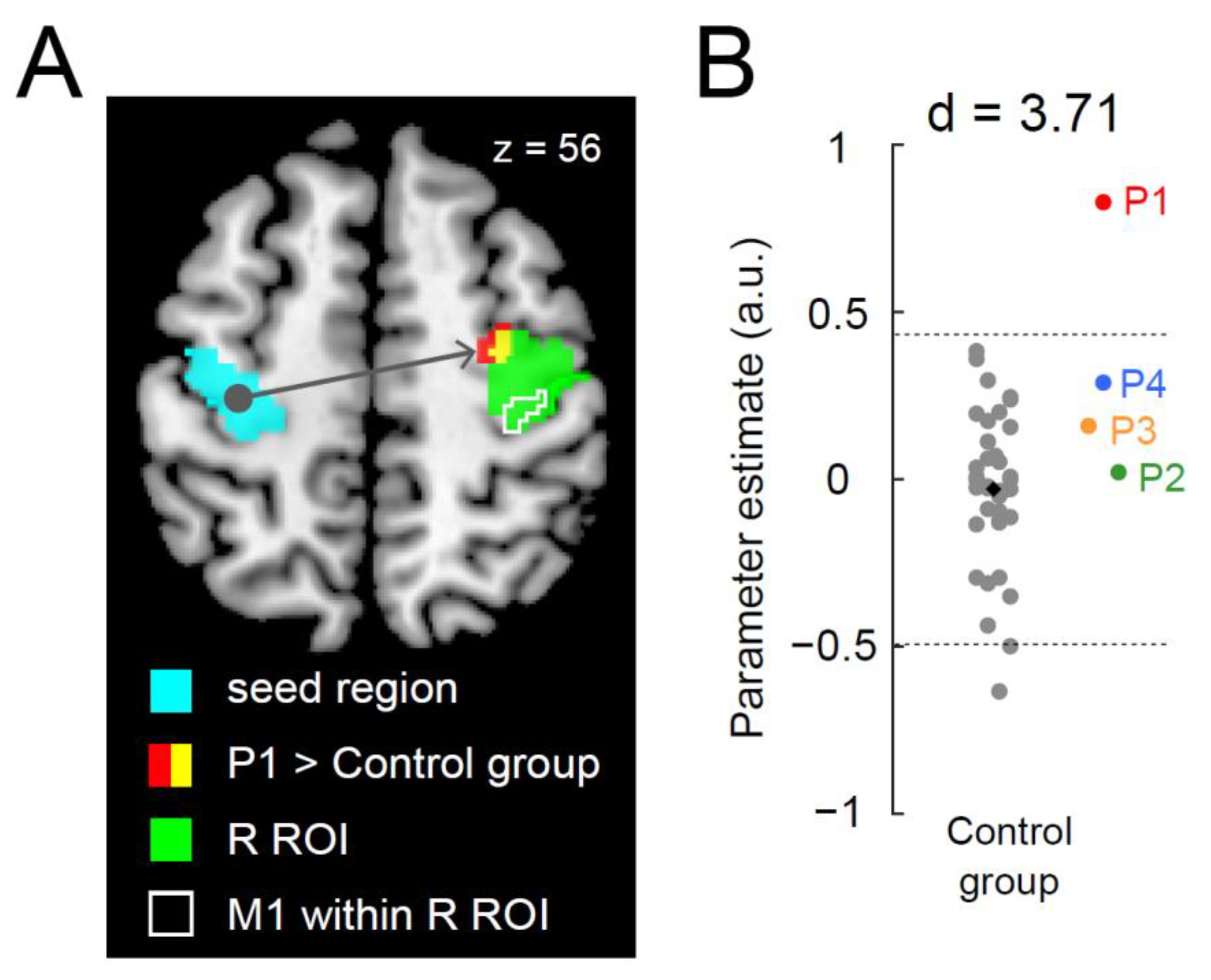
3.2. Greater Functional Connectivity between Bilateral Precentral Hand Sections during the Right-Hand Task in P1
3.3. Microstructural Properties of a Callosal Pathway in P1
4. Discussion
4.1. General
4.2. Ipsilateral Activation in the Right Precentral Motor Regions during a Right-Hand Task
4.3. Development of Transcallosal Pathway in P1
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Callan, D.E.; Naito, E. Neural Processes Distinguishing Elite from Expert and Novice Athletes. Cogn. Behav. Neurol. 2014, 27, 183–188. [Google Scholar] [CrossRef]
- Chang, C.; Chen, Y.; Yen, N. Nonlinear neuroplasticity corresponding to sports experience: A voxel-based morphometry and resting-state functional connectivity study. Hum. Brain Mapp. 2018, 39, 4393–4403. [Google Scholar] [CrossRef]
- Echang, Y. Reorganization and plastic changes of the human brain associated with skill learning and expertise. Front. Hum. Neurosci. 2014, 8, 35. [Google Scholar] [CrossRef]
- Sampaio-Baptista, C.; Khrapitchev, A.A.; Foxley, S.; Schlagheck, T.; Scholz, J.; Jbabdi, S.; DeLuca, G.C.; Miller, K.L.; Taylor, A.; Thomas, N.; et al. Motor Skill Learning Induces Changes in White Matter Microstructure and Myelination. J. Neurosci. 2013, 33, 19499–19503. [Google Scholar] [CrossRef]
- Scholz, J.; Klein, M.C.; Behrens, T.E.J.; Johansen-Berg, H. Training induces changes in white-matter architecture. Nat. Neurosci. 2009, 12, 1370–1371. [Google Scholar] [CrossRef] [PubMed]
- Amunts, K.; Schlaug, G.; Jäncke, L.; Steinmetz, H.; Schleicher, A.; Dabringhaus, A.; Zilles, K. Motor Cortex and Hand Motor Skills: Structural Compliance in the Human Brain. Hum. Brain Mapp. 1997, 5, 206–215. [Google Scholar] [CrossRef]
- Di Paola, M.; Caltagirone, C.; Petrosini, L. Prolonged rock climbing activity induces structural changes in cerebellum and parietal lobe. Hum. Brain Mapp. 2012, 34, 2707–2714. [Google Scholar] [CrossRef]
- Gaser, C.; Schlaug, G. Brain Structures Differ between Musicians and Non-Musicians. J. Neurosci. 2003, 23, 9240–9245. [Google Scholar] [CrossRef]
- Jäncke, L.; Koeneke, S.; Hoppe, A.; Rominger, C.; Hänggi, J. The Architecture of the Golfer’s Brain. PLoS ONE 2009, 4, e4785. [Google Scholar] [CrossRef]
- Lotze, M.; Scheler, G.; Tan, H.-R.M.; Braun, C.; Birbaumer, N. The musician’s brain: Functional imaging of amateurs and professionals during performance and imagery. Neuroimage 2003, 20, 1817–1829. [Google Scholar] [CrossRef]
- Enaito, E.; Ehirose, S. Efficient foot motor control by Neymar’s brain. Front. Hum. Neurosci. 2014, 8, 594. [Google Scholar] [CrossRef]
- Cooper, R.A. Wheelchair racing sports science: A review. J. Rehabil. Res. Dev. 1990, 27, 295–312. [Google Scholar] [CrossRef]
- Allison, J.D.; Meador, K.J.; Loring, D.W.; Figueroa, R.E.; Wright, J.C. Functional MRI cerebral activation and deactivation during finger movement. Neurology 2000, 54, 135. [Google Scholar] [CrossRef]
- Hayashi, M.; Saito, D.N.; Aramaki, Y.; Asai, T.; Fujibayashi, Y.; Sadato, N. Hemispheric Asymmetry of Frequency-Dependent Suppression in the Ipsilateral Primary Motor Cortex during Finger Movement: A Functional Magnetic Resonance Imaging Study. Cereb. Cortex 2008, 18, 2932–2940. [Google Scholar] [CrossRef] [PubMed]
- Marchand, W.R.; Lee, J.N.; Thatcher, J.W.; Thatcher, G.W.; Jensen, C.; Starr, J. Motor deactivation in the human cortex and basal ganglia. Neuroimage 2007, 38, 538–548. [Google Scholar] [CrossRef]
- Morita, T.; Asada, M.; Naito, E. Developmental Changes in Task-Induced Brain Deactivation in Humans Revealed by a Motor Task. Dev. Neurobiol. 2019, 79, 536–558. [Google Scholar] [CrossRef]
- Newton, J.M.; Sunderland, A.; Gowland, P.A. fMRI signal decreases in ipsilateral primary motor cortex during unilateral hand movements are related to duration and side of movement. Neuroimage 2005, 24, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Stefanovic, B.; Warnking, J.M.; Pike, G.B. Hemodynamic and metabolic responses to neuronal inhibition. Neuroimage 2004, 22, 771–778. [Google Scholar] [CrossRef]
- Morita, T.; Asada, M.; Naito, E. Examination of the development and aging of brain deactivation using a unimanual motor task. Adv. Robot. 2021, 35, 842–857. [Google Scholar] [CrossRef]
- Naito, E.; Morita, T.; Hirose, S.; Kimura, N.; Okamoto, H.; Kamimukai, C.; Asada, M. Bimanual digit training improves right-hand dexterity in older adults by reactivating declined ipsilateral motor-cortical inhibition. Sci. Rep. 2021, 11, 22696. [Google Scholar] [CrossRef]
- Ferbert, A.; Priori, A.; Rothwell, J.C.; Day, B.L.; Colebatch, J.G.; Marsden, C.D. Interhemispheric inhibition of the human motor cortex. J. Physiol. 1992, 453, 525–546. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Hutchinson, S.; Schlaug, G.; Pascual-Leone, A. Ipsilateral motor cortex activation on functional magnetic resonance imaging during unilateral hand movements is related to interhemispheric interactions. Neuroimage 2003, 20, 2259–2270. [Google Scholar] [CrossRef] [PubMed]
- Talelli, P.; Ewas, A.; Waddingham, W.; Rothwell, J.C.; Ward, N.S. Neural correlates of age-related changes in cortical neurophysiology. NeuroImage 2008, 40, 1772–1781. [Google Scholar] [CrossRef]
- Ciechanski, P.; Zewdie, E.; Kirton, A. Developmental profile of motor cortex transcallosal inhibition in children and adolescents. J. Neurophysiol. 2017, 118, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Sampaio-Baptista, C.; Johansen-Berg, H. White Matter Plasticity in the Adult Brain. Neuron 2017, 96, 1239–1251. [Google Scholar] [CrossRef]
- Wake, H.; Ortiz, F.C.; Woo, D.H.; Lee, P.R.; Angulo, M.C.; Fields, R.D. Nonsynaptic junctions on myelinating glia promote preferential myelination of electrically active axons. Nat. Commun. 2015, 6, 7844. [Google Scholar] [CrossRef]
- Fields, R.D. A new mechanism of nervous system plasticity: Activity-dependent myelination. Nat. Rev. Neurosci. 2015, 16, 756–767. [Google Scholar] [CrossRef]
- Parlatini, V.; Radua, J.; Dell’acqua, F.; Leslie, A.; Simmons, A.; Murphy, D.G.; Catani, M.; de Schotten, M.T. Functional segregation and integration within fronto-parietal networks. Neuroimage 2017, 146, 367–375. [Google Scholar] [CrossRef]
- Rokem, A.; Takemura, H.; Bock, A.S.; Scherf, K.S.; Behrmann, M.; Wandell, B.A.; Fine, I.; Bridge, H.; Pestilli, F. The visual white matter: The application of diffusion MRI and fiber tractography to vision science. J. Vis. 2017, 17, 4. [Google Scholar] [CrossRef]
- Nozais, V.; Forkel, S.J.; Foulon, C.; Petit, L.; de Schotten, M.T. Functionnectome as a framework to analyse the contribution of brain circuits to fMRI. Commun. Biol. 2021, 4, 1035. [Google Scholar] [CrossRef] [PubMed]
- Grotheer, M.; Kubota, E.; Grill-Spector, K. Establishing the functional relevancy of white matter connections in the visual system and beyond. Anat. Embryol. 2022, 227, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Morita, T.; Hirose, S.; Kimura, N.; Takemura, H.; Asada, M.; Naito, E. Hyper-Adaptation in the Human Brain: Functional and Structural Changes in the Foot Section of the Primary Motor Cortex in a Top Wheelchair Racing Paralympian. Front. Syst. Neurosci. 2022, 16, 780652. [Google Scholar] [CrossRef] [PubMed]
- Morita, T.; Naito, E. Facilitation of Hand Proprioceptive Processing in Paraplegic Individuals with Long-Term Wheelchair Sports Training. Brain Sci. 2022, 12, 1295. [Google Scholar] [CrossRef]
- Moeller, S.; Yacoub, E.; Olman, C.A.; Auerbach, E.; Strupp, J.; Harel, N.; Uğurbil, K. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn. Reson. Med. 2010, 63, 1144–1153. [Google Scholar] [CrossRef]
- Setsompop, K.; Cohen-Adad, J.; Gagoski, B.; Raij, T.; Yendiki, A.; Keil, B.; Wedeen, V.; Wald, L. Improving diffusion MRI using simultaneous multi-slice echo planar imaging. Neuroimage 2012, 63, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.C.; Kamber, M.; Collins, D.L.; MacDonald, D. An MRI-Based Probabilistic Atlas of Neuroanatomy. In Magnetic Resonance Scanning and Epilepsy; Shorvon, S.D., Fish, D.R., Andermann, F., Bydder, G.M., Stefan, H., Eds.; NATO ASI Series; Springer: Boston, MA, USA, 1994; pp. 263–274. ISBN 978-1-4615-2546-2. [Google Scholar]
- Naito, E.; Nakashima, T.; Kito, T.; Aramaki, Y.; Okada, T.; Sadato, N. Human limb-specific and non-limb-specific brain representations during kinesthetic illusory movements of the upper and lower extremities. Eur. J. Neurosci. 2007, 25, 3476–3487. [Google Scholar] [CrossRef] [PubMed]
- Tzoutio-Mazoyera, N.; Landeau, B.; Papathanassiou, D.; Crivello, F.; Etard, O.; Delcroix, N.; Tzourio-Mazoyer, B.; Joliot, M. Automated Anatomical Labeling of Activations in SPM Using a Macroscopic Anatomical Parcellation of the MNI MRI Single-Subject Brain. Neuroimage 2002, 15, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Worsley, K.J.; Marrett, S.; Neelin, P.; Vandal, A.C.; Friston, K.J.; Evans, A.C. A Unified Statistical Approach for Determining Significant Signals in Images of Cerebral Activation. Hum. Brain Mapp. 1996, 4, 58–73. [Google Scholar] [CrossRef]
- Friston, K.; Holmes, A.; Poline, J.-B.; Grasby, P.; Williams, S.; Frackowiak, R.; Turner, R. Analysis of fMRI Time-Series Revisited. Neuroimage 1995, 2, 45–53. [Google Scholar] [CrossRef]
- Worsley, K.; Friston, K. Analysis of fMRI Time-Series Revisited—Again. Neuroimage 1995, 2, 173–181. [Google Scholar] [CrossRef]
- Aguirre, G.; Zarahn, E.; D’Esposito, M. The Inferential Impact of Global Signal Covariates in Functional Neuroimaging Analyses. Neuroimage 1998, 8, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.P.; Friston, K.J. Generalisability, Random Effects and Population Inference. Neuroimage 1998, 7, S754. [Google Scholar] [CrossRef]
- Crawford, J.; Howell, D.C. Comparing an Individual’s Test Score Against Norms Derived from Small Samples. Clin. Neuropsychol. 1998, 12, 482–486. [Google Scholar] [CrossRef]
- Luo, C.; Song, W.; Chen, Q.; Zheng, Z.; Chen, K.; Cao, B.; Yang, J.; Li, J.; Huang, X.; Gong, Q.; et al. Reduced functional connectivity in early-stage drug-naive Parkinson’s disease: A resting-state fMRI study. Neurobiol. Aging 2014, 35, 431–441. [Google Scholar] [CrossRef]
- McLaren, D.G.; Ries, M.L.; Xu, G.; Johnson, S.C. A generalized form of context-dependent psychophysiological interactions (gPPI): A comparison to standard approaches. Neuroimage 2012, 61, 1277–1286. [Google Scholar] [CrossRef]
- Behzadi, Y.; Restom, K.; Liau, J.; Liu, T.T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 2007, 37, 90–101. [Google Scholar] [CrossRef]
- Andersson, J.L.; Skare, S.; Ashburner, J. How to correct susceptibility distortions in spin-echo echo-planar images: Application to diffusion tensor imaging. Neuroimage 2003, 20, 870–888. [Google Scholar] [CrossRef]
- Andersson, J.L.R.; Sotiropoulos, S.N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage 2016, 125, 1063–1078. [Google Scholar] [CrossRef]
- Thomason, M.E.; Thompson, P.M. Diffusion Imaging, White Matter, and Psychopathology. Annu. Rev. Clin. Psychol. 2011, 7, 63–85. [Google Scholar] [CrossRef]
- Jeurissen, B.; Tournier, J.-D.; Dhollander, T.; Connelly, A.; Sijbers, J. Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. Neuroimage 2014, 103, 411–426. [Google Scholar] [CrossRef]
- Tournier, J.-D.; Smith, R.; Raffelt, D.; Tabbara, R.; Dhollander, T.; Pietsch, M.; Christiaens, D.; Jeurissen, B.; Yeh, C.-H.; Connelly, A. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. Neuroimage 2019, 202, 116137. [Google Scholar] [CrossRef] [PubMed]
- Tournier, J.-D.; Calamante, F.; Connelly, A. Improved Probabilistic Streamlines Tractography by 2nd Order Integration over Fibre Orientation Distributions. Proc. Intl. Soc. Mag. Reson. Med. ISMRM 2010, 18. [Google Scholar]
- Takemura, H.; Caiafa, C.F.; Wandell, B.A.; Pestilli, F. Ensemble Tractography. PLoS Comput. Biol. 2016, 12, e1004692. [Google Scholar] [CrossRef] [PubMed]
- Pestilli, F.; Yeatman, J.D.; Rokem, A.; Kay, K.; Wandell, B.A. Evaluation and statistical inference for human connectomes. Nat. Methods 2014, 11, 1058–1063. [Google Scholar] [CrossRef]
- Smith, R.E.; Tournier, J.-D.; Calamante, F.; Connelly, A. Anatomically-constrained tractography: Improved diffusion MRI streamlines tractography through effective use of anatomical information. Neuroimage 2012, 62, 1924–1938. [Google Scholar] [CrossRef]
- Desikan, R.S.; Ségonne, F.; Fischl, B.; Quinn, B.T.; Dickerson, B.C.; Blacker, D.; Buckner, R.L.; Dale, A.M.; Maguire, R.P.; Hyman, B.T.; et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 2006, 31, 968–980. [Google Scholar] [CrossRef]
- Fischl, B. FreeSurfer. Neuroimage 2012, 62, 774–781. [Google Scholar] [CrossRef]
- Yeatman, J.D.; Dougherty, R.F.; Myall, N.J.; Wandell, B.A.; Feldman, H. Tract Profiles of White Matter Properties: Automating Fiber-Tract Quantification. PLoS ONE 2012, 7, e49790. [Google Scholar] [CrossRef]
- Eickhoff, S.B.; Stephan, K.E.; Mohlberg, H.; Grefkes, C.; Fink, G.R.; Amunts, K.; Zilles, K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 2005, 25, 1325–1335. [Google Scholar] [CrossRef]
- Faupin, A.; Borel, B.; Meyer, C.; Gorce, P.; Watelain, E. Effects of synchronous versus asynchronous mode of propulsion on wheelchair basketball sprinting. Disabil. Rehabil. Assist. Technol. 2013, 8, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Forte, P.; Marinho, D.A.; Morais, J.E.; Morouço, P.; Barbosa, T.M. Estimation of mechanical power and energy cost in elite wheelchair racing by analytical procedures and numerical simulations. Comput. Methods Biomech. Biomed. Eng. 2018, 21, 585–592. [Google Scholar] [CrossRef]
- Belyk, M.; Banks, R.; Tendera, A.; Chen, R.; Beal, D.S. Paradoxical facilitation alongside interhemispheric inhibition. Exp. Brain Res. 2021, 239, 3303–3313. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Leodori, G.; Vial, F.; Zhang, Y.; Avram, A.V.; Pajevic, S.; Basser, P.J.; Hallett, M. Measuring latency distribution of transcallosal fibers using transcranial magnetic stimulation. Brain Stimul. 2020, 13, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.; Swayne, O.B.; Vandermeeren, Y.; Camus, M.; Dimyan, M.A.; Harris-Love, M.; Perez, M.A.; Ragert, P.; Rothwell, J.C.; Cohen, L.G. Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. J. Physiol. 2008, 586, 325–351. [Google Scholar] [CrossRef]
- Dum, R.P.; Strick, P.L. Spinal Cord Terminations of the Medial Wall Motor Areas in Macaque Monkeys. J. Neurosci. 1996, 16, 6513–6525. [Google Scholar] [CrossRef]
- Morecraft, R.J.; Ge, J.; Stilwell-Morecraft, K.S.; McNeal, D.W.; Pizzimenti, M.A.; Darling, W.G. Terminal distribution of the corticospinal projection from the hand/arm region of the primary motor cortex to the cervical enlargement in rhesus monkey. J. Comp. Neurol. 2013, 521, 4205–4235. [Google Scholar] [CrossRef]
- Lotze, M.; Markert, J.; Sauseng, P.; Hoppe, J.; Plewnia, C.; Gerloff, C. The Role of Multiple Contralesional Motor Areas for Complex Hand Movements after Internal Capsular Lesion. J. Neurosci. 2006, 26, 6096–6102. [Google Scholar] [CrossRef]
- Steele, C.J.; Bailey, J.A.; Zatorre, R.J.; Penhune, V.B. Early Musical Training and White-Matter Plasticity in the Corpus Callosum: Evidence for a Sensitive Period. J. Neurosci. 2013, 33, 1282–1290. [Google Scholar] [CrossRef]
- Grussu, F.; Schneider, T.; Tur, C.; Yates, R.L.; Tachrount, M.; Ianuş, A.; Yiannakas, M.C.; Newcombe, J.; Zhang, H.; Alexander, D.C.; et al. Neurite dispersion: A new marker of multiple sclerosis spinal cord pathology? Ann. Clin. Transl. Neurol. 2017, 4, 663–679. [Google Scholar] [CrossRef]
- Kelm, N.D.; West, K.; Carson, R.; Gochberg, D.F.; Ess, K.C.; Does, M.D. Evaluation of diffusion kurtosis imaging in ex vivo hypomyelinated mouse brains. Neuroimage 2016, 124, 612–626. [Google Scholar] [CrossRef] [PubMed]
- Moll, N.M.; Rietsch, A.M.; Thomas, S.; Ransohoff, A.J.; Lee, J.-C.; Fox, R.; Chang, A.; Ransohoff, R.M.; Fisher, E. Multiple sclerosis normal-appearing white matter: Pathology-imaging correlations. Ann. Neurol. 2011, 70, 764–773. [Google Scholar] [CrossRef]
- Schmidt, H.; Knösche, T.R. Action potential propagation and synchronisation in myelinated axons. PLoS Comput. Biol. 2019, 15, e1007004. [Google Scholar] [CrossRef] [PubMed]
- Assaf, Y.; Johansen-Berg, H.; de Schotten, M.T. The role of diffusion MRI in neuroscience. NMR Biomed. 2019, 32, e3762. [Google Scholar] [CrossRef] [PubMed]
- Wandell, B.A.; Le, R.K. Diagnosing the Neural Circuitry of Reading. Neuron 2017, 96, 298–311. [Google Scholar] [CrossRef] [PubMed]
- Lazari, A.; Lipp, I. Can MRI measure myelin? Systematic review, qualitative assessment, and meta-analysis of studies validating microstructural imaging with myelin histology. Neuroimage 2021, 230, 117744. [Google Scholar] [CrossRef] [PubMed]
- Yeatman, J.D.; Wandell, B.A.; Mezer, A.A. Lifespan maturation and degeneration of human brain white matter. Nat. Commun. 2014, 5, 4932. [Google Scholar] [CrossRef]
- Kruper, J.; Yeatman, J.D.; Richie-Halford, A.; Bloom, D.; Grotheer, M.; Caffarra, S.; Kiar, G.; Karipidis, I.I.; Roy, E.; Chandio, B.Q.; et al. Evaluating the Reliability of Human Brain White Matter Tractometry. Apert. Neuro 2021, 1, 10.52294/e6198273-b8e3-4b63-babb-6e6b0da10669. [Google Scholar] [CrossRef]
- Song, S.-K.; Suncd, S.W.; Ramsbottom, M.J.; Changc, C.; Russell, J.; Cross, A. Dysmyelination Revealed through MRI as Increased Radial (but Unchanged Axial) Diffusion of Water. Neuroimage 2002, 17, 1429–1436. [Google Scholar] [CrossRef]
- Song, S.-K.; Sun, S.-W.; Ju, W.-K.; Lin, S.-J.; Cross, A.H.; Neufeld, A.H. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 2003, 20, 1714–1722. [Google Scholar] [CrossRef]
- Beaulieu, C. The basis of anisotropic water diffusion in the nervous system—A technical review. NMR Biomed. 2002, 15, 435–455. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.S.; Leppert, I.R.; Narayanan, S.; Boudreau, M.; Duval, T.; Cohen-Adad, J.; Pike, G.B.; Stikov, N. Promise and pitfalls of g-ratio estimation with MRI. Neuroimage 2018, 182, 80–96. [Google Scholar] [CrossRef] [PubMed]
- Filo, S.; Shtangel, O.; Salamon, N.; Kol, A.; Weisinger, B.; Shifman, S.; Mezer, A.A. Disentangling molecular alterations from water-content changes in the aging human brain using quantitative MRI. Nat. Commun. 2019, 10, 3403. [Google Scholar] [CrossRef]
- Mezer, A.; Yeatman, J.D.; Stikov, N.; Kay, K.; Cho, N.-J.; Dougherty, R.F.; Perry, M.L.; Parvizi, J.; Hua, L.; Butts-Pauly, K.; et al. Quantifying the local tissue volume and composition in individual brains with magnetic resonance imaging. Nat. Med. 2013, 19, 1667–1672. [Google Scholar] [CrossRef]
- Weiskopf, N.; Mohammadi, S.; Lutti, A.; Callaghan, M.F. Advances in MRI-based computational neuroanatomy: From Morphometry to in-Vivo Histology. Curr. Opin. Neurol. 2015, 28, 313–322. [Google Scholar] [CrossRef]
- Cubelli, R.; Della Sala, S. Looking back to go forward: Promoting single case studies. Cortex 2017, 97, A1–A3. [Google Scholar] [CrossRef] [PubMed]


| Participant | P1 | P2 | P3 | P4 |
|---|---|---|---|---|
| Age (in years) | 30 | 61 | 54 | 52 |
| Sex | F | M | M | M |
| Upper extremity motor subscores in ASIA | R:25/25 L:25/25 | R:25/25 L:25/25 | R:25/25 L:25/25 | R:25/25 L:25/25 |
| Upper extremity sensory subscores in ASIA | R:10/10 L:10/10 | R:10/10 L:10/10 | R:10/10 L:10/10 | R:10/10 L:10/10 |
| Leg non-use period (in years) | 30 | 60 | 37 | 31 |
| Neurological level | T12 | Cannot be specified | T3 | T8 |
| Cause | * Spina bifida | Poliomyelitis | Spinal cord injury | Spinal cord injury |
| ASIA impairment scale | A | undefined | A | A |
| SCI | Complete | undefined | Complete | Complete |
| FIM | 101/126 | 108/126 | 101/126 | 101/126 |
| Main wheelchair sports (years played) Training period (age), training days/week, training hours/day | Track racing and marathon (23) 8–14 yo, 2 d/w, 8 h/d 15–17 yo, 7 d/w, 1–8 h/d 18–30 yo, 6 d/w, 2 h/d Total 17,600 h | Basketball (42) 17–22 yo, 4–5 d/w, 3 h/d 23–58 yo, 1–2 d/w, 3 h/d Total 12,150 h | Table tennis (27) 28–54 yo, 2–4 d/w, 2.5–8 h/d Total 20,250 h | Basketball (31) 22–35 yo, 5 d/w, 3 h/d 36–52 yo, 1 d/w, 3 h/d Total 13,050 h |
| Sub-wheelchair sports (years played) Training period (age), training days/week, training hours/day | None | Table tennis (4) 15–18 yo, 1 d/w, 2 h/d Total 300 h | None | Marathon (9) 27–35 yo, 2 d/w, 3 h/d Total 2700 h Fencing (9) 27–35 yo, 2 d/w, 3 h/d Total 2700 h |
| Handedness score | 60 | 7 | 100 | 90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morita, T.; Takemura, H.; Naito, E. Functional and Structural Properties of Interhemispheric Interaction between Bilateral Precentral Hand Motor Regions in a Top Wheelchair Racing Paralympian. Brain Sci. 2023, 13, 715. https://doi.org/10.3390/brainsci13050715
Morita T, Takemura H, Naito E. Functional and Structural Properties of Interhemispheric Interaction between Bilateral Precentral Hand Motor Regions in a Top Wheelchair Racing Paralympian. Brain Sciences. 2023; 13(5):715. https://doi.org/10.3390/brainsci13050715
Chicago/Turabian StyleMorita, Tomoyo, Hiromasa Takemura, and Eiichi Naito. 2023. "Functional and Structural Properties of Interhemispheric Interaction between Bilateral Precentral Hand Motor Regions in a Top Wheelchair Racing Paralympian" Brain Sciences 13, no. 5: 715. https://doi.org/10.3390/brainsci13050715
APA StyleMorita, T., Takemura, H., & Naito, E. (2023). Functional and Structural Properties of Interhemispheric Interaction between Bilateral Precentral Hand Motor Regions in a Top Wheelchair Racing Paralympian. Brain Sciences, 13(5), 715. https://doi.org/10.3390/brainsci13050715