The Leading Role of Brain and Abdominal Radiological Features in the Work-Up of Anti-NMDAR Encephalitis in Children: An Up-To-Date Review
Abstract
:1. Introduction
2. Definition, Etiology, and Pathophysiology
3. Clinical Features
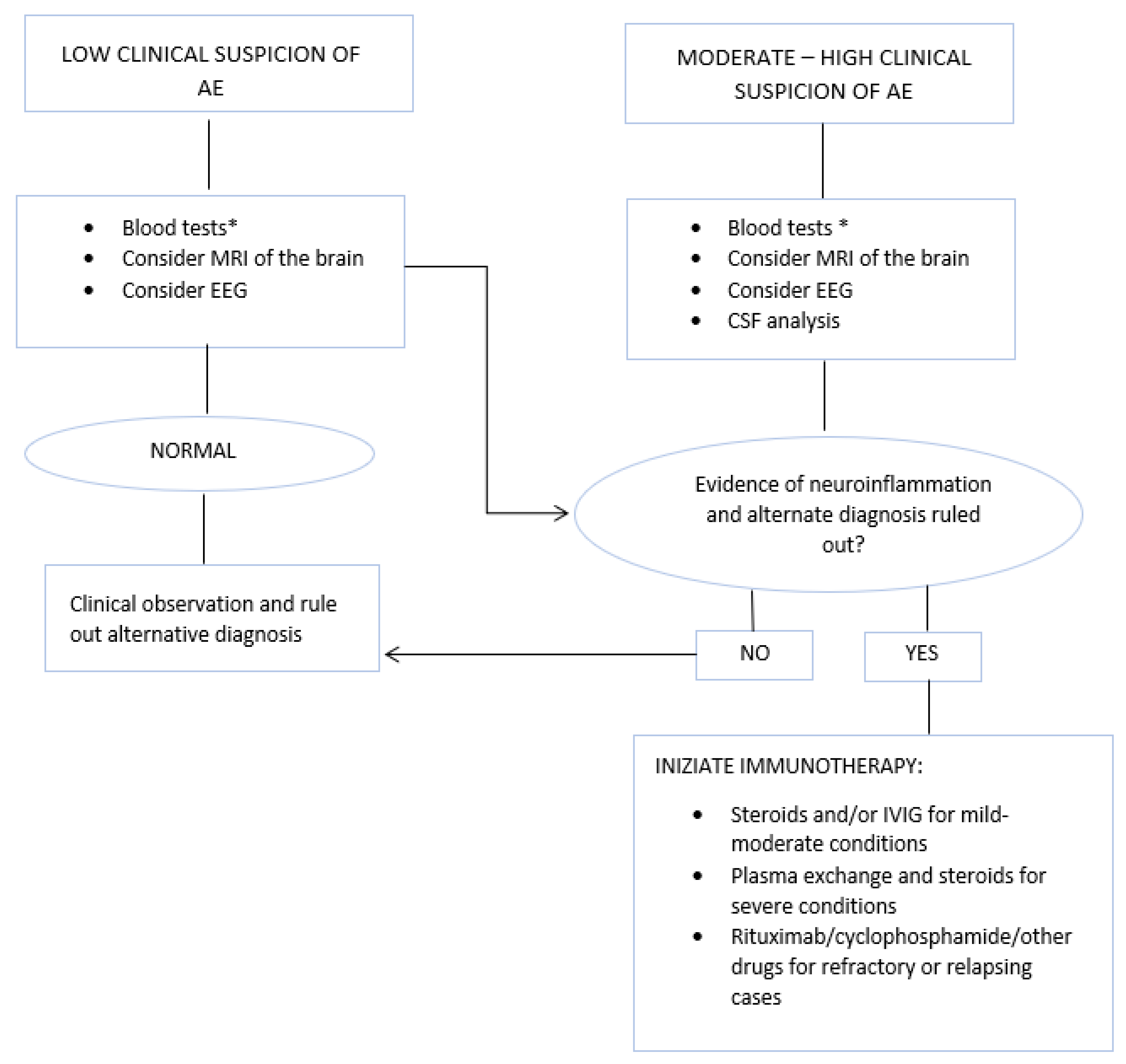
4. Diagnostic Criteria
4.1. Brain MRI Features
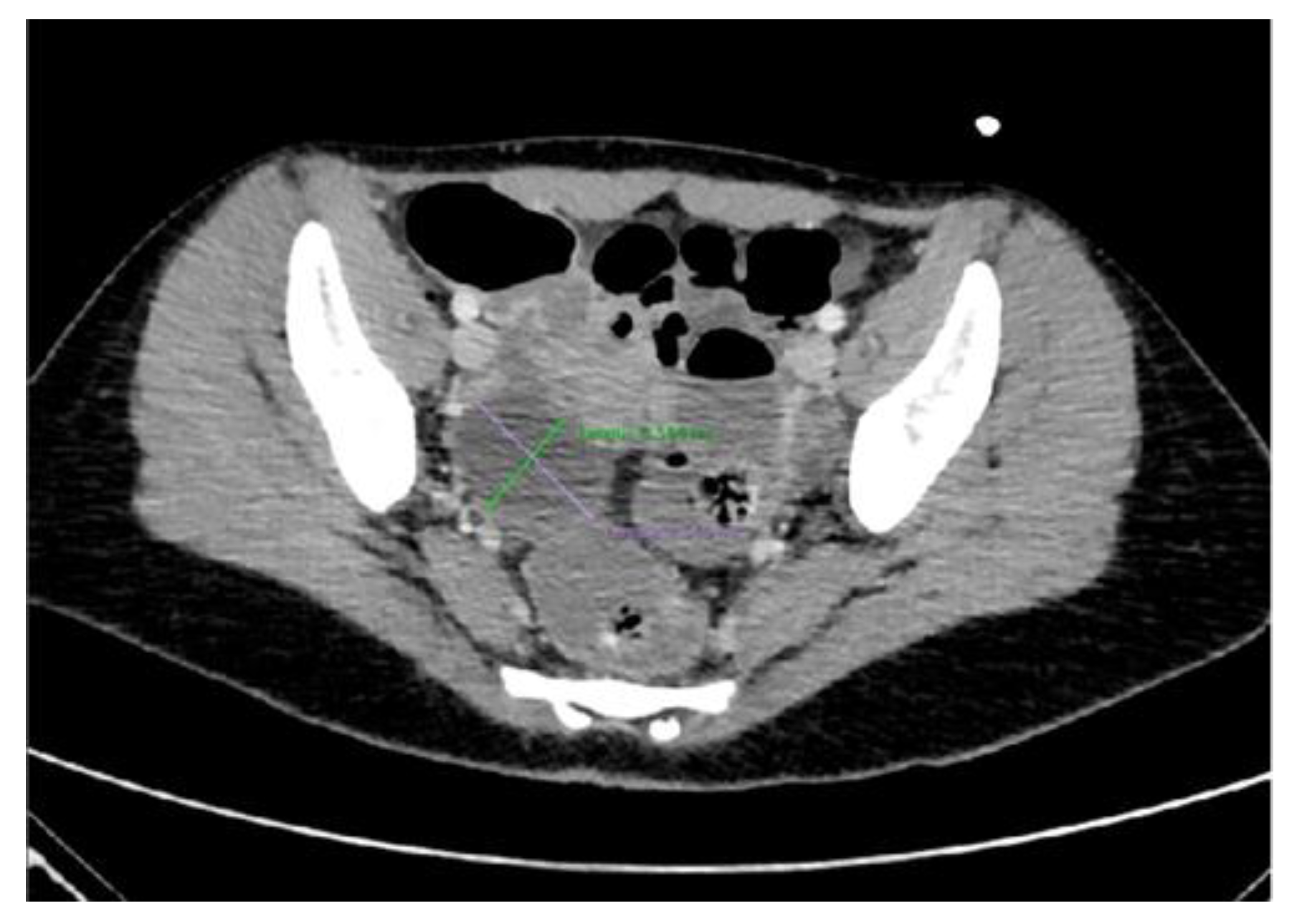
4.2. The Importance of Ultrasonography in NMDARe
5. Treatment
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dalmau, J.; Armangué, T.; Planagumà, J.; Radosevic, M.; Mannara, F.; Leypoldt, F.; Geis, C.; Lancaster, E.; Titulaer, M.J.; Rosenfeld, M.R.; et al. An Update on Anti-NMDA Receptor Encephalitis for Neurologists and Psychiatrists: Mechanisms and Models. Lancet Neurol. 2019, 18, 1045–1057. [Google Scholar] [CrossRef]
- Lynch, D.R.; Rattelle, A.; Dong, Y.N.; Roslin, K.; Gleichman, A.J.; Panzer, J.A. Anti-NMDA Receptor Encephalitis: Clinical Features and Basic Mechanisms. Adv. Pharmacol. 2018, 82, 235–260. [Google Scholar] [PubMed]
- Baizabal-Carvallo, J.F.; Stocco, A.; Muscal, E.; Jankovic, J. The Spectrum of Movement Disorders in Children with Anti-NMDA Receptor Encephalitis. Mov. Disord. 2013, 28, 543–547. [Google Scholar] [CrossRef]
- de Bruijn, M.A.A.M.; Aarsen, F.K.; van Oosterhout, M.P.; van der Knoop, M.M.; Catsman-Berrevoets, C.E.; Schreurs, M.W.J.; Bastiaansen, D.E.M.; Sillevis Smitt, P.A.E.; Neuteboom, R.F.; Titulaer, M.J. Long-Term Neuropsychological Outcome Following Pediatric Anti-NMDAR Encephalitis. Neurology 2018, 90, e1997–e2005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Bruijn, M.A.A.M.; van Sonderen, A.; van Coevorden-Hameete, M.H.; Bastiaansen, A.E.M.; Schreurs, M.W.J.; Rouhl, R.P.W.; van Donselaar, C.A.; Majoie, M.H.J.M.; Neuteboom, R.F.; Sillevis Smitt, P.A.E.; et al. Evaluation of Seizure Treatment in Anti-LGI1, Anti-NMDAR, and Anti-GABABR Encephalitis. Neurology 2019, 92, e2185–e2196. [Google Scholar] [CrossRef] [Green Version]
- Guasp, M.; Giné-Servén, E.; Maudes, E.; Rosa-Justicia, M.; Martínez-Hernández, E.; Boix-Quintana, E.; Bioque, M.; Casado, V.; Módena-Ouarzi, Y.; Guanyabens, N.; et al. Clinical, Neuroimmunologic, and CSF Investigations in First Episode Psychosis. Neurology 2021, 97, e61–e75. [Google Scholar] [CrossRef]
- Graus, F.; Vogrig, A.; Muñiz-Castrillo, S.; Antoine, J.-C.G.; Desestret, V.; Dubey, D.; Giometto, B.; Irani, S.R.; Joubert, B.; Leypoldt, F.; et al. Updated Diagnostic Criteria for Paraneoplastic Neurologic Syndromes. Neurol.-Neuroimmunol. Neuroinflamm. 2021, 8, e1014. [Google Scholar] [CrossRef] [PubMed]
- Dalmau, J.; Lancaster, E.; Martinez-Hernandez, E.; Rosenfeld, M.R.; Balice-Gordon, R. Clinical Experience and Laboratory Investigations in Patients with Anti-NMDAR Encephalitis. Lancet Neurol. 2011, 10, 63–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garg, D.; Mohammad, S.S.; Sharma, S. Autoimmune Encephalitis in Children: An Update. Indian Pediatr. 2020, 57, 662–670. [Google Scholar] [CrossRef]
- Li, J.H.; Milla, S.S.; Gombolay, G.Y. Rate of Anti-NMDA Receptor Encephalitis in Ovarian Teratomas. Neuropediatrics 2022, 53, 133–135. [Google Scholar] [CrossRef]
- Titulaer, M.J.; McCracken, L.; Gabilondo, I.; Armangué, T.; Glaser, C.; Iizuka, T.; Honig, L.S.; Benseler, S.M.; Kawachi, I.; Martinez-Hernandez, E.; et al. Treatment and Prognostic Factors for Long-Term Outcome in Patients with Anti-NMDA Receptor Encephalitis: An Observational Cohort Study. Lancet Neurol. 2013, 12, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Hardy, D. Autoimmune Encephalitis in Children. Pediatr. Neurol. 2022, 132, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Masdeu, J.C.; Dalmau, J.; Berman, K.F. NMDA Receptor Internalization by Autoantibodies: A Reversible Mechanism Underlying Psychosis? Trends Neurosci. 2016, 39, 300–310. [Google Scholar] [CrossRef] [Green Version]
- Balu, D.T. The NMDA Receptor and Schizophrenia. Adv. Pharmacol. 2016, 76, 351–382. [Google Scholar]
- Martinez-Hernandez, E.; Horvath, J.; Shiloh-Malawsky, Y.; Sangha, N.; Martinez-Lage, M.; Dalmau, J. Analysis of Complement and Plasma Cells in the Brain of Patients with Anti-NMDAR Encephalitis. Neurology 2011, 77, 589–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vyas, B.; Dyer, R.B. The “Dot-Dash” Sign. Abdom Imaging 2015, 40, 2901–2902. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, P.; Papadopoulos, G.; Koufou, E.; Parissis, D.; Karacostas, D. Anti-NMDA Receptor Encephalitis Possibly Triggered by Measles Virus. Acta Neurol. Belg. 2015, 115, 801–802. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lee, C.Y.; Park, H.S.; Park, J.; Yun, J.Y. Anti-N-Methyl-D-Aspartate Receptor (NMDAR) Encephalitis Associated With Mediastinal and Ovarian Teratomas: A Case Report. J. Korean Med. Sci. 2023, 38, e31. [Google Scholar] [CrossRef]
- Graus, F.; Titulaer, M.J.; Balu, R.; Benseler, S.; Bien, C.G.; Cellucci, T.; Cortese, I.; Dale, R.C.; Gelfand, J.M.; Geschwind, M.; et al. A Clinical Approach to Diagnosis of Autoimmune Encephalitis. Lancet Neurol. 2016, 15, 391–404. [Google Scholar] [CrossRef] [Green Version]
- Abboud, H.; Probasco, J.C.; Irani, S.; Ances, B.; Benavides, D.R.; Bradshaw, M.; Christo, P.P.; Dale, R.C.; Fernandez-Fournier, M.; Flanagan, E.P.; et al. Autoimmune Encephalitis: Proposed Best Practice Recommendations for Diagnosis and Acute Management. J. Neurol. Neurosurg. Psychiatry 2021, 92, 757–768. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, Y.; Guo, R.; Xu, X.; Zhang, M.; Li, Z.; Xiao, Y.; Cao, W.; Gao, H.; Kong, D.; et al. Anti-NMDA Receptor Encephalitis: Retrospective Analysis of 15 Cases, Literature Review, and Implications for Gynecologists. J. Healthc. Eng. 2022, 2022, 4299791. [Google Scholar] [CrossRef] [PubMed]
- Dalmau, J.; Graus, F. Antibody-Mediated Encephalitis. N. Engl. J. Med. 2018, 378, 840–851. [Google Scholar] [CrossRef] [Green Version]
- Begum, J.; Aziz, Z.; Sahoo, S.K.; Majumder, R.; Sable, M.N. Anti-N-Methyl-D-Aspartate Receptor Encephalitis Associated with Mature Ovarian Teratoma in a Young Adolescent: A Case Report. J. Pediatr. Adolesc. Gynecol. 2022, 35, 400–403. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Martinez-Hernandez, E.; Ariño, H.; Armangué, T.; Spatola, M.; Petit-Pedrol, M.; Saiz, A.; Rosenfeld, M.R.; Graus, F.; Dalmau, J. Clinical and Pathogenic Significance of IgG, IgA, and IgM Antibodies against the NMDA Receptor. Neurology 2018, 90, e1386–e1394. [Google Scholar] [CrossRef] [PubMed]
- Blinder, T.; Lewerenz, J. Cerebrospinal Fluid Findings in Patients with Autoimmune Encephalitis-a Systematic Analysis. Front. Neurol. 2019, 10, 804. [Google Scholar] [CrossRef] [Green Version]
- Gaspard, N.; Foreman, B.P.; Alvarez, V.; Kang, C.C.; Probasco, J.C.; Jongeling, A.C.; Meyers, E.; Espinera, A.; Haas, K.F.; Schmitt, S.E.; et al. New-Onset Refractory Status Epilepticus Etiology, Clinical Features, and Outcome. Neurology 2015, 85, 1604–1613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Titulaer, M.J.; Soffietti, R.; Dalmau, J.; Gilhus, N.E.; Giometto, B.; Graus, F.; Grisold, W.; Honnorat, J.; Sillevis Smitt, P.A.E.; Tanasescu, R.; et al. Screening for Tumours in Paraneoplastic Syndromes: Report of an EFNS Task Force. Eur. J. Neurol. 2011, 18, 19-e3. [Google Scholar] [CrossRef] [Green Version]
- Ni, G.; Lin, W.; Cai, X.; Qin, J.; Feng, L.; Zhu, S.; Zhou, L.; Chen, Z. Associations between Seizures and MRI in Patients with Anti-NMDAR Encephalitis. Acta Neurol. Scand. 2020, 142, 460–465. [Google Scholar] [CrossRef]
- Dalmau, J.; Gleichman, A.J.; Hughes, E.G.; Rossi, J.E.; Peng, X.; Lai, M.; Dessain, S.K.; Rosenfeld, M.R.; Balice-Gordon, R.; Lynch, D.R. Anti-NMDA-Receptor Encephalitis: Case Series and Analysis of the Effects of Antibodies. Lancet Neurol. 2008, 7, 1091–1098. [Google Scholar] [CrossRef] [Green Version]
- Piao, S.; Bao, Y.; Yang, L.; Zhang, Y.; Hu, B.; Li, H.; Geng, D.; Li, Y. Brain MRI Features of Anti-N-Methyl-D-Aspartate (Anti-NMDA) Receptor Encephalitis Secondary to Central Nervous System Infection in Adult Patients. Acta Radiol. 2022, 64, 760–768. [Google Scholar] [CrossRef]
- Huang, Q.; Ma, M.; Wei, X.; Liao, Y.; Qi, H.; Wu, Y.; Wu, Y. Characteristics of Seizure and Antiepileptic Drug Utilization in Outpatients With Autoimmune Encephalitis. Front. Neurol. 2019, 9, 1136. [Google Scholar] [CrossRef] [Green Version]
- Harutyunyan, G.; Hauer, L.; Dünser, M.W.; Moser, T.; Pikija, S.; Leitinger, M.; Novak, H.F.; Aichhorn, W.; Trinka, E.; Sellner, J. Risk Factors for Intensive Care Unit Admission in Patients with Autoimmune Encephalitis. Front. Immunol. 2017, 8, 835. [Google Scholar] [CrossRef] [Green Version]
- Dubey, D.; Samudra, N.; Gupta, P.; Agostini, M.; Ding, K.; van Ness, P.C.; Vernino, S.; Hays, R. Retrospective Case Series of the Clinical Features, Management and Outcomes of Patients with Autoimmune Epilepsy. Seizure 2015, 29, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Dubey, D.; Singh, J.; Britton, J.W.; Pittock, S.J.; Flanagan, E.P.; Lennon, V.A.; Tillema, J.-M.; Wirrell, E.; Shin, C.; So, E.; et al. Predictive Models in the Diagnosis and Treatment of Autoimmune Epilepsy. Epilepsia 2017, 58, 1181–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byun, J.-I.; Lee, S.-T.; Jung, K.-H.; Sunwoo, J.-S.; Moon, J.; Lim, J.-A.; Lee, D.Y.; Shin, Y.-W.; Kim, T.-J.; Lee, K.-J.; et al. Effect of Immunotherapy on Seizure Outcome in Patients with Autoimmune Encephalitis: A Prospective Observational Registry Study. PLoS ONE 2016, 11, e0146455. [Google Scholar] [CrossRef] [PubMed]
- Broadley, J.; Seneviratne, U.; Beech, P.; Buzzard, K.; Butzkueven, H.; O’Brien, T.; Monif, M. Prognosticating Autoimmune Encephalitis: A Systematic Review. J. Autoimmun. 2019, 96, 24–34. [Google Scholar] [CrossRef]
- Singh, T.D.; Fugate, J.E.; Hocker, S.E.; Rabinstein, A.A. Postencephalitic Epilepsy: Clinical Characteristics and Predictors. Epilepsia 2015, 56, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Woodfield, C.A. The Usefulness of Ultrasound Imaging in Gynecologic Oncology. PET Clin. 2018, 13, 143–163. [Google Scholar] [CrossRef]
- Saleh, M.; Bhosale, P.; Menias, C.O.; Ramalingam, P.; Jensen, C.; Iyer, R.; Ganeshan, D. Ovarian Teratomas: Clinical Features, Imaging Findings and Management. Abdom. Radiol. 2021, 46, 2293–2307. [Google Scholar] [CrossRef]
- Quinn, S.F.; Erickson, S.; Black, W.C. Cystic Ovarian Teratomas: The Sonographic Appearance of the Dermoid Plug. Radiology 1985, 155, 477–478. [Google Scholar] [CrossRef]
- Patel, M.D.; Feldstein, V.A.; Lipson, S.D.; Chen, D.C.; Filly, R.A. Cystic Teratomas of the Ovary: Diagnostic Value of Sonography. AJR Am. J. Roentgenol. 1998, 171, 1061–1065. [Google Scholar] [CrossRef] [Green Version]
- Beller, M.J. The “Tip of the Iceberg” Sign. Radiology 1998, 209, 395–396. [Google Scholar] [CrossRef]
- Tongsong, T.; Wanapirak, C.; Khunamornpong, S.; Sukpan, K. Numerous Intracystic Floating Balls as a Sonographic Feature of Benign Cystic Teratoma: Report of 5 Cases. J. Ultrasound Med. 2006, 25, 1587–1591. [Google Scholar] [CrossRef]
- Brammer, H.M.; Buck, J.L.; Hayes, W.S.; Sheth, S.; Tavassoli, F.A. From the Archives of the AFIP. Malignant Germ Cell Tumors of the Ovary: Radiologic-Pathologic Correlation. RadioGraphics 1990, 10, 715–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, S.E.; Lee, J.M.; Rha, S.E.; Byun, J.Y.; Jung, J.I.; Hahn, S.T. CT and MR Imaging of Ovarian Tumors with Emphasis on Differential Diagnosis. RadioGraphics 2002, 22, 1305–1325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levine, D.; Brown, D.L.; Andreotti, R.F.; Benacerraf, B.; Benson, C.B.; Brewster, W.R.; Coleman, B.; DePriest, P.; Doubilet, P.M.; Goldstein, S.R.; et al. Management of Asymptomatic Ovarian and Other Adnexal Cysts Imaged at US: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology 2010, 256, 943–954. [Google Scholar] [CrossRef]
- Patel-Lippmann, K.K.; Sadowski, E.A.; Robbins, J.B.; Paroder, V.; Barroilhet, L.; Maddox, E.; McMahon, T.; Sampene, E.; Wasnik, A.P.; Blaty, A.D.; et al. Comparison of International Ovarian Tumor Analysis Simple Rules to Society of Radiologists in Ultrasound Guidelines for Detection of Malignancy in Adnexal Cysts. Am. J. Roentgenol. 2020, 214, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Mais, V. Transvaginal Ultrasonography in the Diagnosis of Cystic Teratoma. Obstet. Gynecol. 1995, 85, 48–52. [Google Scholar] [CrossRef]
- French, A.V.; Grossmann, L. Early Diagnosis and Treatment Improve Outcomes for Premenarchal Patients with Anti-NMDA Receptor Encephalitis and Ovarian Teratoma. J. Pediatr. Adolesc. Gynecol. 2022, 35, 516–517. [Google Scholar] [CrossRef]
- Nguyen, L.; Wang, C. Anti-NMDA Receptor Autoimmune Encephalitis: Diagnosis and Management Strategies. Int. J. Gen. Med. 2023, 16, 7–21. [Google Scholar] [CrossRef]
- Desena, A.D.; Noland, D.K.; Matevosyan, K.; King, K.; Phillips, L.; Qureshi, S.S.; Greenberg, B.M.; Graves, D. Intravenous Methylprednisolone versus Therapeutic Plasma Exchange for Treatment of Anti-n-Methyl-d-Aspartate Receptor Antibody Encephalitis: A Retrospective Review. J. Clin. Apher 2015, 30, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Nosadini, M.; Thomas, T.; Eyre, M.; Anlar, B.; Armangue, T.; Benseler, S.M.; Cellucci, T.; Deiva, K.; Gallentine, W.; Gombolay, G.; et al. International Consensus Recommendations for the Treatment of Pediatric NMDAR Antibody Encephalitis. Neurol.-Neuroimmunol. Neuroinflamm. 2021, 8, e1052. [Google Scholar] [CrossRef] [PubMed]
- Dalmau, J.; Tüzün, E.; Wu, H.; Masjuan, J.; Rossi, J.E.; Voloschin, A.; Baehring, J.M.; Shimazaki, H.; Koide, R.; King, D.; et al. Paraneoplastic Anti- N -Methyl-D-Aspartate Receptor Encephalitis Associated with Ovarian Teratoma. Ann. Neurol. 2007, 61, 25–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.-Y.; Wu, J.-D.; Chen, C.-C. The Association of Ovarian Teratoma and Anti-N-Methyl-D-Aspartate Receptor Encephalitis: An Updated Integrative Review. Int. J. Mol. Sci. 2021, 22, 10911. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-J.; Yu, M.-H.; Cheng, J.; Bai, W.-X.; Di, W. Ovarian Teratoma Related Anti-N-Methyl-D-Aspartate Receptor Encephalitis: A Case Series and Review of the Literature. World J. Clin. Cases 2022, 10, 5196–5207. [Google Scholar] [CrossRef] [PubMed]
- Nosadini, M.; Eyre, M.; Molteni, E.; Thomas, T.; Irani, S.R.; Dalmau, J.; Dale, R.C.; Lim, M.; International NMDAR Antibody Encephalitis Consensus Group; Anlar, B.; et al. Use and Safety of Immunotherapeutic Management of N-Methyl-d-Aspartate Receptor Antibody Encephalitis: A Meta-Analysis. JAMA Neurol. 2021, 78, 1333–1344. [Google Scholar] [CrossRef]
| Normal Imaging in about Half of Patients |
|---|
| Mostly bilateral brain inflammation |
| Commonly seen bilateral limbic inflammation, especially in hippocampus but also in cingulate and insula |
| Less common bilateral involvement of frontal and temporal lobes |
| Meningeal enhancement, cortical diffusion restriction, and focal or extensive demyelination are rarely present |
| Cortical inflammation seems positively correlated with refractory seizures, and patients with MRI abnormalities have more focal seizures than patients with normal brain MRI |
| The relationship between normal brain MRI at onset and seizure remission is controversial |
| US Finding | Anatomic Counterpart |
|---|---|
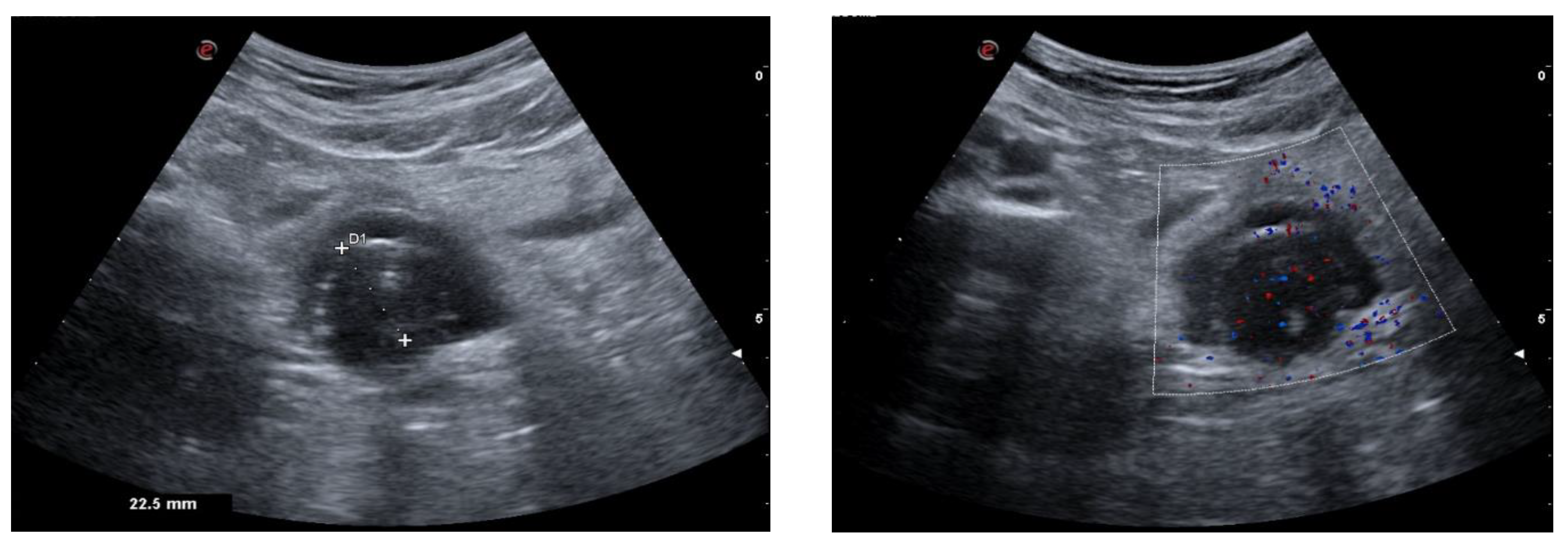
| Single cystic lesion with an echogenic nodule that leads to acoustic shadowing | Unilocular cyst with various septa and a prominent raised protuberance called “Rokitansky nodule”, containing calcifications |
| “Tip of the iceberg” sign, due to markedly echogenic areas with posterior acoustic shadowing | A marked acoustic shadowing masks the true extent of the teratoma due to presence of an echogenic focus, resulting from the presence of hair, cellular debris, fat, teeth, and calcifications |
| “Comet tail” sign | Shadowing without an echogenic focus at the tip, due to the presence of hair balls |
| Fat–fluid and fluid–fluid levels | Different echogenicity between liquid fluid, fat, and sebum |
| “Dot–dash” sign due to hyperechoic lines and dots | Different orientation of floating hair within the cyst, appearing as dots when perpendicular to the imaging plane, and dashes when parallel |
| Highly echogenic avascular mass | Poor vascularization and high presence of fat, hair, and calcifications |
| “Meat/floating balls” sign | Floating hyperechoic balls, composed of sebum, keratin, and hair balls |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guarino, M.; La Bella, S.; Santoro, M.; Caposiena, D.; Di Lembo, E.; Chiarelli, F.; Iannetti, G. The Leading Role of Brain and Abdominal Radiological Features in the Work-Up of Anti-NMDAR Encephalitis in Children: An Up-To-Date Review. Brain Sci. 2023, 13, 662. https://doi.org/10.3390/brainsci13040662
Guarino M, La Bella S, Santoro M, Caposiena D, Di Lembo E, Chiarelli F, Iannetti G. The Leading Role of Brain and Abdominal Radiological Features in the Work-Up of Anti-NMDAR Encephalitis in Children: An Up-To-Date Review. Brain Sciences. 2023; 13(4):662. https://doi.org/10.3390/brainsci13040662
Chicago/Turabian StyleGuarino, Miriana, Saverio La Bella, Marco Santoro, Daniele Caposiena, Enza Di Lembo, Francesco Chiarelli, and Giovanni Iannetti. 2023. "The Leading Role of Brain and Abdominal Radiological Features in the Work-Up of Anti-NMDAR Encephalitis in Children: An Up-To-Date Review" Brain Sciences 13, no. 4: 662. https://doi.org/10.3390/brainsci13040662
APA StyleGuarino, M., La Bella, S., Santoro, M., Caposiena, D., Di Lembo, E., Chiarelli, F., & Iannetti, G. (2023). The Leading Role of Brain and Abdominal Radiological Features in the Work-Up of Anti-NMDAR Encephalitis in Children: An Up-To-Date Review. Brain Sciences, 13(4), 662. https://doi.org/10.3390/brainsci13040662







