Risk Factor Analysis and a Predictive Model of Postoperative Depressive Symptoms in Elderly Patients Undergoing Video-Assisted Thoracoscopic Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Participants
2.3. Ethics Statement
2.4. Anesthesia and Analgesia Protocols
2.5. Data Collection
2.6. Statistical Analysis
3. Results
3.1. Preoperative Basic and Clinical Demographics of Patients
3.2. Intraoperative and Postoperative Characteristics of the Two Groups
3.3. Multivariate Logistic Regression Analysis
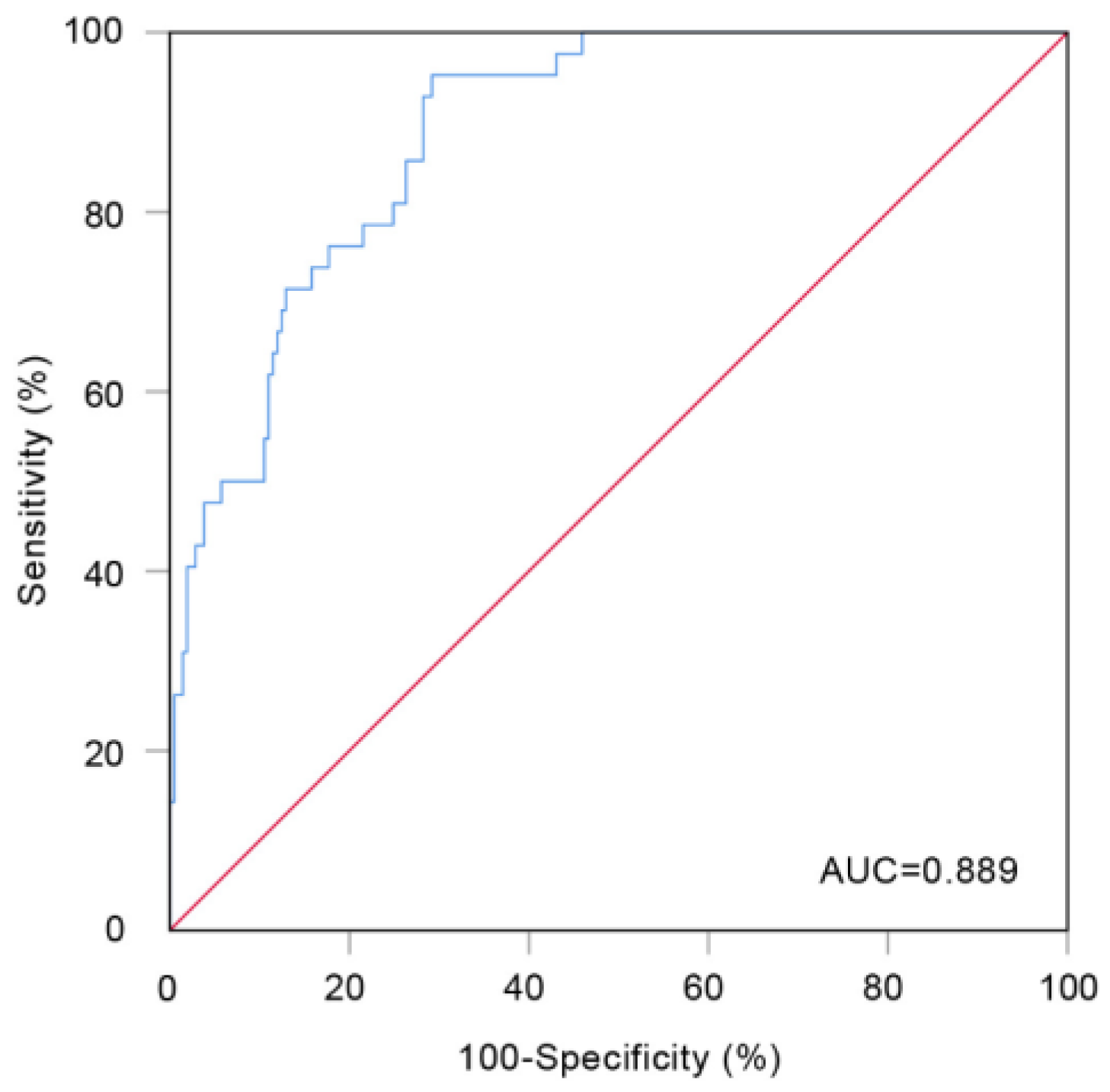
3.4. Evaluation of the Prediction Model
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, D.; Zhang, D.-J.; Shao, J.-J.; Qi, X.-D.; Tian, L. A meta-analysis of the prevalence of depressive symptoms in Chinese older adults. Arch. Gerontol. Geriatr. 2014, 58, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mac Giollabhui, N.; Ng, T.H.; Ellman, L.M.; Alloy, L.B. The longitudinal associations of inflammatory biomarkers and depression revisited: Systematic review, meta-analysis, and meta-regression. Mol. Psychiatry 2021, 26, 3302–3314. [Google Scholar] [CrossRef] [PubMed]
- Zainal, N.H.; Newman, M.G. Increased inflammation predicts nine-year change in major depressive disorder diagnostic status. J. Abnorm. Psychol. 2021, 130, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Schober, J.P.; Stensland, K.D.; Breyer, B.N.; Erickson, B.A.; Myers, J.B.; Voelzke, B.B.; Elliott, S.P.; Buckley, J.C.; Vanni, A.J. TURNS Effect of Urethroplasty on Anxiety and Depression. J. Urol. 2018, 199, 1552–1556. [Google Scholar] [CrossRef] [PubMed]
- Fiske, A.; Wetherell, J.L.; Gatz, M. Depression in Older Adults. Annu. Rev. Clin. Psychol. 2009, 5, 363. [Google Scholar] [CrossRef]
- Alexandra, T.; Suzanne, M.; Rapson, G.; Bridget, B.; Jessica, H. Resiliency among Older Adults: Dispositional Hope as a Protective Factor in the Insomnia-Depressive Symptoms Relation. Aging Ment. Health 2018, 22, 1094–1102. [Google Scholar]
- Li, W.W.L.; Lee, T.W.; Lam, S.S.Y.; Ng, C.S.H.; Sihoe, A.D.L.; Wan, I.Y.P.; Yim, A.P.C. Quality of Life Following Lung Cancer Resection: Video-Assisted Thoracic Surgery vs Thoracotomy. Chest 2002, 122, 584–589. [Google Scholar] [CrossRef]
- Rizk, N.P.; GhanieM, A.; Hsu, M.; Bains, M.S.; Downey, R.J.; Sarkaria, I.S.; Finley, D.J.; Adusumilli, P.S.; Huang, J.; Sima, C.S.; et al. A Prospective Trial Comparing Pain and Quality of Life Measures after Anatomic Lung Resection Using Either Thoracoscopy or Thoracotomy. Ann. Thorac. Surg. 2014, 98, 1160. [Google Scholar] [CrossRef]
- Kaseda, S.; Aoki, T.; Hangai, N.; Shimizu, K. Better pulmonary function and prognosis with video-assisted thoracic surgery than with thoracotomy. Ann. Thorac. Surg. 2000, 70, 1644–1646. [Google Scholar] [CrossRef]
- Avery, K.; Blazeby, J.; Chalmers, K.A.; Batchelor, T.J.P.; Casali, G.; Internullo, E.; Krishnadas, R.; Evans, C.; West, D. Impact on Health-Related Quality of Life of Video-Assisted Thoracoscopic Surgery for Lung Cancer. Ann. Surg. Oncol. 2020, 27, 1259–1271. [Google Scholar] [CrossRef]
- Wildgaard, K.; Ringsted, T.K.; Hansen, H.J.; Petersen, R.H.; Werner, M.U.; Kehlet, H. Quantitative sensory testing of persistent pain after video-assisted thoracic surgery lobectomy. Br. J. Anaesth. 2012, 108, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Caspi-Avissar, N.; Grosman-Rimon, L.; Gohari, J.; Arazi, M.; Granot, D.; Ghanim, D.; Carasso, S.; Shalabi, A.; Sudarsky, D.; Eilat-Adar, S.; et al. Clinical, Surgical, and Sociopsychological Factors and Depression After Cardiothoracic Surgery. Ann. Thorac. Surg. 2021, 111, 1064–1070. [Google Scholar] [CrossRef]
- Bendixen, M.; Jørgensen, O.D.; Kronborg, C.; Andersen, C.; Licht, P.B. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: A andomized controlled trial. Lancet Oncol. 2016, 17, 836–844. [Google Scholar] [CrossRef]
- Hopkins, K.G.; Ferson, P.F.; Shende, M.R.; Christie, N.A.; Schuchert, M.J.; Pennathur, A. Prospective study of quality of life after lung cancer resection. Ann. Transl. Med. 2017, 5, 204. [Google Scholar] [CrossRef]
- Blackwell, M.; Glynn, A.N. How to make causal inferences with time-series cross-sectional data under selection on observables. Stat Methods Med Res. 2018, 112, 1067–1082. [Google Scholar] [CrossRef]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G.M. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD statement. BMJ 2015, 350, g7594. [Google Scholar] [CrossRef] [PubMed]
- Modica, M.; Castiglioni, P.; Minotti, A.; Faini, A.; Racca, V.; Ferratini, M. Psychological Profile in Coronary Artery By-Pass Graft Patients vs. Valve Replacement Patients Entering Cardiac Rehabilitation after Surgery. Sci. Rep. 2018, 8, 14381. [Google Scholar] [CrossRef] [PubMed]
- Hamid, H.; Liu, H.; Cong, X.; Devinsky, O.; Berg, A.T.; Vickrey, B.G.; Sperling, M.R.; Shinnar, S.; Langfitt, J.T.; Walczak, T.S.; et al. Long-term association between seizure outcome and depression after resective epilepsy surgery. Neurology 2011, 77, 1972–1976. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Li, Y.; Xie, J.; Duan, Y.; Gan, G.; Zhou, Y.; Luo, X.; Wang, J.; Chen, Z.; Zhang, Q.; et al. Symptoms of depression are related to sedentary behavior and sleep duration in elderly individuals: A cross-sectional study of 49,317 older Chinese adults. J. Affect. Disord. 2022, 308, 407–412. [Google Scholar] [CrossRef]
- Hawton, K.; Comabella, C.C.I.; Haw, C.; Saunders, K. Risk factors for suicide in individuals with depression: A systematic review. J. Affect. Disord. 2013, 147, 17–28. [Google Scholar] [CrossRef]
- Tsang, A.; Von Korff, M.; Lee, S.; Alonso, J.; Karam, E.; Angermeyer, M.C.; Borges, G.L.G.; Bromet, E.J.; de Girolamo, G.; de Graaf, R.; et al. Common Chronic Pain Conditions in Developed and Developing Countries: Gender and Age Differences and Comorbidity With Depression-Anxiety Disorders. J. Pain 2008, 9, 883–891. [Google Scholar] [CrossRef]
- Kiecolt-Glaser, J.K.; Derry, H.M.; Fagundes, C.P. Inflammation: Depression Fans the Flames and Feasts on the Heat. Am. J. Psychiatry 2015, 172, 1075–1091. [Google Scholar] [CrossRef]
- Ferri, F.; Deschênes, S.S.; Power, N.; Schmitz, N. Associations between cognitive function, metabolic factors and depression: A prospective study in Quebec, Canada. J. Affect. Disord. 2021, 283, 77–83. [Google Scholar] [CrossRef] [PubMed]
- van Grootheest, D.S.; Beekman, A.; van Groenou, M.I.B.; Deeg, D.J.H. Sex differences in depression after widowhood. Do men suffer more? Soc. Psychiatry Psychiatr. Epidemiol. 1999, 34, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Wium-Andersen, M.K.; Ørsted, D.D.; Nordestgaard, B.G. Elevated plasma fibrinogen, psychological distress, antidepressant use, and hospitalization with depression: Two large population-based studies. Psychoneuroendocrinology 2013, 38, 638–647. [Google Scholar] [CrossRef]
- Wium-Andersen, M.K.; Ørsted, D.D.; Nordestgaard, B.G. Association between elevated plasma fibrinogen and psychological distress, and depression in 73 367 individuals from the general population. Mol. Psychiatry 2013, 18, 854–855. [Google Scholar] [CrossRef]
- Matthews, K.A.; Schott, L.L.; Bromberger, J.; Cyranowski, J.; Everson-Rose, S.A.; Sowers, M.F. Associations Between Depressive Symptoms and Inflammatory/Hemostatic Markers in Women During the Menopausal Transition. Psychosom. Med. 2007, 69, 124–130. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, H.; Lan, Y.; Miao, J.; Pan, C.; Sun, W.; Li, G.; Wang, Y.; Zhao, X.; Zhu, Z.; et al. Blood biomarkers of post-stroke depression after minor stroke at three months in males and females. BMC Psychiatry 2022, 22, 162. [Google Scholar] [CrossRef]
- Martins-de-Souza, D.; Maccarrone, G.; Ising, M.; Kloiber, S.; Lucae, S.; Holsboer, F.; Turck, C.W. Plasma Fibrinogen: Now Also an Antidepressant Response Marker? Transl. Psychiatry 2014, 4, e352. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, T.; Shi, S.; Wei, T.; Wang, Q. Potential biomarkers: Differentially expressed proteins of the extrinsic coagulation pathway in plasma samples from patients with depression. Bioengineered 2021, 12, 6318–6331. [Google Scholar] [CrossRef]
- Kierulf, P.; Sandset, P.M.; Klingenberg, O.; Joø, G.B.; Godal, H.C.; Skjønsberg, O.H.; Jensen, T. Fibrinogen and fibrin induce synthesis of proinflammatory cytokines from isolated peripheral blood mononuclear cells. Thromb. Haemost. 2007, 97, 822–829. [Google Scholar] [CrossRef]
- Raison, C.L.; Miller, A.H. Is Depression an Inflammatory Disorder? Curr. Psychiatry Rep. 2011, 13, 467–475. [Google Scholar] [CrossRef]
- Schwieler, L.; Samuelsson, M.; Frye, M.A.; Bhat, M.; Schuppe-Koistinen, I.; Jungholm, O.; Johansson, A.G.; Landén, M.; Sellgren, C.M.; Erhardt, S. Electroconvulsive therapy suppresses the neurotoxic branch of the kynurenine pathway in treatment-resistant depressed patients. J. Neuroinflammation 2016, 13, 51. [Google Scholar] [CrossRef]
- Haroon, E.; Raison, C.L.; Miller, A.H. Psychoneuroimmunology Meets Neuropsychopharmacology: Translational Implications of the Impact of Inflammation on Behavior. Neuropsychopharmacology 2012, 37, 137–162. [Google Scholar] [CrossRef]
- Kupfer, D.J.; Frank, E.; Phillips, M.L. Major depressive disorder: New clinical, neurobiological, and treatment perspectives. Lancet 2012, 379, 1045–1055. [Google Scholar] [CrossRef]
- Barrocas, A.L.; Hankin, B.L. Developmental Pathways to Depressive Symptoms in Adolescence: A Multi-Wave Prospective Study of Negative Emotionality, Stressors, and Anxiety. J. Abnorm. Child Psychol. 2011, 39, 489–500. [Google Scholar] [CrossRef]
- Hettema, J.M.; Kuhn, J.W.; Prescott, C.A.; Kendler, K.S. The impact of generalized anxiety disorder and stressful life events on risk for major depressive episodes. Psychol. Med. 2006, 36, 789–795. [Google Scholar] [CrossRef]
- Kazeminia, M.; Salari, N.; Vaisi-Raygani, A.; Jalali, R.; Abdi, A.; Mohammadi, M.; Daneshkhah, A.; Hosseinian-Far, M.; Shohaimi, S. The effect of exercise on anxiety in the elderly worldwide: A systematic review and meta-analysis. Health Qual. Life Outcomes 2020, 18, 363. [Google Scholar] [CrossRef]
- van der Veen, D.; van Zelst, W.; Schoevers, R.; Comijs, H.; Voshaar, R.O. Comorbid anxiety disorders in late-life depression: Results of a cohort study. Int. Psychogeriatr. 2014, 27, 1157–1165. [Google Scholar] [CrossRef]
- Seo, H.-J.; Jung, Y.-E.; Kim, T.-S.; Kim, J.-B.; Lee, M.-S.; Kim, J.-M.; Lim, H.-W.; Jun, T.-Y. Distinctive Clinical Characteristics and Suicidal Tendencies of Patients With Anxious Depression. J. Nerv. Ment. Dis. 2011, 199, 42–48. [Google Scholar] [CrossRef]
- Yu, J.; Rawtaer, I.; Fam, J.; Jiang, M.-J.; Feng, L.; Kua, E.H.; Mahendran, R. Sleep correlates of depression and anxiety in an elderly Asian population. Psychogeriatr. Off. J. Jpn. Psychogeriatr. Soc. 2015, 16, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, K.; Falvey, C.M.; Hoang, T. Connections between sleep and cognition in older adults. Lancet Neurol. 2014, 13, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Jaussent, I.; Bouyer, J.; Ancelin, M.-L.; Akbaraly, T.; Peres, K.; Ritchie, K.; Besset, A.; Dauvilliers, Y. Insomnia and Daytime Sleepiness Are Risk Factors for Depressive Symptoms in the Elderly. Sleep 2011, 34, 1103–1110. [Google Scholar] [CrossRef]
- Rosenberg-Adamsen, S.; Kehlet, H.; Dodds, C.; Rosenberg, J. Postoperative sleep disturbances: Mechanisms and clinical implications. Br. J. Anaesth. 1996, 76, 552–559. [Google Scholar] [CrossRef]
- Leong, R.W.; Davies, L.J.; Fook-Chong, S.; Ng, S.Y.; Lee, Y.L. Effect of the use of earplugs and eye masks on the quality of sleep after major abdominal surgery: A randomised controlled trial. Anaesthesia 2021, 76, 1482–1491. [Google Scholar] [CrossRef]
- Sun, G.-W.; Yang, Y.-L.; Yang, X.-B.; Wang, Y.-Y.; Cui, X.-J.; Liu, Y.; Xing, C.-Z. Preoperative insomnia and its association with psychological factors, pain and anxiety in Chinese colorectal cancer patients. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2020, 28, 2911–2919. [Google Scholar] [CrossRef]
- Li, X.-R.; Zhang, W.-H.; Williams, J.P.; Li, T.; Yuan, J.-H.; Du, Y.; Liu, J.-D.; Wu, Z.; Xiao, Z.-Y.; Zhang, R.; et al. A multicenter survey of perioperative anxiety in China: Pre- and postoperative associations. J. Psychosom. Res. 2021, 147, 110528. [Google Scholar] [CrossRef]
- Harvey, A.M. Classification of Chronic Pain—Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Clin. J. Pain 1995, 11, 163. [Google Scholar] [CrossRef]
- Qiu, Y.; Ma, Y.; Huang, X. Bidirectional Relationship Between Body Pain and Depressive Symptoms: A Pooled Analysis of Two National Aging Cohort Studies. Front. Psychiatry 2022, 13, 881779. [Google Scholar] [CrossRef]
- Fiorelli, S.; Cioffi, L.; Menna, C.; Ibrahim, M.; De Blasi, R.A.; Rendina, E.A.; Rocco, M.; Massullo, D. Chronic Pain After Lung Resection: Risk Factors, Neuropathic Pain, and Quality of Life. J. Pain Symptom Manag. 2020, 60, 326–335. [Google Scholar] [CrossRef]
- Hilderink, P.H.; Burger, H.; Deeg, D.J.; Beekman, A.T.; Oude Voshaar, R.C. The Temporal Relation between Pain and Depression: Results from the Longitudinal Aging Study Amsterdam. Psychosom. Med. 2012, 74, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C.; Merlo, J. Intermediate and advanced topics in multilevel logistic regression analysis. Stat. Med. 2017, 36, 3257–3277. [Google Scholar] [CrossRef] [PubMed]

| Variable | Depression Group (n = 45) | Nondepression Group(n = 227) | χ2/Z/t Value | p Value |
|---|---|---|---|---|
| Age (years) (IQR) | 69 (67, 72) | 69 (67, 72) | −0.41 | 0.68 |
| Sex (male) (n, %) | 15 (33.33) | 28 (22.05) | 2.26 | 0.16 |
| BMI (kg/m2) (IQR) | 25.39 (23.33, 26.67) | 24.00 (22.31, 26.04) | −2.17 | 0.03 |
| Education level (years > 8) (n, %) | 19 (42.22) | 118 (51.98) | 1.43 | 0.26 |
| Alcohol drinker (n, %) | 12 (26.67) | 75 (33.04) | 0.70 | 0.49 |
| Smoking (n, %) | 11 (24.44) | 81 (35.68) | 2.12 | 0.17 |
| Comorbidities | ||||
| Hypertension (n, %) | 20 (44.44) | 99 (43.61) | 0.01 | >0.99 |
| Diabetes (n, %) | 9 (20.00) | 41 (18.06) | 0.09 | 0.83 |
| COPD (n, %) | 3 (6.67) | 17 (7.49) | 0.00 | >0.99 |
| Coronary heart disease (n, %) | 10 (22.22) | 43 (18.94) | 0.26 | 0.68 |
| Cerebrovascular disease (n, %) | 2 (4.44) | 12 (5.29) | 0.00 | >0.99 |
| Chronic pain (n, %) | 6 (13.33) | 7 (3.08) | 6.56 | 0.01 |
| Exercise tolerance (METs < 5) (n, %) | 11 (24.44) | 37 (16.30) | 1.71 | 0.20 |
| Preoperative fasting time (h) (IQR) | 12 (8, 12) | 10 (8, 13) | −0.41 | 0.68 |
| Laboratory testing | ||||
| Serum albumin (g/L) (SD) | 43.07 (3.12) | 41.86 (3.82) | 2.00 | 0.05 |
| Hemoglobin (g/L) (SD) | 134.51 (15.23) | 135.21 (14.31) | 0.30 | 0.77 |
| Erythrocyte count (×1012/L) (SD) | 4.19 (1.02) | 4.31 (0.75) | 0.87 | 0.39 |
| Leukocyte count (×109/L) (IQR) | 6.32 (5.15, 7.32) | 5.52 (4.58, 6.44) | −2.52 | 0.01 |
| Serum glucose (mmol/L) (IQR) | 5.33 (4.89, 5.88) | 5.19 (4.76, 5.86) | −1.13 | 0.26 |
| Blood urea nitrogen (mmol/L) (IQR)) | 5.48 (4.44, 6.60) | 4.49 (4.56, 6.61) | −0.16 | 0.87 |
| Blood potassium (mmol/L) (SD) | 4.08 (0.34) | 4.08 (0.35) | 0.00 | >0.99 |
| Blood sodium (mmol/L) (IQR) | 141.65 (140.01, 142.88) | 142.00 (140.70, 143.38) | −1.40 | 0.16 |
| Blood chlorine (mmol/L) (SD) | 103.90 (2.98) | 104.45 (2.94) | 1.14 | 0.25 |
| Fibrinogen (g/L) (IQR) | 3.32 (2.95, 3.79) | 2.92 (2.50, 3.31) | −3.93 | <0.001 |
| Prothrombin time (s) (IQR) | 12.75 (11.65, 13.40) | 12.10 (11.00, 13.10) | −2.17 | 0.03 |
| AST (U/L) (IQR) | 18.10 (15.63, 21.88) | 16.30 (14.30, 20.40) | −1.87 | 0.06 |
| ALT (U/L) (IQR) | 15.65 (10.80, 21.78) | 14.90 (11.50, 20.20) | −0.49 | 0.63 |
| Variables | Depression Group (n = 45) | Non-Depression Group (n = 227) | χ2/Z/t Value | p Value |
|---|---|---|---|---|
| ASA physical status (>II), (n, %) | 3 (6.67) | 47 (20.70) | 4.93 | 0.03 |
| Temperature monitor (n, %) | 42 (93.33) | 208 (91.63) | 0.01 | 0.93 |
| Surgery duration (min) (IQR) | 137 (85, 137) | 125 (91, 155) | −0.35 | 0.73 |
| Blood loss (mL) (IQR) | 50 (20, 60) | 50 (20, 50) | −1.19 | 0.23 |
| Urine output (mL) (IQR) | 260 (100, 500) | 300 (100, 500) | −0.17 | 0.87 |
| Infusion volume (mL) (IQR) | 1600 (1100, 1604) | 1100 (1100, 1600) | −2.21 | 0.03 |
| Postoperative analgesia (n, %) | 37 (82.22) | 178 (78.41) | 0.33 | 0.69 |
| Anxiety (n, %) | 12 (26.67) | 9 (3.96) | 24.07 | <0.001 |
| Sleep quality (IQR) | 6.00 (5.00, 7.50) | 8.00 (7.00, 9.00) | −5.53 | <0.001 |
| Pain (n, %) | 31 (68.86) | 87 (38.33) | 14.28 | <0.001 |
| Factor | β | SE | Wald | p Value | OR Value | 95% CI |
|---|---|---|---|---|---|---|
| BMI | 0.10 | 0.07 | 1.94 | 0.16 | 1.10 | 0.96~1.26 |
| Chronic pain | 1.29 | 0.83 | 2.40 | 0.12 | 3.62 | 0.71~18.42 |
| Leukocyte count | 0.08 | 0.15 | 0.30 | 0.59 | 1.08 | 0.81~1.45 |
| Fibrinogen | 0.88 | 0.31 | 7.88 | 0.01 | 2.42 | 1.30~4.47 |
| Prothrombin time | 0.01 | 0.12 | 0.01 | 0.91 | 1.01 | 0.80~1.28 |
| ASA physical status | −0.90 | 0.77 | 1.37 | 0.24 | 0.41 | 0.09~1.84 |
| Infusion volume | 0.00 | 0.00 | 1.26 | 0.26 | 1.00 | 1.00~1.00 |
| Anxiety | 2.49 | 0.65 | 14.74 | <0.001 | 12.05 | 3.38~42.95 |
| Sleep quality | −0.49 | 0.12 | 15.99 | <0.001 | 0.61 | 0.48~0.78 |
| Pain | 1.05 | 0.44 | 5.56 | 0.02 | 2.85 | 1.19~6.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, D.; Guo, X.; Li, Y.; Sheng, Z.; Wang, L.; Liu, L.; Cao, J.; Liu, Y.; Lou, J.; Li, H.; et al. Risk Factor Analysis and a Predictive Model of Postoperative Depressive Symptoms in Elderly Patients Undergoing Video-Assisted Thoracoscopic Surgery. Brain Sci. 2023, 13, 646. https://doi.org/10.3390/brainsci13040646
Xue D, Guo X, Li Y, Sheng Z, Wang L, Liu L, Cao J, Liu Y, Lou J, Li H, et al. Risk Factor Analysis and a Predictive Model of Postoperative Depressive Symptoms in Elderly Patients Undergoing Video-Assisted Thoracoscopic Surgery. Brain Sciences. 2023; 13(4):646. https://doi.org/10.3390/brainsci13040646
Chicago/Turabian StyleXue, Dinghao, Xu Guo, Yanxiang Li, Zhuoqi Sheng, Long Wang, Luyu Liu, Jiangbei Cao, Yanhong Liu, Jingsheng Lou, Hao Li, and et al. 2023. "Risk Factor Analysis and a Predictive Model of Postoperative Depressive Symptoms in Elderly Patients Undergoing Video-Assisted Thoracoscopic Surgery" Brain Sciences 13, no. 4: 646. https://doi.org/10.3390/brainsci13040646
APA StyleXue, D., Guo, X., Li, Y., Sheng, Z., Wang, L., Liu, L., Cao, J., Liu, Y., Lou, J., Li, H., Hao, X., Zhou, Z., & Fu, Q. (2023). Risk Factor Analysis and a Predictive Model of Postoperative Depressive Symptoms in Elderly Patients Undergoing Video-Assisted Thoracoscopic Surgery. Brain Sciences, 13(4), 646. https://doi.org/10.3390/brainsci13040646







