A Projectile Concussive Impact Model Produces Neuroinflammation in Both Mild and Moderate-Severe Traumatic Brain Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
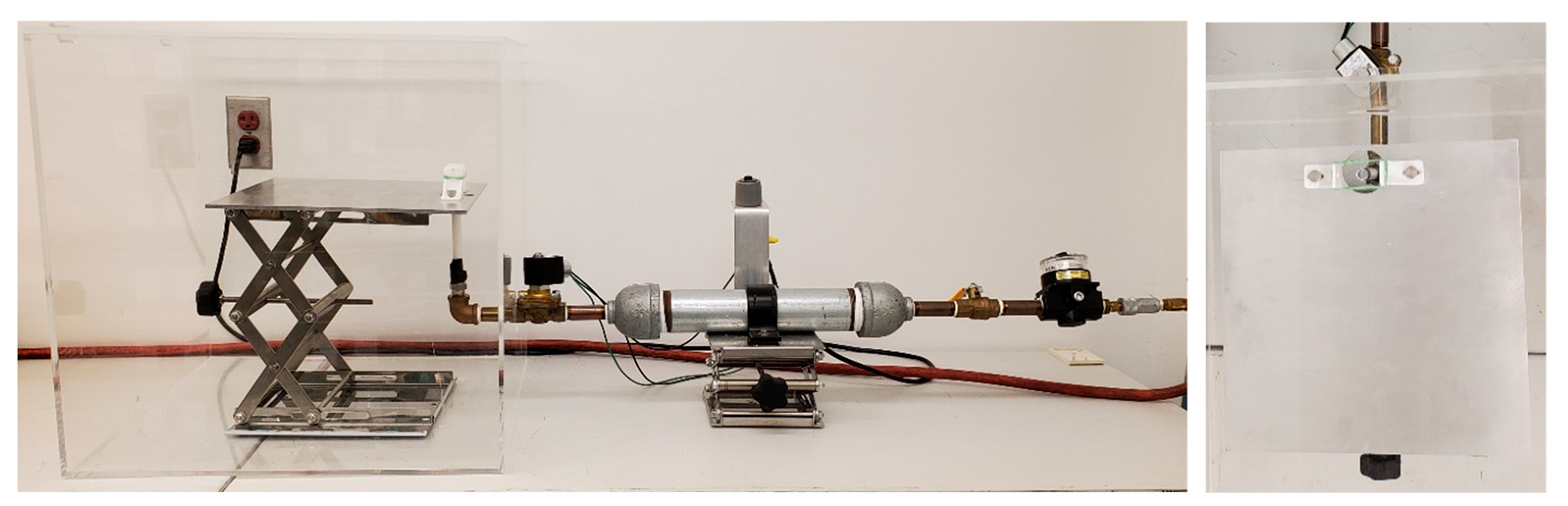
2.2. PCI Model of TBI
2.3. Neurobehavioral Severity Scoring
2.4. Tissue Collection
2.5. Immunofluorescence
2.6. qPCR
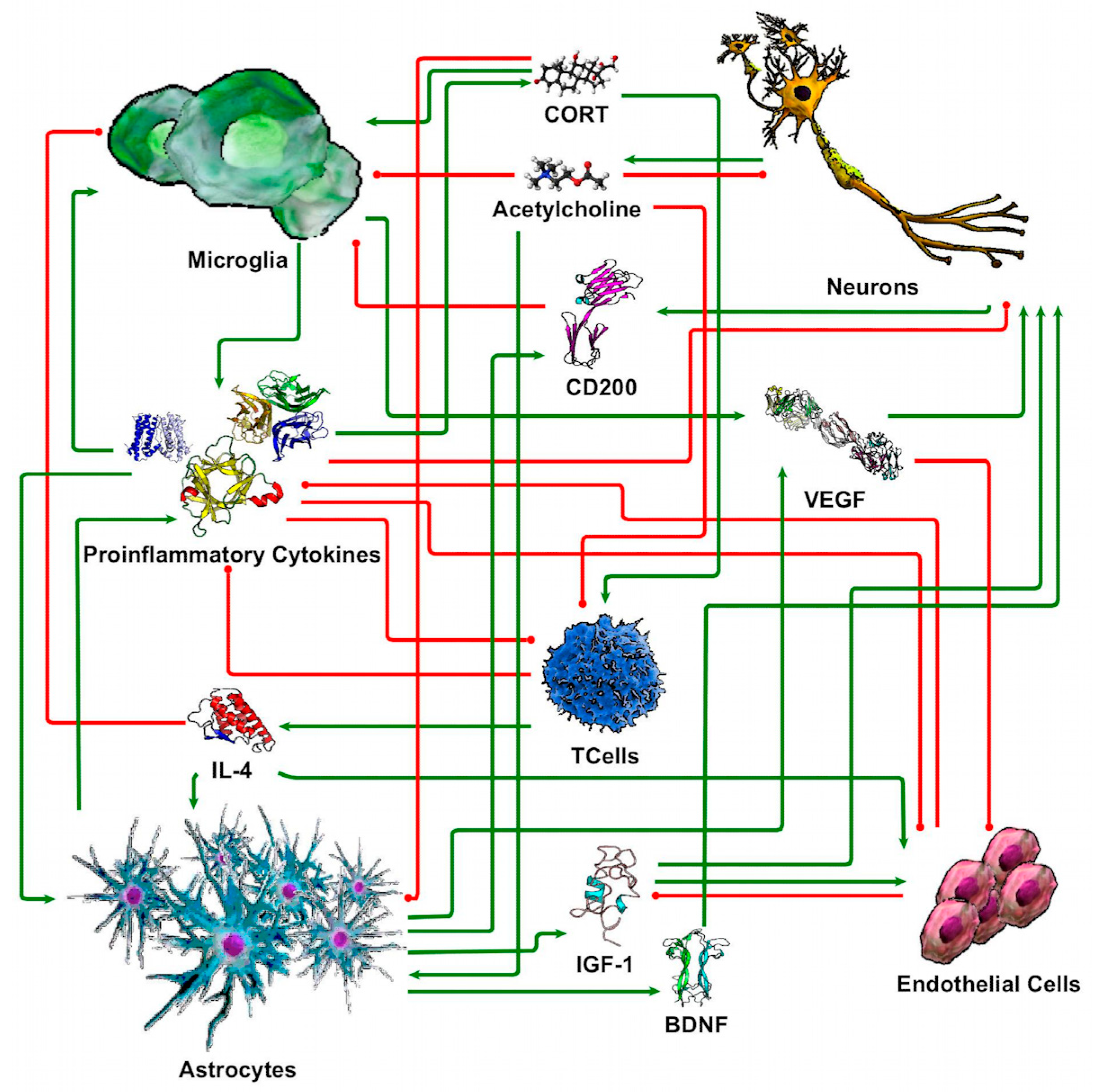
2.7. Discrete Logic Modeling of Neuron-Glia Interaction
2.8. Statistics
3. Results
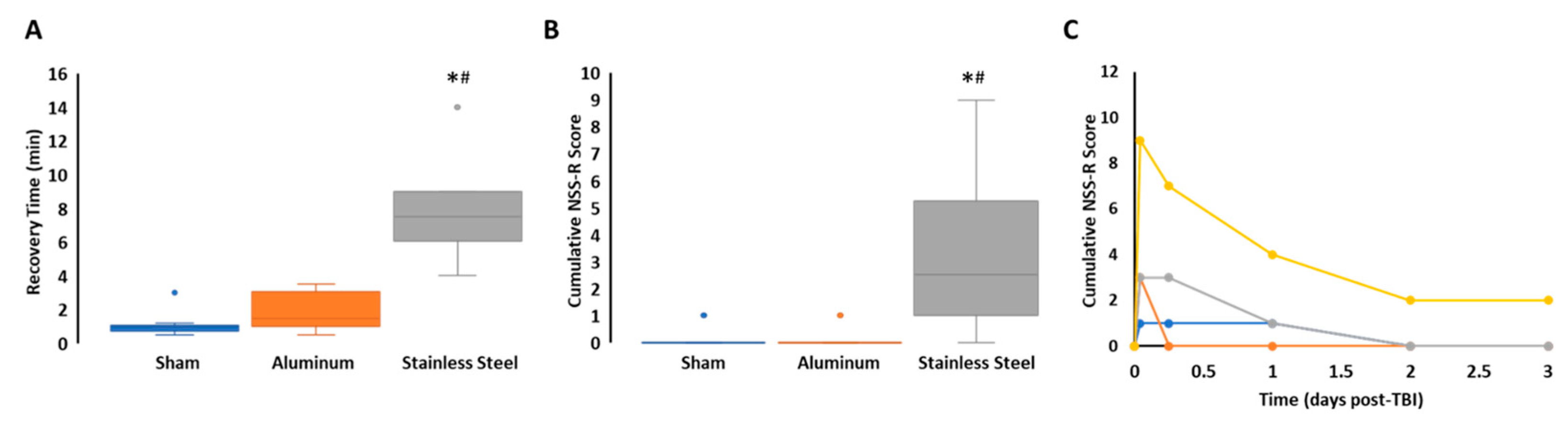
3.1. Impact with a Stainless-Steel Projectile Induced Significantly Prolonged Periods of Unconsciousness and Neurobehavioral Impairment Compared to an Aluminum Projectile
3.2. Impact with a Stainless-Steel Projectile Produced Significant Neuronal Damage and Gliosis Compared to an Aluminum Projectile
3.3. Impact with a Stainless-Steel Projectile Induced Significant Increases in the Expression of Inflammatory Cytokine mRNA in Several Brain Areas Compared to an Aluminum Projectile
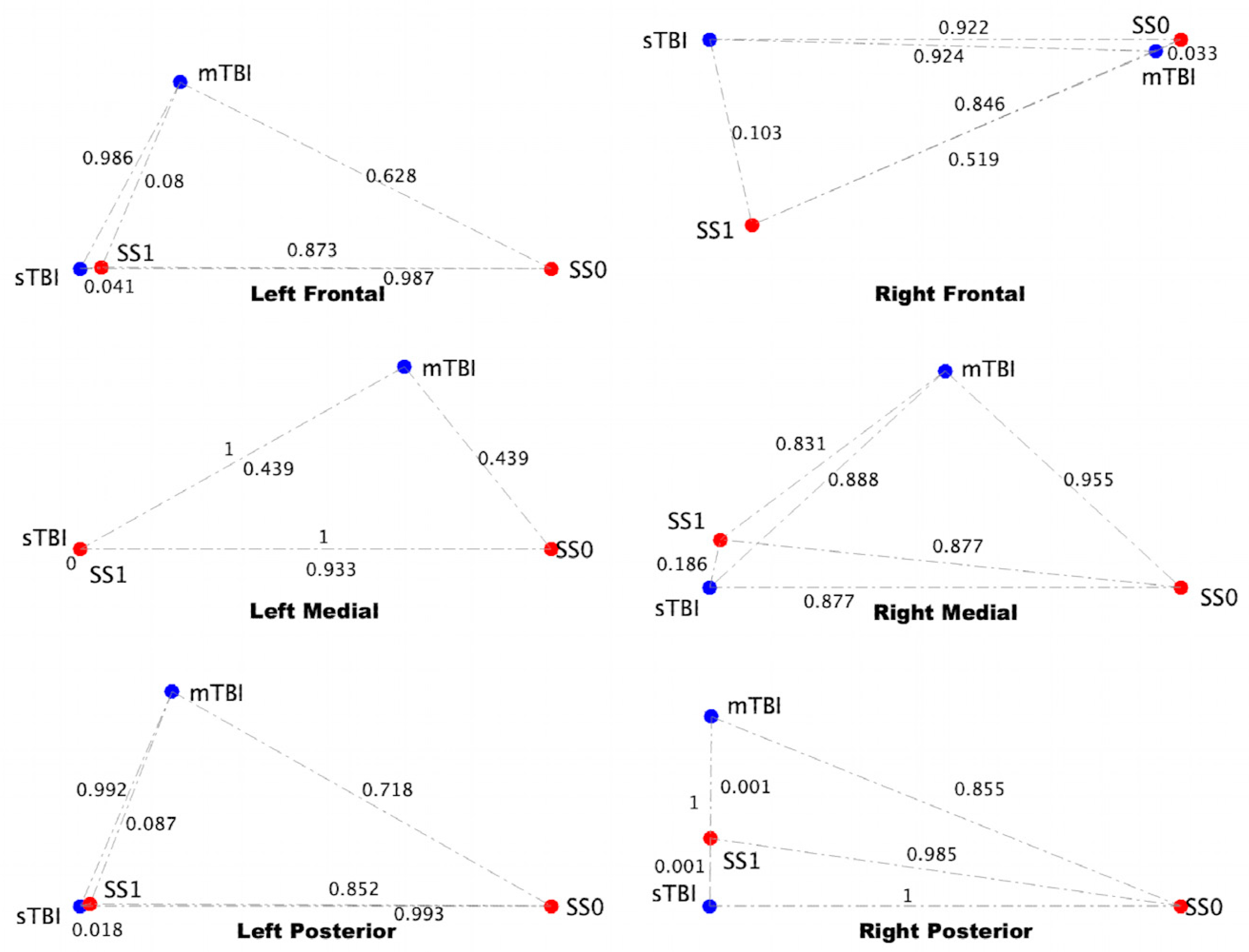
3.4. sTBI Closely Aligns with the Neuroinflammatory State in a Computational Neuron-Glia Interaction Network
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thurman, D.J.; Alverson, C.; Dunn, K.A.; Guerrero, J.; Sniezek, J.E. Traumatic brain injury in the United States: A public health perspective. J. Head Trauma Rehabil. 1999, 14, 602–615. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.C.; Liao, Y.E.; Yang, L.Y.; Wang, J.Y.; Tweedie, D.; Karnati, H.K.; Greig, N.H.; Wang, J.Y. Neuroinflammation in animal models of traumatic brain injury. J. Neurosci. Methods 2016, 272, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Sussman, E.S.; Pendharkar, A.V.; Ho, A.L.; Ghajar, J. Mild traumatic brain injury and concussion: Terminology and classification. Handb. Clin. Neurol. 2018, 158, 21–24. [Google Scholar] [PubMed]
- Centers for Disease Control and Prevention. Surveillance Report of Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths—United States, 2014; Centers for Disease Control and Prevention, U.S. Department of Health and Human Services: Atlanta, GA, USA, 2019.
- Centers for Disease Control and Prevention. “Get the Facts About TBI.” Traumatic Brain Injury & Concussion, Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. 2022. Available online: https://www.cdc.gov/traumaticbraininjury/get_the_facts.html (accessed on 21 March 2022).
- Leo, P.; McCrea, M. Epidemiology. In Translational Research in Traumatic Brain Injury; Laskowitz, D., Grant, G., Eds.; CRC Press/Taylor and Francis Group: Boca Raton, FL, USA, 2016; Chapter 1. [Google Scholar]
- Pavlovic, D.; Pekic, S.; Stojanovic, M.; Popovic, V. Traumatic brain injury: Neuropathological, neurocognitive and neurobehavioral sequelae. Pituitary 2019, 22, 270–282. [Google Scholar] [CrossRef]
- McKee, A.C.; Daneshvar, D.H. The neuropathology of traumatic brain injury. Handb. Clin. Neurol. 2015, 127, 45–66. [Google Scholar]
- Wilson, L.; Stewart, W.; Dams-O’Connor, K.; Diaz-Arrastia, R.; Horton, L.; Menon, D.K.; Polinder, S. The chronic and evolving neurological consequences of traumatic brain injury. Lancet Neurol. 2017, 16, 813–825. [Google Scholar] [CrossRef]
- Kumar, A.; Loane, D.J. Neuroinflammation after traumatic brain injury: Opportunities for therapeutic intervention. Brain Behav. Immun. 2012, 26, 1191–1201. [Google Scholar] [CrossRef]
- Karve, I.P.; Taylor, J.M.; Crack, P.J. The contribution of astrocytes and microglia to traumatic brain injury. Br. J. Pharmacol. 2016, 173, 692–702. [Google Scholar] [CrossRef]
- Sulhan, S.; Lyon, K.A.; Shapiro, L.A.; Huang, J.H. Neuroinflammation and blood-brain barrier disruption following traumatic brain injury: Pathophysiology and potential therapeutic targets. J. Neurosci. Res. 2020, 98, 19–28. [Google Scholar] [CrossRef]
- Brett, B.L.; Gardner, R.C.; Godbout, J.; Dams-O’Connor, K.; Keene, C.D. Traumatic Brain Injury and Risk of Neurodegenerative Disorder. Biol. Psychiatry 2022, 91, 498–507. [Google Scholar] [CrossRef]
- Bell, B.D.; Primeau, M.; Sweet, J.J.; Lofland, K.R. Neuropsychological functioning in migraine headache, nonheadache chronic pain, and mild traumatic brain injury patients. Arch. Clin. Neuropsychol. 1999, 14, 389–399. [Google Scholar] [CrossRef]
- Smith, D.H.; Johnson, V.E.; Stewart, W. Chronic neuropathologies of single and repetitive TBI: Substrates of dementia? Nat. Rev. Neurol. 2013, 9, 211–221. [Google Scholar]
- McKee, A.C.; Robinson, M.E. Military-related traumatic brain injury and neurodegeneration. Alzheimer’s Dement. 2014, 10, S242–S253. [Google Scholar] [CrossRef]
- Stein, T.D.; Alvarez, V.E.; McKee, A.C. Chronic traumatic encephalopathy: A spectrum of neuropathological changes following repetitive brain trauma in athletes and military personnel. Alzheimer’s Res. Ther. 2014, 6, 4. [Google Scholar] [CrossRef]
- Gardner, R.C.; Yaffe, K. Epidemiology of mild traumatic brain injury and neurodegenerative disease. Mol. Cell. Neurosci. 2015, 66, 75–80. [Google Scholar] [CrossRef] [PubMed]
- McInnes, K.; Friesen, C.L.; MacKenzie, D.E.; Westwood, D.A.; Boe, S.G. Mild Traumatic Brain Injury (mTBI) and chronic cognitive impairment: A scoping review. PLoS ONE 2017, 12, e0174847. [Google Scholar] [CrossRef]
- Merritt, V.C.; Clark, A.L.; Crocker, L.D.; Sorg, S.F.; Werhane, M.L.; Bondi, M.W.; Schiehser, D.M.; Delano-Wood, L. Repetitive mild traumatic brain injury in military veterans is associated with increased neuropsychological intra-individual variability. Neuropsychologia 2018, 119, 340–348. [Google Scholar] [CrossRef]
- Pattinson, C.L.; Shahim, P.; Taylor, P.; Dunbar, K.; Guedes, V.A.; Motamedi, V.; Lai, C.; Devoto, C.; Peyer, J.; Roy, M.J.; et al. Elevated Tau in Military Personnel Relates to Chronic Symptoms Following Traumatic Brain Injury. J. Head Trauma Rehabil. 2020, 35, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Kalish, B.T.; Finander, B.; Cao, T.; Jin, G.; Yahya, T.; Levy, E.S.; Kukreja, B.; LaRovere, E.S.; Chung, J.Y.; et al. Repetitive mild closed head injury in adolescent mice is associated with impaired proteostasis, neuroinflammation, and tauopathy. J. Neurosci. 2022, 42, 2418–2432. [Google Scholar] [CrossRef] [PubMed]
- Leung, L.Y.; Larimore, Z.; Holmes, L.; Cartagena, C.; Mountney, A.; Deng-Bryant, Y.; Schmid, K.; Shear, D.; Tortella, F. The WRAIR Projectile Concussive Impact Model of Mild Traumatic Brain Injury: Re-design, Testing and Preclinical Validation. Ann. Biomed. Eng. 2014, 42, 1618–1630. [Google Scholar] [CrossRef] [PubMed]
- Mouzon, B.C.; Bachmeier, C.; Ferro, A.; Ojo, J.O.; Crynen, G.; Acker, C.M.; Davies, P.; Mullan, M.; Stewart, W.; Crawford, F. Chronic neuropathological and neurobehavioral changes in a repetitive mild traumatic brain injury model. Ann. Neurol. 2014, 75, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Mountney, A.; Boutté, A.M.; Cartagena, C.M.; Flerlage, W.F.; Johnson, W.D.; Rho, C.; Lu, X.-C.; Yarnell, A.; Marcsisin, S.; Sousa, J.; et al. Functional and molecular correlates after single and repeated rat closed-head concussion: Indices of vulnerability after brain injury. J. Neurotrauma 2017, 34, 2768–2789. [Google Scholar] [CrossRef] [PubMed]
- Mouzon, B.C.; Bachmeier, C.; Ojo, J.O.; Acker, C.M.; Ferguson, S.; Paris, D.; Ait-Ghezala, G.; Crynen, G.; Davies, P.; Mullan, M.; et al. Lifelong behavioral and neuropathological consequences of repetitive mild traumatic brain injury. Ann. Clin. Transl. Neurol. 2017, 5, 64–80. [Google Scholar] [CrossRef]
- Simon, D.; McGeachy, M.; Bayır, H.; Clark, R.S.B.; Loane, D.J. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat. Rev. Neurol. 2017, 13, 171–219. [Google Scholar] [CrossRef] [PubMed]
- Madathil, S.K.; Wilfred, B.S.; Urankar, S.E.; Yang, W.; Leung, L.Y.; Gilsdorf, J.S.; Shear, D.A. Early microglial activation following closed-head concussive injury is dominated by pro-inflammatory M-1 type. Front. Neurol. 2018, 9, 964. [Google Scholar] [CrossRef]
- Izzy, S.; Brown-Whalen, A.; Yahya, T.; Sarro-Schwartz, A.; Jin, G.; Chung, J.Y.; Lule, S.; Morsett, L.M.; Alquraini, A.; Wu, L.; et al. Repetitive Traumatic Brain Injury Causes Neuroinflammation before Tau Pathology in Adolescent P301S Mice. Int. J. Mol. Sci. 2021, 22, 907. [Google Scholar] [CrossRef]
- Xiong, Y.; Mahmood, A.; Chopp, M. Animal models of traumatic brain injury. Nat. Rev. Neurosci. 2013, 14, 128–142. [Google Scholar] [PubMed]
- Chen, Z.; Leung, L.Y.; Mountney, A.; Liao, Z.; Yang, W.; Lu, X.-C.M.; Dave, J.; Deng-Bryant, Y.; Wei, G.; Schmid, K.; et al. A novel animal model of closed-head concussive-induced mild traumatic brain injury: Development, implementation, and characterization. J. Neurotrauma 2012, 29, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Deng-Bryant, Y.; Leung, L.Y.; Madathil, S.; Flerlage, J.; Yang, F.; Yang, W.; Gilsdorf, J.; Shear, D. Chronic cognitive deficits and associated histopathology following closed-head concussive injury in rats. Front. Neurol. 2019, 10, 699. [Google Scholar] [CrossRef]
- Craddock, T.J.A.; Michalovicz, L.T.; Kelly, K.A.; Rice, M.A.; Miller, D.B., Jr.; Klimas, N.G.; Morris, M.; O’Callaghan, J.P.; Broderick, G. A Logic Model of Neuronal-Glial Interaction Suggests Altered Homeostatic Regulation in the Perpetuation of Neuroinflammation. Front. Cell. Neurosci. 2018, 12, 336. [Google Scholar] [CrossRef]
- Yarnell, A.M.; Barry, E.S.; Mountney, A.; Shear, D.; Tortella, F.; Grunberg, N.E. The revised neurobehavioral severity scale (NSS-R) for rodents. Curr. Protoc. Neurosci. 2016, 75, 9–52. [Google Scholar] [CrossRef] [PubMed]
- Michalovicz, L.T.; Kelly, K.A.; Miller, D.B.; Sullivan, K.; O’Callaghan, J.P. The β-adrenergic receptor blocker and anti-inflammatory drug propranolol mitigates brain cytokine expression in a long-term model of Gulf War Illness. Life Sci. 2021, 285, 119962. [Google Scholar] [CrossRef] [PubMed]
- Locker, A.R.; Michalovicz, L.T.; Kelly, K.A.; Miller, J.V.; Miller, D.B.; O’Callaghan, J.P. Corticosterone primes the neuroinflammatory response to Gulf War Illness-relevant organophosphates independently of acetylcholinesterase inhibition. J. Neurochem. 2017, 142, 444–455. [Google Scholar] [CrossRef]
- Kelly, K.A.; Michalovicz, L.T.; Miller, J.V.; Castranova, V.; Miller, D.B.; O’Callaghan, J.P. Prior exposure to corticosterone markedly enhances and prolongs the neuroinflammatory response to systemic challenge with LPS. PLoS ONE 2018, 13, e0190546. [Google Scholar] [CrossRef]
- Craddock, T.J.; Fritsch, P.; Rice, M.A., Jr.; del Rosario, R.M.; Miller, D.B.; Fletcher, M.A.; Klimas, N.G.; Broderick, G. A role for homeostatic drive in the perpetuation of complex chronic illness: Gulf War Illness and chronic fatigue syndrome. PLoS ONE 2014, 9, e84839. [Google Scholar] [CrossRef]
- Brown, M.B. 400: A method for combining non-independent, one-sided tests of significance. Biometrics 1975, 2, 987–992. [Google Scholar] [CrossRef]
- Craddock, T.J.; Del Rosario, R.R.; Rice, M.; Zysman, J.P.; Fletcher, M.A.; Klimas, N.G.; Broderick, G. Achieving remission in gulf war illness: A simulation-based approach to treatment design. PLoS ONE 2015, 10, e0132774. [Google Scholar] [CrossRef]
- Fritsch, P.; Craddock, T.J.; del Rosario, R.M.; Rice, M.A.; Smylie, A.; Folcik, V.A.; Klimas, N.G.; Broderick, G. Succumbing to the laws of attraction: Exploring the sometimes pathogenic versatility of discrete immune logic. Syst. Biomed. 2013, 1, 179–194. [Google Scholar] [CrossRef]
- Rice, M.A., Jr.; Craddock, T.J.; Folcik, V.A.; del Rosario, R.M.; Barnes, Z.M.; Klimas, N.G.; Fletcher, M.A.; Zysman, J.P.; Broderick, G. Gulf War Illness: Is there lasting damage to the endocrine-immune circuitry? Syst. Biomed. 2014, 2, 80–89. [Google Scholar] [CrossRef]
- Carrera Arias, F.J.; Aenlle, K.; Abreu, M.; Holschbach, M.A.; Michalovicz, L.T.; Kelly, K.A.; Klimas, N.; Craddock, T.J. Modeling neuroimmune interactions in human subjects and animal models to predict subtype-specific multidrug treatments for Gulf War Illness. Int. J. Mol. Sci. 2021, 22, 8546. [Google Scholar] [CrossRef] [PubMed]
- Sammon, J.W. A nonlinear mapping for data structure analysis. IEEE Trans. Comput. 1969, 100, 401–409. [Google Scholar] [CrossRef]
- Johnson, V.E.; Stewart, W.; Smith, D.H. Axonal pathology in traumatic brain injury. Exp. Neurol. 2013, 246, 35–43. [Google Scholar] [CrossRef]
- Omelchenko, A.; Shrirao, A.B.; Bhattiprolu, A.K.; Zahn, J.D.; Schloss, R.S.; Dickson, S.; Meaney, D.F.; Boustany, N.N.; Yarmush, M.L.; Firestein, B.L. Dynamin and reverse-mode sodium calcium exchanger blockade confers neuroprotection from diffuse axonal injury. Cell Death Dis. 2019, 10, 727. [Google Scholar] [CrossRef]
- Schmued, L.C.; Hopkins, K.J. Fluoro-Jade B: A high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000, 874, 123–130. [Google Scholar] [CrossRef]
- Yin, T.C.; Voorhees, J.R.; Genova, R.M.; Davis, K.C.; Madison, A.M.; Britt, J.K.; Cintrón-Pérez, C.J.; McDaniel, L.; Harper, M.M.; Pieper, A.A. Acute Axonal Degeneration Drives Development of Cognitive, Motor, and Visual Deficits after Blast-Mediated Traumatic Brain Injury in Mice. eNeuro 2016, 3, ENEURO.0220-16.2016. [Google Scholar] [CrossRef] [PubMed]
- Grossman, E.J.; Inglese, M. The Role of Thalamic Damage in Mild Traumatic Brain Injury. J. Neurotrauma 2016, 33, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Dolenec, P.; Pilipović, K.; Janković, T.; Župan, G. Pattern of Neuronal and Axonal Damage, Glial Response, and Synaptic Changes in Rat Cerebellum within the First Week following Traumatic Brain Injury. J. Neuropathol. Exp. Neurol. 2020, 79, 1163–1182. [Google Scholar] [CrossRef]
- Matser, J.T.; Kessels, A.G.; Jordan, B.D.; Lezak, M.D.; Troost, J. Chronic traumatic brain injury in professional soccer players. Neurology 1998, 51, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Yee, M.K.; Janulewicz, P.A.; Seichepine, D.R.; Sullivan, K.A.; Proctor, S.P.; Krengel, M.H. Multiple Mild Traumatic Brain Injuries Are Associated with Increased Rates of Health Symptoms and Gulf War Illness in a Cohort of 1990-1991 Gulf War Veterans. Brain Sci. 2017, 7, 79. [Google Scholar] [CrossRef] [PubMed]




| Group | Total N | Mortality 1 | Acute Porphyrin 2 | Hematoma | Fracture |
|---|---|---|---|---|---|
| Sham | 10 | 0 | 0 | 0 | 0 |
| Aluminum | 10 | 0 | 1 | 0 | 0 |
| Stainless Steel | 10 | 2 | 2 | 5 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalovicz, L.T.; Kelly, K.A.; Craddock, T.J.A.; O’Callaghan, J.P. A Projectile Concussive Impact Model Produces Neuroinflammation in Both Mild and Moderate-Severe Traumatic Brain Injury. Brain Sci. 2023, 13, 623. https://doi.org/10.3390/brainsci13040623
Michalovicz LT, Kelly KA, Craddock TJA, O’Callaghan JP. A Projectile Concussive Impact Model Produces Neuroinflammation in Both Mild and Moderate-Severe Traumatic Brain Injury. Brain Sciences. 2023; 13(4):623. https://doi.org/10.3390/brainsci13040623
Chicago/Turabian StyleMichalovicz, Lindsay T., Kimberly A. Kelly, Travis J. A. Craddock, and James P. O’Callaghan. 2023. "A Projectile Concussive Impact Model Produces Neuroinflammation in Both Mild and Moderate-Severe Traumatic Brain Injury" Brain Sciences 13, no. 4: 623. https://doi.org/10.3390/brainsci13040623
APA StyleMichalovicz, L. T., Kelly, K. A., Craddock, T. J. A., & O’Callaghan, J. P. (2023). A Projectile Concussive Impact Model Produces Neuroinflammation in Both Mild and Moderate-Severe Traumatic Brain Injury. Brain Sciences, 13(4), 623. https://doi.org/10.3390/brainsci13040623






