Differentiation of the Contribution of Familiarity and Recollection to the Old/New Effects in Associative Recognition: Insight from Semantic Relation
Abstract
1. Introduction
1.1. Theories for Item Memory and the Associated Old/New Effects
1.2. Associative Memory
1.3. Two Routes to Enhance the Involvement of the Familiarity Process in Associative Memory
1.4. Subtypes of Semantic Relation and the Neural Evidence
1.5. Issues to Explore and Our Current Hypotheses
2. Experimental Procedure
2.1. Participants
2.2. Design
2.3. Materials
2.4. Procedure
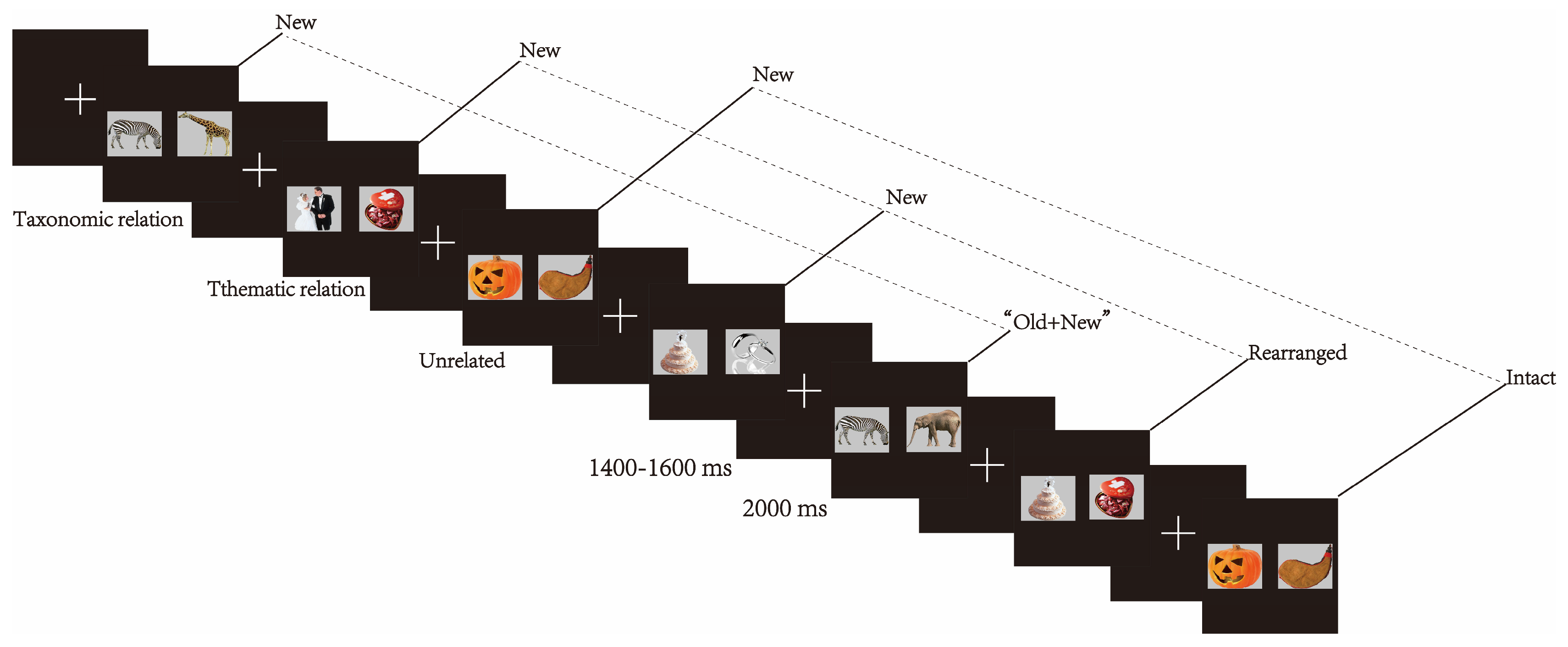
2.5. Electrophysiological Recording
3. Data Analyses and Results
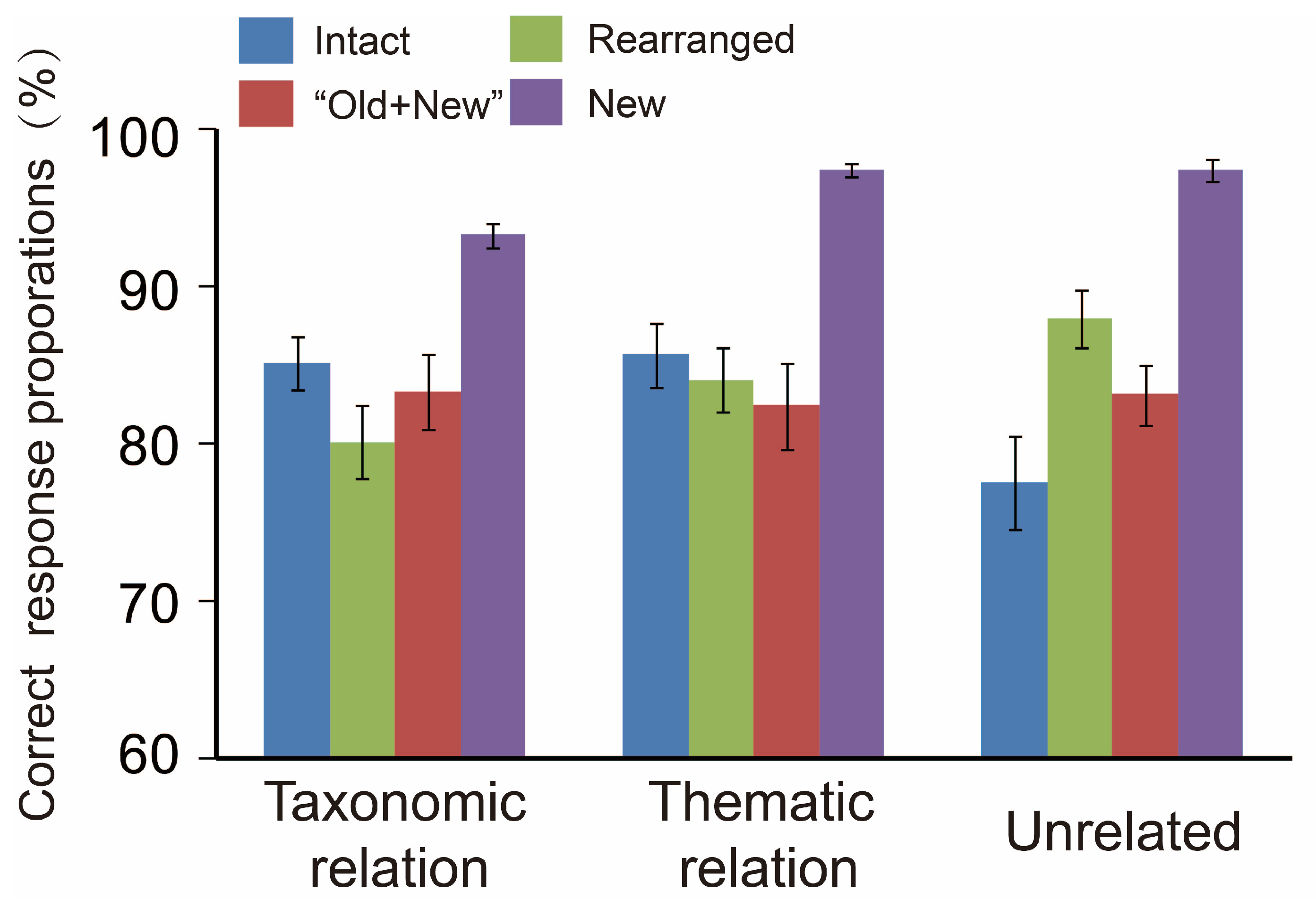
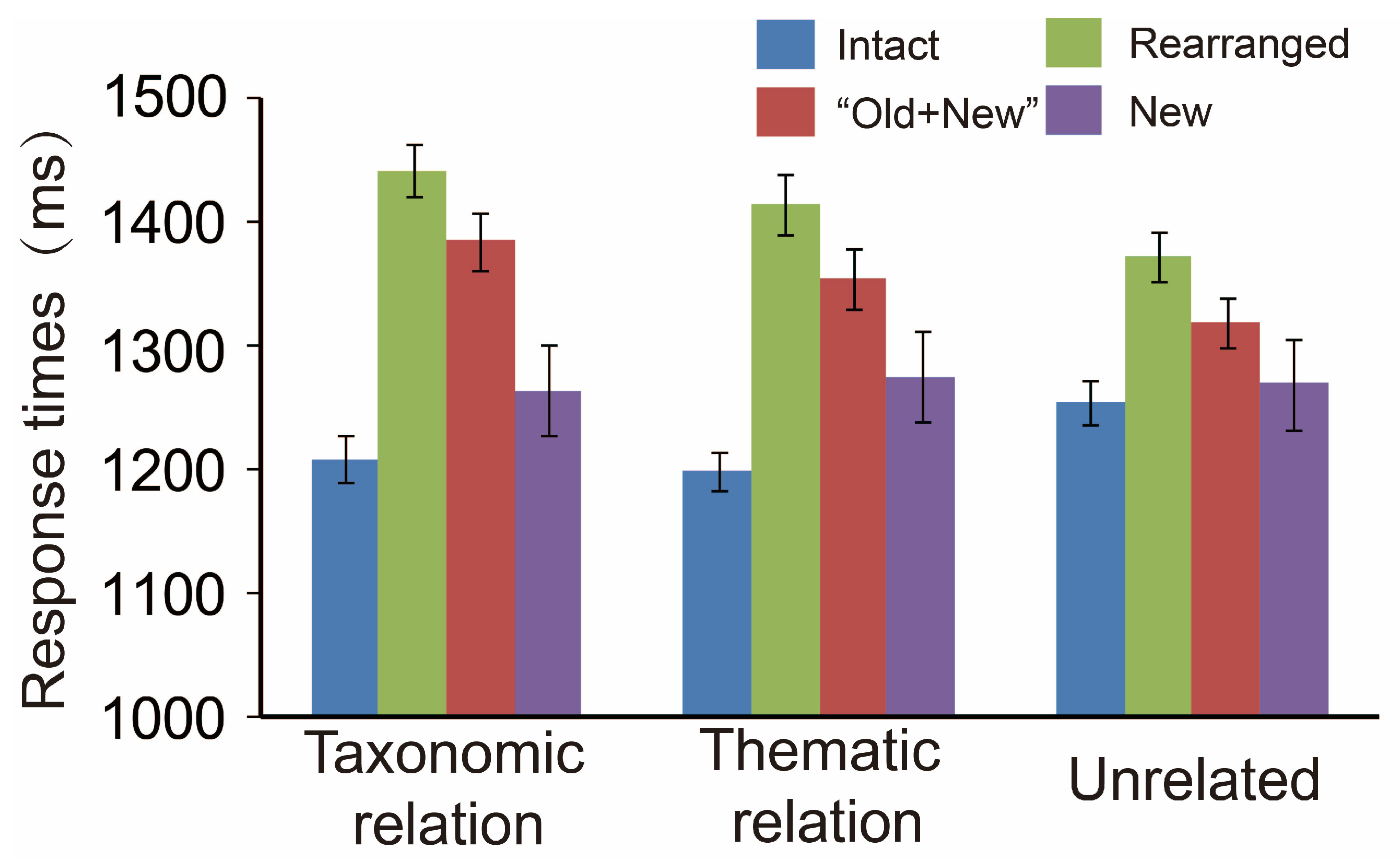
3.1. Behavioral Analyses and the Data
3.2. Electrophysiological Analyses and Results
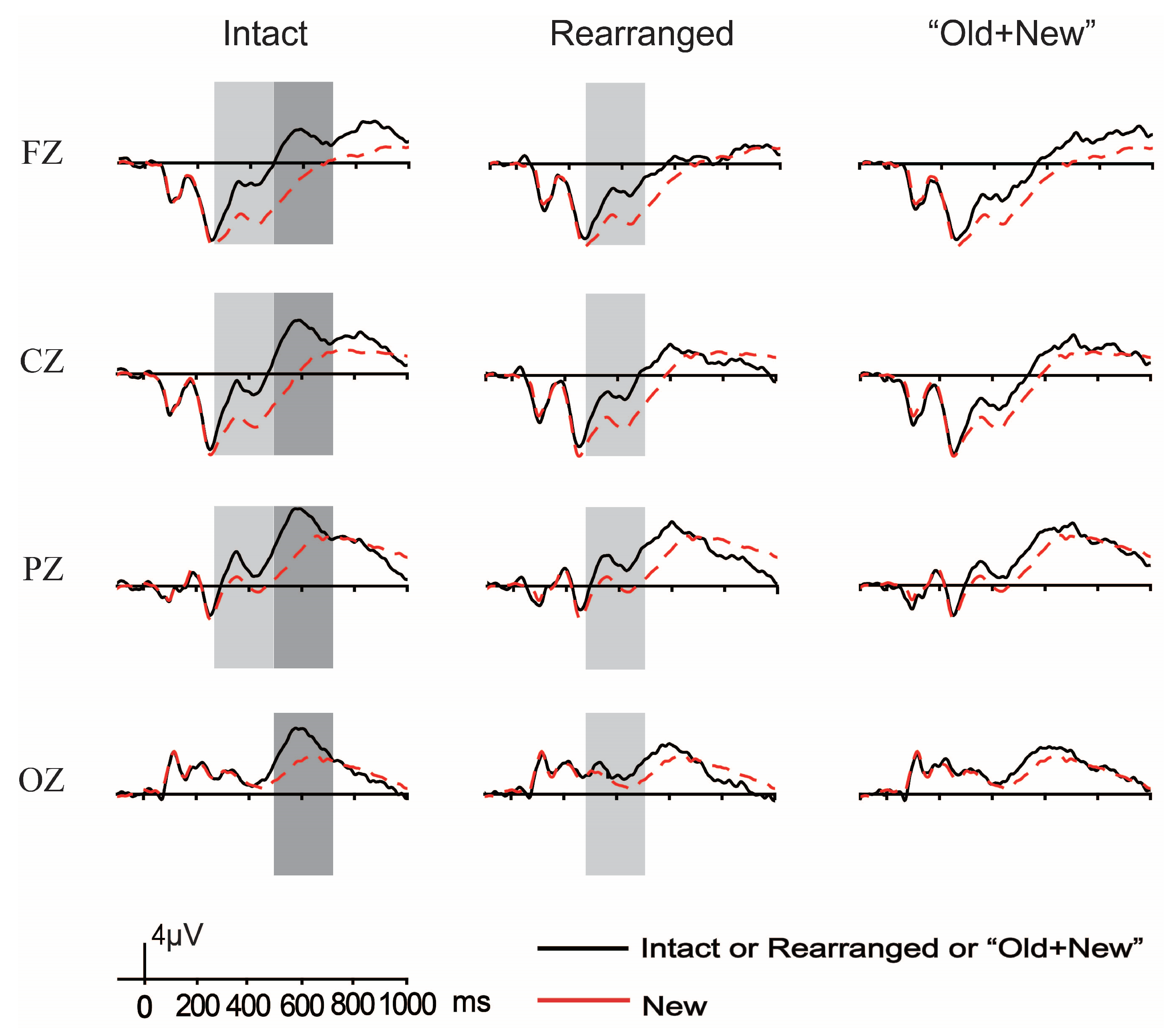
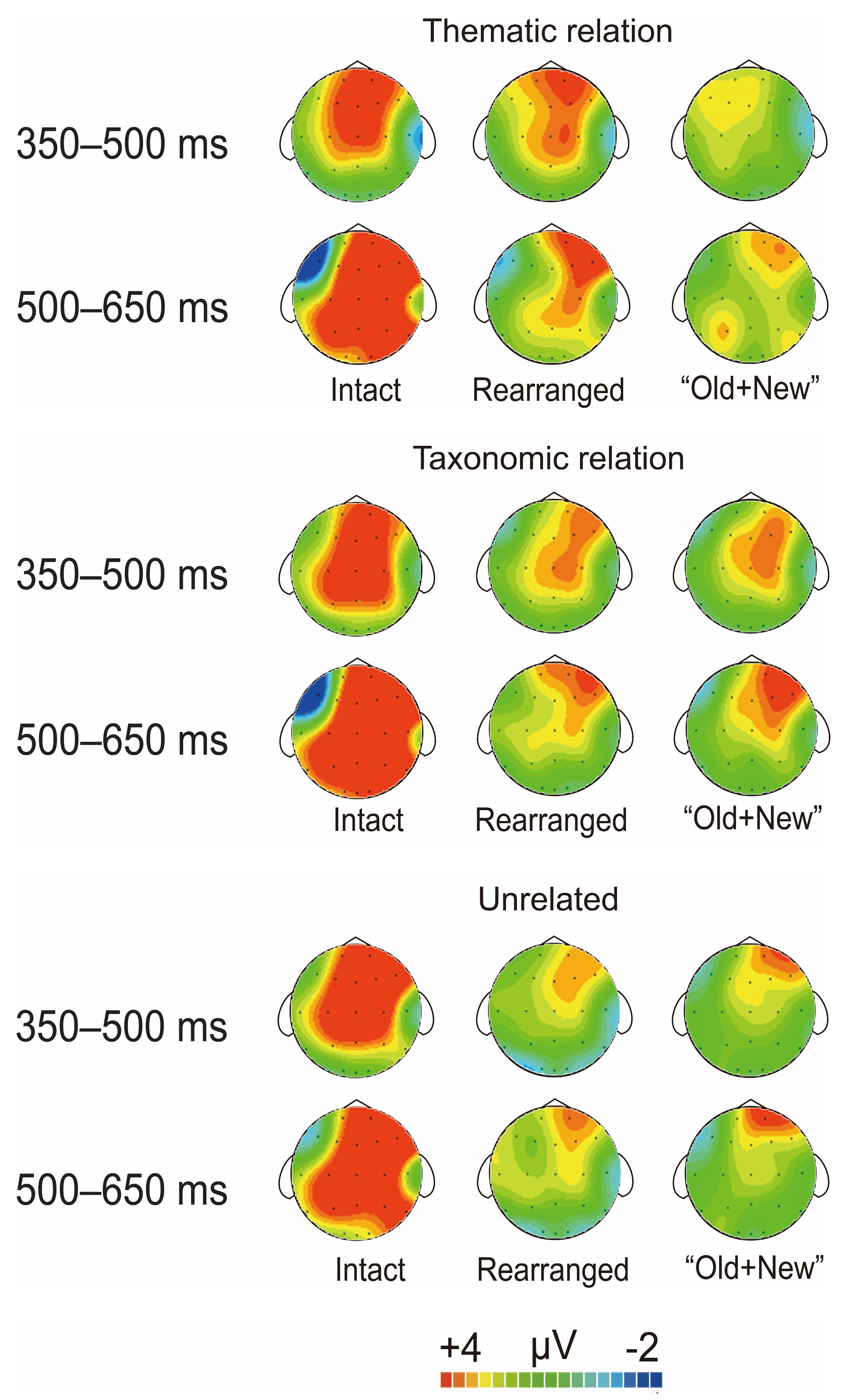
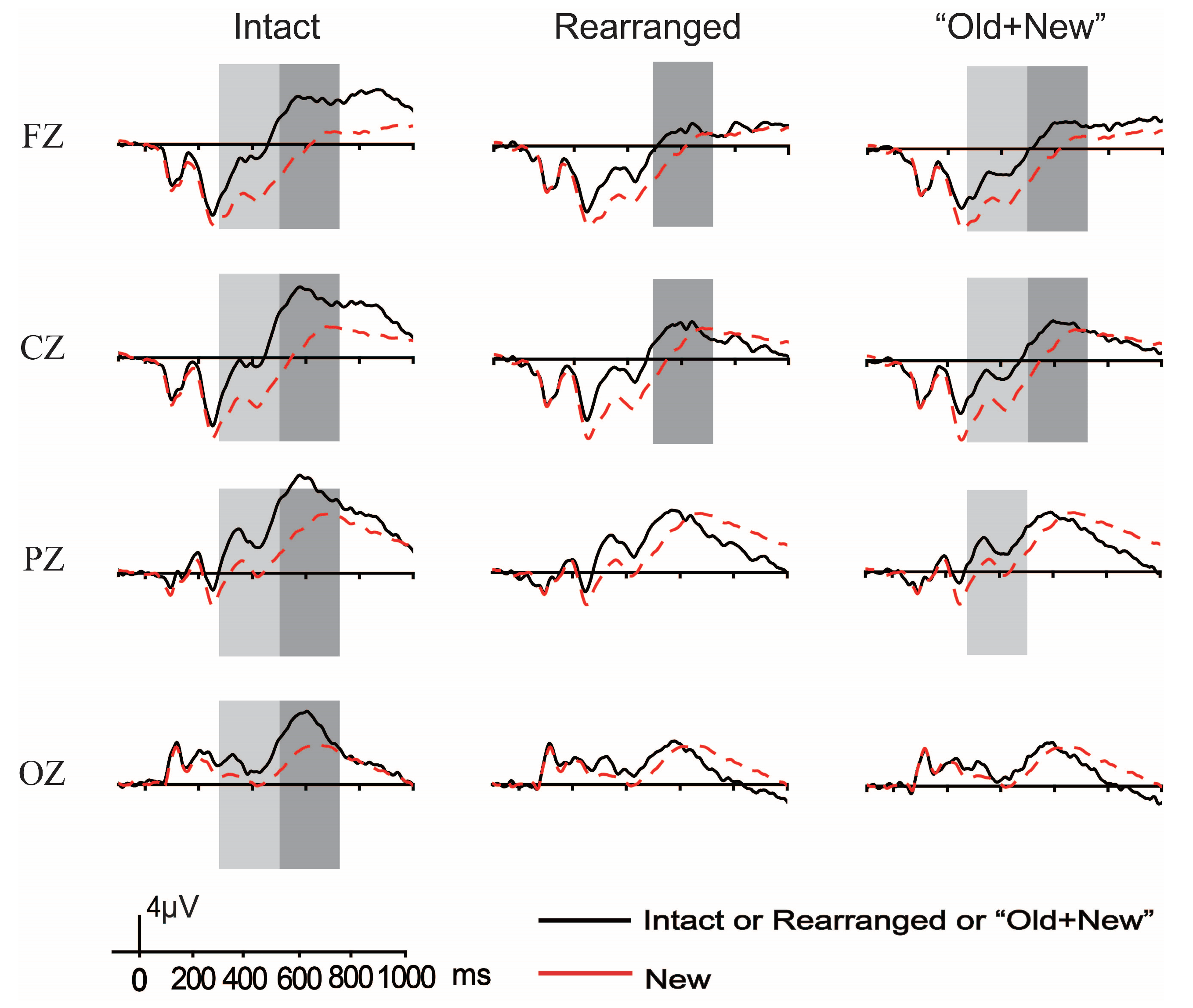
3.2.1. ERP Results of Both FN400 and LPC
The Old/New Effects of Thematic Relation
The Old/New Effects of Taxonomic Relation
3.2.2. Analyses of the Difference Waveforms and Results
Analyses of the Difference Waveforms per Semantic Relation and Results
Analyses of the Difference Waveforms per Pair Type and Results
3.3. Results of the Correlation between Accuracy and Old/New Effects
4. Discussion
4.1. The Neural Activity of Associative Recognition Is Sensitive to the Semantic Relation of Stimuli and Depends More on Stimulus Properties
4.2. The Familiarity of a Single Item can Impact the Neural Activities in Discriminating Associative Pairs
4.3. The Interval Length between Encoding and Test Can Modulate the Familiarity of Unrelated Pairs
5. Limitations and Future Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Isingrini, M.; Sacher, M.; Perrotin, A.; Taconnat, L.; Souchay, C.; Stoehr, H.; Bouazzaoui, B. Episodic feeling-of-knowing relies on noncriterial recollection and familiarity: Evidence using an online remember-know procedure. Conscious. Cogn. 2016, 41, 31–40. [Google Scholar] [CrossRef]
- Malejka, S.; Bröder, A. No source memory for unrecognized items when implicit feedback is avoided. Mem. Cogn. 2016, 44, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Ozubko, J.D.; Seli, P. Forget all that nonsense: The role of meaning during the forgetting of recollective and familiarity-based memories. Neuropsychologia 2016, 90, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Yonelinas, A.P.; Aly, M.; Wang, W.C.; Koen, J.D. Recollection and familiarity: Examining controversial assumptions and new directions. Hippocampus 2010, 20, 1178–1194. [Google Scholar] [CrossRef]
- Yonelinas, A.P.; Parks, C.M. Receiver operating characteristics (ROCs) in recognition memory: A review. Psychol. Bull. 2007, 133, 800–832. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wu, J.; Guo, C. The effect of unitization on associative recognition was not moderated by the unitization-congruence between original and rearranged picture pairs (UC) for picture stimuli. Psychol. Res. 2021, 85, 268–279. [Google Scholar] [CrossRef]
- Maylor, E.A.; Long, H.R.; Newstead, R.A. Differential effects of alcohol on associative versus item memory. Appl. Cogn. Psychol. 2019, 33, 386–392. [Google Scholar] [CrossRef]
- Nie, A.; Jiang, G. Does stimulus emotionality influence associative memory? Insights from directed forgetting. Curr. Psychol. 2021, 40, 4957–4974. [Google Scholar] [CrossRef]
- Zhou, W.; Nie, A.; Xiao, Y.; Liu, S.; Deng, C. Is color source retrieval sensitive to emotion? Electrophysiological evidence from old/new effects. Acta Psychol. 2020, 210, 103156. [Google Scholar] [CrossRef]
- Ventura-Bort, C.; Dolcos, F.; Wendt, J.; Wirkner, J.; Hamm, A.O.; Weymar, M. Item and source memory for emotional associates is mediated by different retrieval processes. Neuropsychologia 2020, 145, 106606. [Google Scholar] [CrossRef]
- Li, M.; Nie, A. Do we prioritise memory for cheaters? Rebuttal evidence from old/new effects in episodic memory. J. Cogn. Psychol. 2021, 33, 247–271. [Google Scholar] [CrossRef]
- Minor, G.; Herzmann, G. Effects of negative emotion on neural correlates of item and source memory during encoding and retrieval. Brain Res. 2019, 1718, 32–45. [Google Scholar] [CrossRef]
- Nie, A.; Griffin, M.; Keinath, A.; Walsh, M.; Dittmann, A.; Reder, L. ERP profiles for face and word recognition are based on their status in semantic memory not their stimulus category. Brain Res. 2014, 1557, 66–73. [Google Scholar] [CrossRef]
- Elward, R.L.; Rugg, M.D. Retrieval goal modulates memory for context. J. Cogn. Neurosci. 2015, 27, 2529–2540. [Google Scholar] [CrossRef]
- Allan, K.; Rugg, M.D. Neural correlates of cued recall with and without retrieval of source memory. Neuroreport 1998, 9, 3463–3466. [Google Scholar] [CrossRef]
- Ye, J.; Nie, A.; Liu, S. How do word frequency and memory task influence directed forgetting: An ERP study. Int. J. Psychophysiol. 2019, 146, 157–172. [Google Scholar] [CrossRef]
- Allan, K.; Wolf, H.A.; Rosenthal, C.R.; Rugg, M.D. The effect of retrieval cues on post-retrieval monitoring in episodic memory: An electrophysiological study. Cogn. Brain Res. 2001, 12, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Morcom, A.M.; Rugg, M.D. Retrieval orientation and the control of recollection: An fMRI study. J. Cogn. Neurosci. 2012, 24, 2372–2384. [Google Scholar] [CrossRef] [PubMed]
- Yick, Y.Y.; Wilding, E.L. Electrophysiological correlates of processes supporting memory for faces. Brain Cogn. 2014, 90, 50–62. [Google Scholar] [CrossRef]
- Gao, C.; Hermiller, M.S.; Voss, J.L.; Guo, C. Basic perceptual changes that alter meaning and neural correlates of recognition memory. Front. Hum. Neurosci. 2015, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Nie, A.; Pan, R.; Shen, H. How processing fluency contribute to the old/new effects of familiarity and recollection: Evidence from the remember/know paradigm. Am. J. Psychol. 2021, 134, 297–319. [Google Scholar] [CrossRef]
- Nie, A.; Guo, C.; Wu, Y.; Qu, N.; Ding, J. Picture encoding and retrieval: An event-related potential study. Chin. Sci. Bull. 2004, 49, 2148–2154. [Google Scholar] [CrossRef]
- Wirkner, J.; Ventura-Bort, C.; Schulz, P.; Hamm, A.O.; Weymar, M. Event-related potentials of emotional and neutral memories: The role of encoding position and delayed testing. Psychophysiology 2018, 55, e13069. [Google Scholar] [CrossRef] [PubMed]
- Baadte, C.; Meinhardt-Injac, B. The picture superiority effect in associative memory: A developmental study. Br. J. Dev. Psychol. 2019, 37, 382–395. [Google Scholar] [CrossRef] [PubMed]
- Bellander, M.; Eschen, A.; Lövdén, M.; Martin, M.; Bäckman, L.; Brehmer, Y. No evidence for improved associative memory performance following process-based associative memory training in older adults. Front. Aging Neurosci. 2017, 8, 326. [Google Scholar] [CrossRef]
- Lucas, H.D.; Gupta, R.S.; Hubbard, R.J.; Federmeier, K.D. Adult age differences in the use of conceptual combination as an associative encoding strategy. Front. Hum. Neurosci. 2019, 13, 339. [Google Scholar] [CrossRef] [PubMed]
- Osth, A.F.; Fox, J. Are associations formed across pairs? A test of learning by temporal contiguity in associative recognition. Psychon. Bull. Rev. 2019, 26, 1650–1656. [Google Scholar] [CrossRef]
- Bridger, E.K.; Kursawe, A.; Bader, R.; Tibon, R.; Gronau, N.; Levy, D.A.; Mecklinger, A. Age effects on associative memory for novel picture pairings. Brain Res. 2017, 1664, 102–115. [Google Scholar] [CrossRef]
- De Brigard, F.; Langella, S.; Stanley, M.L.; Castel, A.D.; Giovanello, K.S. Age-related differences in recognition in associative memory. Aging Neuropsychol. Cogn. 2020, 27, 289–301. [Google Scholar] [CrossRef]
- Delhaye, E.; Folville, A.; Bastin, C. The impact of semantic relatedness on associative memory in aging depending on the semantic relationships between the memoranda. Exp. Aging Res. 2019, 45, 469–479. [Google Scholar] [CrossRef]
- Nie, A.; Jiang, G.; Li, M. Evaluating the contribution of emotional valence to associative memory: Retrieval practice matters. Adv. Cogn. Psychol. 2021, 17, 15–32. [Google Scholar] [CrossRef]
- Buchler, N.G.; Faunce, P.; Light, L.L.; Gottfredson, N.; Reder, L.M. Effects of repetition on associative recognition in young and older adults: Item and associative strengthening. Psychol. Aging 2011, 26, 111–126. [Google Scholar] [CrossRef]
- Kriukova, O.; Bridger, E.; Mecklinger, A. Semantic relations differentially impact associative recognition memory: Electrophysiological evidence. Brain Cogn. 2013, 83, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Cohn, M.; Giannoylis, I.; De Belder, M.; Saint-Cyr, J.A.; McAndrews, M.P. Associative reinstatement memory measures hippocampal function in Parkinson’s Disease. Neuropsychologia 2016, 90, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Liu, Z.; Wang, Y.; Guo, C. The different effects of concept definition and interactive imagery encoding on associative recognition for word and picture stimuli. Int. J. Psychophysiol. 2020, 158, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Mayes, A.; Montaldi, D.; Migo, E. Associative memory and the medial temporal lobes. Trends Cogn. Sci. 2007, 11, 126–135. [Google Scholar] [CrossRef]
- Ahmad, F.N.; Hockley, W.E. Distinguishing familiarity from fluency for the compound word pair effect in associative recognition. Q. J. Exp. Psychol. 2017, 70, 1768–1791. [Google Scholar] [CrossRef]
- Ngo, C.T.; Lloyd, M.E. Familiarity influences on direct and indirect associative memory for objects in scenes. Q. J. Exp. Psychol. 2018, 71, 471–482. [Google Scholar] [CrossRef]
- Parks, C.M.; Yonelinas, A.P. The importance of unitization for familiarity-based learning. J. Exp. Psychol. Learn. Mem. Cogn. 2015, 41, 881–903. [Google Scholar] [CrossRef]
- Robey, A.; Riggins, T. Increasing relational memory in childhood with unitization strategies. Mem. Cogn. 2018, 46, 100–111. [Google Scholar] [CrossRef]
- Tibon, R.; Ben-Zvi, S.; Levy, D.A. Associative recognition processes are modulated by modality relations. J. Cogn. Neurosci. 2014, 26, 1785–1796. [Google Scholar] [CrossRef]
- Tibon, R.; Gronau, N.; Scheuplein, A.L.; Mecklinger, A.; Levy, D.A. Associative recognition processes are modulated by the semantic unitizability of memoranda. Brain Cogn. 2014, 92, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Tibon, R.; Levy, D.A. Temporal texture of associative encoding modulates recall processes. Brain Cogn. 2014, 84, 1–13. [Google Scholar] [CrossRef]
- Bader, R.; Opitz, B.; Reith, W.; Mecklinger, A. Is a novel conceptual unit more than the sum of its parts?: FMRI evidence from an associative recognition memory study. Neuropsychologia 2014, 61, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Bader, R.; Mecklinger, A.; Hoppstädter, M.; Meyer, P. Recognition memory for one-trial-unitized word pairs: Evidence from event-related potentials. NeuroImage 2010, 50, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, I.; Bader, R.; Mecklinger, A. Multiple ways to the prior occurrence of an event: An electrophysiological dissociation of experimental and conceptually driven familiarity in recognition memory. Brain Res. 2010, 1360, 106–118. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, J.; Xiao, F.; Broster, L.S.; Jiang, Y. Electrophysiological evidence for the effects of unitization on associative recognition memory in older adults. Neurobiol. Learn. Mem. 2015, 121, 59–71. [Google Scholar] [CrossRef]
- Wang, Y.; Mao, X.; Li, B.; Lu, B.; Guo, C. Semantic memory influences episodic retrieval by increased familiarity. Neuroreport 2016, 27, 774–782. [Google Scholar] [CrossRef]
- Rhodes, S.M.; Donaldson, D.I. Electrophysiological evidence for the effect of interactive imagery on episodic memory: Encouraging familiarity for non-unitized stimuli during associative recognition. NeuroImage 2008, 39, 873–884. [Google Scholar] [CrossRef]
- Kumar, U. The neural realm of taxonomic and thematic relation: An fMRI study. Lang. Cogn. Neurosci. 2018, 33, 648–658. [Google Scholar] [CrossRef]
- Landrigan, J.F.; Mirman, D. Taxonomic and thematic relatedness ratings for 659 word pairs. J. Open Psychol. Data 2016, 4, e2. [Google Scholar] [CrossRef]
- Savic, O.; Savic, A.M.; Kovic, V. Comparing the temporal dynamics of thematic and taxonomic processing using event-related potentials. PLoS ONE 2017, 12, e0189362. [Google Scholar] [CrossRef]
- Jackson, R.L.; Hoffman, P.; Pobric, G.; Ralph, M.A.L. The nature and neural correlates of semantic association versus conceptual similarity. Cereb. Cortex 2015, 25, 4319–4333. [Google Scholar] [CrossRef]
- Chen, Q.; Li, P.; Xi, L.; Li, F.; Lei, Y.; Li, H. How do taxonomic versus thematic relations impact similarity and difference judgments? An ERP study. Int. J. Psychophysiol. 2013, 90, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.A.; Poeppel, D.; Murphy, G.L. The neural bases of taxonomic and thematic conceptual relations: An MEG study. Neuropsychologia 2015, 68, 176–189. [Google Scholar] [CrossRef]
- Lv, Y.; Liang, J.; Guo, C. The influence of semantic integration between items on associative recognition: Evidence from ERPs study. Acta Psychol. Sin. 2015, 47, 427–438. [Google Scholar] [CrossRef]
- Campeanu, S.; Craik, F.I.M.; Backer, K.C.; Alain, C. Voice reinstatement modulates neural indices of continuous word recognition. Neuropsychologia 2014, 62, 233–244. [Google Scholar] [CrossRef]
- Thézé, R.; Guggisberg, A.G.; Nahum, L.; Schnider, A. Rapid memory stabilization by transient theta coherence in the human medial temporal lobe. Hippocampus 2016, 26, 445–454. [Google Scholar] [CrossRef]
- Yang, N.; Waddington, G.; Adams, R.; Han, J. Translation, cultural adaption, and test-retest reliability of Chinese versions of the Edinburgh Handedness Inventory and Waterloo Footedness Questionnaire. Laterality Asymmetries Body Brain Cogn. 2017, 23, 255–273. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Nie, A.; Li, M.; Ye, J. Lag-length effect on repetition priming of famous and unfamiliar faces: Evidence from N250r and N400. NeuroReport 2016, 27, 755–763. [Google Scholar] [CrossRef]
- Nowagk, R.; Pfeifer, E. Unix implementation of the ERP evaluation package (EEP 3.0). Annu. Rep. Max-Planck-Inst. Cogn. Neurosci. 1996, 124–126. [Google Scholar]
- Ross, R.S.; Smolen, A.; Curran, T.; Nyhus, E. Mao-A phenotype effects response sensitivity and the parietal old/new effect during recognition memory. Front. Hum. Neurosci. 2018, 12, 53. [Google Scholar] [CrossRef] [PubMed]
- Bader, R.; Mecklinger, A. Separating event-related potential effects for conceptual fluency and episodic familiarity. J. Cogn. Neurosci. 2017, 29, 1402–1414. [Google Scholar] [CrossRef] [PubMed]
- Küper, K.; Zimmer, H.D. The impact of perceptual changes to studied items on ERP correlates of familiarity and recollection is subject to hemispheric asymmetries. Brain Cogn. 2018, 122, 17–25. [Google Scholar] [CrossRef]
- Leynes, P.A.; Askin, B.; Landau, J.D. Visual perspective during remembering: ERP evidence of familiarity-based source monitoring. Cortex 2017, 91, 157–168. [Google Scholar] [CrossRef]
- Lin, H.; Liang, J. Contextual effects of angry vocal expressions on the encoding and recognition of emotional faces: An event-related potential (ERP) study. Neuropsychologia 2019, 132, 107147. [Google Scholar] [CrossRef]
- Mecklinger, A.; Rosburg, T.; Johansson, M. Reconstructing the past: The late posterior negativity (LPN) in episodic memory studies. Neurosci. Biobehav. Rev. 2016, 68, 621–638. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, Q.; Li, B.; Guo, C. Dissociable effects of valence and arousal on different subtypes of old/new effect: Evidence from event-related potentials. Front. Hum. Neurosci. 2016, 9, 650. [Google Scholar] [CrossRef]
- Francis, W.S. Repetition priming in picture naming: Sustained learning through the speeding of multiple processes. Psychon. Bull. Rev. 2014, 21, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Caplan, J.B.; Sommer, T.; Madan, C.R.; Fujiwara, E. Reduced associative memory for negative information: Impact of confidence and interactive imagery during study. Cogn. Emot. 2019, 33, 1745–1753. [Google Scholar] [CrossRef] [PubMed]
- Curran, T. Brain potentials of recollection and familiarity. Mem. Cogn. 2000, 28, 923–938. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Taylor, J.R.; Wang, W.; Gao, C.; Guo, C. Electrophysiological signals associated with fluency of different levels of processing reveal multiple contributions to recognition memory. Conscious. Cogn. 2017, 53, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Stróżak, P.; Abedzadeh, D.; Curran, T. Separating the FN400 and N400 potentials across recognition memory experiments. Brain Res. 2016, 1635, 41–60. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, A.; Wu, Y. Differentiation of the Contribution of Familiarity and Recollection to the Old/New Effects in Associative Recognition: Insight from Semantic Relation. Brain Sci. 2023, 13, 553. https://doi.org/10.3390/brainsci13040553
Nie A, Wu Y. Differentiation of the Contribution of Familiarity and Recollection to the Old/New Effects in Associative Recognition: Insight from Semantic Relation. Brain Sciences. 2023; 13(4):553. https://doi.org/10.3390/brainsci13040553
Chicago/Turabian StyleNie, Aiqing, and Yuanying Wu. 2023. "Differentiation of the Contribution of Familiarity and Recollection to the Old/New Effects in Associative Recognition: Insight from Semantic Relation" Brain Sciences 13, no. 4: 553. https://doi.org/10.3390/brainsci13040553
APA StyleNie, A., & Wu, Y. (2023). Differentiation of the Contribution of Familiarity and Recollection to the Old/New Effects in Associative Recognition: Insight from Semantic Relation. Brain Sciences, 13(4), 553. https://doi.org/10.3390/brainsci13040553




