Guillain-Barré Syndrome with Incomplete Oculomotor Nerve Palsy after Traumatic Brain Injury: Case Report and Literature Review
Abstract
1. Introduction
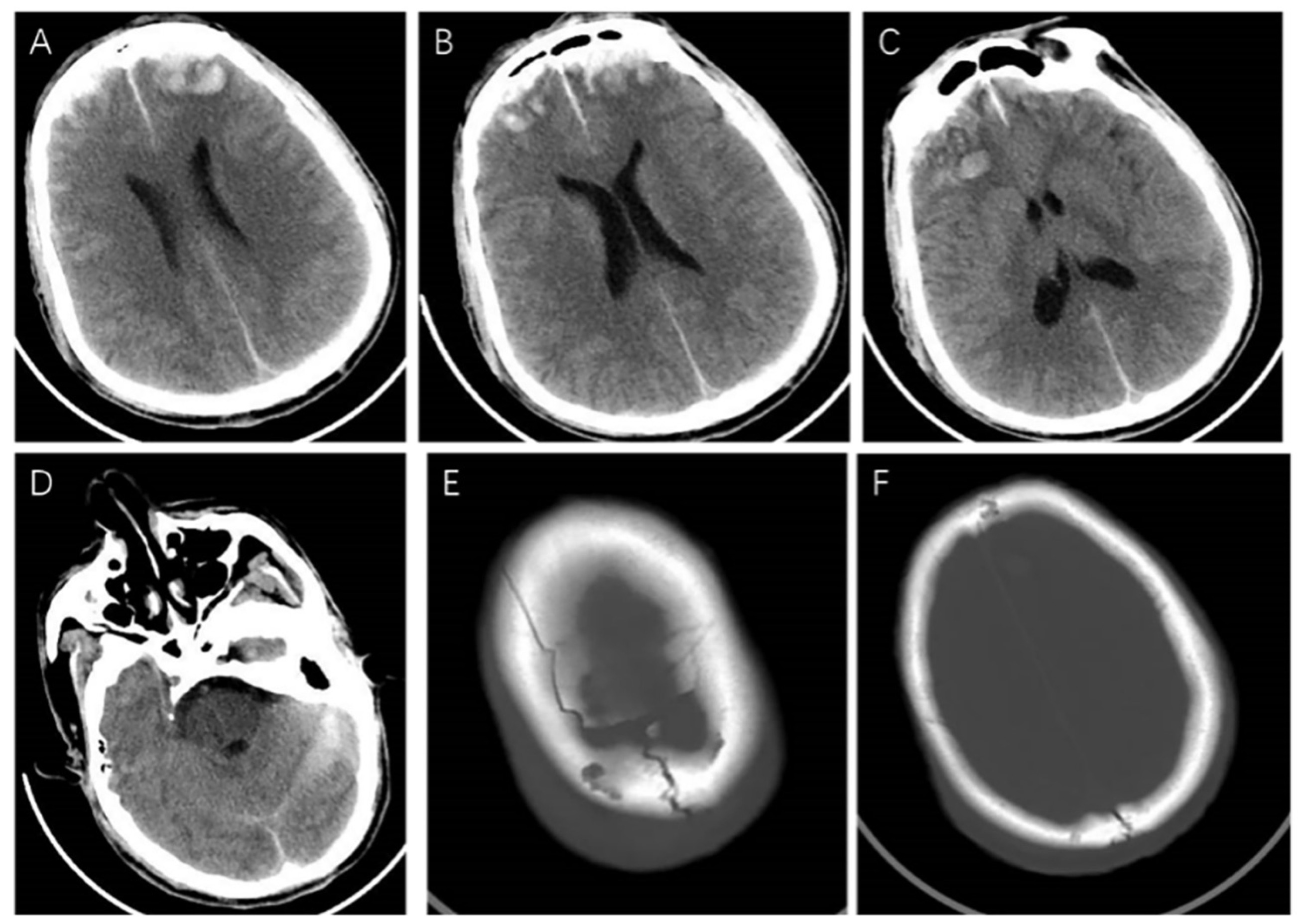
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Esposito, S.; Longo, M.R. Guillain-Barré syndrome. Autoimmun. Rev. 2017, 16, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.; Kennedy, P.G. Guillain-Barré syndrome following acute head trauma. Postgrad. Med. J. 1987, 63, 479–480. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Lian, Y.; Liu, Y.; Wu, B.-Y.; Duan, R.-S. A retrospective analysis of possible triggers of Guillain-Barre syndrome. J. Neuroimmunol. 2016, 293, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Carr, K.R.; Shah, M.; Garvin, R.; Shakir, A.; Jackson, C. Post-Traumatic brain injury (TBI) presenting with Guillain-Barré syndrome and elevated anti-ganglioside antibodies: A case report and review of the literature. Int. J. Neurosci. 2015, 125, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, C.; Lu, W.; Hu, Q. Case Report: Delayed Guillain-Barré syndrome following trauma: A case series and manage considerations. Front. Surg. 2022, 9, 903334. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, Y.; Deng, S.; Ren, Y.; Lu, W. Trauma-Related Guillain-Barré Syndrome: Systematic Review of an Emerging Concept. Front. Neurol. 2020, 11, 588290. [Google Scholar] [CrossRef]
- De Freitas, G.R.; De Freitas, M.R.; Ferreira, M.C. Guillain-Barré syndrome and head trauma. Case report. Arq. Neuropsiquiatr. 1997, 55, 315–318. [Google Scholar] [CrossRef]
- Stojkovic, T.; Verdin, M.; Hurtevent, J.F.; Laureau, E.; Krivosic-Horber, R.; Vermersch, P. Guillain-Barré syndrome resembling brainstem death in a patient with brain injury. J. Neurol. 2001, 248, 430–432. [Google Scholar] [CrossRef]
- Lin, T.-M.; Lee, S.-S.; Lin, R.-T.; Lai, C.-S.; Lin, S.-D. Guillain-Barré syndrome following facial bone fracture. J. Plast. Reconstr. Aesthet. Surg. 2006, 59, 543–546. [Google Scholar] [CrossRef]
- Rivas, S.; Douds, G.L.; Ostdahl, R.H.; Harbaugh, K.S. Fulminant Guillain-Barré syndrome after closed head injury: A potentially reversible cause of an ominous examination. Case report. J. Neurosurg. 2008, 108, 595–600. [Google Scholar] [CrossRef]
- Yardimci, N.; Gulsen, S.; Avci, A.Y.; Altinors, N.; Zileli, T.; Can, U. Can subdural hematoma be a trigger for Guillain-Barré syndrome? Int. J. Neurosci. 2009, 119, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Tan, I.L.; Ng, T.; Vucic, S. Severe Guillain-Barré syndrome following head trauma. J. Clin. Neurosci. 2010, 17, 1452–1454. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, F.; Sevy, A.; Moyse, E.; Roche, P.H. Guillain-Barré syndrome following severe head trauma and spine surgery. Rev. Neurol. 2013, 169, 166–168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-Z.; Li, X.-G. Cerebral trauma, Campylobacter jejuni infection, and monosialotetrahexosylganglioside sodium mediated Guillain-Barré syndrome in a Chinese patient: A rare case event. J. Neuropsychiatry Clin. Neurosci. 2014, 26, E16–E17. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xiao, J.; Ding, Y.; Xu, J.; Li, C.; He, Y.; Zhai, H.; Xie, B.; Hao, J. Clinical and electrophysiological features of post-traumatic Guillain-Barré syndrome. BMC Neurol. 2017, 17, 142. [Google Scholar] [CrossRef]
- Yonekura, S.; Anno, T.; Kobayashi, N. Posterior Reversible Encephalopathy Syndrome and Guillain-Barré Syndrome after Head Injury: Case Report. Neurol. Med. Chir. 2018, 58, 453–458. [Google Scholar] [CrossRef]
- Yilmaz, H.; Akcay, E.; Benek, H.B.; Yurt, A. Guillain-Barre Syndrome After Craniocerebral Gunshot Injury: First Report. World Neurosurg. 2020, 143, 23–25. [Google Scholar] [CrossRef]
- Koga, M.; Yuki, N.; Ariga, T.; Hirata, K. Antibodies to GD3, GT3, and O-acetylated species in Guillain-Barré and Fisher’s syndromes: Their association with cranial nerve dysfunction. J. Neurol. Sci. 1999, 164, 50–55. [Google Scholar] [CrossRef]
- Yuki, N.; Susuki, K.; Koga, M.; Nishimoto, Y.; Odaka, M.; Hirata, K.; Taguchi, K.; Miyatake, T.; Furukawa, K.; Kobata, T.; et al. Carbohydrate mimicry between human ganglioside GM1 and Campylobacter jejuni lipooligosaccharide causes Guillain-Barre syndrome. Proc. Natl. Acad. Sci. USA 2004, 101, 11404–11409. [Google Scholar] [CrossRef]
- Herbert, T.B.; Cohen, S. Stress and immunity in humans: A meta-analytic review. Psychosom. Med. 1993, 55, 364–379. [Google Scholar] [CrossRef]
- Marsland, A.L.; Herbert, T.B.; Muldoon, M.F.; Bachen, E.A.; Patterson, S.; Cohen, S.; Rabin, B.; Manuck, S.B. Lymphocyte subset redistribution during acute laboratory stress in young adults: Mediating effects of hemoconcentration. Health Psychol. 1997, 16, 341–348. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Raad, M.; Nohra, E.; Chams, N.; Itani, M.; Talih, F.; Mondello, S.; Kobeissy, F. Autoantibodies in traumatic brain injury and central nervous system trauma. Neuroscience 2014, 281, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Ankeny, D.P.; Popovich, P.G. B cells and autoantibodies: Complex roles in CNS injury. Trends Immunol. 2010, 31, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.L.; Hayes, K.C.; Dekaban, G.A. Clinical correlates of elevated serum concentrations of cytokines and autoantibodies in patients with spinal cord injury. Arch. Phys. Med. Rehabil. 2007, 88, 1384–1393. [Google Scholar] [CrossRef]
- Skoda, D.; Kranda, K.; Bojar, M.; Glosová, L.; Bäurle, J.; Kenney, J.; Romportl, D.; Pelichovská, M.; Cvachovec, K. Antibody formation against beta-tubulin class III in response to brain trauma. Brain Res. Bull 2006, 68, 213–216. [Google Scholar] [CrossRef]



| Nerve | Site | Latency (ms) | Amplitude (mV) | Conduction Velocity (m/s) | Distance (mm) |
|---|---|---|---|---|---|
| MNCS | |||||
| Left ulnar | Wrist | 3.87 | 1.62 | 45 | |
| Below elbow | 9.35 | 1.47 | 42.9 | 235 | |
| Above elbow | 11.20 | 1.41 | 45.9 | 85 | |
| Right ulnar | Wrist | 5.54 | 0.75 | 30 | |
| Left median | Wrist | 13.50 | 0.40 | 45 | |
| Elbow | 20.20 | 0.33 | 33.6 | 225 | |
| Right median | Wrist | 14.20 | 0.50 | 50 | |
| Left tibial | Ankle | 8.82 | 0.63 | 80 | |
| Right tibial | Ankle | 8.00 | 0.92 | 90 | |
| Left peroneal | Ankle | No response | |||
| Bl Fib.head | No response | ||||
| Right peroneal | Ankle | No response | |||
| Bl Fib.head | No response | ||||
| SNCS | |||||
| Left ulnar | Wrist | No response | |||
| Left median | Wrist | No response | |||
| Right median | Wrist | No response | |||
| Left superficial peroneal | lower leg | 1.82 | 9.80 | 44 | 80 |
| Right superficial peroneal | lower leg | 2.02 | 2.90 | 37.1 | 75 |
| Left sural | middle leg | 1.33 | 7.50 | 56.4 | 75 |
| Right sural | middle leg | No response | |||
| Author/Year | Age | Gender | Activating Events | Interval between Activating Events and GBS | Clinical Features | CSF Examination | Electrophysiology |
|---|---|---|---|---|---|---|---|
| R. Duncan et al., 1987 [2] | 61 | male | TBI after falling off a ladder, brain contusion, and subdural hematoma. | 15 days | Tetraplegia, dysphagia and dyspnea, and bilateral lower motor neurone facial palsies. | Not performed. | Generalized predominantly motor demyelinating peripheral neuropathy. |
| De Freitas G R et al., 1997 [7] | 29 | male | TBI after hitting the head, and subarachnoid hemorrhage. | 7–9 days | Tetraplegia, facial diplegia, deep areflexia, and respiratory failure. | Increased protein concentration. | Increased latency, part of the amplitude was reduced, and no F waves. |
| Stojkovic, T et al., 2001 [8] | 47 | male | TBI, skull fracture, extradural hemorrhage, and multiple intracerebral hematomas. He underwent evacuation of epidural hematoma. | 8 days | Tetraparesis, mydriasis, and respiratory failure. | Protein: 1.97 g/dL; cell count: 2/mm3. | The latency was prolonged, the amplitude was shortened, and the F-wave was absent. |
| Lin, Tsai-Ming et al., 2006 [9] | 22 | female | TBI after a motor vehicle accident, and facial bone fracture. | 10 days | Weakness in all 4 limbs, most severe in the lower extremities, and numbness of both lower legs. | Acellular with a protein of 0.5 g/L. | Absent of F-waves, revealed a severe generalized predominantly motor demyelinating peripheral neuropathy. |
| Rivas, Sharon et al., 2008 [10] | 55 | male | TBI after falling during an alcohol-withdrawal-related seizure, parietal bone fractures, brain contusion, and subdural hematoma | 7 days | Absence of brainstem function, flaccid quadriplegia, absent deep tendon reflexes, and respiratory failure. | Increased protein level with normal cell counts. | All nerves and fibrillation potentials of all muscles are inexcitability. |
| Yardimci, Nilgul et al., 2009 [11] | 75 | female | TBI after a traffic accident, and subdural hematoma | 7 days | Quadriparesis, choreic movements in the right arm, and cerebellar ataxic speech. | Acellular with a high protein level. | Severe generalized, predominantly motor-demyelinating peripheral neuropathy. |
| Tan, Ik Lin et al., 2010 [12] | 44 | male | TBI after a micturition syncope episode, skull fracture, brain contusion, and subarachnoid hemorrhage | 7 days | Areflexic tetraplegia, dysarthria, dysphagia, and respiratory failure. | Albuminocytological dissociation, and the protein level is 1.82 g/L. | Absent motor and sensory responses, absent blink reflexes, and an absence of spontaneous and voluntary activity on electromyography. |
| Battaglia, F et al., 2013 [13] | 73 | female | TBI after falling from a stool, cerebral hemorrhage and lumbar vertebrae fracture, and spinal surgery | 7 days | Dysphagia, asymmetric facial diplegia, the muscle strength of the limbs decreased, and deep and superficial hypoesthesia of both legs. | Albuminocytological dissociation. | An increase of motor distal latencies and temporal dispersion of motor action potential in four limbs, and F-waves latencies were increased in lower limbs. |
| Zhang, Guan-Zhong et al., 2014 [14] | 56 | male | TBI after falling from height, epidural hematoma, and brain contusion | Not mentioned | Tetraplegia, and hoarseness. | Protein level was increased without alteration in cell numbers. | Motor nerve conduction velocity was reduced and F-wave latency was extended. |
| Carr, Kevin R et al., 2015 [4] | 58 | male | TBI after being struck by a fallen branch, brain contusion, subarachnoid hemorrhage, subdural hematoma, and facial bone fractures | 17 days | Bilateral lower extremities and face weakness | Albumincytologic dissociation. | Conduction block in both upper and lower extremity motor nerves, and the latency of F wave was prolonged. |
| Li, Xiaowen et al., 2017 [15] | 48 | female | TBI, unspecified. | 10 days | Weakness on both limbs, deep tendon reflexes were absent, and respiratory muscle involvement. | Albuminocytological dissociation, and the protein level is 0.64 g/L. | CMAP amplitude reduction, and no F waves. |
| Yonekura, Satoru et al., 2018 [16] | 74 | male | TBI after falling on a mountain, subdural hematoma, and subarachnoid hemorrhage | 3 days | Quadriplegia, bulbar palsy, and weakness of respiratory muscles. | Protein: 92 g/dL; cell count: 8/mmc. | Not performed. |
| Yilmaz, Hakan et al., 2020 [17] | 41 | male | TBI after gunshot wound, subdural hematoma, brain contusion and skull fracture, and the patient received emergency surgery. | 14 days | Tetraplegia, dysarthria, dysphagia, and respiratory failure. | Increased protein levels. | The latency of F wave was prolonged, and motor nerve block in the upper and lower extremities. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Tang, F.; Chen, X.; Li, Z. Guillain-Barré Syndrome with Incomplete Oculomotor Nerve Palsy after Traumatic Brain Injury: Case Report and Literature Review. Brain Sci. 2023, 13, 527. https://doi.org/10.3390/brainsci13040527
Liu J, Tang F, Chen X, Li Z. Guillain-Barré Syndrome with Incomplete Oculomotor Nerve Palsy after Traumatic Brain Injury: Case Report and Literature Review. Brain Sciences. 2023; 13(4):527. https://doi.org/10.3390/brainsci13040527
Chicago/Turabian StyleLiu, Jinsheng, Feng Tang, Xinjun Chen, and Zhiqiang Li. 2023. "Guillain-Barré Syndrome with Incomplete Oculomotor Nerve Palsy after Traumatic Brain Injury: Case Report and Literature Review" Brain Sciences 13, no. 4: 527. https://doi.org/10.3390/brainsci13040527
APA StyleLiu, J., Tang, F., Chen, X., & Li, Z. (2023). Guillain-Barré Syndrome with Incomplete Oculomotor Nerve Palsy after Traumatic Brain Injury: Case Report and Literature Review. Brain Sciences, 13(4), 527. https://doi.org/10.3390/brainsci13040527








