Deep Brain Stimulation for Chronic Facial Pain: An Individual Participant Data (IPD) Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Design and Data Focus
2.2. Inclusion and Exclusion Criteria
2.3. Definitions of Primary/Secondary Outcome Measures and the Additional Assessment of Possible Relationships between DBS Outcomes versus Stimulation Parameters and Patient Characteristics
2.4. Statistical Analysis
3. Results
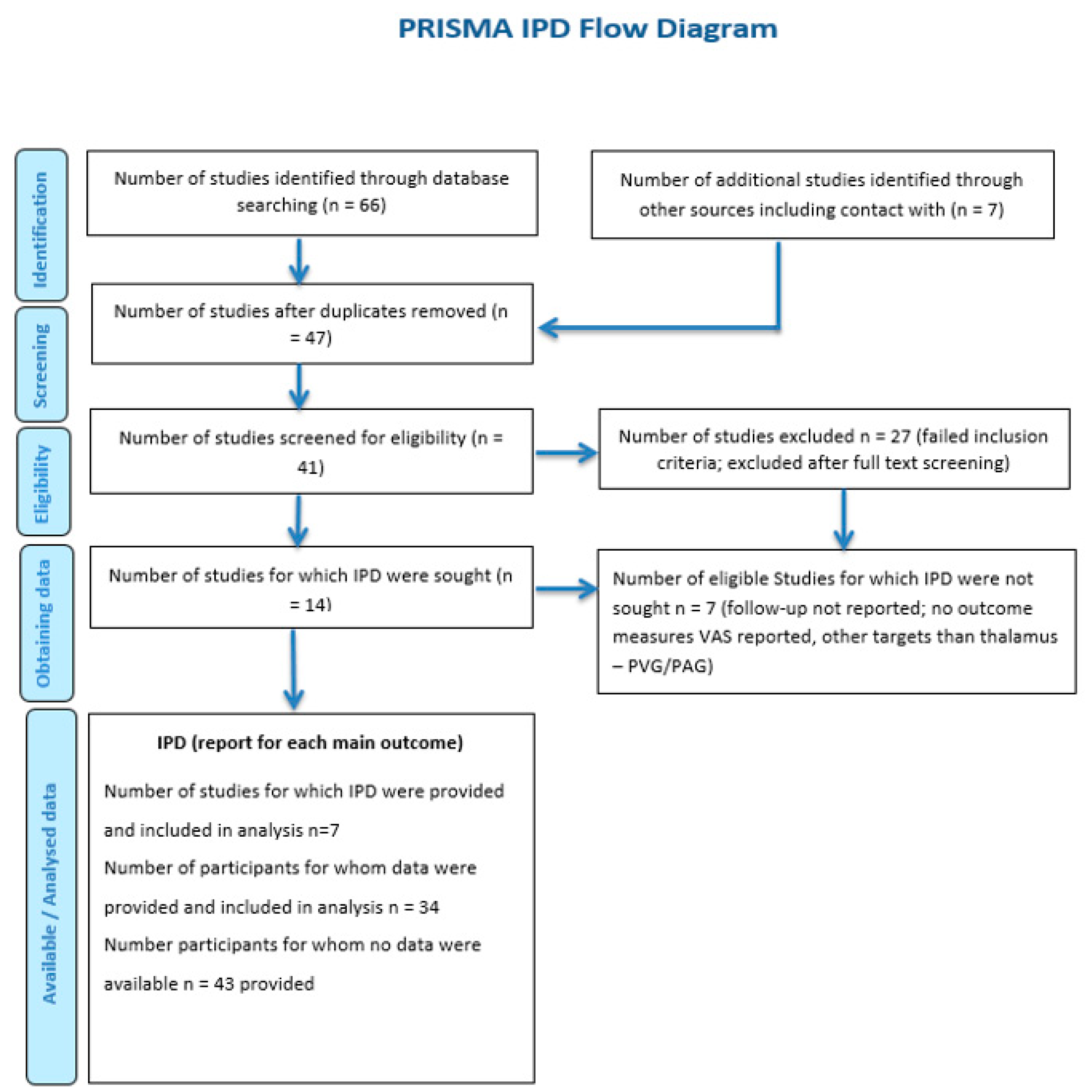
3.1. Baseline Characteristics and the IPD Data Extraction Protocol
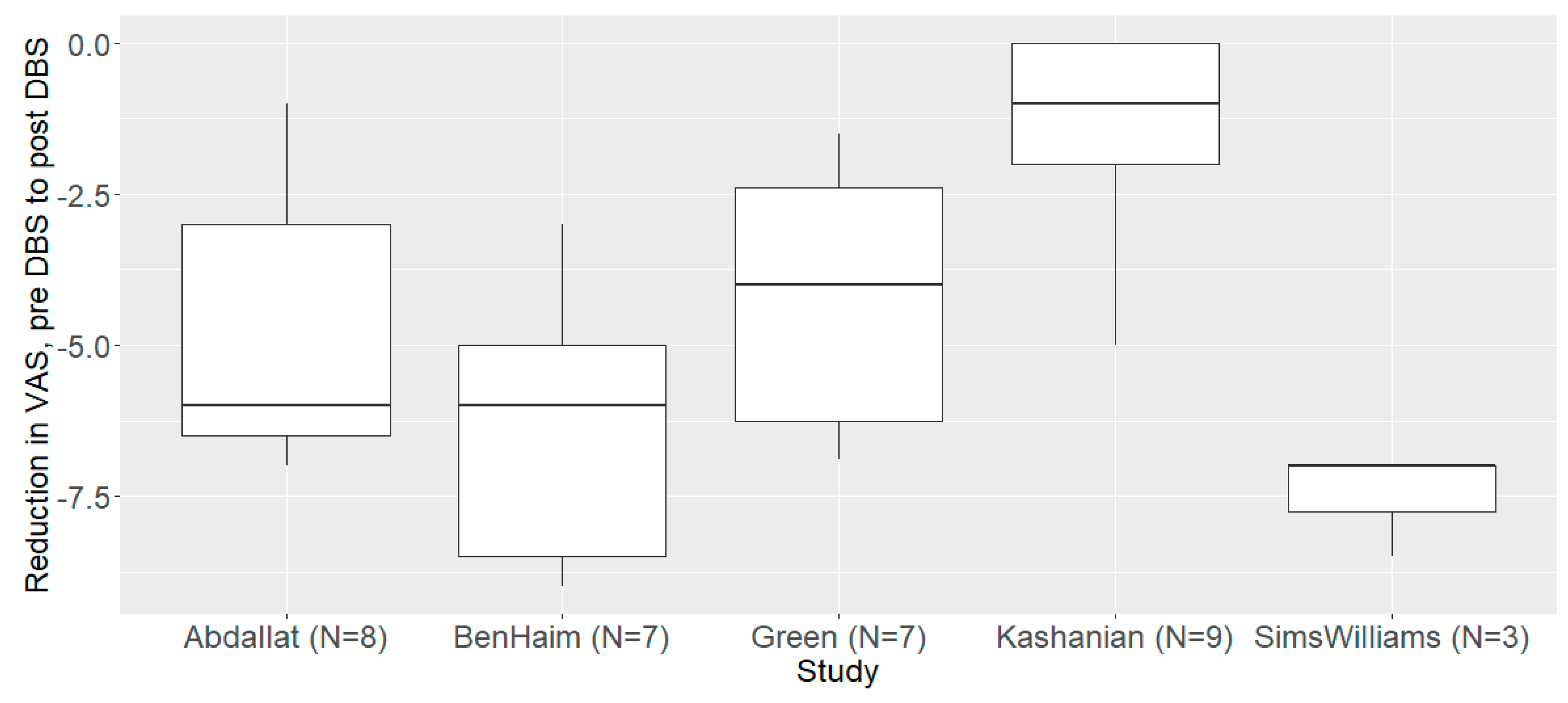
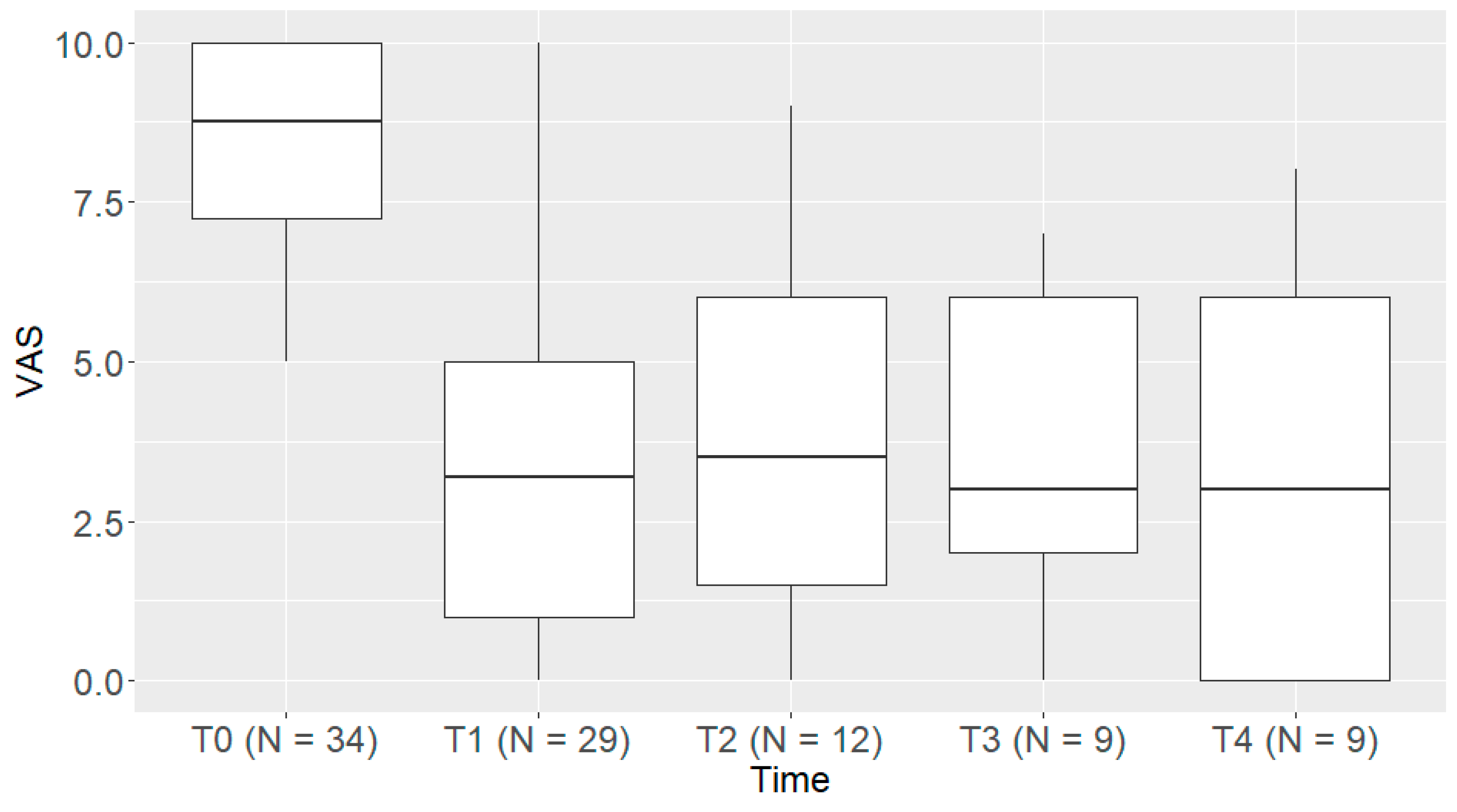
3.2. The Primary Endpoint (a Reduction in PAIN Intensity)
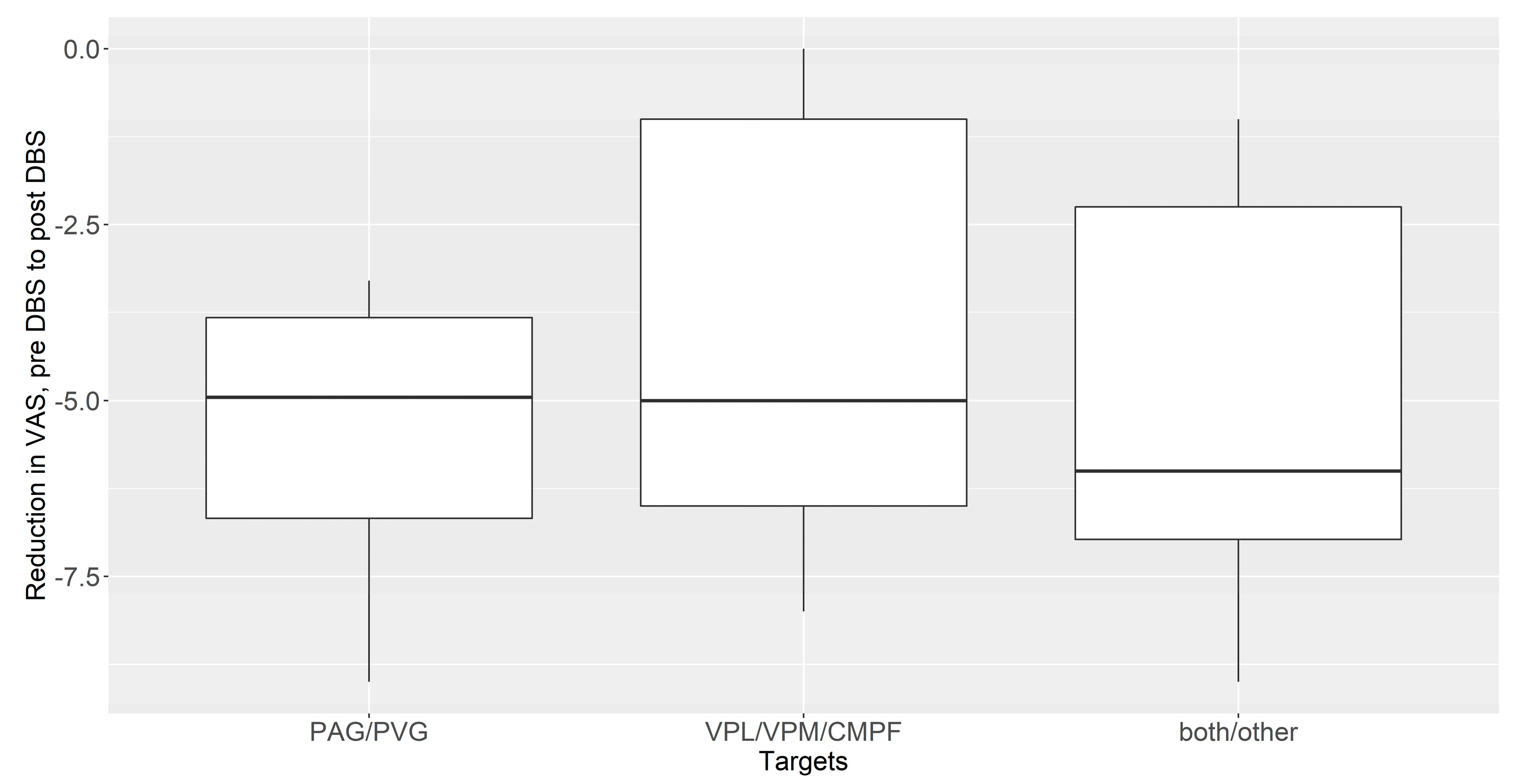
3.3. The Subgroup Analysis between PVG/PAG and Thalamic DBS (VPL/VPM/CmPf)
3.4. The Assessment of Correlations between the DBS Outcome and Age, Stimulation Protocol, Facial Pain Duration, and the DBS Target (Spearman’s Correlation)
3.5. Adverse Events
4. Discussion
4.1. General Remarks on the Use of DBS for Refractory Chronic Facial Pain
4.2. The Impact of DBS on Facial Pain and Functional Capacity
4.3. A Brief Discussion of Long-Term DBS Studies and Alternative DBS Targets (ACC; ALIC/VS)
4.4. The Evidence Level of Less Invasive and Non-Invasive Neurostimulation Therapies for Chronic Pain
4.5. The Impacts of Placebo and Lesion Effects in Deep Brain Stimulation and How to Overcome Them
4.6. Limitations and Prospects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cohen, S.P.; Vase, L.; Hooten, W.M. Chronic pain: An update on burden, best practices, and new advances. Lancet 2021, 397, 2082–2097. [Google Scholar] [CrossRef] [PubMed]
- Leadley, R.M.; Armstrong, N.; Lee, Y.C.; Allen, A.; Kleijnen, J. Chronic diseases in the European Union: The prevalence and health cost implications of chronic pain. J. Pain. Palliat. Care Pharmacother. 2012, 26, 310–325. [Google Scholar] [CrossRef] [PubMed]
- Karra, R.; Holten-Rossing, S.; Mohammed, D.; Parmeggiani, L.; Heine, M.; Namnun, O.C. Unmet needs in the management of functional impairment in patients with chronic pain: A multinational survey. Pain. Manag. 2021, 11, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.L.; Ehrhardt, K.P.; Tolba, R. Atypical Facial Pain: A Comprehensive, Evidence-Based Review. Curr. Pain Headache Rep. 2017, 21, 8. [Google Scholar] [CrossRef] [PubMed]
- Hupe, C.A.G.; Slavin, K.V. Classification of Facial Pain: A Clinician’s Perspective. Prog. Neurol. Surg. 2020, 35, 1–17. [Google Scholar]
- Zubrzycki, M.; Stasiolek, M.; Zubrzycka, M. Opioid and endocannabinoid system in orofacial pain. Physiol. Res. 2019, 68, 705–715. [Google Scholar] [CrossRef]
- Yin, D.; Slavin, K.V. Gasserian Ganglion Stimulation for Facial Pain. Prog. Neurol. Surg. 2020, 35, 96–104. [Google Scholar]
- Stokey, B.G.; Weiner, R.L.; Slavin, K.V.; Hayek, S.M. Peripheral Nerve Stimulation for Facial Pain Using Wireless Devices. Prog. Neurol. Surg. 2020, 35, 75–84. [Google Scholar]
- Magown, P.; Becker, W.J.; Kiss, Z.H. Outcomes of Occipital Nerve Stimulation for Craniofacial Pain Syndromes. Can. J. Neurol. Sci. 2021, 48, 690–697. [Google Scholar] [CrossRef]
- Brown, J.A.; Pilitsis, J.G. Motor cortex stimulation for central and neuropathic facial pain: A prospective study of 10 patients and observations of enhanced sensory and motor function during stimulation. Neurosurgery 2005, 56, 290–297, discussion 290–297. [Google Scholar] [CrossRef]
- Nuti, C.; Peyron, R.; Garcia-Larrea, L.; Brunon, J.; Laurent, B.; Sindou, M.; Mertens, P. Motor cortex stimulation for refractory neuropathic pain: Four year outcome and predictors of efficacy. Pain 2005, 118, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Abdallat, M.; Saryyeva, A.; Blahak, C.; Wolf, M.E.; Weigel, R.; Loher, T.J.; Runge, J.; Heissler, H.E.; Kinfe, T.M.; Krauss, J.K. Centromedian-Parafascicular and Somatosensory Thalamic Deep Brain Stimulation for Treatment of Chronic Neuropathic Pain: A Contemporary Series of 40 Patients. Biomedicines 2021, 9, 731. [Google Scholar] [CrossRef] [PubMed]
- Rasche, D.; Rinaldi, P.C.; Young, R.F.; Tronnier, V.M. Deep brain stimulation for the treatment of various chronic pain syndromes. Neurosurg. Focus. 2006, 21, E8. [Google Scholar] [CrossRef] [PubMed]
- Antony, A.B.; Mazzola, A.J.; Dhaliwal, G.S.; Hunter, C.W. Neurostimulation for the Treatment of Chronic Head and Facial Pain: A Literature Review. Pain. Physician 2019, 22, 447–477. [Google Scholar] [CrossRef]
- Ben-Haim, S.; Mirzadeh, Z.; Rosenberg, W.S. Deep brain stimulation for intractable neuropathic facial pain. Neurosurg. Focus. 2018, 45, E15. [Google Scholar] [CrossRef]
- Constantoyannis, C.; Kumar, A.; Stoessl, A.J.; Honey, C.R. Tremor induced by thalamic deep brain stimulation in patients with complex regional facial pain. Mov. Disord. 2004, 19, 933–936. [Google Scholar] [CrossRef]
- Evidente, V.G.H.; Ponce, F.A.; Evidente, M.H.; Garrett, R.; Lambert, M. Short-Lasting Unilateral Neuralgiform Headache With Conjunctival Injection and Tearing (SUNCT) Improves With Bilateral Ventral Tegmental Area Deep Brain Stimulation. Headache 2020, 60, 2548–2554. [Google Scholar] [CrossRef]
- Franzini, A.; Leone, M.; Messina, G.; Cordella, R.; Marras, C.; Bussone, G.; Broggi, G. Neuromodulation in treatment of refractory headaches. Neurol. Sci. 2008, 29 (Suppl. S1), S65–S68. [Google Scholar] [CrossRef]
- Green, A.L.; Owen, S.L.F.; Davies, P.; Moir, L.; Aziz, T.Z. Deep brain stimulation for neuropathic cephalalgia. Cephalalgia 2006, 26, 561–567. [Google Scholar] [CrossRef]
- Kashanian, A.; DiCesare, J.A.; Rohatgi, P.; Albano, L.; Krahl, S.E.; Bari, A.; De Salles, A.; Pouratian, N. Case Series: Deep Brain Stimulation for Facial Pain. Oper. Neurosurg. (Hagerstown) 2020, 19, 510–517. [Google Scholar] [CrossRef]
- Kumar, K.; Toth, C.; Nath, R.K. Deep brain stimulation for intractable pain: A 15-year experience. Neurosurgery 1997, 40, 736–746, discussion 746–747. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.B.; MacDougall, K.W. New-Onset Stutter after Electrode Insertion in the Ventrocaudalis Nucleus for Face Pain. World Neurosurg. 2016, 90, 703.e7–703.e10. [Google Scholar] [CrossRef]
- Owen, S.L.; Green, A.L.; Stein, J.F.; Aziz, T.Z. Deep brain stimulation for the alleviation of post-stroke neuropathic pain. Pain 2006, 120, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.A.; Wang, S.; Owen, S.L.; Aziz, T.Z.; Green, A.L. Human periventricular grey somatosensory evoked potentials suggest rostrocaudally inverted somatotopy. Stereotact. Funct. Neurosurg. 2013, 91, 290–297. [Google Scholar] [CrossRef]
- Sims-Williams, H.P.; Javed, S.; Pickering, A.E.; Patel, N.K. Characterising the Analgesic Effect of Different Targets for Deep Brain Stimulation in Trigeminal Anaesthesia Dolorosa. Stereotact. Funct. Neurosurg. 2016, 94, 174–181. [Google Scholar] [CrossRef]
- Yamgoue, Y.; Pralong, E.; Levivier, M.; Bloch, J. Deep Brain Stimulation of the Ventroposteromedial (VPM) Thalamus 10 Years after VPM Thalamotomy to Treat a Recurrent Facial Pain. Stereotact. Funct. Neurosurg. 2016, 94, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Singleton, W.G.B.; Ashida, R.; Patel, N.K. Deep brain stimulation for facial pain. Prog. Neurol. Surg. 2020, 35, 141–161. [Google Scholar] [PubMed]
- Debray, T.P.; Moons, K.G.; van Valkenhoef, G.; Efthimiou, O.; Hummel, N.; Groenwold, R.H.; Reitsma, J.B.; GetReal Methods Review Group. Reitsma. Get. Real in Individual Participant Data (IPD) Meta-Analysis: A Review of the methodology. Res. Synth. Methods 2015, 6, 293–309. [Google Scholar] [CrossRef]
- de Jong, V.M.; Moons, K.G.; Riley, R.D.; Tudur Smith, C.; Marson, A.G.; Eijkemans, M.J.; Debray, T.P. Individual participant data meta-analysis of intervention studies with time-to-event outcomes: A review of the methodology and an applied example. Res. Synth. Methods 2020, 11, 148–168. [Google Scholar] [CrossRef]
- Coffey, R.J. Deep brain stimulation for chronic pain: Results from two multicenter trials and a structured review. Pain Med. 2001, 2, 183–192. [Google Scholar] [CrossRef]
- Nüssel, M.; Zhao, Y.; Knorr, C.; Regensburger, M.; Stadlbauer, A.; Buchfelder, M.; Del Vecchio, A.; Kinfe, T. Deep Brain Stimulation, Stereotactic Radiosurgery and High-Intensity Focused Ultrasound Targeting the Limbic Pain Matrix: A Comprehensive Review. Pain Ther. 2022, 11, 459–476. [Google Scholar] [CrossRef] [PubMed]
- De Ridder, D.; Vanneste, S.; Smith, M.; Adhia, D. Pain, and the Triple Network Model. Front. Neurol. 2022, 13, 757241. [Google Scholar] [CrossRef] [PubMed]
- Boccard, S.G.; Pereira, E.A.; Moir, L.; Aziz, T.Z.; Green, A.L. Long-term Outcomes of Deep Brain Stimulation for Neuropathic Pain. Neurosurgery 2013, 72, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.; Deer, T.R.; Henderson, J. Intracranial neurostimulation for pain control: A review. Pain. Physician 2010, 13, 157–165. [Google Scholar] [PubMed]
- Knotkova, H.; Hamani, C.; Sivanesan, E.; Le Beuffe, M.F.E.; Moon, J.Y.; Cohen, S.P.; Huntoon, M.A. Neuromodulation for chronic pain. Lancet 2021, 397, 2111–2124. [Google Scholar] [CrossRef] [PubMed]
- Moisset, X.; Lanteri-Minet, M.; Fontaine, D. Neurostimulation methods in the treatment of chronic pain. J. Neural Transm. (Vienna) 2020, 127, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Cruccu, G.; Garcia-Larrea, L.; Hansson, P.; Keindl, M.; Lefaucheur, J.P.; Paulus, W.; Taylor, R.; Tronnier, V.; Truini, A.; Attal, N. EAN guidelines on central neurostimulation therapy in chronic pain conditions. Eur. J. Neurol. 2016, 23, 1489–1499. [Google Scholar] [CrossRef]
- Deer, T.R.; Mekhail, N.; Provenzano, D.; Pope, J.; Krames, E.; Leong, M.; Levy, R.M.; Abejon, D.; Buchser, E.; Burton, A.; et al. The Appropriate Use of Neurostimulation of the Spinal Cord and Peripheral Nervous System for the Treatment of Chronic Pain and Ischemic Diseases: The Neuromodulation Appropriateness Consensus Committee. Neuromodulation Technol. Neural Interface 2014, 6, 515–550. [Google Scholar] [CrossRef]
- Mestre, T.A.; Lang, A.E.; Okun, M.S. Factors influencing the outcome of deep brain stimulation: Placebo, nocebo, lessebo, and lesion effects. Mov. Disord. 2016, 31, 290–296. [Google Scholar] [CrossRef]
- Benedetti, F.; Frisaldi, E.; Carlino, E.; Giudetti, L.; Pampallona, A.; Zibetti, M.; Lanotte, M.; Lopiano, L. Teaching neurons to respond to placebos. J. Physiol. 2016, 594, 5647–5660. [Google Scholar] [CrossRef]
- Wang, Y.; Tricou, C.; Raghuraman, N.; Akintola, T.; Haycock, N.R.; Blasini, M.; Phillips, J.; Zhu, S.; Colloca, L. Modeling Learning Patterns to Predict Placebo Analgesic Effects in Healthy and Chronic Orofacial Pain Participants. Front. Psychiatry 2020, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Vase, L.; Wartolowska, K. Pain, placebo, and test of treatment efficacy: A narrative review. Br. J. Anaesth. 2019, 123, e254–e262. [Google Scholar] [CrossRef] [PubMed]

| Data Extracted Prior to IPD Analysis | |
|---|---|
| Eligible/Screened | 34/77 |
| Age (years) | |
| Mean (SD) | 54 ± 14 |
| Gender | |
| F | 25 (32%) |
| M | 38 (49%) |
| (missing) | 14 (19%) |
| Facial Pain origin | |
| Stroke | 25 (32%) |
| Trigeminal. | 12 (16%) |
| Post-infection | 9 (12%) |
| Post-Surgical | 17 (22%) |
| (missing) | 14 (18%) |
| DBS target | |
| VPL/VPM/CmPF | 31 (40%) |
| PAG/PVG | 24 (31%) |
| Combined | 15 (20%) |
| others | 7 (9%) |
| Study | Estimate | Std.Err. | t Value | p Value |
|---|---|---|---|---|
| Source Abdallat | 8.00 | 4.10 | 1.95 | 0.057 |
| Source BenHaim | 8.70 | 2.74 | 3.18 | 0.003 |
| Source Green | 8.74 | 2.37 | 3.68 | <0.001 |
| Source Kashanian | 11.35 | 3.95 | 2.87 | 0.006 |
| Source Sims Williams | 9.03 | 3.22 | 2.81 | 0.007 |
| VAS pre vs. post-DBS | −4.64 | 0.54 | −8.63 | <0.001 |
| Age | 0.00 | 0.02 | 0.09 | 0.930 |
| Lead_Conf. bipolar | −0.60 | 1.11 | −0.54 | 0.593 |
| Intensity_max | −0.09 | 0.33 | −0.28 | 0.780 |
| Freq. max | −0.01 | 0.02 | −0.60 | 0.548 |
| Amplitud.max | 0.00 | 0.01 | 0.27 | 0.789 |
| Facial pain Duration months | 0.00 | 0.01 | −0.53 | 0.600 |
| Target VPL/VPM CmPf | 1.04 | 1.43 | 0.72 | 0.474 |
| Targets both/other | 0.08 | 0.92 | 0.08 | 0.934 |
| DBS Targets | Estimate | SE | df | t Ratio | p Value |
|---|---|---|---|---|---|
| PAG/PVG vs. VPL/VPM/CmPf | −1.37 | 1.67 | 26 | −0.82 | 0.42 |
| PAG/PVG alone vs. both targets | −0.55 | 1.62 | 26 | −0.34 | 0.74 |
| VPL/VPM/CmPf alone vs. both targets | 0.82 | 1.15 | 26 | 0.71 | 0.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qassim, H.; Zhao, Y.; Ströbel, A.; Regensburger, M.; Buchfelder, M.; de Oliveira, D.S.; Del Vecchio, A.; Kinfe, T. Deep Brain Stimulation for Chronic Facial Pain: An Individual Participant Data (IPD) Meta-Analysis. Brain Sci. 2023, 13, 492. https://doi.org/10.3390/brainsci13030492
Qassim H, Zhao Y, Ströbel A, Regensburger M, Buchfelder M, de Oliveira DS, Del Vecchio A, Kinfe T. Deep Brain Stimulation for Chronic Facial Pain: An Individual Participant Data (IPD) Meta-Analysis. Brain Sciences. 2023; 13(3):492. https://doi.org/10.3390/brainsci13030492
Chicago/Turabian StyleQassim, Hebatallah, Yining Zhao, Armin Ströbel, Martin Regensburger, Michael Buchfelder, Daniela Souza de Oliveira, Alessandro Del Vecchio, and Thomas Kinfe. 2023. "Deep Brain Stimulation for Chronic Facial Pain: An Individual Participant Data (IPD) Meta-Analysis" Brain Sciences 13, no. 3: 492. https://doi.org/10.3390/brainsci13030492
APA StyleQassim, H., Zhao, Y., Ströbel, A., Regensburger, M., Buchfelder, M., de Oliveira, D. S., Del Vecchio, A., & Kinfe, T. (2023). Deep Brain Stimulation for Chronic Facial Pain: An Individual Participant Data (IPD) Meta-Analysis. Brain Sciences, 13(3), 492. https://doi.org/10.3390/brainsci13030492






