Abstract
While research has consistently demonstrated how autobiographical memory triggers visual exploration, prior studies did not investigate gender differences in this domain. We thus compared eye movement between women and men while performing an autobiographical retrieval task. We invited 35 women and 35 men to retrieve autobiographical memories while their gaze was monitored by an eye tracker. We further investigated gender differences in eye movement and autobiographical specificity, that is, the ability to retrieve detailed memories. The analysis demonstrated shorter fixations, larger duration and amplitude of saccades, and higher autobiographical specificity in women than in men. The significant gender differences in eye movement disappeared after controlling for autobiographical specificity. When retrieving autobiographical memory, female participants generated a large scan with short fixation and high saccade amplitude, while male participants increased their fixation duration and showed poorer gaze scan. The large saccades in women during autobiographical retrieval may constitute an exploratory gaze behavior enabling better autobiographical memory functioning, which is reflected by the larger number of autobiographical details retrieved compared to men.
1. Introduction
Gender differences have been reported in cognitive functioning [1,2,3], especially regarding visual perception [4,5]. Given that biological and neural processes of visual perception can vary between males and females [4,5], different gaze patterns or visual exploration behavioral differences could be observed between women and men. Based on this hypothesis, we investigated gender differences in eye movement during autobiographical retrieval. As explained below, prior research has demonstrated how the retrieval of autobiographical memory can trigger eye movement and even how this eye movement can influence autobiographical retrieval. However, prior research has not assessed gender differences in this domain.
Compared to other memory systems, autobiographical memory is key for understanding the intersection between memory, cognition, and social interactions. While autobiographical memory supports the self [6], it also supports directive and social functions, as the retrieval of past personal experiences simulates and guides future behavior [7,8,9]. Beyond this directive function, autobiographical memory has a social goal, as the retrieval of past personal experiences allows us to communicate these experiences [10] and promotes intimacy [11,12,13]. Autobiographical memory can thus be defined as a dynamic system supporting personal memories as constructed to serve a given personal, social, and/or directive goal [14,15].
Little is known about how autobiographical retrieval can activate eye movement. One exception is a study by El Haj, Delerue [16], who recorded fixations and saccades while participants retrieved autobiographical memories and during a control condition in which participants counted aloud. Their analysis showed longer amplitudes and longer durations of saccades under the autobiographical memory condition than under the control condition. According to El Haj, Delerue [16], these eye movements reflect the attempts by the visual system to reconstruct the visual scene of the retrieved memories. Similar results were reported by El Haj, Nandrino [17], who instructed participants to remember neutral and emotional memories. Their results demonstrated fewer fixations and saccades but longer fixation duration in the retrieval of neutral memories than in the retrieval of emotional memories. Other research has investigated differences in eye movement between autobiographical retrieval and future thinking [18], demonstrating how future thinking triggers fewer fixations and saccades than past thinking. Eye movement during autobiographical retrieval may also differ depending on the temporal distribution of memories, as demonstrated by a study showing that the retrieval of remote autobiographical memories activates fewer, but longer, fixations compared to the retrieval of recent memories [19]. While eye movement can be influenced by the characteristics of autobiographical memories, the reverse pattern has also been reported, as research has demonstrated how eye movement can influence autobiographical retrieval. The latter issue was investigated by Lenoble, Janssen [20] who asked participants to remember autobiographical events while fixating on a cross on a screen or while exploring the screen without constraints. Their results demonstrated more detailed and faster memories when retrieved in the free-gaze condition than when retrieved in the maintained fixation condition. These studies show how retrieval of autobiographical memory can activate fixations and saccades and how these eye movements may mirror the generation and manipulation of mental representations in the visual system [21]. Thus, eye movements as activated by autobiographical retrieval would reflect mental imagery operations underlying autobiographical information recollection.
While the above-mentioned studies have shown how the retrieval of autobiographical memory can trigger eye movement, the impact of gender on these eye movement patterns has not yet, to our knowledge, been explored. The present study addresses this issue by comparing fixations and saccades between women and men during autobiographical retrieval. Besides being inspired by research on eye movement as activated by autobiographical memory, the present study is inspired by research demonstrating gender differences in visual processing.
One of the research topics in gender differences and eye movement is the well-known eye-tracking research demonstrating that compared to women, men tend to fixate significantly earlier and longer on women’s breasts during the visual processing of pictures depicting women’s bodies, regardless of whether the women are fully clothed [22], wearing bathing suits [23], or nude [24]. Other research has uncovered important gender differences regarding gaze, such as that women demonstrate more visual exploratory scanning strategy than men. This finding is supported by the study of Coutrot, Binetti [25], who assessed the dynamics of gaze when participants watched videos of another person. Their results demonstrated much more exploratory gaze in women than men. A similar finding was made by Gomez, von Gunten [26], who demonstrated that when viewing pictures depicting neutral and emotional scenes, women demonstrated a more exploratory scanning behavior compared to men. In a similar vein, Heisz, Pottruff [27] reported higher scanning behavior in the encoding of faces in women than in men, and that this higher scanning behavior was associated with high recognition memory in women. High explorative gaze in women was also reported by Sammaknejad, Pouretemad [28]. They analyzed regions of interest when women and men processed pictures of faces. Their analysis demonstrated that women showed a significant increase in transitions (i.e., saccades) from the regions of interest to the eyes. High explorative gaze behavior in women was also reported by Abdi Sargezeh, Tavakoli [29], who observed that, compared to women, men demonstrated less explorative gaze, as mirrored by smaller saccade amplitudes and slower scan paths, when exploring indoor scenes (e.g., a living room). Taken together, prior research has demonstrated that, compared to men, women tend to demonstrate more exploratory scanning behavior. Accordingly, in the current study, this exploratory scan pattern will, for the first time, be examined for autobiographical retrieval.
To summarize, while research has demonstrated how autobiographical memory can trigger visual exploration [17,18,20,21,30,31], this prior research has not investigated gender differences. Because gender differences have been observed for visual processing [22,24], we investigated gender differences in eye movement during autobiographical retrieval. Accordingly, we recorded fixations and saccades in women and men during autobiographical retrieval. In light of research reporting how women show high explorative gaze behavior [25,26,28,29], we predicted that we would observe a similar behavior during autobiographical retrieval. More specifically, we expected that compared to men, women would demonstrate larger saccades when retrieving autobiographical memories, such that women may tend to explore/scan the retrieved memories more than men. We further assessed the specificity of memories, in order to investigate whether gender differences in eye movement as activated by autobiographical retrieval would be influenced by the specificity of memories.
2. Method
2.1. Participants
The current study included seventy undergraduate/graduate students at the University of Nantes. Thirty-five participants were female (M age = 20.46 years, SD = 3.01, M education = 13.21 years, SD = 4.21) and thirty-five were male (M age = 20.98 years, SD = 3.14, M education = 13.54 years, SD = 4.44). No significant differences were observed between the two groups regarding age [t(68) = 0.73, p = 0.46] or educational level [t(68) = 0.32, p = 0.75].
We estimated the sample size using G*Power [32] for independent t-tests because our protocol involved two-group comparisons (i.e., women vs. men). This estimation involved 95% power, a probability of making Type I error of 0.05, and a large effect size of 0.80 [33]. This calculation suggested that 35 participants would be necessary in each of the two groups to obtain sufficient statistical power, i.e., a total sample size of 70 participants. Note, however, that we initially recruited a larger sample of 87 participants, from which 17 participants were excluded for several reasons, described below. The protocol was validated by the ethics committee of the University of Nantes (reference IORG0011023).
Because our study included a verbal assessment of autobiographical memory, the general verbal memory performances of participants were controlled for using the test of Grober and Buschke [34], on which participants must recall as many previously studied words as possible, with the maximum score being 16 points. The mean score of women was 12.89 (SD = 2.73) and that of men was 10.46 (SD = 2.83) [t(68) = 3.65, p = 0.001]. Note that we excluded four subjects from the original sample (n = 87) because their scores were two standard deviations below the expected range for their age. We excluded six other participants as they declared a history of psychiatric and/or neurological disorders. We also excluded seven participants due to difficulties with eye movement processing, including failures with the calibration of one participant and recording quality below 70% for six other participants.
2.2. Procedures
Autobiographical Memory and Eye Movement Recording
We instructed participants to verbally retrieve three autobiographical memories while wearing eye-tracking glasses and looking at a white wall. We explained that the events had to be experienced and that the events had to be specific and precise (e.g., involving spatiotemporal details). A time of two minutes was allocated to each of the three autobiographical events. No significant differences were observed between women (M = 87087.14 msec, SD = 4143.947) and men (M = 83108.14 msec, SD = 27119.93) regarding the duration of description of memories [t(68) = 0.64, p = 0.52]. Note that the instructions were repeated for each of the three memories and that, for the three memories, the participants were free to retrieve any event. We thus did not provide cue words; we rather used the same instructions for the three memories to ensure that the memories were triggered with the same instructions.
We invited the participants to remember the memory out loud while wearing eye-tracking glasses. These remote pupil-tracking glasses (Pupil Lab) had a gaze position accuracy of <0.1° and 200 Hertz sampling rate. During the experiment, we closed the blinds and kept the lightness of the room (60-watt fluorescent lamp) constant to avoid variations in retinal illumination.
2.3. Eye Movement Variables
The variables were the number of fixations (i.e., total number of fixations per minute), mean duration of fixations in milliseconds, number of saccades (i.e., total number of saccades per minute), mean duration of saccades in milliseconds, average amplitude of saccades (i.e., the average angle covered by saccades), and total amplitude size of saccades (i.e., the total angle of the saccades). Note that, for each variable, we considered the mean score for the three memories. Regarding blinks, we excluded them once the horizontal deviation of gaze exceeded 2° (5.5% of our dataset).
Autobiographical Memory Analysis
To control for whether potential gender differences in eye movement are influenced by the specificity of retrieval (e.g., whether women may retrieve more specific memories than men), we analyzed autobiographical specificity using a procedure proposed by Piolino, Desgranges [35]. For each event, zero was attributed when no memory was provided or when only general information was given about a theme; one point was attributed when an extended or a repeated event was provided; two points were attributed when events described memories with spatiotemporal details; three points were attributed when events referred to those lasting less than 24 h and situated in time and space; and four points were attributed when events referred to specific ones with spatiotemporal detail and with phenomenological information (e.g., emotion). We retained the mean scores of the three memories with a maximum score of four points.
3. Results
We compared the number of fixations, duration of fixations, number of saccades, duration of saccades, average and total saccade amplitude, and autobiographical specificity between women and men using independent t-tests. To investigate whether any significant gender differences in eye movement were influenced by autobiographical specificity, we carried out an analysis of covariance (ANCOVA) with eye movement as the dependent variable, gender as the independent variable, and autobiographical specificity as the covariate variable.
3.1. Shorter Fixations but Larger Saccades in Women Than in Men
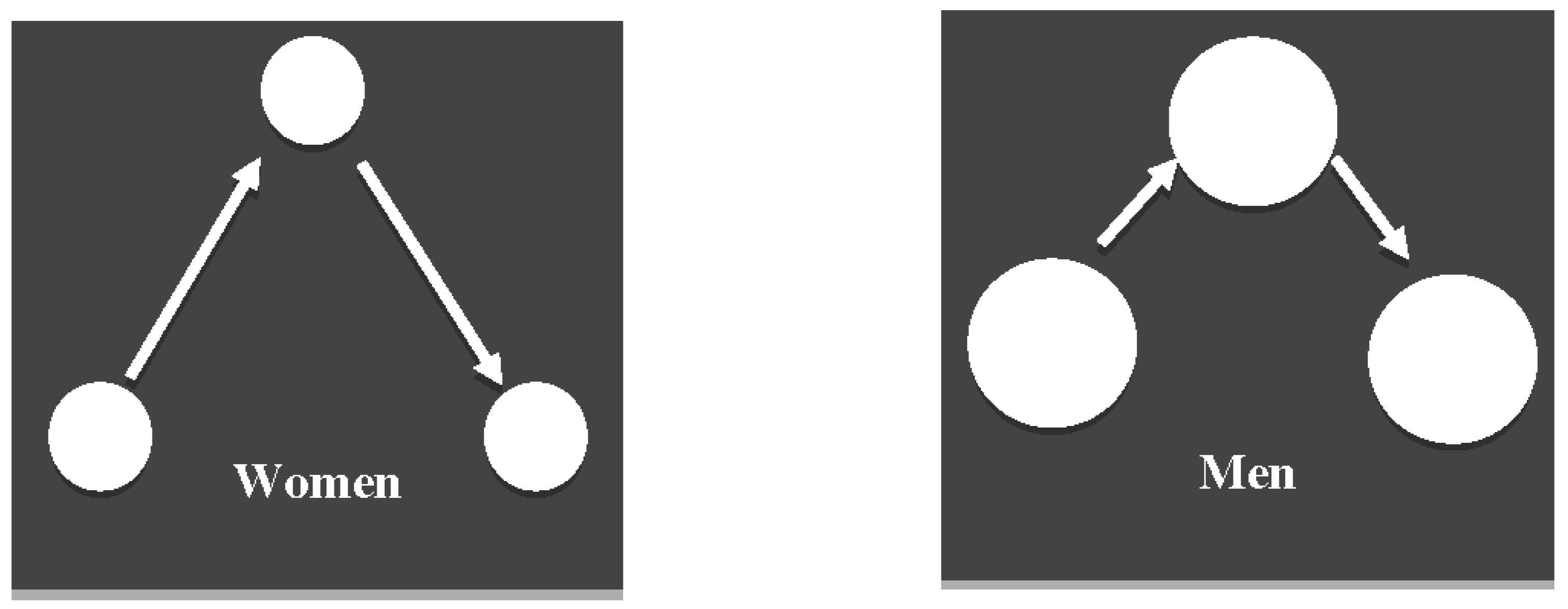
Data are provided in Table 1. The analyses demonstrated no significant effect of gender differences regarding the number of fixations [t(68) = 0.68, p = 0.50, Cohen’s d = 0.16] or the number of saccades [t(68) = 0.56, p = 0.58, Cohen’s d = 0.13]. However, as illustrated in Figure 1, women demonstrated shorter fixations [t(68) = 3.10, p = 0.003, Cohen’s d = 0.74] but larger duration of saccades [t(68) = 2.87, p = 0.005, Cohen’s d = 0.69] and larger average [t(68) = 2.65, p = 0.01, Cohen’s d = 0.61] and total [t(68) = 3.53, p = 0.001, Cohen’s d = 0.84] amplitude of saccades compared to men.

Table 1.
Characteristics of eye movements under both conditions.
Figure 1.
A schematic illustration of gaze during autobiographical retrieval: women demonstrated shorter fixations (i.e., smaller circles) but longer saccades (i.e., longer arrows) compared to men.
3.2. Higher Autobiographical Specificity in Women and Men
The analyses demonstrated higher autobiographical specificity in women (M = 3.50, SD = 0.44) than in men (M = 3.12, SD = 0.62) [t(68) = 2.89, p = 0.005, Cohen’s d = 0.69].
3.3. Relationship between Autobiographical Specificity and Eye Movement
The covariate analysis demonstrated no significant gender differences regarding fixation duration [F(1, 67) = 0.41, p = 0.52, ηp2 = 0.092], saccade duration [F(1, 67) = 0.33, p = 0.57, ηp2 = 0.073], or average [F(1, 67) = 0.88, p = 0.35, ηp2 = 0.15] or total [F(1, 67) = 0.76, p = 0.39, ηp2 = 0.10] amplitude of saccades after controlling for autobiographical specificity. Thus, the significant gender differences in eye movement disappeared after controlling for autobiographical specificity. For convenience, we carried out a covariance analysis for the remaining eye movement variable and found no significant gender differences regarding the number of fixations [F(1, 67) = 0.01, p = 0.92, ηp2 = 0.052] or saccades [F(1, 67) = 0.42, p = 0.52, ηp2 = 0.10].
3.4. Additional Analyses
We further carried out covariance analysis for verbal episodic memory (i.e., performance on the task of Grober and Buschke). The analysis demonstrated that after controlling for verbal episodic memory, women demonstrated significantly shorter fixation [F(1, 67) = 10.72, p = 0.002, ηp2 = 0.90] but larger duration [F(1, 67) = 5.61, p = 0.021, ηp2 = 0.65] and larger average [F(1, 67) = 0.49, p = 0.49, ηp2 = 0.11] and total [F(1, 67) = 8.42, p = 0.005, ηp2 = 0.82] amplitude of saccades than men. Thus, gender differences in eye movement were not influenced by verbal episodic memory. For convenience, we carried out covariance analysis for the two remaining eye movement variables and found no significant effect of gender regarding the number of fixations [F(1, 67) = 0.04, p = 0.84, ηp2 = 0.055] or saccades [F(1, 67) = 0.79, p = 0.38, ηp2 = 0.14].
4. Discussion
We, for the first time, investigated gender differences in eye movement during autobiographical retrieval. While no significant gender differences were found regarding the numbers of fixations and saccades, shorter fixations and larger saccades were observed in women than in men.
The shorter fixations and larger saccades, as activated by autobiographical retrieval in women compared to men, demonstrated that women tend to generate a larger gaze scan compared to men, who tend to fixate more, when retrieving autobiographical memories. The large gaze in women may mirror an exploratory gaze strategy involving the creation of larger visual scenes compared to men, who generate narrower visual scenes but with longer fixations. The large gaze in women, as observed in the current study, aligns with research demonstrating that women show high explorative gaze behavior, expressed by large saccades, when exploring scenes [25,26,28,29]. In a similar vein, Mercer Moss, Baddeley [36] demonstrated that the fixation distributions of women are larger than those of men when exploring pictures of art pieces and social interactions. Further, the explorative gaze in women as observed in our study aligns with hypothesis of selective information processing [37,38]. This hypothesis proposes that, compared to women, men are more selective processors as they consider only a subset of the available information when visually processing an environment. Taken together, the larger saccades during autobiographical retrieval may mirror the exploratory gaze behavior in women.
An alternative, but complementary, account for the significant gender differences in eye movement is autobiographical specificity. As demonstrated by our covariate analysis, the significant gender differences regarding fixation and saccade duration and amplitude were not present after controlling for autobiographical specificity. Thus, the shorter fixations and larger saccades in women than in men can be attributed to the women’s ability to retrieve detailed autobiographical memories. More specifically, we suggest that the exploratory gaze behavior in women may mirror the retrieval of a higher number of autobiographical details compared to men. Note that the higher autobiographical specificity, as observed in women in our study, mirrors previous research demonstrating that, compared to women, men retrieve events with less richness of details, such as how the event occurred [39,40] and even regarding factual details, such as where the event occurred [41,42] or to whom a message was previously told [1]. Taken together, the exploratory gaze behavior in women may mirror the richness of spatiotemporal/factual details during autobiographical retrieval.
Besides autobiographical specificity, the large saccades in women can be influenced by phenomenological characteristics of retrieval, especially by the vividness of mental imagery. Mental imagery refers to the ability to generate and manipulate mental images of the retrieved memories [43], and this ability is closely linked to the subjective experience of autobiographical memory: the more vivid the constructed image, the stronger the subjective experience [44,45]. Mental imagery is also closely associated with eye movement during autobiographical retrieval [16,17,18,21]. The role of visual imagery was shown by Lenoble, Janssen [20], who invited participants to remember autobiographical events while fixating on a cross in the center of a screen or while exploring the screen without constraints. This study showed that memories retrieved in the free-gaze condition triggered higher mental imagery than did those retrieved in the exploration-without-constraints condition. Thus, compared to those in men, the shorter fixations in women, as observed in the present study, can be associated with higher visual imagery. Besides mental imagery, the shorter fixations in women can be attributed to stronger emotional experience compared to that in men. This hypothesis is supported by a study showing that women express more affect than men when retrieving autobiographical memories [41]. While appealing, this assumption should be interpreted with some caution because our study design did not involve an assessment of emotion or mental imagery, or even the phenomenological characteristics of retrieval in general. This is a limitation of our study design that should be further investigated in future work.
Unlike the effect of autobiographical specificity, gender differences in eye movement in our study were independent of verbal episodic memory. As demonstrated by the covariance analysis, women demonstrated significantly shorter fixation and larger saccades even after controlling for scores on the task of Grober and Buschke. The lack of effects of verbal episodic memory on eye movement in our study can be attributed to the fact that we assessed verbal episodic memory using a general word list, while autobiographical specificity was assessed regarding the ability to retrieve personal episodic details. Hence, the short fixations but large saccades in women can be attributed to the ability to retrieve detailed personal information (i.e., where and when a personal event has occurred), rather than to the ability to retrieve general/semantic verbal information. However, the high verbal episodic memory in women, as observed on Grober and Buschke’s test in our study, mirrors research demonstrating high item memory in women [46,47], especially regarding the retention of verbal information [48].
Strengths and Limitations
One limitation of the present study may be the lack of assessment of phenomenological characteristics of memories, such as whether differences in eye movement would be observed between men and women regarding the emotional characteristics of memories. Future research may thus assess gender differences for these characteristics. Another suggestion would be the assessment of gender differences regarding the temporal distribution of memories in light of research demonstrating how eye movement may differ between recent and old memories [19]. However, regardless of these limitations, our study has the merit to shed light on gender differences in eye movement during autobiographical retrieval in general.
5. Conclusions
To summarize, one behavior often made in everyday life by humans is gaze. Gaze can be triggered by external or even internal stimuli, as demonstrated by research showing how the retrieval of autobiographical memory triggers gaze. Our study expands this research by shedding light on gender differences. In fact, women exhibited larger saccades and smaller fixation than men, reflecting a larger gaze scan, which could reflect an exploratory gaze strategy. Interestingly, these eye movement pattern differences related to gender were not observed when autobiographical specificity was controlled for. This suggests that some cognitive processes (e.g., visual imagery, detail richness) underlying autobiographical specificity could account for the larger gaze scan pattern observed in women. Thus, this study contributes not only to the emergent research on eye movement during autobiographical memory, but also to the well-established research on cognitive and physiological processes related to gender differences.
Author Contributions
Data acquisition: M.E.H. and L.G.S., Analysis: M.E.H., L.G.S. and Q.L. Interpretation: C.B.-B., A.A.M., E.S., G.C. and A.N., Writing: M.E.H. and A.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was approved by the ethics board of the University of Nantes (reference IORG0011023).
Informed Consent Statement
All participants provided informed consent.
Data Availability Statement
Raw data are available upon request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- El Haj, M.; Allain, P.; Lucenet, J.; Ndobo, A. Better Destination Memory in Females. Adv. Cogn. Psychol. 2020, 16, 242–247. [Google Scholar] [CrossRef] [PubMed]
- El Haj, M.; Nandrino, J.-L.; Kessels, R.P.C.; Ndobo, A. High emotional experience during autobiographical retrieval in women with Korsakoff syndrome. Cogn. Neuropsychiatry 2021, 26, 136–148. [Google Scholar] [CrossRef]
- El Haj, M.; Boutoleau-Bretonnière, C.; Gallouj, K. The Past as Seen by Women and Men With Alzheimer Disease: Sex Differences in Autobiographical Memory. Alzheimer Dis. Assoc. Disord. 2020, 34, 170–174. [Google Scholar] [CrossRef]
- Ino, T.; Nakai, R.; Azuma, T.; Kimura, T.; Fukuyama, H. Gender differences in brain activation during encoding and recognition of male and female faces. Brain Imaging Behav. 2010, 4, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Vanston, J.E.; Strother, L. Sex differences in the human visual system. J. Neurosci. Res. 2017, 95, 617–625. [Google Scholar] [CrossRef]
- Conway, M.A. Memory and the self. J. Mem. Lang. 2005, 53, 594–628. [Google Scholar] [CrossRef]
- Pillemer, D.B. Directive functions of autobiographical memory: The guiding power of the specific episode. Memory 2003, 11, 193–202. [Google Scholar] [CrossRef]
- Bluck, S.; Alea, N.; Habermas, T.; Rubin, D.C. A tale of three functions: The Self–Reported Uses of Autobiographical Memory. Soc. Cogn. 2005, 23, 91–117. [Google Scholar] [CrossRef]
- Schacter, D.L.; Addis, D.R. Constructive memory: The ghosts of past and future. Nature 2007, 445, 27. [Google Scholar] [CrossRef]
- Fivush, R.; Habermas, T.; Waters, T.E.; Zaman, W. The making of autobiographical memory: Intersections of culture, narratives and identity. Int. J. Psychol. 2011, 46, 321–345. [Google Scholar] [CrossRef] [PubMed]
- Alea, N.; Bluck, S. Why are you telling me that? A conceptual model of the social function of autobiographical memory. Memory 2003, 11, 165–178. [Google Scholar] [CrossRef]
- Cappeliez, P. Neglected issues and new orientations for research and practice in reminiscence and life review. Int. J. Reminisc. Life Rev. 2013, 1, 19–25. [Google Scholar]
- Westerhof, G.J.; Bohlmeijer, E.; Webster, J.D. Reminiscence and mental health: A review of recent progress in theory, research and interventions. Ageing Soc. 2010, 30, 697–721. [Google Scholar] [CrossRef]
- Conway, A.R.; Kane, M.J.; Bunting, M.F.; Hambrick, D.Z.; Wilhelm, O.; Engle, R.W. Working memory span tasks: A methodological review and user’s guide. Psychon. Bull. Rev. 2005, 12, 769–786. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.C. A basic-systems approach to autobiographical memory. Curr. Dir. Psychol. Sci. 2005, 14, 79–83. [Google Scholar] [CrossRef]
- El Haj, M.; Delerue, C.; Omigie, D.; Antoine, P.; Nandrino, J.L.; Boucart, M. Autobiographical recall triggers visual exploration. J. Eye Mov. Res. 2014, 7, 1–7. [Google Scholar] [CrossRef]
- El Haj, M.; Nandrino, J.L.; Antoine, P.; Boucart, M.; Lenoble, Q. Eye movement during retrieval of emotional autobiographical memories. Acta Psychol. (Amst.) 2017, 174, 54–58. [Google Scholar] [CrossRef]
- El Haj, M.; Lenoble, Q. Eying the future: Eye movement in past and future thinking. Cortex 2018, 105, 97–103. [Google Scholar] [CrossRef]
- El Haj, M.; Boutoleau-Bretonnière, C.; Janssen, S.M.J. Eye movements of recent and remote autobiographical memories: Fewer and longer lasting fixations during the retrieval of childhood memories. Psychol. Res. 2020, 85, 2466–2473. [Google Scholar] [CrossRef]
- Lenoble, Q.; Janssen, S.M.J.; El Haj, M. Don’t stare, unless you don’t want to remember: Maintaining fixation compromises autobiographical memory retrieval. Memory 2018, 27, 231–238. [Google Scholar] [CrossRef]
- Lenoble, Q.; El Haj, M. “Look at Me”—Eye Movements During Autobiographical Retrieval in Face-to-Face Interactions. J. Psychophysiol. 2021, 35, 237–242. [Google Scholar] [CrossRef]
- Hewig, J.; Trippe, R.H.; Hecht, H.; Straube, T.; Miltner, W.H.R. Gender Differences for Specific Body Regions When Looking at Men and Women. J. Nonverbal Behav. 2008, 32, 67–78. [Google Scholar] [CrossRef]
- Suschinsky, K.D.; Elias, L.J.; Krupp, D.B. Looking for Ms. Right: Allocating Attention to Facilitate Mate Choice Decisions. Evol. Psychol. 2007, 5, 147470490700500214. [Google Scholar] [CrossRef]
- Dixson, B.J.; Grimshaw, G.M.; Linklater, W.L.; Dixson, A.F. Eye-Tracking of Men’s Preferences for Waist-to-Hip Ratio and Breast Size of Women. Arch. Sex. Behav. 2011, 40, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Coutrot, A.; Binetti, N.; Harrison, C.; Mareschal, I.; Johnston, A. Face exploration dynamics differentiate men and women. J. Vis. 2016, 16, 16. [Google Scholar] [CrossRef] [PubMed]
- Gomez, P.; von Gunten, A.; Danuser, B. Eye gaze behavior during affective picture viewing: Effects of motivational significance, gender, age, and repeated exposure. Biol. Psychol. 2019, 146, 107713. [Google Scholar] [CrossRef]
- Heisz, J.J.; Pottruff, M.M.; Shore, D.I. Females scan more than males: A potential mechanism for sex differences in recognition memory. Psychol. Sci. 2013, 24, 1157–1163. [Google Scholar] [CrossRef]
- Sammaknejad, N.; Pouretemad, H.; Eslahchi, C.; Salahirad, A.; Alinejad, A. Gender Classification Based on Eye Movements: A Processing Effect During Passive Face Viewing. Adv. Cogn. Psychol. 2017, 13, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Abdi Sargezeh, B.; Tavakoli, N.; Daliri, M.R. sGender-based eye movement differences in passive indoor picture viewing: An eye-tracking study. Physiol. Behav. 2019, 206, 43–50. [Google Scholar] [CrossRef]
- Gautier, J.; Sastoque, L.G.; Chapelet, G.; Boutoleau-Bretonnière, C.; El Haj, M. “Look at the future”: Maintained fixation impoverishes future thinking. Conscious. Cogn. 2022, 105, 103398. [Google Scholar] [CrossRef]
- Gautier, J.; El Haj, M. Eyes don’t lie: Eye movements differ during covert and overt autobiographical recall. Cognition 2023, 235, 105416. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis. Curr. Dir. Psychol. Sci. 1992, 1, 98–101. [Google Scholar] [CrossRef]
- Grober, E.; Buschke, H. Genuine memory deficits in dementia. Dev. Neuropsychol. 1987, 3, 13–36. [Google Scholar] [CrossRef]
- Piolino, P.; Desgranges, B.; Benali, K.; Eustache, F. Episodic and semantic remote autobiographical memory in ageing. Memory 2002, 10, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Mercer Moss, F.J.; Baddeley, R.; Canagarajah, N. Eye Movements to Natural Images as a Function of Sex and Personality. PLoS ONE 2012, 7, e47870. [Google Scholar] [CrossRef] [PubMed]
- Darley, W.K.; Smith, R.E. Gender Differences in Information Processing Strategies: An Empirical Test of the Selectivity Model in Advertising Response. J. Advert. 1995, 24, 41–56. [Google Scholar] [CrossRef]
- Meyers-Levy, J. Gender differences in information processing: A selectivity interpetation. In Cognitive and Affective Responses to Advertising; Cafferata, P., Tybout, A.M., Tybout, A., Eds.; Lexington Books: Lexington, MA, USA, 1989; pp. 219–260. [Google Scholar]
- Nahari, G.; Pazuelo, M. Telling a convincing story: Richness in detail as a function of gender and information. J. Appl. Res. Mem. Cogn. 2015, 4, 363–367. [Google Scholar] [CrossRef]
- Pasupathi, M.; Wainryb, C. On telling the whole story: Facts and interpretations in autobiographical memory narratives from childhood through midadolescence. Dev. Psychol. 2010, 46, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Grysman, A.; Fivush, R.; Merrill, N.A.; Graci, M. The influence of gender and gender typicality on autobiographical memory across event types and age groups. Mem. Cognit. 2016, 44, 856–868. [Google Scholar] [CrossRef]
- Grysman, A. Gender Differences in Episodic Encoding of Autobiographical Memory. J. Appl. Res. Mem. Cogn. 2017, 6, 51–59. [Google Scholar] [CrossRef]
- Kosslyn, S.M.; Ganis, G.; Thompson, W.L. Neural foundations of imagery. Nat. Rev. Neurosci. 2001, 2, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.C. The Basic-Systems Model of Episodic Memory. Perspect. Psychol. Sci. 2006, 1, 277–311. [Google Scholar] [CrossRef] [PubMed]
- Vannucci, M.; Pelagatti, C.; Chiorri, C.; Mazzoni, G. Visual object imagery and autobiographical memory: Object Imagers are better at remembering their personal past. Memory 2016, 24, 455–470. [Google Scholar] [CrossRef]
- Rehnman, J.; Herlitz, A. Women remember more faces than men do. Acta Psychol. 2007, 124, 344–355. [Google Scholar] [CrossRef]
- McBain, R.; Norton, D.; Chen, Y. Females excel at basic face perception. Acta Psychol. 2009, 130, 168–173. [Google Scholar] [CrossRef]
- Ullman, M.T.; Miranda, R.A.; Travers, M.L. Sex differences in the neurocognition of language. In Sex Differences in the Brain from Genes to Behavior; Becker, J.B., Berklry, K.J., Geary, N., Hampson, E., Herman, J.P., Young, E.A., Eds.; Oxford University Press: Oxford, UK, 2008; pp. 291–309. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).