The Latest Cellular and Molecular Mechanisms of COVID-19 on Non-Lung Organs
Abstract
1. Introduction
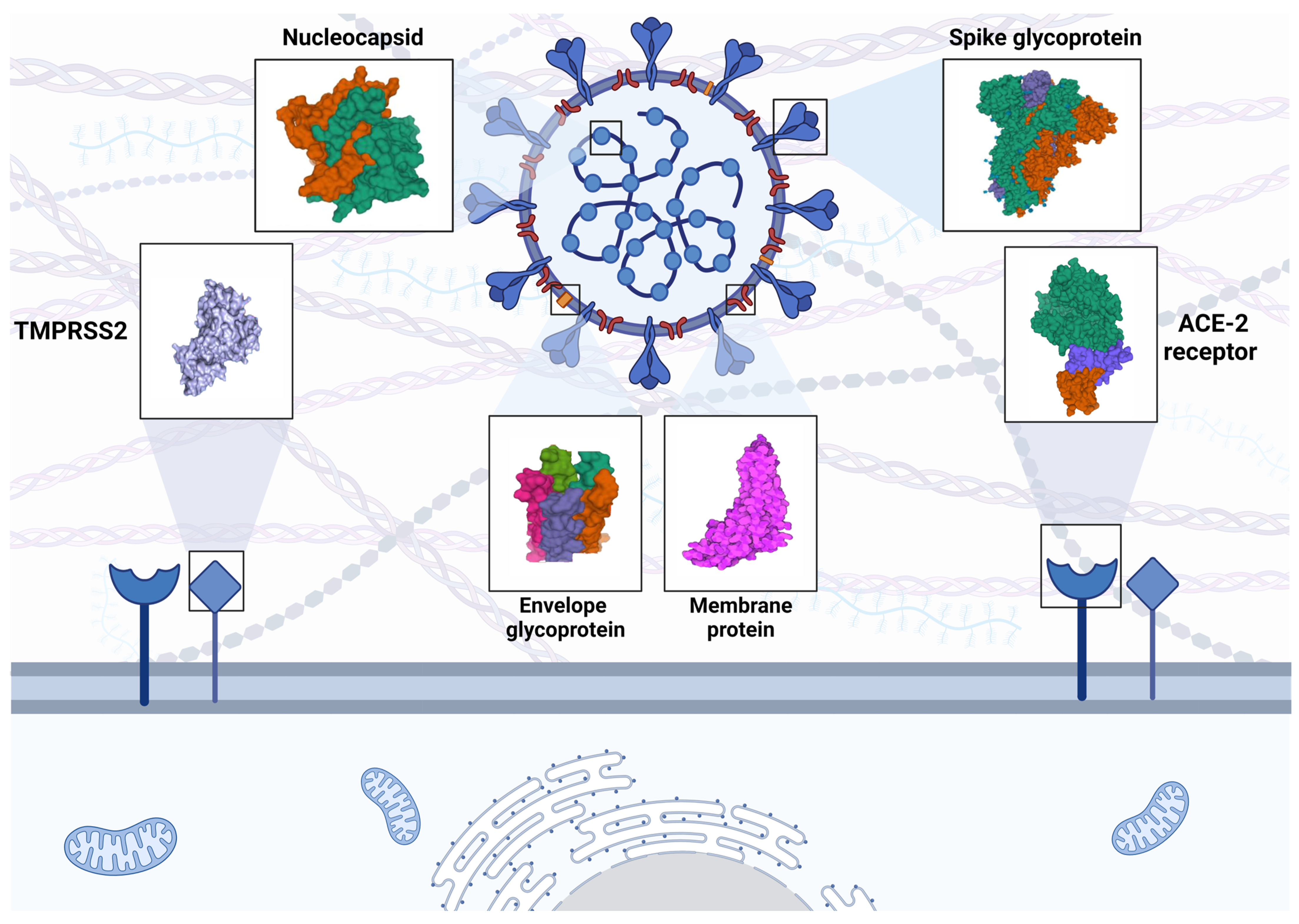
2. SARS-CoV-2 Characteristics
3. Investigating Neurological Disorders
3.1. Potential SARS-CoV-2-Mediated Brain Injury Mechanisms
3.2. COVID-19 Associated Neurological Symptoms
3.2.1. Cerebrovascular Diseases
3.2.2. Encephalitis
3.2.3. Seizures
3.2.4. Guillain–Barré Syndrome (GBS)
3.2.5. Neurodegenerative and Demyelinating Disorders
4. Complications of Skeletal Muscle and Neuromuscular Junction
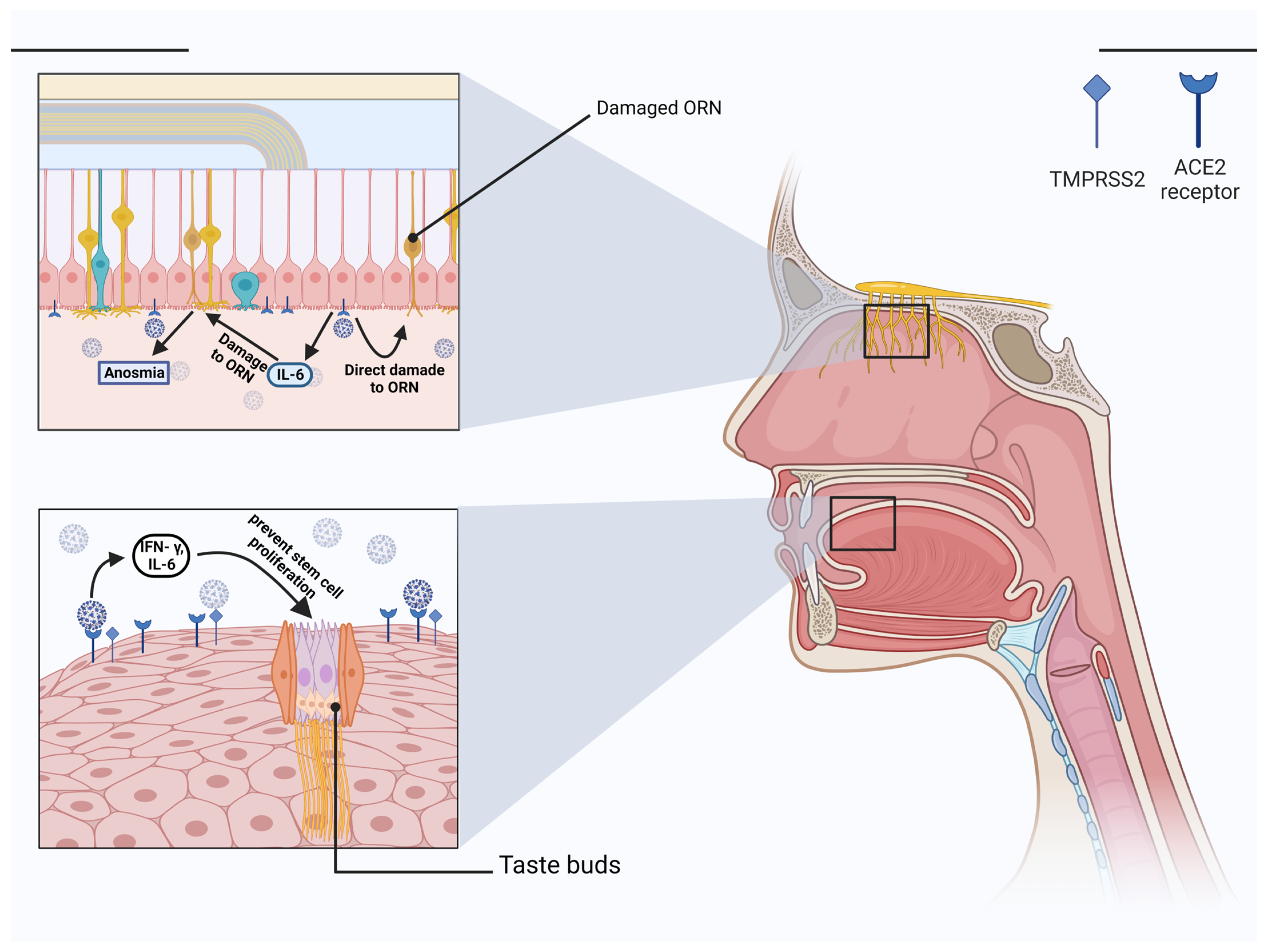
5. Involvement of the Olfactory Nerve in SARS-CoV-2 Infection
6. Ophthalmic Manifestation in COVID-19
7. Taste Dysfunction in COVID-19
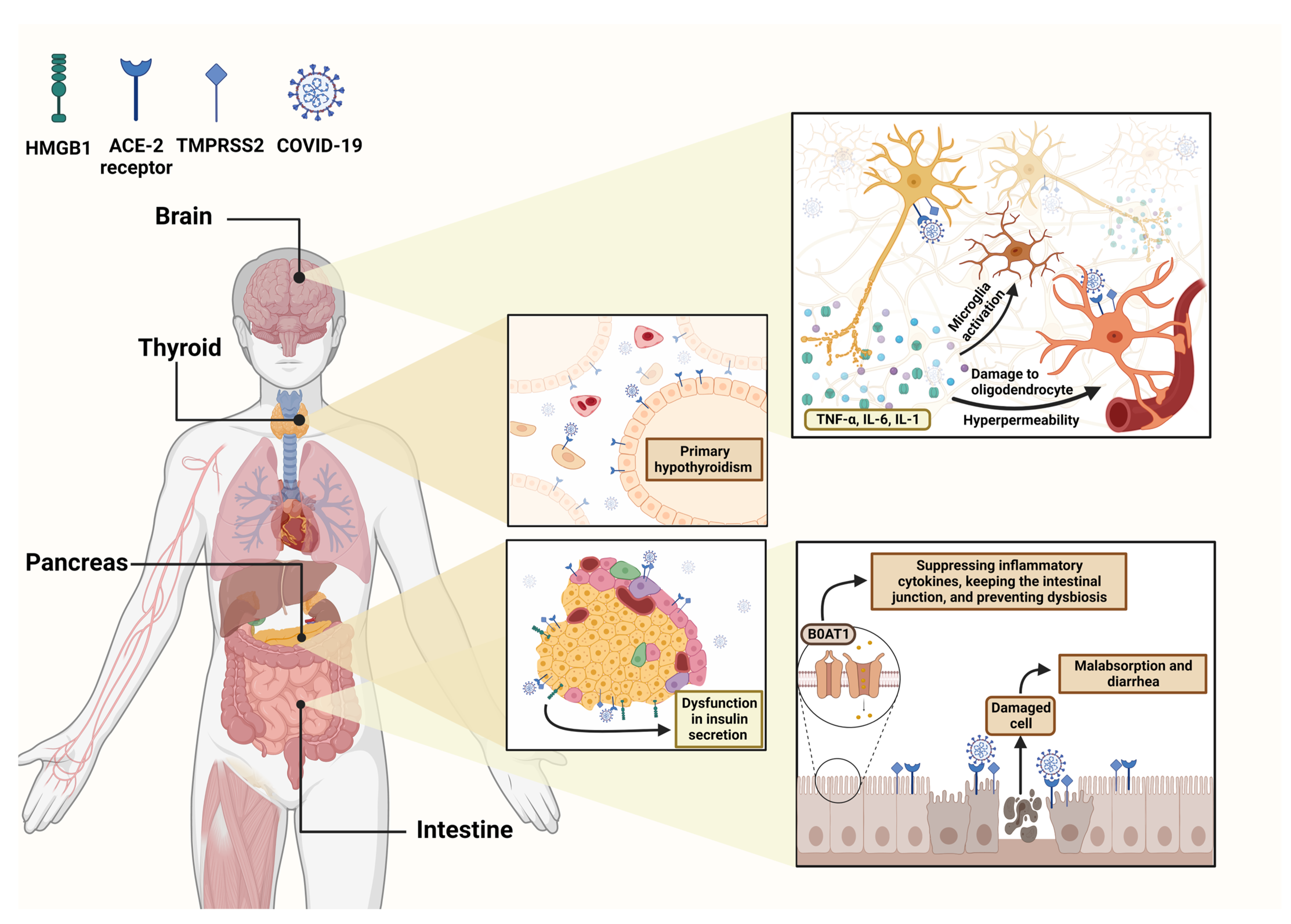
8. COVID-19 and the Endocrine System
8.1. Hypothalamic-Pituitary Axis (HPA)
8.2. Thyroid
8.3. Pancreas
8.4. Adrenal Gland
9. Gastrointestinal (GI) Disorders of COVID19
9.1. Direct Injury
9.2. Enteric Nervous System (ENS) Dysfunction
9.3. Immune-Mediated Injury
9.4. Liver Injury
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Coronavirus Disease 2019 | COVID-19 |
| Angiotensin Converting Enzyme-2 | ACE-2 |
| Cellular Transmembrane Serine Protease 2 | TMPRSS2 |
| Severe acute respiratory syndrome coronavirus 2 | SARS-CoV-2 |
| Open Reading Frames | ORF |
| Nonstructural proteins | nsp |
| Middle East respiratory syndrome coronavirus | MERS-CoV |
| Acute Respiratory Distress Syndrome | ARDS |
| Pattern Recognition Receptors | PRRs |
| Pathogen-Associated Molecular Patterns | PAMP |
| Damage-Associated Molecular Patterns | DAMP |
| Toll-Like Receptors | TLR |
| RIG-I-Like Receptors | RLR |
| Scavenger Receptors | SR |
| Receptor-Binding Domain | RBD |
| Cluster of Differentiation | CD |
| C-type lectin CD209L | L-SIGN |
| Similar protein CD209 | DC-SIGN |
| NOD Like-Receptor Protein | NLRP3 |
| Interleukin | IL |
| High Density Lipoprotein | HDL |
| NOD like Receptor | NLR |
| NOD-Like Receptor Protein | NLRP |
| Human Coronaviruses | HCoV |
| Central Nervous System | CNS |
| Reverse Transcription-Polymerase Chain Reaction | RT-PCR |
| Tumor Necrosis Factor | TNF |
| Neutrophil-to-Lymphocyte Ratio | NLR |
| C-reactive Protein | CRP |
| Neutrophil Extracellular Traps | NETs |
| Nitric Oxide Synthase | NOS |
| Intensive Care Units | ICUs |
| Cerebro-Spinal Fluid | CSF |
| Herpes Simplex Virus | HSV |
| Electroencephalography | EEG |
| Magnetic Resonance Imaging | MRI |
| Cytomegalovirus | CMV |
| acute neurological event | ANE |
| Guillain–Barré Syndrome | GBS |
| Multiple Sclerosis | MS |
| Alzheimer’s disease | AD |
| Parkinson’s disease | PD |
| Peripheral Nervous System | PNS |
| Olfactory Receptor Neurons | ORN |
| Interferon | IFN |
| Monocyte Chemoattractant Protein | MCP |
| Interferon-inducible Protein | IP |
| Photorefractive Keratectomy | PRK |
| Renin–Angiotensin–Aldosterone System | RAAS |
| Hypothalamic-Pituitary Axis | HPA |
| Corticotroph Releasing Hormone | CRH |
| Adrenocorticotropic Hormone | ACTH |
| G-Protein-Coupled-Receptor | GPCR |
| Luteinizing Hormone | LH |
| Follicle Stimulating Hormone | FSH |
| Thyroid Stimulating Hormone | TSH |
| NonThyroidal Illness Syndrome | NTIS |
| High mobility group box 1 | HMGB1 |
| Nuclear Factor Kappa B | NFKB |
| C-X-C motif chemokine Ligand | CXCL |
| Signal Transducer and Activator of Transcription | STAT |
| Adrenal insufficiency | AI |
| Gastrointestinal | GI |
| Aspartate Transaminase | AST |
| Alanine Transaminase | ALT |
| Gamma-Glutamyl Transferase | GGT |
| Alkaline phosphatase | ALP |
| Small Intestinal Epithelial Cells | iPSC-SIECs |
| Enteric Nervous System | ENS |
| CC Chemokine Receptor 9 | CCR9+ |
| CC motif Chemokine Ligand | CCL |
References
- Ozma, M.A.; Maroufi, P.; Khodadadi, E.; Köse, Ş.; Esposito, I.; Ganbarov, K.; Dao, S.; Esposito, S.; Dal, T.; Zeinalzadeh, E. Clinical manifestation, diagnosis, prevention and control of SARS-CoV-2 (COVID-19) during the outbreak period. Infez. Med. 2020, 28, 153–165. [Google Scholar] [PubMed]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280.e278. [Google Scholar] [CrossRef] [PubMed]
- Varatharaj, A.; Thomas, N.; Ellul, M.A.; Davies, N.W.S.; Pollak, T.A.; Tenorio, E.L.; Sultan, M.; Easton, A.; Breen, G.; Zandi, M.; et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: A UK-wide surveillance study. Lancet Psychiat. 2020, 7, 875–882. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Ceban, F.; Ling, S.; Lui, L.M.W.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav. Immun. 2022, 101, 93–135. [Google Scholar] [CrossRef]
- Ercoli, T.; Masala, C.; Pinna, I.; Orofino, G.; Solla, P.; Rocchi, L.; Defazio, G. Qualitative smell/taste disorders as sequelae of acute COVID-19. Neurol. Sci. 2021, 42, 4921–4926. [Google Scholar] [CrossRef]
- Wee, L.E.; Chan, Y.F.Z.; Teo, N.W.Y.; Cherng, B.P.Z.; Thien, S.Y.; Wong, H.M.; Wijaya, L.; Toh, S.T.; Tan, T.T. The role of self-reported olfactory and gustatory dysfunction as a screening criterion for suspected COVID-19. Eur. Arch. Otorhinolaryngol. 2020, 277, 2389–2390. [Google Scholar] [CrossRef]
- Lin, L.; Jiang, X.; Zhang, Z.; Huang, S.; Zhang, Z.; Fang, Z.; Gu, Z.; Gao, L.; Shi, H.; Mai, L. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut 2020, 69, 997–1001. [Google Scholar] [CrossRef]
- Puig-Domingo, M.; Marazuela, M.; Giustina, A. COVID-19 and endocrine diseases. A statement from the European Society of Endocrinology. Endocrine 2020, 68, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.U.; Shafique, L.; Ihsan, A.; Liu, Q. Evolutionary Trajectory for the Emergence of Novel Coronavirus SARS-CoV-2. Pathogens 2020, 9, 240. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Li, X.; Ge, C.; Ding, Y.; Zhang, T.; Cao, S.; Meng, L.; Lu, S. Molecular detection of SARS-CoV-2 being challenged by virus variation and asymptomatic infection. J. Pharm. Anal. 2021, 11, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Chilamakuri, R.; Agarwal, S. COVID-19: Characteristics and Therapeutics. Cells 2021, 10, 206. [Google Scholar] [CrossRef] [PubMed]
- Gorkhali, R.; Koirala, P.; Rijal, S.; Mainali, A.; Baral, A.; Bhattarai, H.K. Structure and Function of Major SARS-CoV-2 and SARS-CoV Proteins. Bioinform. Biol. Insights 2021, 15, 1–32. [Google Scholar] [CrossRef]
- Hassan, S.S.; Attrish, D.; Ghosh, S.; Choudhury, P.P.; Uversky, V.N.; Aljabali, A.A.A.; Lundstrom, K.; Uhal, B.D.; Rezaei, N.; Seyran, M.; et al. Notable sequence homology of the ORF10 protein introspects the architecture of SARS-CoV-2. Int. J. Biol. Macromol. 2021, 181, 801–809. [Google Scholar] [CrossRef]
- Bakhshandeh, B.; Jahanafrooz, Z.; Abbasi, A.; Goli, M.B.; Sadeghi, M.; Mottaqi, M.S.; Zamani, M. Mutations in SARS-CoV-2; Consequences in structure, function, and pathogenicity of the virus. Microb. Pathog. 2021, 154, 104831. [Google Scholar] [CrossRef]
- Camporota, L.; Chiumello, D.; Busana, M.; Gattinoni, L.; Marini, J.J. Pathophysiology of COVID-19-associated acute respiratory distress syndrome. Lancet Respir. Med. 2021, 9, e1. [Google Scholar] [CrossRef]
- Okamoto, M.; Tsukamoto, H.; Kouwaki, T.; Seya, T.; Oshiumi, H. Recognition of Viral RNA by Pattern Recognition Receptors in the Induction of Innate Immunity and Excessive Inflammation During Respiratory Viral Infections. Viral Immunol. 2017, 30, 408–420. [Google Scholar] [CrossRef]
- PrabhuDas, M.R.; Baldwin, C.L.; Bollyky, P.L.; Bowdish, D.M.E.; Drickamer, K.; Febbraio, M.; Herz, J.; Kobzik, L.; Krieger, M.; Loike, J.; et al. A Consensus Definitive Classification of Scavenger Receptors and Their Roles in Health and Disease. J. Immunol. 2017, 198, 3775–3789. [Google Scholar] [CrossRef]
- Gusev, E.Y.; Zotova, N.; Zhuravleva, Y.A.; Chereshnev, V. Physiological and pathogenic role of scavenger receptors in humans. Med. Immunol. 2020, 22, 7–48. [Google Scholar] [CrossRef]
- Wei, C.; Wan, L.; Yan, Q.; Wang, X.; Zhang, J.; Yang, X.; Zhang, Y.; Fan, C.; Li, D.; Deng, Y.; et al. HDL-scavenger receptor B type 1 facilitates SARS-CoV-2 entry. Nat. Metab. 2020, 2, 1391–1400. [Google Scholar] [CrossRef]
- Li, F. Structure, Function, and Evolution of Coronavirus Spike Proteins. Annu. Rev. Virol. 2016, 3, 237–261. [Google Scholar] [CrossRef]
- Harrison, A.G.; Lin, T.; Wang, P. Mechanisms of SARS-CoV-2 Transmission and Pathogenesis. Trends Immunol. 2020, 41, 1100–1115. [Google Scholar] [CrossRef]
- Ji, H.L.; Zhao, R.; Matalon, S.; Matthay, M.A. Elevated Plasmin(ogen) as a Common Risk Factor for COVID-19 Susceptibility. Physiol. Rev. 2020, 100, 1065–1075. [Google Scholar] [CrossRef]
- Evans, J.P.; Liu, S.L. Role of host factors in SARS-CoV-2 entry. J. Biol. Chem. 2021, 297, 100847. [Google Scholar] [CrossRef]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef]
- Hatmal, M.M.; Alshaer, W.; Al-Hatamleh, M.A.I.; Hatmal, M.; Smadi, O.; Taha, M.O.; Oweida, A.J.; Boer, J.C.; Mohamud, R.; Plebanski, M. Comprehensive Structural and Molecular Comparison of Spike Proteins of SARS-CoV-2, SARS-CoV and MERS-CoV, and Their Interactions with ACE2. Cells 2020, 9, 2638. [Google Scholar] [CrossRef]
- Shang, C.; Zhuang, X.; Zhang, H.; Li, Y.; Zhu, Y.; Lu, J.; Ge, C.; Cong, J.; Li, T.; Tian, M.; et al. Inhibitors of endosomal acidification suppress SARS-CoV-2 replication and relieve viral pneumonia in hACE2 transgenic mice. Virol. J. 2021, 18, 46. [Google Scholar] [CrossRef]
- Lokhande, K.B.; Apte, G.R.; Shrivastava, A.; Singh, A.; Pal, J.K.; Swamy, K.V.; Gupta, R.K. Sensing the interactions between carbohydrate-binding agents and N-linked glycans of SARS-CoV-2 spike glycoprotein using molecular docking and simulation studies. J. Biomol. Struct. Dyn. 2022, 40, 3880–3898. [Google Scholar] [CrossRef]
- Verma, J.; Subbarao, N. A comparative study of human betacoronavirus spike proteins: Structure, function and therapeutics. Arch. Virol. 2021, 166, 697–714. [Google Scholar] [CrossRef] [PubMed]
- Mycroft-West, C.J.; Su, D.; Pagani, I.; Rudd, T.R.; Elli, S.; Gandhi, N.S.; Guimond, S.E.; Miller, G.J.; Meneghetti, M.C.Z.; Nader, H.B.; et al. Heparin Inhibits Cellular Invasion by SARS-CoV-2: Structural Dependence of the Interaction of the Spike S1 Receptor-Binding Domain with Heparin. Thromb. Haemost. 2020, 120, 1700–1715. [Google Scholar] [CrossRef] [PubMed]
- Clausen, T.M.; Sandoval, D.R.; Spliid, C.B.; Pihl, J.; Perrett, H.R.; Painter, C.D.; Narayanan, A.; Majowicz, S.A.; Kwong, E.M.; McVicar, R.N.; et al. SARS-CoV-2 Infection Depends on Cellular Heparan Sulfate and ACE2. Cell 2020, 183, 1043–1057. [Google Scholar] [CrossRef] [PubMed]
- Cagno, V.; Tseligka, E.D.; Jones, S.T.; Tapparel, C. Heparan Sulfate Proteoglycans and Viral Attachment: True Receptors or Adaptation Bias? Viruses 2019, 11, 596. [Google Scholar] [CrossRef] [PubMed]
- Seyran, M.; Takayama, K.; Uversky, V.N.; Lundstrom, K.; Palù, G.; Sherchan, S.P.; Attrish, D.; Rezaei, N.; Aljabali, A.A.A.; Ghosh, S.; et al. The structural basis of accelerated host cell entry by SARS-CoV-2. Febs. J. 2021, 288, 5010–5020. [Google Scholar] [CrossRef]
- Dakal, T.C. SARS-CoV-2 attachment to host cells is possibly mediated via RGD-integrin interaction in a calcium-dependent manner and suggests pulmonary EDTA chelation therapy as a novel treatment for COVID 19. Immunobiology 2021, 226, 152021. [Google Scholar] [CrossRef]
- Hassanpour, M.; Rezaie, J.; Nouri, M.; Panahi, Y. The role of extracellular vesicles in COVID-19 virus infection. Infect. Genet Evol. 2020, 85, 104422. [Google Scholar] [CrossRef]
- Elrashdy, F.; Aljaddawi, A.A.; Redwan, E.M.; Uversky, V.N. On the potential role of exosomes in the COVID-19 reinfection/reactivation opportunity. J. Biomol. Struct. Dyn. 2021, 39, 5831–5842. [Google Scholar] [CrossRef]
- Xia, X.; Yuan, P.; Liu, Y.; Wang, Y.; Cao, W.; Zheng, J.C. Emerging roles of extracellular vesicles in COVID-19, a double-edged sword? Immunology 2021, 163, 416–430. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.H.; Kim, J.H. Diverse Effects of Exosomes on COVID-19: A Perspective of Progress From Transmission to Therapeutic Developments. Front. Immunol. 2021, 12, 716407. [Google Scholar] [CrossRef]
- Fenizia, C.; Galbiati, S.; Vanetti, C.; Vago, R.; Clerici, M.; Tacchetti, C.; Daniele, T. SARS-CoV-2 Entry: At the Crossroads of CD147 and ACE2. Cells 2021, 10, 1434. [Google Scholar] [CrossRef]
- Krejner-Bienias, A.; Grzela, K.; Grzela, T. DPP4 Inhibitors and COVID-19-Holy Grail or Another Dead End? Arch. Immunol. Ther. Exp. 2021, 69, 1. [Google Scholar] [CrossRef]
- Kočar, E.; Režen, T.; Rozman, D. Cholesterol, lipoproteins, and COVID-19: Basic concepts and clinical applications. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158849. [Google Scholar] [CrossRef]
- Amraei, R.; Yin, W.; Napoleon, M.A.; Suder, E.L.; Berrigan, J.; Zhao, Q.; Olejnik, J.; Chandler, K.B.; Xia, C.; Feldman, J.; et al. CD209L/L-SIGN and CD209/DC-SIGN Act as Receptors for SARS-CoV-2. ACS Cent Sci. 2021, 7, 1156–1165. [Google Scholar] [CrossRef]
- Lo, M.W.; Kemper, C.; Woodruff, T.M. COVID-19: Complement, Coagulation, and Collateral Damage. J. Immunol. 2020, 205, 1488–1495. [Google Scholar] [CrossRef]
- Perico, L.; Benigni, A.; Casiraghi, F.; Ng, L.F.P.; Renia, L.; Remuzzi, G. Immunity, endothelial injury and complement-induced coagulopathy in COVID-19. Nat. Rev. Nephrol. 2021, 17, 46–64. [Google Scholar] [CrossRef]
- Aboudounya, M.M.; Heads, R.J. COVID-19 and Toll-Like Receptor 4 (TLR4): SARS-CoV-2 May Bind and Activate TLR4 to Increase ACE2 Expression, Facilitating Entry and Causing Hyperinflammation. Mediat. Inflamm. 2021, 2021, 8874339. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.; Mukherjee, S. In silico studies on the comparative characterization of the interactions of SARS-CoV-2 spike glycoprotein with ACE-2 receptor homologs and human TLRs. J. Med. Viro.l 2020, 92, 2105–2113. [Google Scholar] [CrossRef]
- Moreno-Eutimio, M.A.; López-Macías, C.; Pastelin-Palacios, R. Bioinformatic analysis and identification of single-stranded RNA sequences recognized by TLR7/8 in the SARS-CoV-2, SARS-CoV, and MERS-CoV genomes. Microbes Infect. 2020, 22, 226–229. [Google Scholar] [CrossRef]
- de Marcken, M.; Dhaliwal, K.; Danielsen, A.C.; Gautron, A.S.; Dominguez-Villar, M. TLR7 and TLR8 activate distinct pathways in monocytes during RNA virus infection. Sci. Signal. 2019, 12, eaaw1347. [Google Scholar] [CrossRef]
- Onomoto, K.; Onoguchi, K.; Yoneyama, M. Regulation of RIG-I-like receptor-mediated signaling: Interaction between host and viral factors. Cell Mol. Immunol. 2021, 18, 539–555. [Google Scholar] [CrossRef]
- Rodrigues, T.S.; de Sá, K.S.G.; Ishimoto, A.Y.; Becerra, A.; Oliveira, S.; Almeida, L.; Gonçalves, A.V.; Perucello, D.B.; Andrade, W.A.; Castro, R.; et al. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J. Exp. Med. 2021, 218, e20201707. [Google Scholar] [CrossRef] [PubMed]
- Gusev, E.Y.; Zotova, N.V. Cellular Stress and General Pathological Processes. Curr. Pharm. Des. 2019, 25, 251–297. [Google Scholar] [CrossRef] [PubMed]
- Gusev, E.; Sarapultsev, A.; Hu, D.; Chereshnev, V. Problems of Pathogenesis and Pathogenetic Therapy of COVID-19 from the Perspective of the General Theory of Pathological Systems (General Pathological Processes). Int. J. Mol. Sci. 2021, 22, 7582. [Google Scholar] [CrossRef]
- Papanikolaou, V.; Chrysovergis, A.; Ragos, V.; Tsiambas, E.; Katsinis, S.; Manoli, A.; Papouliakos, S.; Roukas, D.; Mastronikolis, S.; Peschos, D.; et al. From delta to Omicron: S1-RBD/S2 mutation/deletion equilibrium in SARS-CoV-2 defined variants. Gene 2022, 814, 146134. [Google Scholar] [CrossRef]
- Sardu, C.; Gambardella, J.; Morelli, M.B.; Wang, X.; Marfella, R.; Santulli, G. Hypertension, Thrombosis, Kidney Failure, and Diabetes: Is COVID-19 an Endothelial Disease? A Comprehensive Evaluation of Clinical and Basic Evidence. J. Clin. Med. 2020, 9, 1417. [Google Scholar] [CrossRef]
- Ding, Y.; He, L.; Zhang, Q.; Huang, Z.; Che, X.; Hou, J.; Wang, H.; Shen, H.; Qiu, L.; Li, Z.; et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: Implications for pathogenesis and virus transmission pathways. J. Pathol. 2004, 203, 622–630. [Google Scholar] [CrossRef]
- Arbour, N.; Day, R.; Newcombe, J.; Talbot, P.J. Neuroinvasion by human respiratory coronaviruses. J. Virol. 2000, 74, 8913–8921. [Google Scholar] [CrossRef]
- Edwards, J.A.; Denis, F.; Talbot, P.J. Activation of glial cells by human coronavirus OC43 infection. J. Neuroimmunol. 2000, 108, 73–81. [Google Scholar] [CrossRef]
- Stamatovic, S.M.; Shakui, P.; Keep, R.F.; Moore, B.B.; Kunkel, S.L.; Van Rooijen, N.; Andjelkovic, A.V. Monocyte chemoattractant protein-1 regulation of blood-brain barrier permeability. J. Cereb. Blood Flow. Metab. 2005, 25, 593–606. [Google Scholar] [CrossRef]
- Glass, W.G.; Subbarao, K.; Murphy, B.; Murphy, P.M. Mechanisms of host defense following severe acute respiratory syndrome-coronavirus (SARS-CoV) pulmonary infection of mice. J. Immunol. 2004, 173, 4030–4039. [Google Scholar] [CrossRef] [PubMed]
- Kandemirli, S.G.; Dogan, L.; Sarikaya, Z.T.; Kara, S.; Akinci, C.; Kaya, D.; Kaya, Y.; Yildirim, D.; Tuzuner, F.; Yildirim, M.S.; et al. Brain MRI Findings in Patients in the Intensive Care Unit with COVID-19 Infection. Radiology 2020, 297, E232–E235. [Google Scholar] [CrossRef]
- Torabi, A.; Mohammadbagheri, E.; Akbari Dilmaghani, N.; Bayat, A.H.; Fathi, M.; Vakili, K.; Alizadeh, R.; Rezaeimirghaed, O.; Hajiesmaeili, M.; Ramezani, M.; et al. Proinflammatory Cytokines in the Olfactory Mucosa Result in COVID-19 Induced Anosmia. ACS Chem. Neurosci. 2020, 11, 1909–1913. [Google Scholar] [CrossRef]
- Solomon, I.H.; Normandin, E.; Bhattacharyya, S.; Mukerji, S.S.; Keller, K.; Ali, A.S.; Adams, G.; Hornick, J.L.; Padera, R.F., Jr.; Sabeti, P. Neuropathological Features of COVID-19. N. Engl. J. Med. 2020, 383, 989–992. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.S.; Brown, B.; Brian, D.; Cabirac, G.F. Detection of coronavirus RNA and antigen in multiple sclerosis brain. Ann. Neurol. 1992, 31, 525–533. [Google Scholar] [CrossRef]
- Netland, J.; Meyerholz, D.K.; Moore, S.; Cassell, M.; Perlman, S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J. Virol. 2008, 82, 7264–7275. [Google Scholar] [CrossRef]
- Jacomy, H.; Fragoso, G.; Almazan, G.; Mushynski, W.E.; Talbot, P.J. Human coronavirus OC43 infection induces chronic encephalitis leading to disabilities in BALB/C mice. Virology 2006, 349, 335–346. [Google Scholar] [CrossRef]
- Robertson, J.; Beaulieu, J.M.; Doroudchi, M.M.; Durham, H.D.; Julien, J.P.; Mushynski, W.E. Apoptotic death of neurons exhibiting peripherin aggregates is mediated by the proinflammatory cytokine tumor necrosis factor-alpha. J. Cell Biol. 2001, 155, 217–226. [Google Scholar] [CrossRef]
- Wan, Y.; Shang, J.; Graham, R.; Baric, R.S.; Li, F. Receptor Recognition by the Novel Coronavirus from Wuhan: An Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J. Virol. 2020, 94, e00127-20. [Google Scholar] [CrossRef]
- Monteil, V.; Kwon, H.; Prado, P.; Hagelkrüys, A.; Wimmer, R.A.; Stahl, M.; Leopoldi, A.; Garreta, E.; Hurtado Del Pozo, C.; Prosper, F.; et al. Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Cell 2020, 181, 905–913. [Google Scholar] [CrossRef]
- Yang, X.H.; Deng, W.; Tong, Z.; Liu, Y.X.; Zhang, L.F.; Zhu, H.; Gao, H.; Huang, L.; Liu, Y.L.; Ma, C.M.; et al. Mice transgenic for human angiotensin-converting enzyme 2 provide a model for SARS coronavirus infection. Comp. Med. 2007, 57, 450–459. [Google Scholar] [PubMed]
- Wang, S.; Le, T.Q.; Kurihara, N.; Chida, J.; Cisse, Y.; Yano, M.; Kido, H. Influenza virus-cytokine-protease cycle in the pathogenesis of vascular hyperpermeability in severe influenza. J. Infect. Dis. 2010, 202, 991–1001. [Google Scholar] [CrossRef]
- Ouattara, L.A.; Barin, F.; Barthez, M.A.; Bonnaud, B.; Roingeard, P.; Goudeau, A.; Castelnau, P.; Vernet, G.; Paranhos-Baccalà, G.; Komurian-Pradel, F. Novel human reovirus isolated from children with acute necrotizing encephalopathy. Emerg. Infect. Dis. 2011, 17, 1436–1444. [Google Scholar] [CrossRef]
- Allan, S.M.; Rothwell, N.J. Cytokines and acute neurodegeneration. Nat. Rev. Neurosci. 2001, 2, 734–744. [Google Scholar] [CrossRef]
- Ahmad, I.; Rathore, F.A. Neurological manifestations and complications of COVID-19: A literature review. J. Clin. Neurosci. 2020, 77, 8–12. [Google Scholar] [CrossRef]
- Andalib, S.; Biller, J.; Di Napoli, M.; Moghimi, N.; McCullough, L.D.; Rubinos, C.A.; O’Hana Nobleza, C.; Azarpazhooh, M.R.; Catanese, L.; Elicer, I.; et al. Peripheral Nervous System Manifestations Associated with COVID-19. Curr. Neurol. Neurosci. Rep. 2021, 21, 9. [Google Scholar] [CrossRef]
- Yaghi, S.; Ishida, K.; Torres, J.; Mac Grory, B.; Raz, E.; Humbert, K.; Henninger, N.; Trivedi, T.; Lillemoe, K.; Alam, S.; et al. SARS-CoV-2 and Stroke in a New York Healthcare System. Stroke 2020, 51, 2002–2011. [Google Scholar] [CrossRef]
- Klok, F.A.; Kruip, M.; van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.; Kant, K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.A.M.; Huisman, M.V.; et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020, 191, 145–147. [Google Scholar] [CrossRef]
- Fois, A.G.; Paliogiannis, P.; Scano, V.; Cau, S.; Babudieri, S.; Perra, R.; Ruzzittu, G.; Zinellu, E.; Pirina, P.; Carru, C.; et al. The Systemic Inflammation Index on Admission Predicts In-Hospital Mortality in COVID-19 Patients. Molecules 2020, 25, 5725. [Google Scholar] [CrossRef]
- Middleton, E.A.; He, X.Y.; Denorme, F.; Campbell, R.A.; Ng, D.; Salvatore, S.P.; Mostyka, M.; Baxter-Stoltzfus, A.; Borczuk, A.C.; Loda, M.; et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood 2020, 136, 1169–1179. [Google Scholar] [CrossRef]
- Akerström, S.; Gunalan, V.; Keng, C.T.; Tan, Y.J.; Mirazimi, A. Dual effect of nitric oxide on SARS-CoV replication: Viral RNA production and palmitoylation of the S protein are affected. Virology 2009, 395, 1–9. [Google Scholar] [CrossRef]
- Kochi, A.N.; Tagliari, A.P.; Forleo, G.B.; Fassini, G.M.; Tondo, C. Cardiac and arrhythmic complications in patients with COVID-19. J. Cardiovasc. Electrophysiol. 2020, 31, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Sharifian-Dorche, M.; Huot, P.; Osherov, M.; Wen, D.; Saveriano, A.; Giacomini, P.S.; Antel, J.P.; Mowla, A. Neurological complications of coronavirus infection; a comparative review and lessons learned during the COVID-19 pandemic. J. Neurol. Sci. 2020, 417, 117085. [Google Scholar] [CrossRef] [PubMed]
- Oxley, T.J.; Mocco, J.; Majidi, S.; Kellner, C.P.; Shoirah, H.; Singh, I.P.; De Leacy, R.A.; Shigematsu, T.; Ladner, T.R.; Yaeger, K.A.; et al. Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. N. Engl. J. Med. 2020, 382, e60. [Google Scholar] [CrossRef] [PubMed]
- Meyfroidt, G.; Kurtz, P.; Sonneville, R. Critical care management of infectious meningitis and encephalitis. Intensive Care Med. 2020, 46, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xu, X.; Chen, Z.; Duan, J.; Hashimoto, K.; Yang, L.; Liu, C.; Yang, C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020, 87, 18–22. [Google Scholar] [CrossRef]
- Benameur, K.; Agarwal, A.; Auld, S.C.; Butters, M.P.; Webster, A.S.; Ozturk, T.; Howell, J.C.; Bassit, L.C.; Velasquez, A.; Schinazi, R.F.; et al. Encephalopathy and Encephalitis Associated with Cerebrospinal Fluid Cytokine Alterations and Coronavirus Disease, Atlanta, Georgia, USA, 2020. Emerg. Infect. Dis. 2020, 26, 2016–2021. [Google Scholar] [CrossRef]
- Cag, Y.; Erdem, H.; Leib, S.; Defres, S.; Kaya, S.; Larsen, L.; Poljak, M.; Ozturk-Engin, D.; Barsic, B.; Argemi, X.; et al. Managing atypical and typical herpetic central nervous system infections: Results of a multinational study. Clin. Microbiol. Infect. 2016, 22, 568.e9–568.e17. [Google Scholar] [CrossRef]
- Hepburn, M.; Mullaguri, N.; George, P.; Hantus, S.; Punia, V.; Bhimraj, A.; Newey, C.R. Acute Symptomatic Seizures in Critically Ill Patients with COVID-19: Is There an Association? Neurocrit. Care 2021, 34, 139–143. [Google Scholar] [CrossRef]
- Lu, L.; Xiong, W.; Liu, D.; Liu, J.; Yang, D.; Li, N.; Mu, J.; Guo, J.; Li, W.; Wang, G.; et al. New onset acute symptomatic seizure and risk factors in coronavirus disease 2019: A retrospective multicenter study. Epilepsia 2020, 61, e49–e53. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Taylor, R.S.; Renwanz Boyle, A.G. Guillain Barre Syndrome (Nursing); StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Rahimi, K. Guillain-Barre syndrome during COVID-19 pandemic: An overview of the reports. Neurol. Sci. 2020, 41, 3149–3156. [Google Scholar] [CrossRef]
- Sancho-Saldaña, A.; Lambea-Gil, Á.; Liesa, J.L.C.; Caballo, M.R.B.; Garay, M.H.; Celada, D.R.; Serrano-Ponz, M. Guillain-Barré syndrome associated with leptomeningeal enhancement following SARS-CoV-2 infection. Clin. Med. 2020, 20, e93–e94. [Google Scholar] [CrossRef]
- Tatu, L.; Nono, S.; Grácio, S.; Koçer, S. Guillain-Barré syndrome in the COVID-19 era: Another occasional cluster? J. Neurol. 2021, 268, 1198–1200. [Google Scholar] [CrossRef]
- Wang, F.; Kream, R.M.; Stefano, G.B. Long-Term Respiratory and Neurological Sequelae of COVID-19. Med. Sci. Monit. 2020, 26, e928996. [Google Scholar] [CrossRef]
- Lukiw, W.J.; Pogue, A.; Hill, J.M. SARS-CoV-2 Infectivity and Neurological Targets in the Brain. Cell Mol. Neurobiol. 2022, 42, 217–224. [Google Scholar] [CrossRef]
- Lassmann, H. Multiple Sclerosis Pathology. Cold Spring Harb. Perspect. Med. 2018, 8, a028936. [Google Scholar] [CrossRef]
- Kempuraj, D.; Selvakumar, G.P.; Ahmed, M.E.; Raikwar, S.P.; Thangavel, R.; Khan, A.; Zaheer, S.A.; Iyer, S.S.; Burton, C.; James, D.; et al. COVID-19, Mast Cells, Cytokine Storm, Psychological Stress, and Neuroinflammation. Neuroscientist 2020, 26, 402–414. [Google Scholar] [CrossRef]
- Zanin, L.; Saraceno, G.; Panciani, P.P.; Renisi, G.; Signorini, L.; Migliorati, K.; Fontanella, M.M. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir. 2020, 162, 1491–1494. [Google Scholar] [CrossRef]
- Boziki, M.K.; Mentis, A.A.; Shumilina, M.; Makshakov, G.; Evdoshenko, E.; Grigoriadis, N. COVID-19 Immunopathology and the Central Nervous System: Implication for Multiple Sclerosis and Other Autoimmune Diseases with Associated Demyelination. Brain Sci. 2020, 10, 345. [Google Scholar] [CrossRef]
- Sormani, M.P. An Italian programme for COVID-19 infection in multiple sclerosis. Lancet Neurol. 2020, 19, 481–482. [Google Scholar] [CrossRef]
- Fuchs, V.; Kutza, M.; Wischnewski, S.; Deigendesch, N.; Lutz, L.; Kulsvehagen, L.; Ricken, G.; Kappos, L.; Tzankov, A.; Hametner, S.; et al. Presence of SARS-CoV-2 Transcripts in the Choroid Plexus of MS and Non-MS Patients With COVID-19. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e957. [Google Scholar] [CrossRef] [PubMed]
- Krajcovicova, L.; Klobusiakova, P.; Rektorova, I. Gray Matter Changes in Parkinson’s and Alzheimer’s Disease and Relation to Cognition. Curr. Neurol. Neurosci. Rep. 2019, 19, 85. [Google Scholar] [CrossRef] [PubMed]
- McAlpine, L.S.; Fesharaki-Zadeh, A.; Spudich, S. Coronavirus disease 2019 and neurodegenerative disease: What will the future bring? Curr. Opin. Psychiatry 2021, 34, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Beitz, J.M. Parkinson’s disease: A review. Front. Biosci. (Schol. Ed.) 2014, 6, 65–74. [Google Scholar] [CrossRef]
- Bernaus, A.; Blanco, S.; Sevilla, A. Glia Crosstalk in Neuroinflammatory Diseases. Front. Cell Neurosci. 2020, 14, 209. [Google Scholar] [CrossRef]
- Van Bulck, M.; Sierra-Magro, A.; Alarcon-Gil, J.; Perez-Castillo, A.; Morales-Garcia, J.A. Novel Approaches for the Treatment of Alzheimer’s and Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 719. [Google Scholar] [CrossRef]
- Ponsen, M.M.; Stoffers, D.; Booij, J.; van Eck-Smit, B.L.; Wolters, E.; Berendse, H.W. Idiopathic hyposmia as a preclinical sign of Parkinson’s disease. Ann. Neurol. 2004, 56, 173–181. [Google Scholar] [CrossRef]
- Welch, C.; Greig, C.; Masud, T.; Wilson, D.; Jackson, T.A. COVID-19 and Acute Sarcopenia. Aging Dis. 2020, 11, 1345–1351. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, L.; Luo, T.; Wu, F.; Zhao, B.; Li, X. The etiology of Bell’s palsy: A review. J. Neurol. 2020, 267, 1896–1905. [Google Scholar] [CrossRef]
- Koc, G.; Odabasi, Z.; Tan, E. Myasthenic Syndrome Caused by Hydroxychloroquine Used for COVID-19 Prophylaxis. J. Clin. Neuromuscul Dis 2020, 22, 60–62. [Google Scholar] [CrossRef]
- Agyeman, A.A.; Chin, K.L.; Landersdorfer, C.B.; Liew, D.; Ofori-Asenso, R. Smell and taste dysfunction in patients with COVID-19: A systematic review and meta-analysis. Mayo Clin. Proc. 2020, 95, 1621–1631. [Google Scholar] [CrossRef]
- Meng, X.; Deng, Y.; Dai, Z.; Meng, Z. COVID-19 and anosmia: A review based on up-to-date knowledge. Am. J. Otolaryngol. 2020, 41, 102581. [Google Scholar] [CrossRef]
- Zou, L.; Ruan, F.; Huang, M.; Liang, L.; Huang, H.; Hong, Z.; Yu, J.; Kang, M.; Song, Y.; Xia, J. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020, 382, 1177–1179. [Google Scholar] [CrossRef]
- Sungnak, W.; Huang, N.; Bécavin, C.; Berg, M.; Queen, R.; Litvinukova, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; Sampaziotis, F. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020, 26, 681–687. [Google Scholar] [CrossRef]
- Colavita, F.; Lapa, D.; Carletti, F.; Lalle, E.; Bordi, L.; Marsella, P.; Nicastri, E.; Bevilacqua, N.; Giancola, M.L.; Corpolongo, A. SARS-CoV-2 isolation from ocular secretions of a patient with COVID-19 in Italy with prolonged viral RNA detection. Ann. Intern. Med. 2020, 173, 242–243. [Google Scholar] [CrossRef]
- Ou, X.; Liu, Y.; Lei, X.; Li, P.; Mi, D.; Ren, L.; Guo, L.; Guo, R.; Chen, T.; Hu, J. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020, 11, 2144. [Google Scholar] [CrossRef]
- Reznik, G.K. Comparative anatomy, physiology, and function of the upper respiratory tract. Environ. Health Perspecives 1990, 85, 171–176. [Google Scholar]
- Suzuki, Y.; Schafer, J.; Farbman, A.I. Phagocytic cells in the rat olfactory epithelium after bulbectomy. Exp. Neurol. 1995, 136, 225–233. [Google Scholar] [CrossRef]
- Menini, A.; Lagostena, L.; Boccaccio, A. Olfaction: From odorant molecules to the olfactory cortex. News. Physiol. Sci. 2004, 19, 101–104. [Google Scholar] [CrossRef]
- Brann, D.H.; Tsukahara, T.; Weinreb, C.; Lipovsek, M.; Van den Berge, K.; Gong, B.; Chance, R.; Macaulay, I.C.; Chou, H.-J.; Fletcher, R.B. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci. Adv. 2020, 6, eabc5801. [Google Scholar] [CrossRef] [PubMed]
- Shirai, T.; Takase, D.; Yokoyama, J.; Nakanishi, K.; Uehara, C.; Saito, N.; Kato-Namba, A.; Yoshikawa, K. Functions of human olfactory mucus and age-dependent changes. Sci. Rep. 2023, 13, 971. [Google Scholar] [CrossRef] [PubMed]
- Eshraghi, A.A.; Mirsaeidi, M.; Davies, C.; Telischi, F.F.; Chaudhari, N.; Mittal, R. Potential mechanisms for COVID-19 induced anosmia and dysgeusia. Front Physiol. 2020, 11, 1039. [Google Scholar] [CrossRef] [PubMed]
- Bilinska, K.; Butowt, R. Anosmia in COVID-19: A bumpy road to establishing a cellular mechanism. ACS Chem. Neurosci. 2020, 11, 2152–2155. [Google Scholar] [CrossRef] [PubMed]
- Butowt, R.; von Bartheld, C.S. Anosmia in COVID-19: Underlying mechanisms and assessment of an olfactory route to brain infection. Neuroscientist 2021, 27, 582–603. [Google Scholar] [CrossRef] [PubMed]
- Cazzolla, A.P.; Lovero, R.; Lo Muzio, L.; Testa, N.F.; Schirinzi, A.; Palmieri, G.; Pozzessere, P.; Procacci, V.; Di Comite, M.; Ciavarella, D.; et al. Taste and smell disorders in COVID-19 patients: Role of interleukin-6. ACS Chem. Neurosci. 2020, 11, 2774–2781. [Google Scholar] [CrossRef]
- Mastrangelo, A.; Bonato, M.; Cinque, P. Smell and taste disorders in COVID-19: From pathogenesis to clinical features and outcomes. Neurosci. Lett. 2021, 748, 135694. [Google Scholar] [CrossRef]
- Ohkubo, K.; Lee, C.H.; Baraniuk, J.N.; Merida, M.; Hausfeld, J.N.; Kaliner, M.A. Angiotensin-converting enzyme in the human nasal mucosa. Am. J. Respir. Cell Mol. Biol. 1994, 11, 173–180. [Google Scholar] [CrossRef]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef]
- He, L.; Ding, Y.; Zhang, Q.; Che, X.; He, Y.; Shen, H.; Wang, H.; Li, Z.; Zhao, L.; Geng, J. Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+ cells in SARS patients: Relation to the acute lung injury and pathogenesis of SARS. J. Pathol. 2006, 210, 288–297. [Google Scholar] [CrossRef]
- Hamming, I.; Timens, W.; Bulthuis, M.; Lely, A.; Navis, G.V.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, Z.; Castiglione, G.M.; Soiberman, U.S.; Eberhart, C.G.; Duh, E.J. ACE2 and TMPRSS2 are expressed on the human ocular surface, suggesting susceptibility to SARS-CoV-2 infection. Ocul. Surf. 2020, 18, 537–544. [Google Scholar] [CrossRef]
- Lange, C.; Wolf, J.; Auw-Haedrich, C.; Schlecht, A.; Boneva, S.; Lapp, T.; Horres, R.; Agostini, H.; Martin, G.; Reinhard, T. Expression of the COVID-19 receptor ACE2 in the human conjunctiva. J. Med. Virol. 2020, 92, 2081–2086. [Google Scholar] [CrossRef]
- Wu, P.; Duan, F.; Luo, C.; Liu, Q.; Qu, X.; Liang, L.; Wu, K. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020, 138, 575–578. [Google Scholar] [CrossRef]
- Seah, I.; Agrawal, R. Can the coronavirus disease 2019 (COVID-19) affect the eyes? A review of coronaviruses and ocular implications in humans and animals. Ocul. Immunol. Inflamm. 2020, 28, 391–395. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, C.B. Conjunctiva is not a preferred gateway of entry for SARS-CoV-2 to infect respiratory tract. J. Med. Virol. 2020, 92, 1410–1412. [Google Scholar] [CrossRef]
- Wang, K.; Chen, W.; Zhou, Y.-S.; Lian, J.-Q.; Zhang, Z.; Du, P.; Gong, L.; Zhang, Y.; Cui, H.-Y.; Geng, J.-J. CD147-spike proteinis a novel route for SARS-CoV-2 infection to host cells. Signal Transduct Target Ther 2020, 283, 1–10. [Google Scholar]
- Marinho, P.M.; Marcos, A.A.; Romano, A.C.; Nascimento, H.; Belfort, R. Retinal findings in patients with COVID-19. Lancet 2020, 395, 1610. [Google Scholar] [CrossRef]
- Usami, Y.; Hirose, K.; Okumura, M.; Toyosawa, S.; Sakai, T. Brief communication: Immunohistochemical detection of ACE2 in human salivary gland. Oral. Sci. Int. 2021, 18, 101–104. [Google Scholar] [CrossRef]
- Lechien, J.R.; Radulesco, T.; Calvo-Henriquez, C.; Chiesa-Estomba, C.M.; Hans, S.; Barillari, M.R.; Cammaroto, G.; Descamps, G.; Hsieh, J.; Vaira, L. ACE2 & TMPRSS2 expressions in head & neck tissues: A systematic review. Head Neck Pathol. 2021, 15, 225–235. [Google Scholar]
- Milanetti, E.; Miotto, M.; Di Rienzo, L.; Nagaraj, M.; Monti, M.; Golbek, T.W.; Gosti, G.; Roeters, S.J.; Weidner, T.; Otzen, D.E. In-silico evidence for a two receptor based strategy of SARS-CoV-2. Front. Mol. Biosci. 2021, 8, 690655. [Google Scholar] [CrossRef] [PubMed]
- Small, D.M.; Prescott, J. Odor/taste integration and the perception of flavor. Exp. Brain Res. 2005, 166, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Roper, S.D.; Chaudhari, N. Taste buds: Cells, signals and synapses. Nat. Rev. Neurosci. 2017, 18, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Roper, S.D. Taste buds as peripheral chemosensory processors. Semin Cell Dev. Biol. 2013, 24, 71–79. [Google Scholar] [CrossRef]
- Breslin, P.A. An evolutionary perspective on food and human taste. Curr. Biol. 2013, 23, R409–R418. [Google Scholar] [CrossRef]
- Finsterer, J.; Stollberger, C. Causes of hypogeusia/hyposmia in SARS-CoV2 infected patients. J. Med. Virol. 2020, 92, 1793. [Google Scholar] [CrossRef]
- Cohn, Z.J.; Kim, A.; Huang, L.; Brand, J.; Wang, H. Lipopolysaccharide-induced inflammation attenuates taste progenitor cell proliferation and shortens the life span of taste bud cells. BMC Neurosci. 2010, 11, 72. [Google Scholar] [CrossRef]
- Xu, H.; Zhong, L.; Deng, J.; Peng, J.; Dan, H.; Zeng, X. High expression of ACE2 receptor of 2019-nCoV onthe epitelial cells of oral mucosa. Int. J. Oral. Sci. 2020, 12, 8. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, M.; Brand, J.; Huang, L. Inflammation and taste disorders: Mechanisms in taste buds. Ann. N. Y Acad. Sci. 2009, 1170, 596–603. [Google Scholar] [CrossRef]
- Clark, H.L.; Jhingran, A.; Sun, Y.; Vareechon, C.; de Jesus Carrion, S.; Skaar, E.P.; Chazin, W.J.; Calera, J.A.; Hohl, T.M.; Pearlman, E. Zinc and manganese chelation by neutrophil S100A8/A9 (calprotectin) limits extracellular Aspergillus fumigatus hyphal growth and corneal infection. J. Immunol. 2016, 196, 336–344. [Google Scholar] [CrossRef]
- Esmaeilzadeh, A.; Elahi, R.; Siahmansouri, A.; Maleki, A.J.; Moradi, A. Endocrine and metabolic complications of COVID-19: Lessons learned and future prospects. J. Mol. Endocrinol. 2022, 69, R125–R150. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.; Hosni, A. Insights into the possible impact of COVID-19 on the endocrine system. Arch. Physiol. Biochem. 2021, 1–9. [Google Scholar] [CrossRef]
- Lechien, J.R.; Chiesa-Estomba, C.M.; De Siati, D.R.; Horoi, M.; Le Bon, S.D.; Rodriguez, A.; Dequanter, D.; Blecic, S.; El Afia, F.; Distinguin, L. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multicenter European study. Eur. Arch. Otorhinolaryngol. 2020, 277, 2251–2261. [Google Scholar] [CrossRef]
- Garg, M.K.; Gopalakrishnan, M.; Yadav, P.; Misra, S. Endocrine Involvement in COVID-19: Mechanisms, Clinical Features, and Implications for Care. Indian J. Endocrinol. Metab. 2020, 24, 381–386. [Google Scholar] [CrossRef]
- Hornick, M.G.; Olson, M.E.; Jadhav, A.L. SARS-CoV-2 Psychiatric Sequelae: A Review of Neuroendocrine Mechanisms and Therapeutic Strategies. Int. J. Neuropsychopharmacol. 2022, 25, 1–12. [Google Scholar] [CrossRef]
- Elkazzaz, M.; Ahmed, A.; Abo-Amer, Y.E.; Hydara, T.; Haikal, A.; Razek, D.; Eltayb, W.A.; Wang, X.; Karpiński, T.M.; Hamza, D.; et al. In Silico Discovery of GPCRs and GnRHRs as Novel Binding Receptors of SARS-CoV-2 Spike Protein Could Explain Neuroendocrine Disorders in COVID-19. Vaccines 2022, 10, 1500. [Google Scholar] [CrossRef]
- Piticchio, T.; Le Moli, R.; Tumino, D.; Frasca, F. Relationship between betacoronaviruses and the endocrine system: A new key to understand the COVID-19 pandemic-A comprehensive review. J. Endocrinol. Investig. 2021, 44, 1553–1570. [Google Scholar] [CrossRef]
- Chen, W.; Tian, Y.; Li, Z.; Zhu, J.; Wei, T.; Lei, J. Potential Interaction Between SARS-CoV-2 and Thyroid: A Review. Endocrinology 2021, 162, bqab004. [Google Scholar] [CrossRef]
- Shaharuddin, S.H.; Wang, V.; Santos, R.S.; Gross, A.; Wang, Y.; Jawanda, H.; Zhang, Y.; Hasan, W.; Garcia, G., Jr.; Arumugaswami, V.; et al. Deleterious Effects of SARS-CoV-2 Infection on Human Pancreatic Cells. Front. Cell Infect. Microbiol. 2021, 11, 678482. [Google Scholar] [CrossRef]
- Saeed, U.; Piracha, Z.Z.; Uppal, S.R.; Waheed, Y.; Uppal, R. SARS-CoV-2 induced hepatic injuries and liver complications. Front. Cell Infect. Microbiol. 2022, 12, 726263. [Google Scholar] [CrossRef]
- Campbell, P.T.; Fix, O.K. Coronavirus Disease-2019 and Implications on the Liver. Clin. Liver Dis. 2023, 27, 27–45. [Google Scholar] [CrossRef]
- Alnamshan, M.M. Potential histopathological and immunological effects of SARS-CoV-2 on the liver. Braz. J. Biol. 2022, 82, e262008. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhou, Y.; Yan, D.; Wan, Y. An Update on the Mutual Impact between SARS-CoV-2 Infection and Gut Microbiota. Viruses 2022, 14, 1774. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Noda, T.; Okabe, K.; Yanagida, S.; Nishida, M.; Kanda, Y. SARS-CoV-2 induces barrier damage and inflammatory responses in the human iPSC-derived intestinal epithelium. J. Pharmacol. Sci. 2022, 149, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Marasco, G.; Lenti, M.V.; Cremon, C.; Barbaro, M.R.; Stanghellini, V.; Di Sabatino, A.; Barbara, G. Implications of SARS-CoV-2 infection for neurogastroenterology. Neurogastroenterol. Motil. 2021, 33, e14104. [Google Scholar] [CrossRef]
- Chen, T.H.; Hsu, M.T.; Lee, M.Y.; Chou, C.K. Gastrointestinal Involvement in SARS-CoV-2 Infection. Viruses 2022, 14, 1188. [Google Scholar] [CrossRef]
- Zhong, P.; Xu, J.; Yang, D.; Shen, Y.; Wang, L.; Feng, Y.; Du, C.; Song, Y.; Wu, C.; Hu, X.; et al. COVID-19-associated gastrointestinal and liver injury: Clinical features and potential mechanisms. Signal. Transduct. Target Ther. 2020, 5, 256. [Google Scholar] [CrossRef]
- Dawood, R.M.; Maher Salum, G.; Abd El-Meguid, M. The Impact of COVID-19 on Liver Injury. Am J Med Sci. 2022, 363, 94–103. [Google Scholar] [CrossRef]
- Willcox, M.D.; Walsh, K.; Nichols, J.J.; Morgan, P.B.; Jones, L.W. The ocular surface, coronaviruses and COVID-19. Clin. Exp. Optom. 2020, 103, 418–424. [Google Scholar] [CrossRef]
- Lei, H.Y.; Ding, Y.H.; Nie, K.; Dong, Y.M.; Xu, J.H.; Yang, M.L.; Liu, M.Q.; Wei, L.; Nasser, M.I.; Xu, L.Y.; et al. Potential effects of SARS-CoV-2 on the gastrointestinal tract and liver. Biomed. Pharm. 2021, 133, 111064. [Google Scholar] [CrossRef]
| Systems (Disorders) | Factors Involved | Mechanisms | Reference |
|---|---|---|---|
| CNS (Cerebrovascular diseases) | MCP-1, chemokine, IL-6, IL-1β, TNF-α, and NETs |
| [61,68,80] |
| Skeletal muscle and Neuromuscular Junction (Myasthenia gravis) | Proinflammatory cytokines |
| [109] |
| Olfactory Nerve (smell dysfunction) | IL-6, IFN-γ, MCP-1, and IP-10 |
| [160] |
| Ophthalmic manifestation | ACE-2 receptors, TMPRSS2, CD147, |
| [170] |
| Taste manifestation | IFN- γ, and IL-6 |
| [142,148,151] |
| Endocrine System (sick euthyroid syndrome, hypothyroidism, diabetes and hyperglycemia) | IL-1, IL-6, and TNFα, the spike protein, MCP-1, inducible protein-10, and inter-leukin-1β |
| [152,153,155,156,157,160] |
| GI System (diarrhea, loss of appetite, nausea, vomiting, and abdominal pain) | CCL2, CCL3, CCL5, CXCL10, IL-6, and IL-1β, IFN-γ, IL-2, IL-7, and ΤΝF-α |
| [162,163,166,171] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Askari, H.; Rabiei, F.; Lohrasbi, F.; Ghadir, S.; Ghasemi-Kasman, M. The Latest Cellular and Molecular Mechanisms of COVID-19 on Non-Lung Organs. Brain Sci. 2023, 13, 415. https://doi.org/10.3390/brainsci13030415
Askari H, Rabiei F, Lohrasbi F, Ghadir S, Ghasemi-Kasman M. The Latest Cellular and Molecular Mechanisms of COVID-19 on Non-Lung Organs. Brain Sciences. 2023; 13(3):415. https://doi.org/10.3390/brainsci13030415
Chicago/Turabian StyleAskari, Hamid, Fatemeh Rabiei, Fatemeh Lohrasbi, Sara Ghadir, and Maryam Ghasemi-Kasman. 2023. "The Latest Cellular and Molecular Mechanisms of COVID-19 on Non-Lung Organs" Brain Sciences 13, no. 3: 415. https://doi.org/10.3390/brainsci13030415
APA StyleAskari, H., Rabiei, F., Lohrasbi, F., Ghadir, S., & Ghasemi-Kasman, M. (2023). The Latest Cellular and Molecular Mechanisms of COVID-19 on Non-Lung Organs. Brain Sciences, 13(3), 415. https://doi.org/10.3390/brainsci13030415









