Exogenous Cannabinoids Impair Effort-Related Decision-Making via Affecting Neural Synchronization between the Anterior Cingulate Cortex and Nucleus Accumbens
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal
2.2. Drugs
2.3. Apparatus
2.4. Behavioral Training
2.5. Experimental Design
2.6. General and Surgical Procedures
2.7. Neural Recording and Wavelet Analysis
Wavelet Analysis
2.8. Statistics
3. Results
3.1. Effects of the Administration of Exogenous Cannabinoid in the NAc Core on High Remuneration Choice (HRC) Percentage in Endeavor-Related Decision-Making the Task
3.2. A High Degree of Correlation between the ACC and NAc Core in the DMSO-Control Group While Making an Endeavor-Related Decision and Choosing a High Endeavor/High Remuneration Arm
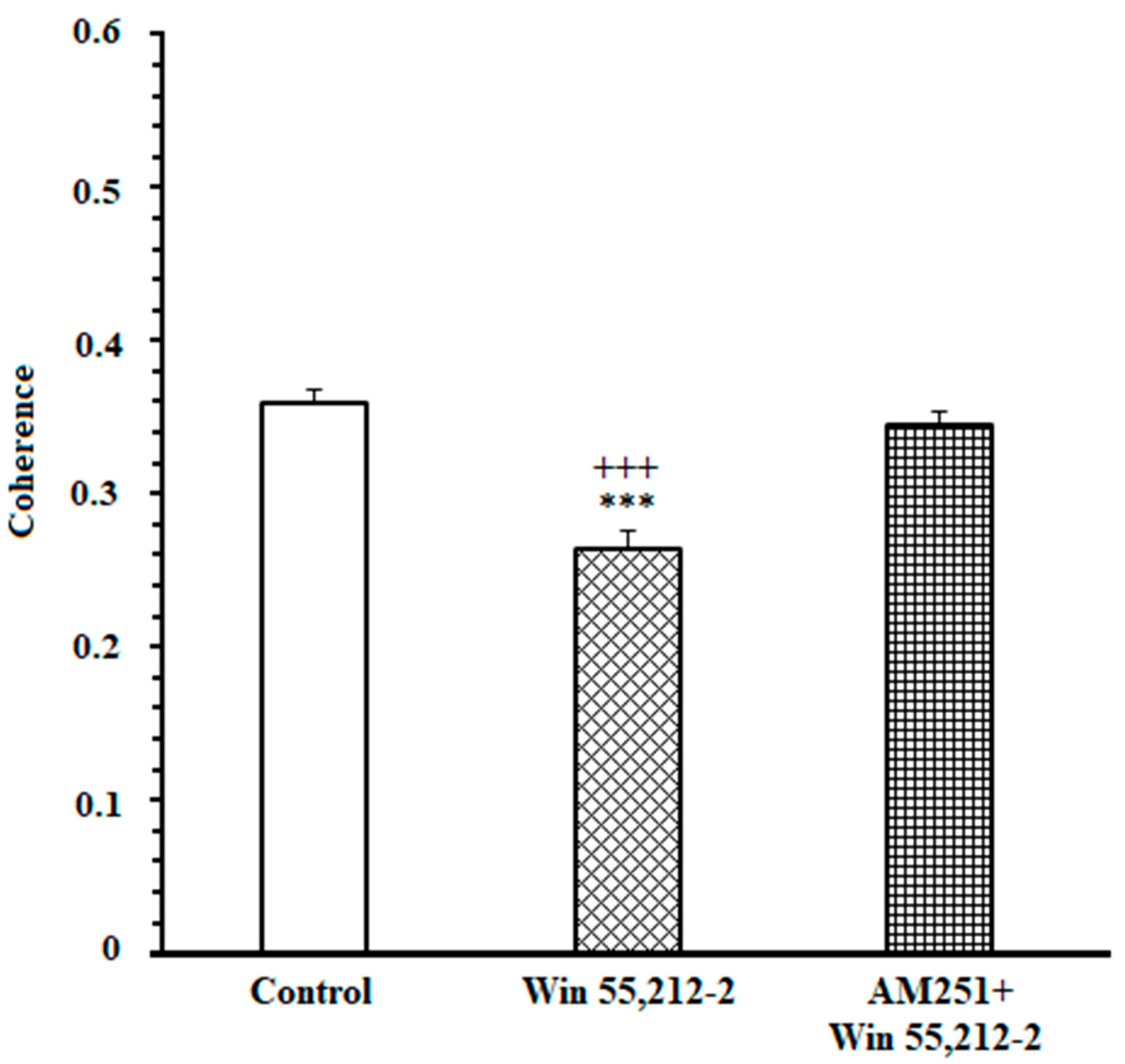
3.3. A Poor Correlation between the ACC and NAc Core in the Cannabinoid Agonist-Treated Group While Making an Endeavor-Related Decision and Choosing Low Endeavor/Low Remuneration Arm
3.4. A High Correlation between the ACC and NAc Core in the Antagonist-Treated Group While Making an Endeavor-Based Decision and Choosing a High Remuneration/High Endeavor Arm
3.5. A High Degree of Correlation between the ACC and NAc Core in the DMSO-Control Group While Reaching the Remuneration
3.6. Poor Correlation between the ACC and NAc Core in the Agonist-Treated Animals While Reaching the Remuneration
3.7. A High Correlation between the ACC and NAc Core in the Antagonist-Treated Group While Reaching the Remuneration
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hosking, J.G.; Floresco, S.B.; Winstanley, C.A. Dopamine antagonism decreases willingness to expend physical, but not cognitive, effort: A comparison of two rodent cost/benefit decision-making tasks. Neuropsychopharmacology 2015, 40, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Hampson, R.E.; Sweatt, A.J.; Goonawardena, A.V.; Song, D.; Chan, R.H.; Marmarelis, V.Z.; Berger, T.W.; Deadwyler, S.A. Memory encoding in hippocampal ensembles is negatively influenced by cannabinoid CB1 receptors. Behav. Pharmacol. 2011, 22, 335–346. [Google Scholar] [CrossRef]
- Hill, M.N.; Campolongo, P.; Yehuda, R.; Patel, S. Integrating endocannabinoid signaling and cannabinoids into the biology and treatment of posttraumatic stress disorder. Neuropsychopharmacology 2018, 43, 80–102. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.C.; Ploner, M.; Wiech, K.; Bingel, U.; Wanigasekera, V.; Brooks, J.; Menon, D.K.; Tracey, I. Amygdala activity contributes to the dissociative effect of cannabis on pain perception. PAIN® 2013, 154, 124–134. [Google Scholar] [CrossRef]
- Hernandez, G.; Cheer, J.F. To act or not to act: Endocannabinoid/dopamine interactions in decision-making. Front. Behav. Neurosci. 2015, 9, 336. [Google Scholar] [CrossRef] [PubMed]
- Fatahi, Z.; Haghparast, A. Activation of the cannabinoid system in the nucleus accumbens affects effort-based decision making. Pharmacol. Biochem. Behav. 2018, 165, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Fatahi, Z.; Sadeghi, B.; Haghparast, A. Involvement of cannabinoid system in the nucleus accumbens on delay-based decision making in the rat. Behav. Brain Res. 2018, 337, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Silveira, M.M.; Adams, W.K.; Morena, M.; Hill, M.N.; Winstanley, C.A. Δ9-Tetrahydrocannabinol decreases willingness to exert cognitive effort in male rats. J. Psychiatry Neurosci. 2017, 42, 131–138. [Google Scholar] [CrossRef]
- Hill, K.P.; Gold, M.S.; Nemeroff, C.B.; McDonald, W.; Grzenda, A.; Widge, A.S.; Rodriguez, C.; Kraguljac, N.V.; Krystal, J.H.; Carpenter, L.L. Risks and benefits of cannabis and cannabinoids in psychiatry. Am. J. Psychiatry 2022, 179, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Compton, W.M.; Einstein, E.B.; Volkow, N.D. Associations of suicidality trends with cannabis use as a function of sex and depression status. JAMA Netw. Open 2021, 4, e2113025. [Google Scholar] [CrossRef] [PubMed]
- Gruber, S.A.; Dahlgren, M.K.; Sagar, K.A.; Gönenc, A.; Killgore, W. Age of onset of marijuana use impacts inhibitory processing. Neurosci. Lett. 2012, 511, 89–94. [Google Scholar] [CrossRef]
- Cohen, K.; Weizman, A.; Weinstein, A. Modulatory effects of cannabinoids on brain neurotransmission. Eur. J. Neurosci. 2019, 50, 2322–2345. [Google Scholar] [CrossRef] [PubMed]
- Hosking, J.G.; Cocker, P.J.; Winstanley, C.A. Dissociable contributions of anterior cingulate cortex and basolateral amygdala on a rodent cost/benefit decision-making task of cognitive effort. Neuropsychopharmacology 2014, 39, 1558–1567. [Google Scholar] [CrossRef] [PubMed]
- Pistis, M.; Muntoni, A.L.; Pillolla, G.; Gessa, G.L. Cannabinoids inhibit excitatory inputs to neurons in the shell of the nucleus accumbens: An in vivo electrophysiological study. Eur. J. Neurosci. 2002, 15, 1795–1802. [Google Scholar] [CrossRef]
- Schweimer, J.; Hauber, W. Dopamine D1 receptors in the anterior cingulate cortex regulate effort-based decision making. Learn. Mem. 2006, 13, 777–782. [Google Scholar] [CrossRef]
- Nestler, E.J.; Carlezon, W.A., Jr. The mesolimbic dopamine reward circuit in depression. Biol. Psychiatry 2006, 59, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Raymond, D.R.; Paneto, A.; Yoder, K.K.; O’Donnell, B.F.; Brown, J.W.; Hetrick, W.P.; Newman, S.D. Does Chronic Cannabis Use Impact Risky Decision-Making: An Examination of fMRI Activation and Effective Connectivity? Front. Psychiatry 2020, 11, 1340. [Google Scholar] [CrossRef] [PubMed]
- Rudebeck, P.H.; Walton, M.E.; Smyth, A.N.; Bannerman, D.M.; Rushworth, M.F. Separate neural pathways process different decision costs. Nat. Neurosci. 2006, 9, 1161–1168. [Google Scholar] [CrossRef]
- Albrechet-Souza, L.; Carvalho, M.C.; Brandao, M.L. D1-like receptors in the nucleus accumbens shell regulate the expression of contextual fear conditioning and activity of the anterior cingulate cortex in rats. Int. J. Neuropsychopharmacol. 2013, 16, 1045–1057. [Google Scholar] [CrossRef]
- Walton, M.E.; Groves, J.; Jennings, K.A.; Croxson, P.L.; Sharp, T.; Rushworth, M.F.; Bannerman, D.M. Comparing the role of the anterior cingulate cortex and 6-hydroxydopamine nucleus accumbens lesions on operant effort-based decision making. Eur. J. Neurosci. 2009, 29, 1678–1691. [Google Scholar] [CrossRef]
- Mai, B.; Sommer, S.; Hauber, W. Motivational states influence effort-based decision making in rats: The role of dopamine in the nucleus accumbens. Cogn. Affect. Behav. Neurosci. 2012, 12, 74–84. [Google Scholar] [CrossRef]
- Saalmann, Y.B.; Pigarev, I.N.; Vidyasaga, T.R. Neural mechanisms of visual attention: How top-down feedback highlights relevant locations. Science 2007, 316, 1612–1615. [Google Scholar] [CrossRef] [PubMed]
- Brincat, S.L.; Miller, E.K. Frequency-specific hippocampal-prefrontal interactions during associative learning. Nat. Neurosci. 2015, 18, 576–581. [Google Scholar] [CrossRef]
- Neubert, F.X.; Mars, R.B.; Salle, J.; Rushworth, M.F. Connectivity reveals relationship of brain areas for reward-guided learning and decision making in human and monkey frontal cortex. Proc. Natl. Acad. Sci. USA 2015, 112, E2695–E2704. [Google Scholar] [CrossRef]
- Khani, A.; Kermani, M.; Hesam, S.; Haghparast, A.; Argandoña, E.G.; Rainer, G. Activation of cannabinoid system in anterior cingulate cortex and orbitofrontal cortex modulates cost-benefit decision making. Psychopharmacology 2015, 232, 2097–2112. [Google Scholar] [CrossRef]
- Fatahi, Z.; Haghparast, A.; Khani, A.; Kermani, M. Functional connectivity between anterior cingulate cortex and orbitofrontal cortex during value-based decision making. Neurobiol. Learn. Mem. 2018, 147, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Fatahi, Z.; Ghorbani, A.; Zibaii, M.I.; Haghparast, A. Neural synchronization between the anterior cingulate and orbitofrontal cortices during effort-based decision making. Neurobiol. Learn. Mem. 2020, 175, 107320. [Google Scholar] [CrossRef] [PubMed]
- Denk, F.; Walton, M.E.; Jennings, K.A.; Sharp, T.; Rushworth, M.F.; Bannerman, D.M. Differential involvement of serotonin and dopamine systems in cost-benefit decisions about delay or effort. Psychopharmacology 2005, 179, 587–596. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Grinsted, A.; Moore, J.C.; Jevrejeva, S. Application of the cross wavelet transform and wavelet coherence to geophysical time series. Nonlinear Process. Geophys. 2004, 11, 561–566. [Google Scholar] [CrossRef]
- Maraun, D.; Kurths, J. Cross wavelet analysis: Significance testing and pitfalls. Nonlinear Process. Geophys. 2004, 11, 505–514. [Google Scholar] [CrossRef]
- Bastos, A.M.; Schoffelen, J. A tutorial review of functional connectivity analysis methods and their interpretational pitfalls. Front. Syst. Neurosci. 2016, 9, 175. [Google Scholar] [CrossRef]
- Volkow, N.D.; Han, B.; Compton, W.M.; McCance-Katz, E.F. Self-reported medical and nonmedical cannabis use among pregnant women in the United States. JAMA 2019, 322, 167–169. [Google Scholar] [CrossRef]
- Luk, C.H.; Wallis, J.D. Choice coding in frontal cortex during stimulus-guided or action-guided decision-making. J. Neurosci. 2013, 33, 1864–1871. [Google Scholar] [CrossRef]
- Hu, Y.; van Wingerden, M.; Sellitto, M.; Schäble, S.; Kalenscher, T. Anterior cingulate cortex lesions abolish budget effects on effort-based decision-making in rat consumers. J. Neurosci. 2021, 41, 4448–4460. [Google Scholar] [CrossRef]
- Bryce, C.A.; Floresco, S.B. Alterations in effort-related decision-making induced by stimulation of dopamine D1, D2, D3, and corticotropin-releasing factor receptors in nucleus accumbens subregions. Psychopharmacology 2019, 236, 2699–2712. [Google Scholar] [CrossRef]
- Ghods-Sharifi, S. Dissociable Involvement of the Nucleus Accumbens Subregions in Effort-Based Decision Making. Ph.D. Thesis, The University of British Columbia, Vancouver, BC, Canada, 2009. [Google Scholar]
- Cohen, M.X.; Heller, A.S.; Ranganath, C. Functional connectivity with anterior cingulate and orbitofrontal cortices during decision-making. Cogn. Brain Res. 2005, 23, 61–70. [Google Scholar] [CrossRef] [PubMed]
- McCairn, K.W.; Nagai, Y.; Hori, Y.; Ninomiya, T.; Kikuchi, E.; Lee, J.Y.; Suhara, T.; Iriki, A.; Minamimoto, T.; Takada, M.; et al. A primary role for nucleus accumbens and related limbic network in vocal tics. Neuron 2016, 89, 300–307. [Google Scholar] [CrossRef]
- Wang, Z.; Fang, J.; Liu, J.; Rong, P.; Jorgenson, K.; Park, J.; Lang, C.; Hong, Y.; Zhu, B.; Kong, J. Frequency-dependent functional connectivity of the nucleus accumbens during continuous transcutaneous vagus nerve stimulation in major depressive disorder. J. Psychiatr. Res. 2018, 102, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, H.L.; Xiang, X.H.; Zhao, Y. The role of glutamate and its receptors in mesocorticolimbic dopaminergic regions in opioid addiction. Neurosci. Biobehav. Rev. 2009, 33, 864–873. [Google Scholar] [CrossRef]
- Kest, K.; Cruz, I.; Chen, D.H.; Galaj, E.; Ranaldi, R. A food associated CS activates c-Fos in VTA DA neurons and elicits conditioned approach. Behav. Brain Res. 2012, 235, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Hilairet, S.; Bouaboula, M.; Carriere, D.; Le Fur, G.; Casellas, P. Hypersensitization of the orexin 1 receptor by the CB1 receptor evidence for cross-talk blocked by the specific CB1 antagonist, SR141716. J. Biol. Chem. 2003, 278, 23731–23737. [Google Scholar] [CrossRef] [PubMed]
- Layer, R.T.; Uretsky, N.J.; Wallace, L.J. Effects of the AMPA/kainite receptor antagonist DNQX in the nucleus accumbens on drug induced conditioned place preference. Brain Res. 1993, 617, 267–273. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatahi, Z.; Fatahi, M.; Mirramezani Alizamini, M.; Ghorbani, A.; Zibaii, M.I.; Haghparast, A. Exogenous Cannabinoids Impair Effort-Related Decision-Making via Affecting Neural Synchronization between the Anterior Cingulate Cortex and Nucleus Accumbens. Brain Sci. 2023, 13, 413. https://doi.org/10.3390/brainsci13030413
Fatahi Z, Fatahi M, Mirramezani Alizamini M, Ghorbani A, Zibaii MI, Haghparast A. Exogenous Cannabinoids Impair Effort-Related Decision-Making via Affecting Neural Synchronization between the Anterior Cingulate Cortex and Nucleus Accumbens. Brain Sciences. 2023; 13(3):413. https://doi.org/10.3390/brainsci13030413
Chicago/Turabian StyleFatahi, Zahra, Mohammad Fatahi, Mirmohammadali Mirramezani Alizamini, Ahmad Ghorbani, Mohammad Ismail Zibaii, and Abbas Haghparast. 2023. "Exogenous Cannabinoids Impair Effort-Related Decision-Making via Affecting Neural Synchronization between the Anterior Cingulate Cortex and Nucleus Accumbens" Brain Sciences 13, no. 3: 413. https://doi.org/10.3390/brainsci13030413
APA StyleFatahi, Z., Fatahi, M., Mirramezani Alizamini, M., Ghorbani, A., Zibaii, M. I., & Haghparast, A. (2023). Exogenous Cannabinoids Impair Effort-Related Decision-Making via Affecting Neural Synchronization between the Anterior Cingulate Cortex and Nucleus Accumbens. Brain Sciences, 13(3), 413. https://doi.org/10.3390/brainsci13030413






