Differences in Gray Matter Volume in Cerebral Small Vessel Disease Patients with and without Sleep Disturbance
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. MRI Acquisition
2.3. Assessment of MRI Markers of CSVD
2.4. MRI Data Processing
2.5. Statistical Analysis
3. Results
3.1. Demographic and Clinical Data
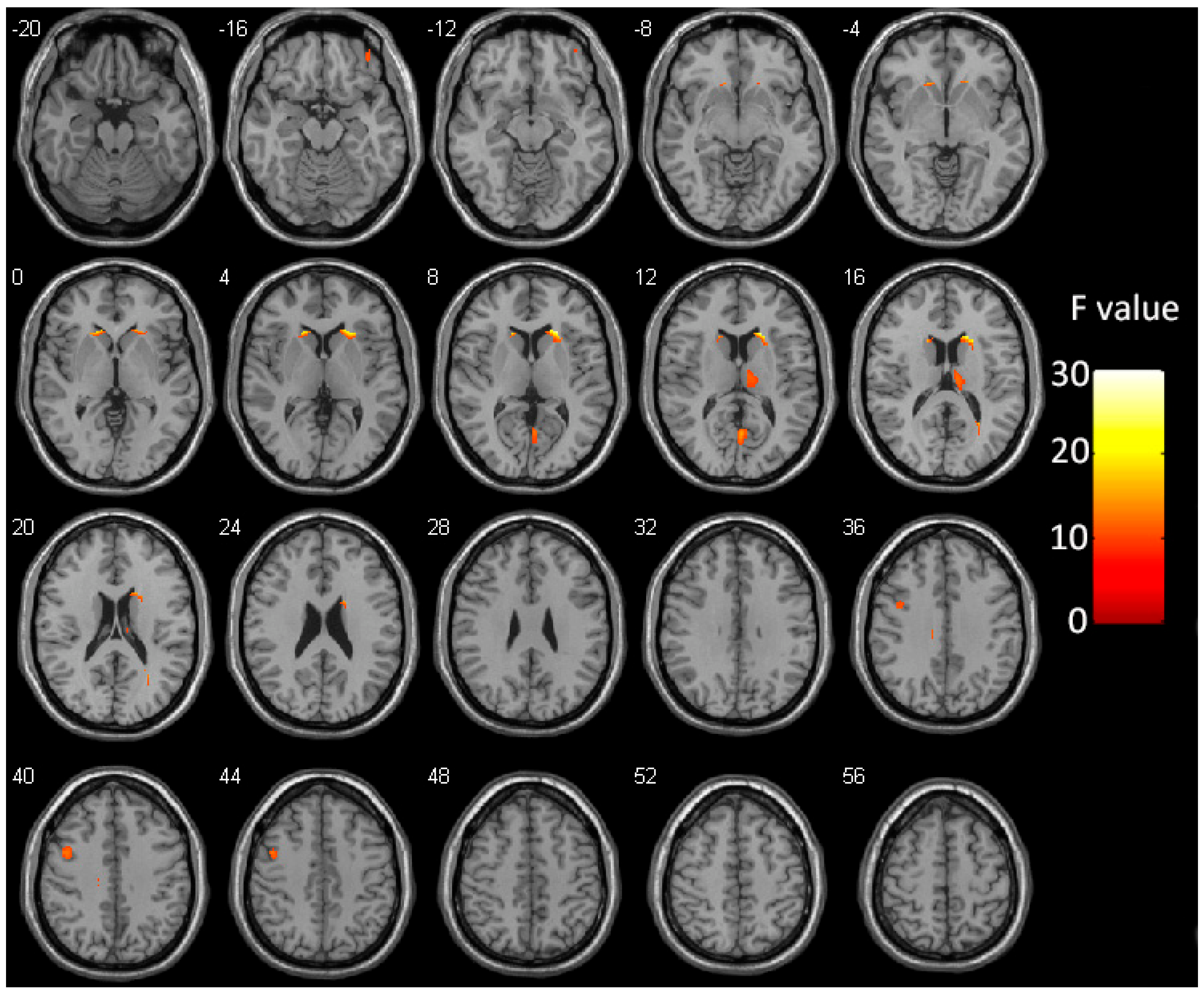
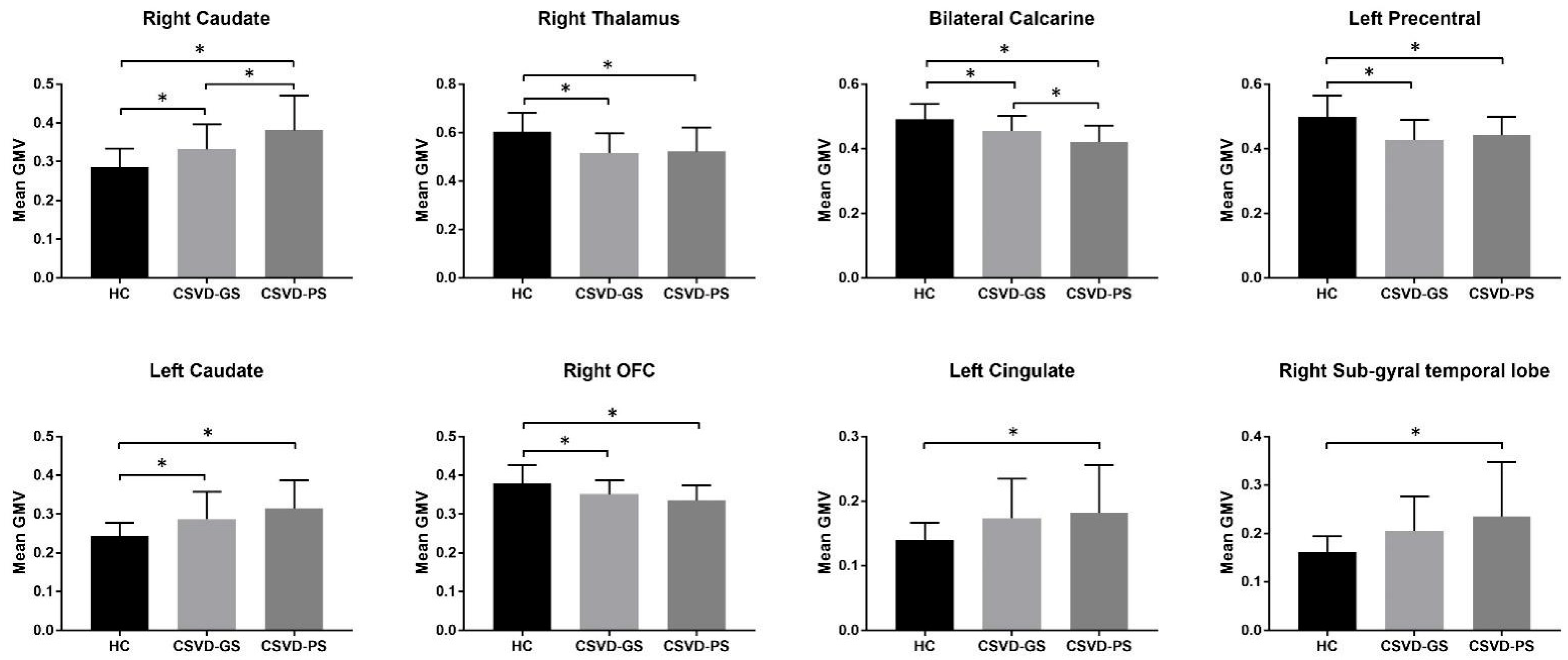
3.2. Group Differences in GMV
3.3. Associations among Sleep Quality, GMV, and WMH in CSVD Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wardlaw, J.M.; Smith, C.; Dichgans, M. Small vessel disease: Mechanisms and clinical implications. Lancet Neurol. 2019, 18, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, J.M.; Smith, E.E.; Biessels, G.J.; Cordonnier, C.; Fazekas, F.; Frayne, R.; Lindley, R.I.; O’Brien, J.T.; Barkhof, F.; Benavente, O.R.; et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013, 12, 822–838. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, J.M. Prevalence of cerebral white matter lesions in elderly people: A population based magnetic resonance imaging study: The Rotterdam Scan Study. J. Neurol. Neurosurg. Psychiatry 2001, 70, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Kong, Q.; Wang, M.; Huang, H.; Zhou, X.; Guo, Y.; Zhang, Y.; Wu, L.; Yu, Z.; Luo, X. Association of Excessive Daytime Sleepiness with Cerebral Small Vessel Disease in Community-Dwelling Older Adults. Nat. Sci. Sleep 2022, 14, 765–773. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X.; Liao, J.; Zhou, L.; Liao, S.; Shan, Y.; Lu, Z.; Tao, J. The influence of non-breathing-related sleep fragmentation on cognitive function in patients with cerebral small vessel disease. Neuropsychiatr. Dis. Treat. 2019, 15, 1009–1014. [Google Scholar] [CrossRef]
- Del Brutto, O.H.; Mera, R.M.; Zambrano, M.; Lama, J.; Del Brutto, V.J.; Castillo, P.R. Poor sleep quality and silent markers of cerebral small vessel disease: A population-based study in community-dwelling older adults (The Atahualpa Project). Sleep Med. 2015, 16, 428–431. [Google Scholar] [CrossRef]
- Chokesuwattanaskul, A.; Lertjitbanjong, P.; Thongprayoon, C.; Bathini, T.; Sharma, K.; Mao, M.A.; Cheungpasitporn, W.; Chokesuwattanaskul, R. Impact of obstructive sleep apnea on silent cerebral small vessel disease: A systematic review and meta-analysis. Sleep Med. 2020, 68, 80–88. [Google Scholar] [CrossRef]
- Kang, M.K.; Koo, D.L.; Shin, J.H.; Kwon, H.-M.; Nam, H. Association between periodic limb movements during sleep and cerebral small vessel disease. Sleep Med. 2018, 51, 47–52. [Google Scholar] [CrossRef]
- Phillips, B.; Ancoli-Israel, S. Sleep disorders in the elderly. Sleep Med. 2001, 2, 99–114. [Google Scholar] [CrossRef]
- Gulia, K.K.; Kumar, V.M. Sleep disorders in the elderly: A growing challenge. Psychogeriatrics 2018, 18, 155–165. [Google Scholar] [CrossRef]
- Kaur, S.; Banerjee, N.; Miranda, M.; Slugh, M.; Sun-Suslow, N.; McInerney, K.F.; Sun, X.; Ramos, A.R.; Rundek, T.; Sacco, R.L.; et al. Sleep quality mediates the relationship between frailty and cognitive dysfunction in non-demented middle aged to older adults. Int. Psychogeriatr. 2020, 32, 663. [Google Scholar] [CrossRef]
- Del Brutto, O.H.; Mera, R.M.; Del Brutto, V.J.; Castillo, P.R. Enlarged basal ganglia perivascular spaces and sleep parameters. A population-based study. Clin. Neurol. Neurosurg. 2019, 182, 53–57. [Google Scholar] [CrossRef]
- Kanda, A.; Matsui, T.; Ebihara, S.; Arai, H.; Sasaki, H. Periventricular white matter lesions and sleep alteration in older people. J. Am. Geriatr. Soc. 2003, 51, 432–433. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, D.-M.; Zhang, C.; Zhang, Y.; Wang, C.; Zhang, B.; Zhao, W.; Zhu, J.; Yu, Y. Brain Structural and Functional Alterations Specific to Low Sleep Efficiency in Major Depressive Disorder. Front. Neurosci. 2020, 14, 50. [Google Scholar] [CrossRef]
- Ashburner, J.; Friston, K.J. Voxel-based morphometry—The methods. Neuroimage 2000, 11 Pt 1, 805–821. [Google Scholar] [CrossRef]
- Zhang, M.; Yu, H.; Tang, W.; Ding, D.; Tang, J.; Liu, N.; Xue, Y.; Ren, X.; Shi, L.; Fu, J. Decreased nocturnal heart rate variability and potentially related brain regions in arteriosclerotic cerebral small vessel disease. BMC Neurol. 2021, 21, 361. [Google Scholar] [CrossRef]
- Liu, R.; Chen, H.; Qin, R.; Gu, Y.; Chen, X.; Zou, J.; Jiang, Y.; Li, W.; Bai, F.; Zhang, B.; et al. The Altered Reconfiguration Pattern of Brain Modular Architecture Regulates Cognitive Function in Cerebral Small Vessel Disease. Front. Neurol. 2019, 10, 324. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Lund, H.G.; Reider, B.D.; Whiting, A.B.; Prichard, J.R. Sleep Patterns and Predictors of Disturbed Sleep in a Large Population of College Students. J. Adolesc. Health 2010, 46, 124–132. [Google Scholar] [CrossRef]
- Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef]
- Fazekas, F.; Chawluk, J.B.; Alavi, A.; Hurtig, H.I.; Zimmerman, R.A. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am. J. Roentgenol. 1987, 149, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Huang, H.; Yang, S.; Luo, X.; Zhu, W.; Xu, S.; Meng, Q.; Zuo, C.; Liu, Y.; Wang, W.; et al. Cortical and Subcortical Grey Matter Abnormalities in White Matter Hyperintensities and Subsequent Cognitive Impairment. Neurosci. Bull. 2021, 37, 789–803. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liang, Y.; Chen, H.; Wang, W.; Wang, Y.; Liang, Y.; Zhang, Y. Structural changes in white matter lesion patients and their correlation with cognitive impairment. Neuropsychiatr. Dis. Treat. 2019, 15, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.-Q.; Peng, Y.; Li, S.-H.; Liu, X.; Zhuang, Y.; Wu, L.; Gong, H.; Liu, D. Density abnormalities in normal-appearing gray matter in the middle-aged brain with white matter hyperintense lesions: A DARTEL-enhanced voxel-based morphometry study. Clin. Interv. Aging 2016, 11, 615–622. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, C.; Li, L.; Zhang, Y.; Zhang, W.; Yin, W.; Yu, X.; Zhu, X.; Qian, Y.; Sun, Z. Altered Brain Function in Cerebral Small Vessel Disease Patients with Gait Disorders: A Resting-State Functional MRI Study. Front. Aging Neurosci. 2020, 12, 234. [Google Scholar] [CrossRef]
- Porter, J.N.; Roy, A.K.; Benson, B.; Carlisi, C.; Collins, P.F.; Leibenluft, E.; Pine, D.S.; Luciana, M.; Ernst, M. Age-related changes in the intrinsic functional connectivity of the human ventral vs. dorsal striatum from childhood to middle age. Dev. Cogn. Neurosci. 2015, 11, 83–95. [Google Scholar] [CrossRef]
- Dong, H.; Chen, Z.-K.; Guo, H.; Yuan, X.-S.; Liu, C.-W.; Qu, W.-M.; Huang, Z.-L. Striatal neurons expressing dopamine D1 receptor promote wakefulness in mice. Curr. Biol. 2022, 32, 600–613.e4. [Google Scholar] [CrossRef]
- Villablanca, J.R.; Marcus, R.J.; Olmstead, C.E. Effects of caudate nuclei or frontal cortical ablations in cats. I. Neurology and gross behavior. Exp. Neurol. 1976, 52, 389–420. [Google Scholar] [CrossRef]
- Chkhenkeli, S.A.; Šramka, M.; Lortkipanidze, G.S.; Rakviashvili, T.N.; Bregvadze, E.S.; Magalashvili, G.E.; Gagoshidze, T.S.; Chkhenkeli, I.S. Electrophysiological effects and clinical results of direct brain stimulation for intractable epilepsy. Clin. Neurol. Neurosurg. 2004, 106, 318–329. [Google Scholar] [CrossRef]
- Jalbrzikowski, M.; Hayes, R.A.; Scully, K.E.; Franzen, P.L.; Hasler, B.P.; Siegle, G.J.; Buysse, D.J.; Dahl, R.E.; Forbes, E.E.; Ladouceur, C.D.; et al. Associations between brain structure and sleep patterns across adolescent development. Sleep 2021, 44, zsab120. [Google Scholar] [CrossRef]
- Grau-Rivera, O.; for the ALFA Study; Operto, G.; Falcón, C.; Sánchez-Benavides, G.; Cacciaglia, R.; Brugulat-Serrat, A.; Gramunt, N.; Salvadó, G.; Suárez-Calvet, M.; et al. Association between insomnia and cognitive performance, gray matter volume, and white matter microstructure in cognitively unimpaired adults. Alzheimer’s Res. Ther. 2020, 12, 1–14. [Google Scholar] [CrossRef]
- Sanjari Moghaddam, H.; Mohammadi, E.; Dolatshahi, M.; Mohebi, F.; Ashrafi, A.; Khazaie, H.; Aarabi, M.H. White matter microstructural abnormalities in primary insomnia: A systematic review of diffusion tensor imaging studies. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 105, 110132. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, M.; Zeng, S.; Ma, X.; Yan, J.; Lin, C.; Xu, G.; Li, G.; Yin, Y.; Fu, S.; et al. Abnormal Topology of the Structural Connectome in the Limbic Cortico-Basal-Ganglia Circuit and Default-Mode Network Among Primary Insomnia Patients. Front. Neurosci. 2018, 12, 860. [Google Scholar] [CrossRef]
- Jespersen, K.V.; Stevner, A.; Fernandes, H.; Sørensen, S.D.; Van Someren, E.; Kringelbach, M.; Vuust, P. Reduced structural connectivity in Insomnia Disorder. J. Sleep Res. 2020, 29, e12901. [Google Scholar] [CrossRef]
- Shao, Z.; Xu, Y.; Chen, L.; Wang, S.; Zhang, M.; Liu, S.; Wen, X.; Yu, D.; Yuan, K. Dysfunction of the NAc-mPFC circuit in insomnia disorder. Neuroimage Clin. 2020, 28, 102474. [Google Scholar] [CrossRef]
- Ding, K.; Liu, Y.; Yan, X.; Lin, X.; Jiang, T. Altered Functional Connectivity of the Primary Visual Cortex in Subjects with Amblyopia. Neural Plast. 2013, 2013, 612086. [Google Scholar] [CrossRef]
- Levenson, J.C.; Kay, D.B.; Buysse, D.J. The Pathophysiology of Insomnia. Chest 2015, 147, 1179–1192. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, N.; Yoo, J.; Kim, H.-K.; Son, Y.-D.; Kim, Y.-B.; Oh, S.M.; Kim, S.; Lee, H.; Jeon, J.E.; et al. Multitask fMRI and machine learning approach improve prediction of differential brain activity pattern in patients with insomnia disorder. Sci. Rep. 2021, 11, 9402. [Google Scholar] [CrossRef]
- Tao, S.W.; Chattun, M.R.; Yan, R.; Geng, J.T.; Zhu, R.X.; Shao, J.N.; Lu, Q.; Yao, Z.J. TPH-2 Gene Polymorphism in Major Depressive Disorder Patients with Early-Wakening Symptom. Front. Neurosci. 2018, 12, 827. [Google Scholar] [CrossRef]
- Ran, Q.; Chen, J.; Li, C.; Wen, L.; Yue, F.; Shu, T.; Mi, J.; Wang, G.; Zhang, L.; Gao, D.; et al. Abnormal amplitude of low-frequency fluctuations associated with rapid-eye movement in chronic primary insomnia patients. Oncotarget 2017, 8, 84877–84888. [Google Scholar] [CrossRef]
- Plante, D.T.; Jensen, J.E.; Schoerning, L.; Winkelman, J.W. Reduced gamma-aminobutyric acid in occipital and anterior cingulate cortices in primary insomnia: A link to major depressive disorder? Neuropsychopharmacology 2012, 37, 1548–1557. [Google Scholar] [CrossRef]
- McKinnon, A.C.; Hickie, I.B.; Scott, J.; Duffy, S.L.; Norrie, L.; Terpening, Z.; Grunstein, R.R.; Lagopoulos, J.; Batchelor, J.; Lewis, S.J.; et al. Current sleep disturbance in older people with a lifetime history of depression is associated with increased connectivity in the Default Mode Network. J. Affect. Disord. 2018, 229, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.L.; Bajada, C.J.; Rice, G.E.; Cloutman, L.L.; Ralph, M.A.L. An emergent functional parcellation of the temporal cortex. Neuroimage 2018, 170, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Parra, M.A.; Pattan, V.; Wong, D.; Beaglehole, A.; Lonie, J.; Wan, H.I.; Honey, G.; Hall, J.; Whalley, H.C.; Lawrie, S.M. Medial temporal lobe function during emotional memory in early Alzheimer’s disease, mild cognitive impairment and healthy ageing: An fMRI study. BMC Psychiatry 2013, 13, 76. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.K.; Shi, X.H.; Wang, Y.Y.; Zhang, X.; Liu, H.Y.; Wang, X.T.; Mang, J.; Xu, Z.-X. Evaluation of the age-related and gender-related differences in patients with primary insomnia by fractional amplitude of low-frequency fluctuation: A resting-state functional magnetic resonance imaging study. Medicine 2020, 99, e18786. [Google Scholar] [CrossRef] [PubMed]
- Jingliang, C.; Liu, L.; Wang, E.; Zhang, H.; Dou, S.; Tong, L.; Cheng, J.; Chen, C.; Shi, D. Abnormal Neural Network of Primary Insomnia: Evidence from Spatial Working Memory Task fMRI. Eur. Neurol. 2016, 75, 48–57. [Google Scholar]
- Dou, S.; Wang, E.; Zhang, H.; Tong, L.; Zhang, X.; Shi, D.; Cheng, J.; Li, Y. Application of spatial working memory task fMRI in evaluation of primary insomnia patient’s cognitive dysfunction. Zhonghua Yi Xue Za Zhi 2015, 95, 1677–1680. [Google Scholar]
- Li, S.; Wang, B.A.; Li, C.; Feng, Y.; Li, M.; Wang, T.; Nie, L.; Li, C.; Hua, W.; Lin, C.; et al. Progressive gray matter hypertrophy with severity stages of insomnia disorder and its relevance for mood symptoms. Eur. Radiol. 2021, 31, 6312–6322. [Google Scholar] [CrossRef]
- Bellesi, M.; Pfister-Genskow, M.; Maret, S.; Keles, S.; Tononi, G.; Cirelli, C. Effects of Sleep and Wake on Oligodendrocytes and Their Precursors. J. Neurosci. 2013, 33, 14288–14300. [Google Scholar] [CrossRef]
- Benedetti, F.; Melloni, E.M.; Dallaspezia, S.; Bollettini, I.; Locatelli, C.; Poletti, S.; Colombo, C. Night sleep influences white matter microstructure in bipolar depression. J. Affect. Disord. 2017, 218, 380–387. [Google Scholar] [CrossRef]
- Bellesi, M. Sleep and Oligodendrocyte Functions. Curr. Sleep Med. Rep. 2015, 1, 20–26. [Google Scholar] [CrossRef]
- Grumbach, P.; Opel, N.; Martin, S.; Meinert, S.; Leehr, E.J.; Redlich, R.; Enneking, V.; Goltermann, J.; Baune, B.T.; Dannlowski, U.; et al. Sleep duration is associated with white matter microstructure and cognitive performance in healthy adults. Hum. Brain Mapp. 2020, 41, 4397–4405. [Google Scholar] [CrossRef]
- Falgàs, N.; Illán-Gala, I.; Allen, I.E.; Mumford, P.; Essanaa, Y.M.; Le, M.M.; You, M.; Grinberg, L.T.; Rosen, H.J.; Neylan, T.C.; et al. Specific cortical and subcortical grey matter regions are associated with insomnia severity. PLoS ONE 2021, 16, e0252076. [Google Scholar] [CrossRef]
- Mackiewicz, M.; Shockley, K.R.; Romer, M.A.; Galante, R.J.; Zimmerman, J.E.; Naidoo, N.; Baldwin, D.A.; Jensen, S.T.; Churchill, G.A.; Pack, A.I. Macromolecule biosynthesis: A key function of sleep. Physiol. Genom. 2007, 31, 441–457. [Google Scholar] [CrossRef]
- Cirelli, C.; Gutierrez, C.M.; Tononi, G. Extensive and Divergent Effects of Sleep and Wakefulness on Brain Gene Expression. Neuron 2004, 41, 35–43. [Google Scholar] [CrossRef]
- Cheng, C.-Y.; Tsai, C.-F.; Wang, S.-J.; Hsu, C.-Y.; Fuh, J.-L. Sleep Disturbance Correlates with White Matter Hyperintensity in Patients with Subcortical Ischemic Vascular Dementia. J. Geriatr. Psychiatry Neurol. 2013, 26, 158–164. [Google Scholar] [CrossRef]
- Lee, S.-J.; Kim, J.-S.; Song, I.-U.; An, J.-Y.; Kim, Y.-I.; Lee, K.-S. Poststroke restless legs syndrome and lesion location: Anatomical considerations. Mov. Disord. 2009, 24, 77–84. [Google Scholar] [CrossRef]
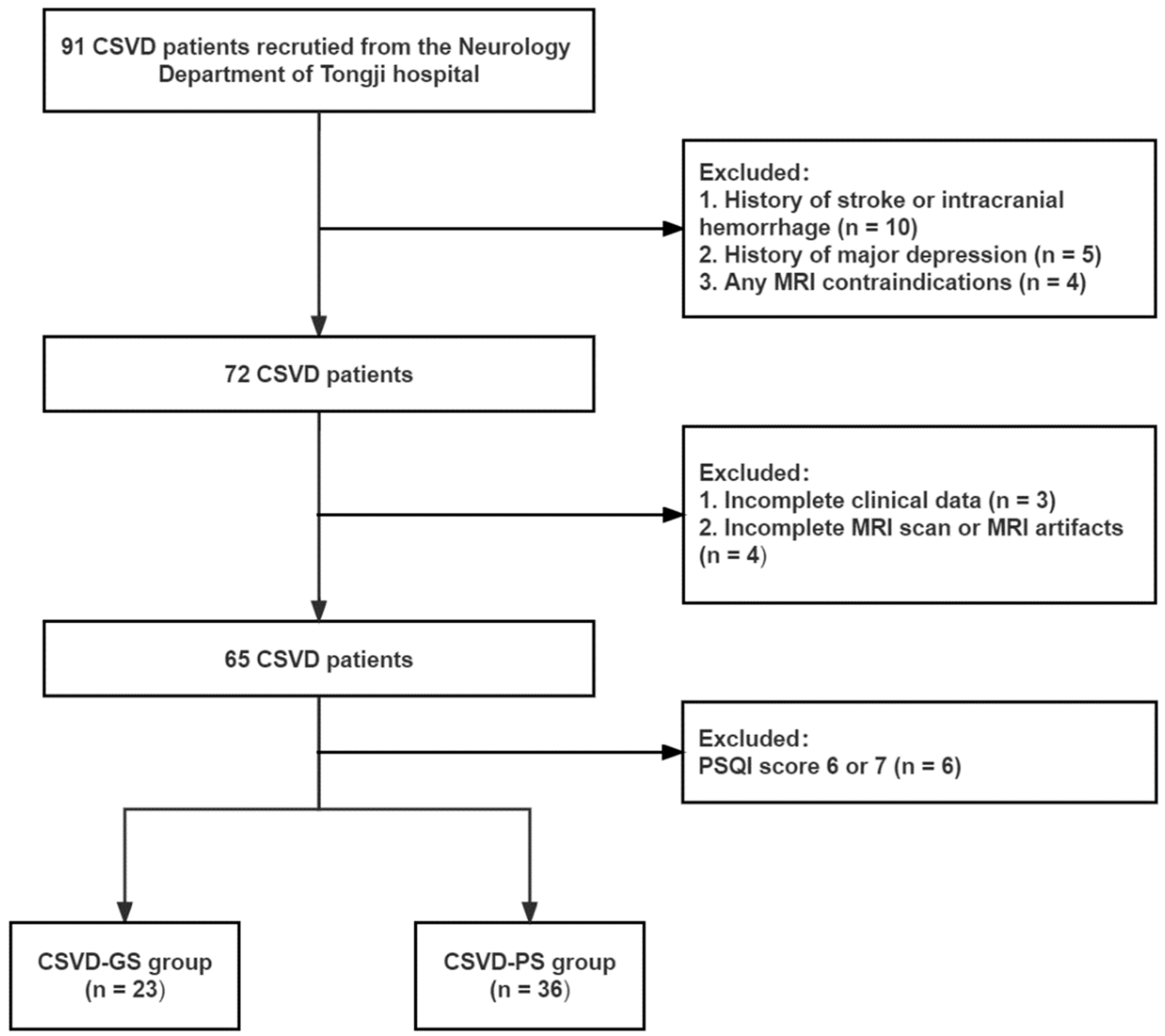
| Characteristics | HC (n = 40) | CSVD-GS (n = 23) | CSVD-PS (n = 36) | Overall p Value |
|---|---|---|---|---|
| Age (years) | 61.53 ± 6.07 | 64.91 ± 6.40 | 64.31 ± 7.09 | 0.079 |
| Male, n (%) | 25 (62.5%) | 15 (65.2%) | 16 (44.4%) | 0.180 |
| Education (years) | 9.73 ± 3.87 | 9.65 ± 4.58 | 9.03 ± 4.16 | 0.740 |
| Hypertension, n (%) | 17 (42.5%) | 14 (60.9%) | 23 (63.9%) | 0.137 |
| Diabetes, n (%) | 7 (17.5%) | 5 (21.7%) | 8 (22.2%) | 0.854 |
| Hyperlipidemia, n (%) | 8 (20.0%) | 8 (34.8%) | 12 (33.3%) | 0.319 |
| Smoking status, n (%) | 11 (27.5%) | 7 (30.4%) | 8 (22.2%) | 0.840 |
| PSQI | 3.88 ± 1.28 | 3.61 ± 1.16 | 12.36 ± 2.97 | <0.001 a,b |
| HAMD | 4.60 ± 2.81 | 3.43 ± 3.22 | 6 ± 2.11 | 0.002 a,b |
| TIV (cm3) | 1524.14 ± 138.74 | 1528.61 ± 115.41 | 1520.06 ± 160.02 | 0.975 |
| Total Fazekas score | 1.08 ± 0.83 | 4.39 ± 1.12 | 4.64 ± 1.20 | <0.001 a,c |
| Total WMH volume (cm3) | 1.12 ± 1.37 | 15.99 ± 4.51 | 22.10 ± 22.63 | <0.001 a,c |
| Lacunes, n (%) | 0 | 6 (26.1%) | 10 (27.8%) | <0.001 a,c |
| Microbleeds, n (%) | 0 | 8 (34.8%) | 14 (38.9%) | <0.001 a,c |
| >10 EPVS_BG, n (%) | 2 (5.0%) | 8 (34.8%) | 15 (41.7%) | <0.001 a,c |
| Brain Region | Hemisphere | Cluster Size | MNI Coordinates | Peak F Values | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Caudate | R | 387 | 21 | 22.5 | 10.5 | 28.14 |
| Thalamus | R | 282 | 9 | −16.5 | 15 | 12.24 |
| Calcarine cortex | Bilateral | 198 | 1.5 | −64.5 | 10.5 | 15.34 |
| Precentral gyrus | L | 177 | −46.5 | 4.5 | 42 | 12.84 |
| Caudate | L | 148 | −18 | 24 | 4.5 | 21.13 |
| Orbitofrontal cortex | R | 61 | 37.5 | 45 | −18 | 11.16 |
| Cingulate gyrus | L | 58 | −12 | −24 | 33 | 12.57 |
| Sub-gyral of the temporal lobe | R | 45 | 27 | −55.5 | 18 | 16.86 |
| Independent Variable | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | |
| Right Caudate | 0.399 (0.147; 0.650) | 0.002 | 0.362 (0.128; 0.595) | 0.003 | 0.315 (0.048; 0.583) | 0.022 |
| Right Thalamus | −0.156 (−0.449; 0.137) | 0.290 | −0.075 (−0.352; 0.202) | 0.588 | −0.045 (−0.317; 0.227) | 0.740 |
| Bilateral Calcarine cortex | −0.421 (−0.672; −0.171) | 0.001 | −0.397 (−0.627; −0.167) | 0.001 | −0.354 (−0.598; −0.109) | 0.005 |
| Left Precentral gyrus | 0.240 (−0.043; 0.523) | 0.095 | 0.232 (−0.028; 0.492) | 0.079 | 0.216 (−0.038; 0.470) | 0.094 |
| Left Caudate | 0.301 (0.039; 0.564) | 0.025 | 0.260 (0.015; 0.505) | 0.038 | 0.187 (−0.084; 0.457) | 0.172 |
| Right Orbitofrontal cortex | −0.089 (−0.383; 0.204) | 0.544 | −0.064 (−0.335; 0.207) | 0.638 | −0.086 (−0.351; 0.178) | 0.517 |
| Left Cingulate gyrus | 0.321 (−0.009; 0.650) | 0.056 | 0.282 (−0.023; 0.588) | 0.069 | 0.161 (−0.204; 0.526) | 0.380 |
| Right Sub-gyral of the temporal lobe | 0.399 (0.094; 0.704) | 0.011 | 0.261 (−0.050; 0.571) | 0.098 | 0.142 (−0.210; 0.495) | 0.421 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Kong, Q.; Zhou, X.; Zhang, Y.; Yu, Z.; Qu, W.; Huang, H.; Luo, X. Differences in Gray Matter Volume in Cerebral Small Vessel Disease Patients with and without Sleep Disturbance. Brain Sci. 2023, 13, 294. https://doi.org/10.3390/brainsci13020294
Zhao J, Kong Q, Zhou X, Zhang Y, Yu Z, Qu W, Huang H, Luo X. Differences in Gray Matter Volume in Cerebral Small Vessel Disease Patients with and without Sleep Disturbance. Brain Sciences. 2023; 13(2):294. https://doi.org/10.3390/brainsci13020294
Chicago/Turabian StyleZhao, Jing, Qianqian Kong, Xirui Zhou, Yi Zhang, Zhiyuan Yu, Wensheng Qu, Hao Huang, and Xiang Luo. 2023. "Differences in Gray Matter Volume in Cerebral Small Vessel Disease Patients with and without Sleep Disturbance" Brain Sciences 13, no. 2: 294. https://doi.org/10.3390/brainsci13020294
APA StyleZhao, J., Kong, Q., Zhou, X., Zhang, Y., Yu, Z., Qu, W., Huang, H., & Luo, X. (2023). Differences in Gray Matter Volume in Cerebral Small Vessel Disease Patients with and without Sleep Disturbance. Brain Sciences, 13(2), 294. https://doi.org/10.3390/brainsci13020294






