Abstract
Repetitive transcranial magnetic stimulation (rTMS) with intensive occupational therapy improves upper limb motor paralysis and activities of daily living after stroke; however, the degree of improvement according to paralysis severity remains unverified. Target activities of daily living using upper limb functions can be established by predicting the amount of change after treatment for each paralysis severity level to further aid practice planning. We estimated post-treatment score changes for each severity level of motor paralysis (no, poor, limited, notable, and full), stratified according to Action Research Arm Test (ARAT) scores before combined rTMS and intensive occupational therapy. Motor paralysis severity was the fixed factor for the analysis of covariance; the delta (post-pre) of the scores was the dependent variable. Ordinal logistic regression analysis was used to compare changes in ARAT subscores according to paralysis severity before treatment. We implemented a longitudinal, prospective, interventional, uncontrolled, and multicenter cohort design and analyzed a dataset of 907 patients with stroke hemiplegia. The largest treatment-related changes were observed in the Limited recovery group for upper limb motor paralysis and the Full recovery group for quality-of-life activities using the paralyzed upper limb. These results will help predict treatment effects and determine exercises and goal movements for occupational therapy after rTMS.
1. Introduction
Motor paralysis after stroke limits patients’ activities of daily living (ADL) and reduces their quality of life [1,2]. Recently, noninvasive brain stimulation therapy has been developed to improve patients’ motor paralysis and ADL, and its effectiveness has been demonstrated [3,4]. The treatment of upper limb motor paralysis involves modulation of interhemispheric inhibition and induction of neuroplasticity in the cerebrum. A novel intervention using repetitive transcranial magnetic stimulation (rTMS) in combination with intensive occupational therapy (NEURO) has recently been developed [5]. In patients with stroke hemiplegia, high-frequency rTMS has been applied to the hemisphere ipsilateral to the paralysis to increase excitability [6], and low-frequency rTMS has been applied to the contralateral hemisphere to decrease interhemispheric inhibitory connections [7,8] with the damaged cortex [9]; thus, both high-frequency rTMS and low-frequency rTMS have been applied [10]. Repetitive currents are induced in the brain cortex to produce long-term changes in cortical excitability. In acute patients, high-frequency (10 Hz) rTMS applied to the impaired motor cortex activates it, improving paralysis [11,12]. In occupational therapy after rTMS, the patients in whom the activation of the interhemispheric inhibitory motor cortex has been adjusted are prescribed repetitive joint movements. The aim is to promote use-dependent plasticity in the brain and to subsequently restore motor paralysis and improve ADL [13]. NEURO is an effective treatment for improving upper limb dysfunction and impairments in ADL in chronic stroke patients 6 months after stroke onset. Its therapeutic effect has been shown to be unaffected by stroke type (cerebral hemorrhage or cerebral infarction) [14].
The goal of NEURO is to improve the quality of movement of the patient’s paralyzed upper limb by allowing it to be used in ADL. Since the effectiveness of NEURO depends on the severity of motor paralysis, therapists determine the exercises and target movements based on the patient’s pre-treatment upper limb function assessment score. The Fugl–Meyer Assessment of the Upper Extremity (FMAUE) and the Action Research Arm Test (ARAT) are used to assess upper limb motor function outcomes in NEURO [15]. These evaluation methods have been shown to have high accuracy and clinical usefulness. A previous study has been conducted to estimate post-treatment scores from the pre-NEURO FMAUE score [16]. The ARAT is a functional upper limb assessment tool used in patients with post-stroke hemiplegia and is characterized by its ability to reflect the patient’s activity [17]. Since the ARAT consists of object manipulation and reaching tasks, the occupational therapist (OT) plans exercises by estimating the ADLs in which the patient can use their hands based on the obtained assessment results. As the ARAT score correlates with the Motor Activity Log, which investigates the use of the paralyzed limb in ADLs, OTs helping patients improve their activity limitations can use it as a reference value for exercises and goal-setting [18,19]. Therefore, it can be inferred that predicting treatment effects with ARAT is more advantageous than using FMAUE in setting treatment goals and planning effective ADL exercises for patients. If ARAT scores are found to improve with NEURO, it will be easier for OTs to pre-determine the content of ADL exercises and develop achievable ADL goals.
Patients with mild-to-moderate motor paralysis with FMAUE scores ≥43 have higher interhemispheric inhibition from the healthy hemisphere to the affected hemisphere. It is predicted that the therapeutic effect of upper limb practice in the presence of rTMS-induced changes in synaptic transmission efficiency is dependent on motor paralysis severity [20]. If the post-treatment effects according to motor paralysis severity can be predicted using pre-treatment ARAT scores, the target movements for patients could be set with high accuracy. Recently, a treatment method using a brain-computer interface (BCI) was developed for the rehabilitation of stroke patients, and its effectiveness has been reported [21,22]. Even for new intervention methods, it is better to formulate exercises adapted to the severity of paralysis and recovery. Therefore, the results obtained in this study can be used as data to plan the most appropriate practice for patients in terms of future new intervention methods. As a result, this study aimed to estimate the amount of change in ARAT scores for each level of motor paralysis severity, classified according to the ARAT score before NEURO.
2. Materials and Methods
2.1. Study Design
In this multicenter, longitudinal, prospective, interventional, uncontrolled study, we reviewed the medical records of patients with stroke from February 2017 to March 2021 from 6 different hospitals in Japan certified as NEURO implementation facilities. These included Izumi Memorial Hospital, Shimizu Hospital, Nishi-Hiroshima Rehabilitation Hospital, Tokyo General Hospital, Kyoto O’Hara Memorial Hospital, and Tokyo Jikei University Hospital. Izumi Memorial Hospital is located in Tokyo and is certified as a community rehabilitation support center. Shimizu Hospital is located in the Tottori Prefecture, about 900 km west of Tokyo, and mainly provides orthopedic surgery and rehabilitation medicine treatments. Nishi-Hiroshima Rehabilitation Hospital is located in the Hiroshima prefecture, about 800 km west of Tokyo, and specializes in rehabilitation for patients who have passed the acute stroke phase. Tokyo General Hospital is located in Tokyo and is a general hospital with 30 departments. Kyoto O’Hara Memorial Hospital is located in the Kyoto prefecture, about 500 km west of Tokyo, and is a specialized facility for rehabilitation. Tokyo Jikei University Hospital is located in Tokyo and is a university hospital that provides advanced medical treatment. We evaluated the therapeutic effects of NEURO by providing patients with selected functional exercises based on the severity of their motor paralysis. We also attempted to define a research protocol in this study.
2.2. Ethics Statements
Patients were not required to provide informed consent because the analysis used anonymous clinical data obtained after each patient had agreed to undergo NEURO by providing written consent. This study was approved by the Jikei University School of Medicine Ethics Committee (approval number 24-295-7061).
2.3. Participants
In this study, we selected patients who presented at an accredited facility due to wishing to undergo NEURO. Patients who received NEURO according to the rTMS guidelines during the study period, who were aged ≥18 years, had been diagnosed with stroke for >12 months and had no cognitive impairment (pre-treatment Mini-Mental State Examination score >26) were included [23]. The exclusion criteria were as follows: no ARAT score data, subarachnoid hemorrhage, arteriovenous malformation, brain tumor, diagnosis of childhood paralysis, and bilateral motor paralysis.
2.4. Sample Size
The ARAT score was used as a quantitative variable, and variations in the ARAT scores among the five groups (see Statistical analysis for pre-treatment severity) were compared using G*power with analysis of covariance (ANCOVA; F-test, main effects) using the following values: effect size f = 0.25, α = 0.05, 1 − β = 0.80, number of groups = 5, and number of covariates = 5. Therefore, the total number of patients required was 242 (49 × 5 groups).
2.5. rTMS Combined with Occupational Therapy
NEURO was performed at an accredited facility in Japan, where physicians and therapists who had completed the prescribed training treated the patients according to the NEURO protocol. All patients were hospitalized for 15 days and received rTMS and occupational therapy [5]. Patients received a maximum of 6 sessions of occupational therapy per day, with each session lasting 20 min. Physiotherapy was occasionally prescribed for two to three of the 6 sessions, depending on the patient’s complaints and state of physical function. Physiotherapy mainly consisted of muscle stretching, stimulating exercises, balance exercises, ADL exercises, and gait exercises, with the goal of improving the patient’s ability to mainly walk, stand, and perform basic movements. The allocation of occupational and physical therapy sessions was determined by the physician in charge.
Occupational therapy was conducted as one-to-one training with the goal of regaining the use of the paralyzed upper limb in daily life. The OT determined the target movements together with the patient, based on the patient’s wishes and the results of the physical function assessment. To achieve the goal, the OT prescribed the following to the patients: functional exercises for the proximal and distal parts of the upper limb, skillful movement exercises using objects, daily living exercises to use the paralyzed side, lifestyle guidance to promote the use of the paralyzed side, and self-guided exercises to improve motor paralysis. The exercises were individualized, taking into account the severity of the motor paralysis and the patient’s goals. However, the main treatment modalities were as follows: for patients with severe motor paralysis and severe spasticity, muscle stretching, joint mobilization exercises, and proximal upper extremity training were prioritized to allow the patient the use of the affected hand as an adjunct in ADLs. Patients with moderate motor paralysis were given exercises to promote isolated movement and real-life activities to restore motor function and improve ADLs. Patients with mild motor paralysis were given coordination and manipulation exercises to enable them to perform more challenging ADLs requiring arm-hand coordination [13,24]. Treatments used in conjunction with occupational therapy included practice with visual stimulation of mirror images, transcutaneous electrical nerve stimulation, repetitive peripheral sensory stimulation, and muscle-tendon vibration [25,26,27,28].
Patients received rTMS daily, excluding holidays [5,13,24]. A 70-mm figure-eight coil, attached to a MagPro R100 stimulator (MagVenture Company, Farum, Denmark), was used for rTMS during each session, with one of the following methods: (1) focal 1-Hz rTMS applied to the contralesional hemisphere over the primary motor area, as described by previous studies, (2) rTMS over the hand area of the ipsilesional primary motor cortex (M1) for a duration of 30 trains of 50 pulses with 25-s intervals at 10 Hz and 90% resting motor threshold (RMT) (total, 1500 pulses/day), (3) bilateral sequential stimulation involving low-frequency (1 Hz) contralesional stimulation followed immediately by high-frequency (10 Hz) stimulation in the ipsilesional primary motor cortex, or (4) theta-burst stimulation. The protocol consisted of bursts containing 3 pulses at 50 Hz, repeated at 5 Hz intervals (20 ms between each stimulus) but applied in 2-s trains repeated every 10 s for a total of 190 s (600 pulses in total). The attending physician defined the stimulation intensity as the lowest intensity, which was set to 90% of the RMT for the first dorsal interosseous muscle.
2.6. Outcomes
The change in ARAT scores was used to assess the primary outcome. The ARAT is an upper extremity function assessment developed based on the Upper Extremity Function Test [29]. The ARAT consists of 4 subtests: grasp, grip, pinch, and gross movement [30]. For the grasp subtest, a block, cricket ball, and grinding stone are used; for the grip subtest, a glass, cylinder, and washer are used; and for the pinch subtest, a metal ball and marble are used. In these subtests, the patient moves an object to a specified position or performs a movement with an object according to the instructions. In gross movement, patients reach toward the back of their head, the top of their head, and the mouth with their hands. The ARAT is scored on a 4-point ordinal scale, wherein: 0 = unable to perform any part of the test, 1 = able to perform test partially, 2 = able to complete the test but takes an abnormally long time or has great difficulty, and 3 = able to perform test normally. With reference to a 57-point scale, ARAT scores of 0–10, 11–21, 22–42, 43–54, and 55–57 are construed to represent no, poor, limited, notable, and full recovery capacity, respectively [31]. Muscle spasticity of the paretic arm was assessed using the modified Ashworth Scale (MAS).
Changes in Jikei Assessment Scale for Motor Impairment in Daily Living (JASMID) scores were used as the secondary outcome. The JASMID is a patient-reported measure for investigating paralyzed side upper-limb use in ADL among patients with stroke hemiplegia. It is an assessment measure that has previously been validated for reliability and validity [32]. A similar assessment is the Motor Activity Log; however, the JASMID includes questions adapted to the Japanese lifestyle [33]. Patients are asked to respond to each question on a 5-point quantitative scale (0 = never, 3 = sometimes, and 5 = always) to determine the frequency of paralyzed upper limb use and on another 5-point qualitative scale (0 = almost no use, 3 = experiencing moderate difficulty, and 5 = experiencing no difficulty at all) to determine ability to use of the upper limb). The quantity and quality scores are then calculated based on the scores of a total of 20 questions.
2.7. Statistical Analysis
Patients’ ARAT scores were divided into five groups (no, poor, limited, notable, and full) according to pre-treatment severity and used as fixed factors in the ANCOVA. The severity of paralysis is a predictor of ARAT scores [31]. ARAT scores were converted from the total grasp, grip, pinch, and gross movement subscores into a delta value (post-treatment minus pre-treatment), which was used as a dependent variable in the ANCOVA. To estimate post-treatment recovery from pre-treatment paralysis severity, a multinomial logistic regression analysis was performed using delta values of ARAT and JASMID as dependent variables and pre-treatment motor paralysis severity as a predictor. Ordinal logistic regression analysis was used to compare changes in ARAT subscores in patients categorized according to the pre-treatment paralysis severity.
Recovery of upper limb motor paralysis is affected by neuromodulation with rTMS and spasticity treatment [34]. Age, sex, side of paralysis, and time since onset were included as potential confounders when comparing the effects of NEURO between groups in a previous study [35]. Therefore, we also included these factors as confounders in the covariates of the ANCOVA and ordinal logistic regression analysis. JASP 0.16 (https://jasp-stats.org/ accessed on 1 August 2022) software was used for statistical analysis. A p-value of <0.05 was considered statistically significant.
3. Results
3.1. Participants
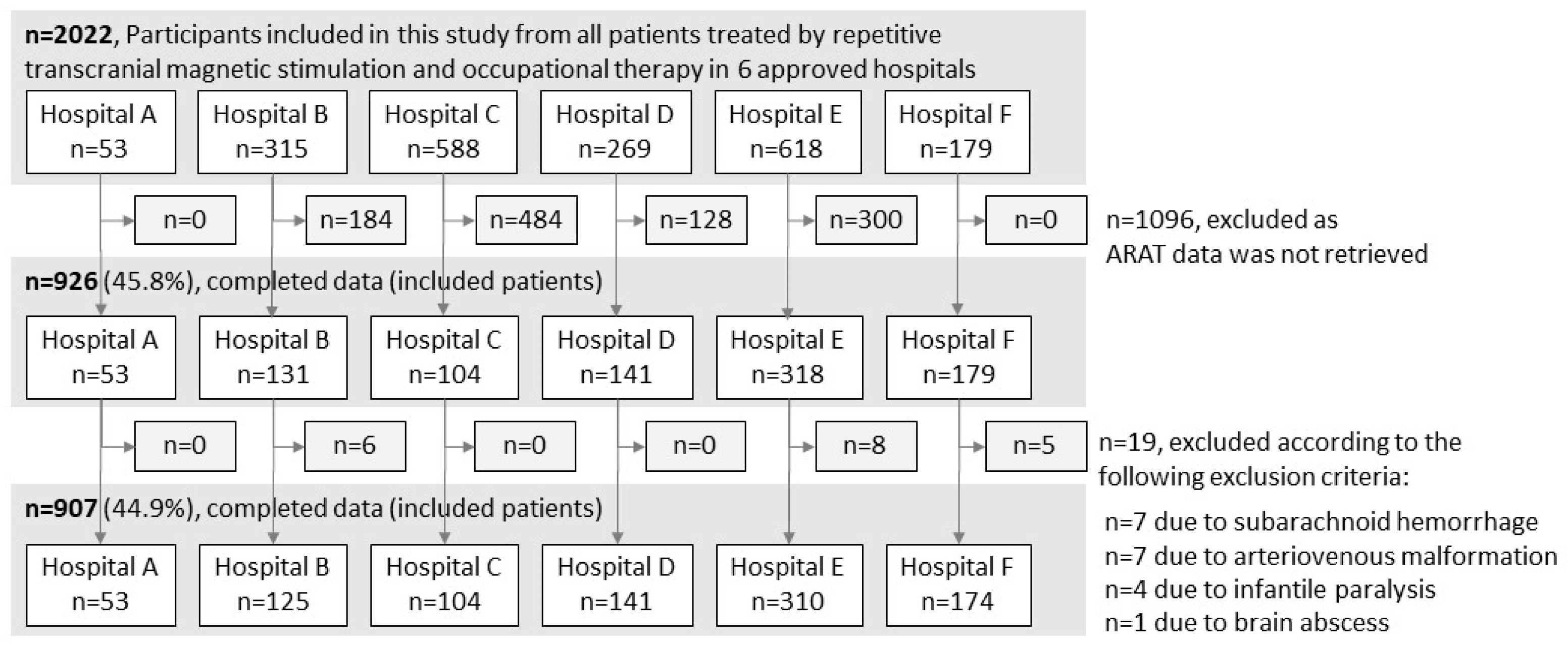
A total of 2022 patients with stroke who met the NEURO eligibility criteria were treated at the six hospitals. ARAT data were unavailable for 1096 patients, and 19 patients who met the exclusion criteria were excluded. Therefore, the final analysis included 907 patients (Figure 1). Variations in the total ARAT scores (mean ± standard deviation) before and after NEURO for each severity group were as follows: hospital A, 3.6 ± 4.2; hospital B, 1.7 ± 4.9; hospital C, 3.2 ± 4.0; hospital D, 2.8 ± 4.4; hospital E, 3.4 ± 5.1; and hospital F, 3.7 ± 5.1.
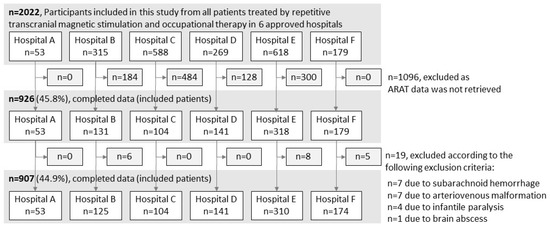
Figure 1.
Patient selection procedure. ARAT, Action Research Arm Test.
3.2. Descriptive Data
The patients’ clinical characteristics are presented in Table 1. Patients were categorized into the “No” recovery group, 275 patients (30%); “Poor” recovery group, 167 patients (18%); “Limited” recovery group, 269 patients (30%); “Notable” recovery group, 84 patients (9%), and “Full” recovery group, 112 patients (12%). rTMS included low-frequency stimulation on the intact brain hemispheres in 708 patients (77.9%), high-frequency rTMS on the lesion-side hemisphere in one patient (0.1%), and theta burst stimulation on the intact hemisphere in 198 patients (21.8%). Seven patients (0.8%) were treated with botulinum toxin A or xylocaine during NEURO.

Table 1.
Clinical characteristics of analyzed patients.
3.3. Outcome Data
The patients’ clinical characteristics according to ARAT severity classifications are presented in Table 2. The total ARAT scores (median (25th, 75th percentile)) before treatment were 4 (3, 6) in the No recovery group, 16 (13, 18) in the Poor recovery group, 30 (26, 36) in the Limited recovery group, 48 (45, 51) in the Notable recovery group, and 57 (56, 57) in the Full recovery group. The JASMID quantity and quality scores were highest in the Full recovery and lowest in the No recovery groups. The results of changes in ARAT and JASMID scores for patients classified by ARAT severity are shown in Table 3.

Table 2.
Characteristics of analyzed patients according to ARAT severity classification.

Table 3.
Outcome scores according to ARAT severity classification.
3.4. Main Results
Delta values of the total ARAT scores before and after NEURO were analyzed according to the severity of paralysis using ANCOVA. The results are displayed in Table 4. The delta values (mean ± standard deviation) were 2.4 ± 4.2 in the No recovery group, 3.9 ± 4.8 in the Poor recovery group, 4.6 ± 5.8 in the Limited recovery group, 3.0 ± 4.2 in the Notable recovery group, and 0.3 ± 1.3 in the Full recovery group (Table 3), indicating a significant main effect by group (F = 18.677, p < 0.001, η2 = 0.077) age, sex, laterality in the paretic and dominant hands, and time since onset were adjusted for as covariates. This main effect remained unchanged when adjusted for the pre-treatment ARAT total score, indicating a significant main effect (F = 18.545, p < 0.001, η2 = 0.076). A post-test for the change in total ARAT scores showed that the Limited and Notable recovery groups displayed a more significant change in total ARAT scores than the Full recovery group (p = 0.024 and p = 0.022). Because muscle spasticity influenced upper extremity paraplegia recovery, the patients’ pretreatment data were reanalyzed with the main outcome as a covariate, and this did not affect the results (MASelbow; F = 2.278, p = 0.132, η2 = 0.002).

Table 4.
Differences between pre- and post-treatment ARAT and JASMID scores using analysis of covariance.
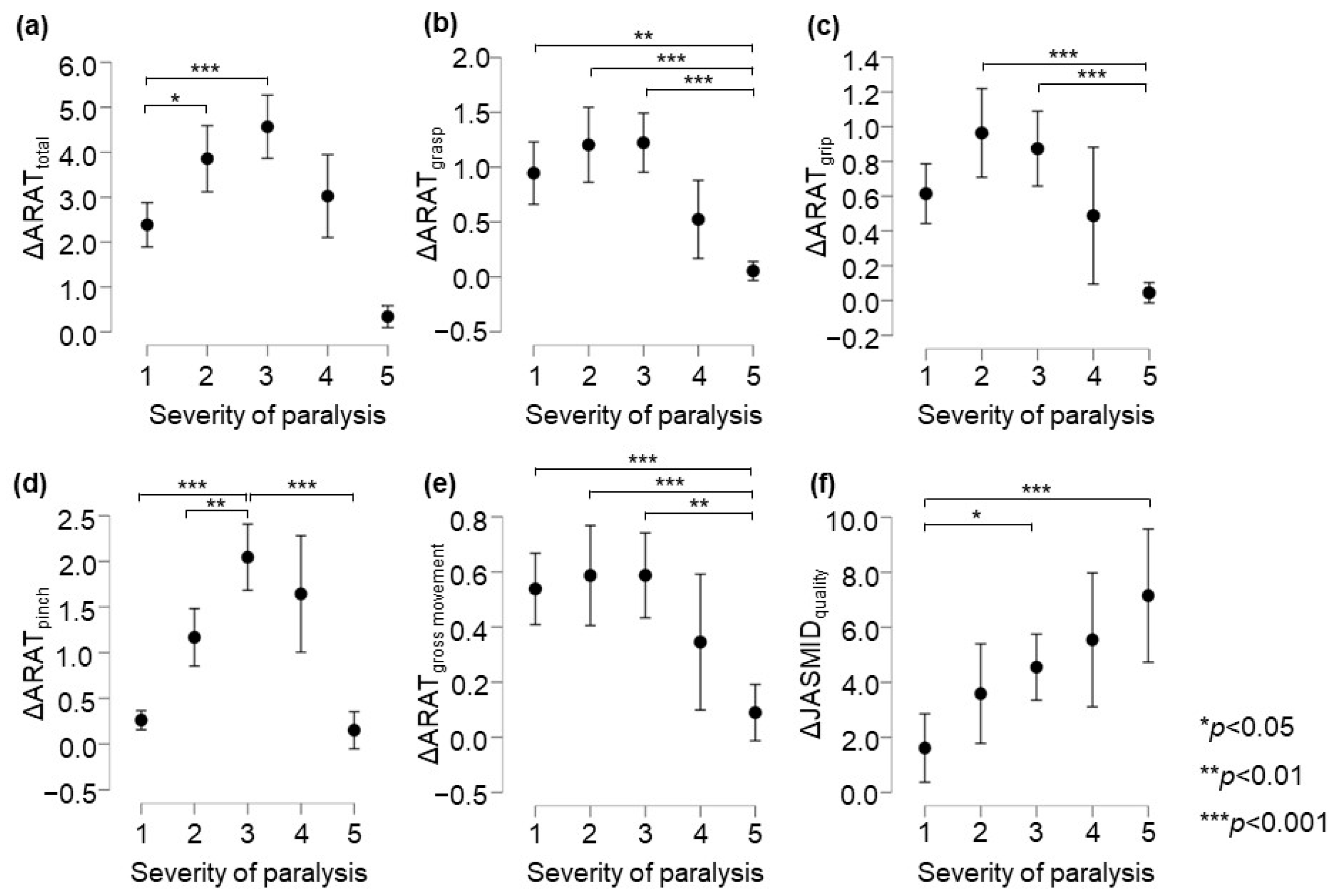
In the ARAT subscore analysis, there was a significant main effect of the group on the delta values for subscores A–D (p < 0.05, adjusted for all covariates; Table 4). The post-test results showed that in terms of ARAT total scores, the changes in the Poor and the Limited recovery groups were significantly greater than that in the No recovery group (p = 0.034 and p < 0.001, respectively, Figure 2a). The change in the Notable recovery group was significantly greater than that in the Full recovery group (p = 0.003). Regarding the ARAT grasp score, the changes in the No, Poor, and Limited recovery groups were significantly greater than that in the Full recovery group (p = 0.005, p < 0.001, and p < 0.001, respectively, Figure 2b). Further, in terms of the ARAT grip score, the changes in the Poor and Limited recovery groups were significantly greater than that in the Full recovery group (both p < 0.001, respectively, Figure 2c). In terms of the ARAT pinch score, the change in the Limited recovery group was significantly greater than that in the No, Poor, and Full recovery groups (p < 0.001, p = 0.002, and p < 0.001, respectively, Figure 2d). The change in the Notable recovery group was significantly greater than that in the No and Full recovery groups (both p < 0.001). Regarding the ARAT gross movement scores, the changes in the No, Poor, and Limited recovery groups were significantly greater than that in the Full recovery group (p = 0.014, p = 0.012, and p = 0.004, respectively, Figure 2e). In terms of the post-test ARAT subscores, the change in the Notable recovery group was not significantly different from those in the Poor and Limited recovery groups. The JASMID score of hand usage (quantity, F = 2.02, p = 0.089, η2 = 0.009) had no main effect of the group on the delta value. On the other hand, the JASMID score of satisfaction did have a main effect of the group on the delta values (quality, F = 6.66, p < 0.001, η2 = 0.028, Table 4). According to the multiple comparisons test, the JASMID score of quality had a significantly greater main effect of group on the delta value in the Limited and Full recovery groups than in the No recovery group (p = 0.032 and p < 0.001, respectively, Figure 2f).
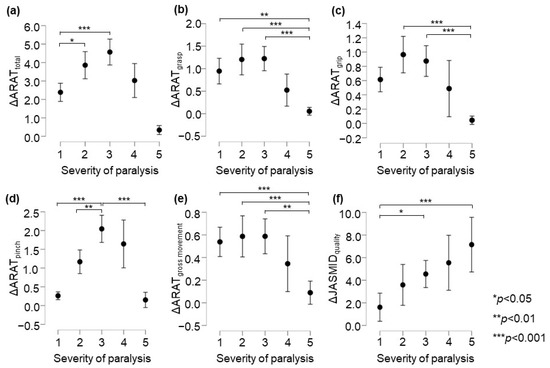
Figure 2.
Comparison of changes in upper extremity function according to ARAT and JASMID scores. Panels are displayed delta score (post − pre) by severity of paralysis; (a) ARAT total, (b) ARAT grasp, (c) ARAT grip, (d) ARAT pinch, (e) ARAT gross movement, (f) JASMID quality. Statistical significance was set at p < 0.05 for Scheffé’s multiple comparisons (n = 907). ARAT, Action Research Arm Test; JASMID, Jikei Assessment Scale for Motor Impairment in Daily Living.
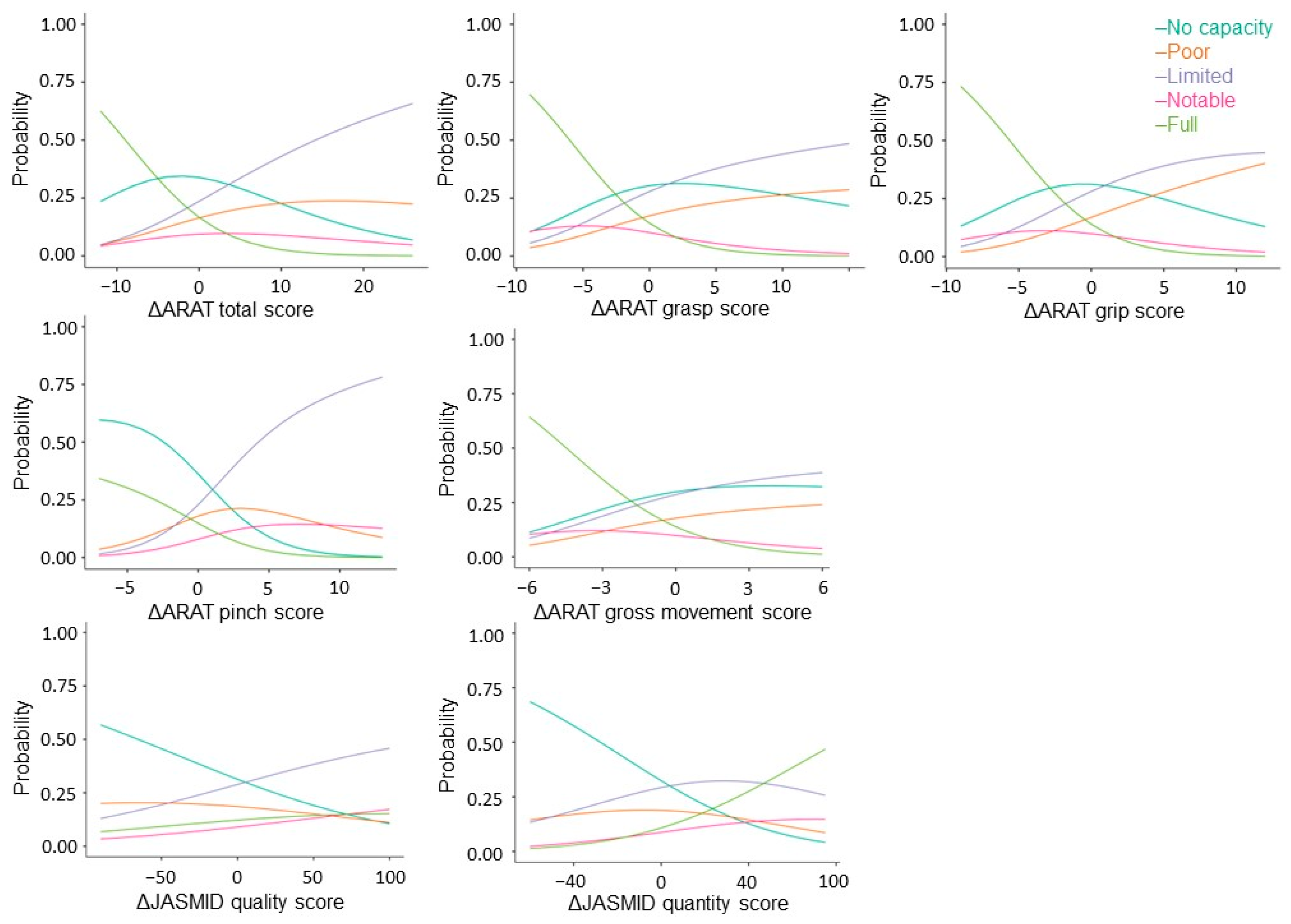
Next, stratified analysis was performed using multinomial regression to factor changes in ARAT and JASMID scores into the severity of motor paralysis prior to treatment. Akaike’s Information Criterion (AIC) = 2677, grasp (x2 = 36.2, p < 0.001, AIC = 2724), grip (x2 = 31.9, p < 0.001, AIC = 2728), pinch (x2 = 124, p < 0.001, AIC = 2635), gross movement (x2 = 21.1, p < 0.001, AIC = 2739), and JASMID quality (x2 = 24.8, p < 0.001, AIC = 2735) showed a significant fit to the model by the severity of motor paralysis, but none in JASMID quantity (x2 = 6.52, p = 0.16, AIC = 2753). Coefficients of variation and odds ratios were calculated for the ARAT total score, subscores A–D, and the change in JASMID quantity and quality scores with respect to the No recovery group data for each severity level (Figure 3).
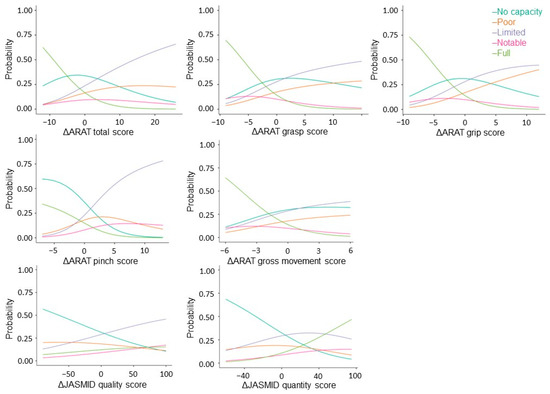
Figure 3.
Multinomial logistic probability plots showing the association between the level of agreement for delta ARAT and JASMID score. Logistic curves were discriminated using the probability of being grouped by pre-treatment ARAT scores: scores of 0–10 indicate no upper limb capacity; scores of 11–21 represent poor capacity; scores of 22–42 represent limited capacity; scores of 43–54 represent notable capacity; and scores of 55–67 represent full upper limb capacity. ARAT, Action Research Arm Test; JASMID, Jikei Assessment Scale for Motor Impairment in Daily Living.
Regarding total ΔARAT, when the coefficient of variation of the No recovery group was 1, that of the Poor recovery group was 0.9, that of the Limited recovery group was 1.1, and that of the Notable recovery group was 0.8; meanwhile, the odds ratio was estimated to be 2.53 times that of the No recovery group for the Poor recovery group, 3.08 times that of the No recovery group for the Limited recovery group, and 2.22 times that of the No recovery group for the Notable recovery group. The coefficient of variation for the Full recovery group was −0.9, and the odds ratio was 0.42. Regarding ΔJASMID, when the coefficient of variation of the No recovery group was 1, that of the Limited recovery group was 0.6, that of the Notable recovery group was 0.8, and that of the Full recovery group was 0.8; meanwhile, the odds ratio was estimated to be 1.84 times that of the No recovery group for the Limited recovery group, 2.30 times that of the No recovery group for the Notable recovery group, and 2.23 times that of the No recovery group for the Full recovery group (see Appendix A).
4. Discussion
In this study, the amount of change in ARAT was calculated for each level of motor paralysis severity, classified according to the ARAT score before NEURO. The results indicated that patients in the Limited recovery group experienced the most significant improvement in upper limb motor paralysis with NEURO. In a previous study, patients with FMAUE scores ≥43 had higher interhemispheric inhibition from the healthy hemisphere to the affected hemisphere [20]. In the present study, all the patients were irradiated with rTMS, and in 99% of them, the irradiation site was the primary motor cortex of the intact hemisphere. The higher interhemispheric inhibition in the Limited recovery group and the higher excitability of the primary and supplementary motor cortexes on the lesion side achieved with rTMS may have caused the greater upper limb motor paralysis caused by the subsequent occupational therapy [20,36].
In the present study, the degree of change in the frequency of hand use did not differ according to motor paralysis severity; however, the quality of movement was most greatly improved in the Full recovery group. A phenomenon termed “Learned non-use hand” that affects the paretic arm or hand occurs in patients with stroke paraplegia due to the continuous disuse of the paralyzed upper limb in their daily lives [37,38]. In the present study, the Full recovery group had the highest scores in the pre-treatment evaluation and had less functional impairment of the upper limb than the other groups. In the intensive inpatient rehabilitation program, in which OTs provided appropriate practice and instructions for performing ADLs, the degree of improvement in the frequency of hand use did not differ by severity; nevertheless, the Full recovery group was presumably more satisfied with the use of their hands and had greater qualitative improvement than the other groups. The effectiveness of rehabilitation is enhanced when patients themselves are aware of the motor functions they need to perform to carry out the ADL set as a goal by them and the therapist [39,40]. The results obtained in this study may be used as a reference for determining the content of occupational therapy exercises and ADL goals for use with rTMS; this data is summarized in Appendix B.
The No recovery group was predicted to have a more significant improvement in the ARAT grasp and gross movement scores by NEURO than the other groups. However, the quality of paralyzed upper limb movement was predicted to show a smaller improvement. Our results are consistent with the findings of previous studies reporting that patients with severe motor paralysis regained function in the proximal part of the upper limb [41,42]. Regarding NEURO occupational therapy, it can be inferred that ADL exercises that frequently use shoulder and elbow movements are suitable for patients with severe motor paralysis, as they improve the function of the proximal part of the upper limbs. In addition, it is recommended to practice movements with the paralyzed upper limb according to the patient’s wishes and to set up target movements to improve the quality of hand use. In the ARAT, the grasp task includes rotation of the forearm, and the gross movement task includes reaching the patient’s own body. For the target daily activities, it is suggested to use the proximal part of the upper limb to hold in place a plate, paper, or a book on a desk, a task that involves inward movement of the forearm. Similarly, even in cases of reduced hand dexterity, if the patient can reach their own body, they can aim to acquire movements such as lifting an upper garment when opening and closing a zipper, washing the upper limb and fingers of the non-paralyzed side, smoothing creases in clothes, and removing dust from clothes [43,44].
The Poor recovery group was more likely to show improvement in ARAT grasp and grip scores using NEURO. However, these patients were less likely to show improvement in ARAT pinch scores. It is assumed that improvement in hand function is necessary to improve the use of the paralyzed upper limb and the movement quality of the Poor recovery group patients. The ARAT grip task involves holding the forearm in the middle position or moving it from the medial to the external rotation position. Using occupational therapy, we expect that the grip score will improve. The target movements include grasping a plastic bottle, opening and closing a sliding door, holding a toothbrush while applying toothpaste, flipping a switch or pressing an elevator button within reach using the paralyzed limb, and grasping a cell phone with the paralyzed hand [45,46].
Since the Limited recovery group showed the most promising improvement in ARAT scores with NEURO, the target movement practices should be defined by estimating the target degree of improvement in grasp, grip, gross movement, and pinch. In rehabilitation, an improvement in motor function score does not always directly lead to an improvement in the amount of use or quality of movement possible with the paralyzed upper limb [47]. This may be because patients can perform compensatory movements with the paralyzed fingers and upper limbs and do not use the improved hand functions properly. Therefore, daily living exercises are essential in occupational therapy. Learned bad use of the paretic limb reinforces abnormal compensatory movement strategies at the expense of normal movement patterns, making it difficult to improve movement performance [48,49]. In occupational therapy, it is important to provide appropriate feedback for abnormal joint movements and inhibit learned bad use so that patients can appropriately use their paralyzed upper limbs in their ADLs. Although this point has not been tested, the patient’s target movements should be incorporated into the practice. The patient should be motivated to thoroughly practice using the paralyzed upper limb for ADLs so that they can perform the function when their motor function improves. The suggested target activities include manipulating a spoon or fork, zipping and unzipping clothes, buttoning and unbuttoning clothes, putting on socks, tying shoelaces, washing one’s face, writing one’s signature, and other activities requiring fine motor skills [50,51].
The Notable recovery group had fewer ARAT and JASMID subscores showing significant improvement compared to the other groups. Use-dependent plasticity is a phenomenon in which the same pattern of activity tends to occur when specific neurons are repeatedly activated; this is the aim of repetitive practice in rehabilitation [52,53]. In recent years, the practice time and the number of joint movements required to achieve recovery of motor function have been assessed [54,55]. In occupational therapy included in NEURO, it is important to promote use-dependent plasticity and improve motor function and ADL by providing a sufficient amount of challenging movement exercises for patients with Notable-level scores. For patients with Notable-level scores and good proximal and distal function, we propose the following ADLs as goals: drinking water from a cup, drying laundry, washing hair, tying hair, manipulating chopsticks, and tying a necktie [44,56,57].
In the Full recovery group, the change in the ARAT score was small, but the change in JASMID quality was expected to be high. The recovery of motor paralysis improved in terms of motor speed and coordination after patients regained joint movements, experienced weakening spasticity and gained the ability to perform isolated movements. Studies examining the difficulty level of detailed FMAUE items also support this recovery process [58,59,60]. A method using a motion analyzer to detect angular and velocity changes in joints is recommended for evaluating functional impairment and determining treatment effects in patients with mild hemiplegia [61]. The Box and Block Test and the Wolf motor function test can assess the coordination of movement and speed of joint movement based on the number of blocks carried and the time required to perform the task [62,63,64]. However, the ARAT is scored on an ordinal scale, and it is difficult to evaluate these factors in detail. In other words, the Full recovery group is expected to show improved quality of movement by improving the speed of movement and coordination of joint movements, which would be challenging to evaluate using the ARAT. Therefore, occupational therapy in NEURO is recommended to provide more challenging exercises, such as those for adjusting the speed of joint movements, for complex movements that require multiple joints, and for resistance exercises. Regarding ADL, it is possible to aim to acquire activities that require hand dexterity and upper limb motor coordination, such as cooking, brushing teeth, operating smartphones and PCs, and putting on and taking off necklaces and earrings [65,66]. In addition, this can be expected to improve the quality of ADLs to a level desired by the patient.
The results of this study can be applied to new interventions, including BCI, when planning exercises appropriate to the severity of the patient’s condition. For example, a treatment in which BCI was applied to exercise therapy provided by therapists was reported to result in greater improvement in patients’ motor paralysis [67,68]. Although the therapeutic effects of BCI have been demonstrated, a method to predict patient recovery and to set motor tasks appropriate for their severity has not been formulated [69]. The results of this study provided data to predict the amount of recovery after treatment for patients to whom newly developed interventions will be applied and to plan effective exercise tasks according to the severity of the patient’s illness.
This study has several limitations. The ARAT used for the main outcome reportedly has a ceiling effect, and it is inferred that changes in patients with mild motor paralysis may be underestimated [70]. Sensory perception, muscle tone, and joint range of motion are involved in the ability to manipulate objects [71,72,73,74]. The present study does not clarify the effects of the presence or absence of these symptoms on the degree of change in ARAT scores. Since the JASMID questionnaire was designed for the Japanese lifestyle, it is unclear whether the same results would be obtained if it was administered to patients from other countries [32]. In this study, customized rTMS methods were applied to individual patients. This was a factor that influenced our results since the effects of rTMS depend on the irradiation method implemented. In addition, occupational therapy in NEURO was provided by therapists affiliated with NEURO-accredited facilities based on a pre-defined concept. Therefore, it was expected that treatment equivalence had been ensured; however, we were unable to confirm this point as part of this study. The generalization of the results is limited because the treatment effect will not be the same if the therapy is provided outside of a NEURO-accredited facility. The content and number of exercises provided to patients influence motor paralysis recovery [54,55]. Since the patients in the present analysis received NEURO at an accredited facility because they had chosen to undergo NEURO, it was inferred that they were highly motivated to undergo rehabilitation. Patient motivation and effort during inpatient treatment are confounding factors that influence treatment efficacy [75]. In the present study, we could not investigate the specific content and amount of exercise provided by the therapists nor the effort capacity of the patients. Physiotherapy sessions were assigned at the discretion of the attending physicians, but we have not been able to verify whether the sessions had an influence on the treatment effect of upper limb motor function. In the present study, patients for whom at least 6 months had elapsed since the onset of illness, which is the criterion for application of NEURO, were included in the study. In the acute phase, ipsilesional facilitation is performed by HF-rTMS [76]. In the chronic phase, rTMS was applied for interhemispheric inhibition, and the irradiation method differed from that in the acute phase according to the purpose of neuromodulation. The effectiveness of treatment for patients in the early stages of disease onset should be verified by other studies. In addition, in the present study, brain imaging data were not examined in detail. Given that some previous studies have shown that stroke subtype is a confounding factor for recovery, a detailed analysis of the neurological characteristics of patients receiving NEURO should be conducted to fully understand this issue [77].
5. Conclusions
This study estimated the level of hand and upper limb function restoration resulting from NEURO treatment, according to the severity of motor paralysis assessed in pre-treatment ARAT scores. The results of the present study can be used to suggest patient-desired ADL exercises in occupational therapy after rTMS in accordance with the functional recovery of the paralyzed upper limb. The benefits of the paralyzed upper limb functional recovery were estimated, and the ADL exercises appropriate for functional recovery need to be verified in future studies. The results of this study may be applied in rehabilitation therapy using BCI, which has been developed in recent years, to set motor tasks according to patients’ motor paralysis. We expect that providing patients with motor tasks based on pre-intervention severity to predict post-treatment recovery will only be used in newly developed therapies in the future.
Author Contributions
Conceptualization, D.S., T.H. and M.A.; methodology, D.S., T.H. and K.M.; software, T.H.; validation, H.I. and Y.N.; formal analysis, D.S. and T.H.; investigation, D.S., K.M. and H.I.; resources, Y.N. and M.A.; data curation, K.M. and T.H.; writing—original draft preparation, D.S.; writing—review and editing, T.H. and M.A.; visualization, K.M.; supervision, Y.N. and M.A.; project administration, M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Jikei University School of Medicine Ethics Committee (approval number 24-295-7061).
Informed Consent Statement
The requirement for informed consent was waived because the analysis used anonymous clinical data obtained after each patient agreed to undergo NEURO by written consent.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Acknowledgments
We thank the physicians and occupational therapists at Izumi Memorial Hospital, Shimizu Hospital, Nishi-Hiroshima Rehabilitation Hospital, Tokyo General Hospital, Kyoto O’Hara Memorial Hospital, and Tokyo Jikei University Hospital for their cooperation in obtaining and providing data for this study.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Comparison of post-treatment changes in ARAT and JASMID scores using ordinal logistic regression analysis.
Table A1.
Comparison of post-treatment changes in ARAT and JASMID scores using ordinal logistic regression analysis.
| Index of Measurements | Predictors | Coefficient | p | Odds Ratio | 95% Confidence Interval | ||
| Lower | Upper | ||||||
| ARAT | Total | Poor | 0.9 | <0.001 | 2.53 | 1.79 | 3.56 |
| Limited | 1.1 | <0.001 | 3.08 | 2.26 | 4.21 | ||
| Notable | 0.8 | <0.001 | 2.22 | 1.45 | 3.40 | ||
| Full | −0.9 | <0.001 | 0.42 | 0.29 | 0.61 | ||
| A. grasp | Poor | 0.6 | 0.002 | 1.80 | 1.25 | 2.59 | |
| Limited | 0.6 | <0.001 | 1.79 | 1.30 | 2.47 | ||
| Notable | 0.1 | 0.734 | 1.08 | 0.69 | 1.70 | ||
| Full | −0.8 | <0.001 | 0.44 | 0.29 | 0.67 | ||
| B. grip | Poor | 0.6 | 0.001 | 1.86 | 1.28 | 2.70 | |
| Limited | 0.6 | <0.001 | 1.82 | 1.31 | 2.53 | ||
| Notable | 0.2 | 0.382 | 1.24 | 0.76 | 1.99 | ||
| Full | −0.8 | <0.001 | 0.45 | 0.29 | 0.69 | ||
| C. pinch | Poor | 1.0 | <0.001 | 2.75 | 1.90 | 3.99 | |
| Limited | 1.7 | <0.001 | 5.43 | 3.86 | 7.67 | ||
| Notable | 1.5 | <0.001 | 4.52 | 2.80 | 7.26 | ||
| Full | −0.1 | 0.600 | 0.89 | 0.57 | 1.38 | ||
| D. gross movement | Poor | 0.2 | 0.344 | 1.21 | 0.82 | 1.77 | |
| Limited | 0.3 | 0.120 | 1.31 | 0.93 | 1.84 | ||
| Notable | −0.3 | 0.325 | 0.77 | 0.46 | 1.28 | ||
| Full | −1.0 | <0.001 | 0.38 | 0.23 | 0.61 | ||
| JASMID | Quantity | Poor | 0.3 | 0.047 | 1.40 | 1.00 | 1.96 |
| Limited | 0.5 | 0.001 | 1.64 | 1.22 | 2.20 | ||
| Notable | 0.8 | <0.001 | 2.16 | 1.38 | 3.36 | ||
| Full | 0.3 | 0.173 | 1.32 | 0.89 | 1.99 | ||
| Quality | Poor | 0.3 | 0.055 | 1.39 | 0.99 | 1.96 | |
| Limited | 0.6 | <0.001 | 1.84 | 1.37 | 2.47 | ||
| Notable | 0.8 | <0.001 | 2.30 | 1.49 | 3.55 | ||
| Full | 0.8 | <0.001 | 2.23 | 1.49 | 3.34 | ||
Ordinal logistic regression analysis was used. Statistical significance was set at 0.05 (n = 907). Data from the No recovery group was used as a reference for the other groups. ARAT, Action Research Arm Test; JASMID, Jikei Assessment Scale for Motor Impairment in Daily Living. Pre-treatment ARAT scores of 0–10 indicate no upper limb capacity; scores of 11–21 represent poor capacity; scores of 22–42 represent limited capacity; scores of 43–54 represent notable capacity; and scores of 55–67 represent full upper limb capacity.
Appendix B

Table A2.
Goal-setting and practice content according to the severity of motor paralysis for patients undergoing combined repetitive transcranial magnetic stimulation and occupational therapy.
Table A2.
Goal-setting and practice content according to the severity of motor paralysis for patients undergoing combined repetitive transcranial magnetic stimulation and occupational therapy.
| Recovery Capacity | Scores Predicted to Change Significantly | Goals for ADLs Using the Paralyzed Upper Limb and Fingers | Main Required Joint Movements |
| No: 0–10 | ARAT: Grasp, Gross movement | Holding a plate in place on a desk. | Shoulder flexion, adduction and internal rotation, forearm pronation. |
| Holding in place a paper or a book on a desk. | Shoulder flexion, adduction, and internal rotation, forearm pronation. | ||
| Lifting an upper garment when opening and closing a zipper. | Shoulder adduction and internal rotation, elbow extension, forearm pronation. | ||
| Washing the upper limb and fingers of the non-paralyzed side. | Shoulder flexion, adduction, abduction, elbow flexion, elbow extension. | ||
| Smoothing creases in clothes. Removing dust from clothes. | Shoulder flexion, adduction, abduction, elbow flexion, elbow extension. | ||
| Poor: 11–21 | ARAT: Grasp, Grip | Grasping a plastic bottle with the paralyzed limb. | Elbow flexion, Forearm supination, finger flexion. |
| Opening and closing a sliding door. | Shoulder flexion, extension, adduction, abduction, elbow extension. | ||
| Holding a toothbrush with the paralyzed limb when applying toothpaste. | Elbow flexion, forearm supination, finger flexion. | ||
| Flipping a switch or pressing an elevator button within reach using the paralyzed limb. | Shoulder flexion and extension, elbow flexion and extension. | ||
| Grasping a cell phone with the paralyzed hand. | Elbow flexion, forearm rotation, finger flexion. | ||
| Limited: 22–42 | ARAT: Grasp, Grip, Pinch, Gross movement | Manipulating a spoon or fork. | Forearm rotation, wrist flexion/extension, finger dexterity exercises. |
| Zipping and unzipping clothes; buttoning and unbuttoning clothes | Forearm rotation, wrist flexion/extension, finger dexterity exercises. | ||
| Putting on socks. Tying shoelaces. | Forearm rotation, wrist flexion/extension, finger dexterity exercises. | ||
| Washing one’s face. | Elbow flexion, external forearm rotation, wrist extension, finger extension. | ||
| Writing one’s signature. | Wrist flexion, wrist extension, finger dexterity exercises. | ||
| Notable: 43–54 | ARAT: Pinch | Drinking water from a cup. | Shoulder flexion/extension, elbow flexion, forearm rotation, finger flexion. |
| Drying laundry. | Shoulder flexion, elbow extension, forearm rotation, finger flexion and extension. | ||
| Washing hair. Tying hair. | Shoulder flexion, rotation, forearm rotation, and finger dexterity exercises. | ||
| Manipulating chopsticks. | Wrist flexion/extension, forearm rotation, finger dexterity exercises. | ||
| Tying a necktie. | Wrist flexion/extension, forearm rotation, finger dexterity exercises. | ||
| Full: 55–57 | JASMID: Quality | Cooking. | Wrist flexion/extension, forearm rotation, hand dexterity exercises. |
| Brushing teeth. | Elbow flexion/extension, forearm rotation, hand dexterity exercises. | ||
| Operating smartphones and PCs. | Forearm rotation, wrist flexion/extension, hand dexterity exercises. | ||
| Putting on and taking off necklaces and earrings. | Shoulder flexion/rotation, forearm rotation, and hand dexterity exercises. | ||
| The quality of ADLs improved to meet the patient’s desire. | Complex movements requiring multiple joints. |
ARAT, Action Research Arm Test; JASMID, Jikei Assessment Scale for Motor Impairment in Daily Living; ADL, activities of daily living; rTMS, repetitive transcranial magnetic stimulation. Pre-treatment ARAT scores of 0–10, 11–21, 22–42, 43–54, and 55–57 indicated no, poor, limited, notable, and full recovery capacity, respectively.
References
- Carlsson, H.; Gard, G.; Brogardh, C. Upper-limb sensory impairments after stroke: Self-reported experiences of daily life and rehabilitation. J. Rehabil. Med. 2018, 50, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.H.; van Wijck, F.; Joice, S.; Donaghy, M. Predicting health related quality of life 6 months after stroke: The role of anxiety and upper limb dysfunction. Disabil. Rehabil. 2013, 35, 291–299. [Google Scholar] [CrossRef]
- Ahmed, I.; Mustafaoglu, R.; Benkhalifa, N.; Yakhoub, Y.H. Does noninvasive brain stimulation combined with other therapies improve upper extremity motor impairment, functional performance, and participation in activities of daily living after stroke? A systematic review and meta-analysis of randomized controlled trial. Top. Stroke Rehabil. 2022, 3, 1–22. [Google Scholar]
- Hatem, S.M.; Saussez, G.; Della Faille, M.; Prist, V.; Zhang, X.; Dispa, D.; Bleyenheuft, Y. Rehabilitation of motor function after stroke: A multiple systematic review focused on techniques to stimulate upper extremity recovery. Front. Hum. Neurosci. 2016, 10, 442. [Google Scholar] [CrossRef]
- Kakuda, W.; Abo, M.; Kobayashi, K.; Momosaki, R.; Yokoi, A.; Fukuda, A.; Ishikawa, A.; Ito, H.; Tominaga, A. Low-frequency repetitive transcranial magnetic stimulation and intensive occupational therapy for poststroke patients with upper limb hemiparesis: Preliminary study of a 15-day protocol. Int. J. Rehabil. Res. 2010, 33, 339–345. [Google Scholar] [CrossRef]
- Blesneag, A.V.; Slăvoacă, D.F.; Popa, L.; Stan, A.D.; Jemna, N.; Isai Moldovan, F.; Mureșanu, D.F. Low-frequency rTMS in patients with subacute ischemic stroke: Clinical evaluation of short and long-term outcomes and neurophysiological assessment of cortical excitability. J. Med. Life 2015, 8, 378–387. [Google Scholar]
- Murase, N.; Duque, J.; Mazzocchio, R.; Cohen, L.G. Influence of interhemispheric interactions on motor function in chronic stroke. Ann. Neurol. 2004, 55, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Ferbert, A.; Priori, A.; Rothwell, J.C.; Day, B.L.; Colebatch, J.G.; Marsden, C.D. Interhemispheric inhibition of the human motor cortex. J. Physiol. 1992, 453, 525–546. [Google Scholar] [CrossRef]
- Adeyemo, B.O.; Simis, M.; Macea, D.D.; Fregni, F. Systematic review of parameters of stimulation, clinical trial design characteristics, and motor outcomes in noninvasive brain stimulation in stroke. Front. Psychiatry 2012, 3, 88. [Google Scholar] [CrossRef]
- Momosaki, R.; Abo, M.; Kakuda, W. Bilateral repetitive transcranial magnetic stimulation combined with intensive swallowing rehabilitation for chronic stroke Dysphagia: A case series study. Case Rep. Neurol. 2014, 6, 60–67. [Google Scholar] [CrossRef]
- Moslemi Haghighi, F.; Kordi Yoosefinejad, A.; Razeghi, M.; Shariat, A.; Bagheri, Z.; Rezaei, K. The effect of high-frequency repetitive transcranial magnetic stimulation on functional indices of affected upper limb in patients with subacute stroke. J. Biomed. Phys. Eng. 2021, 11, 175–184. [Google Scholar] [CrossRef]
- Guan, Y.Z.; Li, J.; Zhang, X.W.; Wu, S.; Du, H.; Cui, L.Y.; Zhang, W.H. Effectiveness of repetitive transcranial magnetic stimulation (rTMS) after acute stroke: A one-year longitudinal randomized trial. CNS Neurosci. Ther. 2017, 23, 940–946. [Google Scholar] [CrossRef]
- Kakuda, W.; Abo, M.; Shimizu, M.; Sasanuma, J.; Okamoto, T.; Yokoi, A.; Taguchi, K.; Mitani, S.; Harashima, H.; Urushidani, N.; et al. A multicenter study on low-frequency rTMS combined with intensive occupational therapy for upper limb hemiparesis in post-stroke patients. J. Neuroeng. Rehabil. 2012, 9, 4. [Google Scholar] [CrossRef]
- Tatsuno, H.; Hamaguchi, T.; Sasanuma, J.; Kakita, K.; Okamoto, T.; Shimizu, M.; Nakaya, N.; Abo, M. Does a combination treatment of repetitive transcranial magnetic stimulation and occupational therapy improve upper limb muscle paralysis equally in patients with chronic stroke caused by cerebral hemorrhage and infarction? A retrospective cohort study. Medicine 2021, 100, e26339. [Google Scholar] [CrossRef]
- Alt Murphy, M.; Resteghini, C.; Feys, P.; Lamers, I. An overview of systematic reviews on upper extremity outcome measures after stroke. BMC Neurol. 2015, 15, 29. [Google Scholar] [CrossRef]
- Hamaguchi, T.; Yamada, N.; Hada, T.; Abo, M. Prediction of motor recovery in the upper extremity for repetitive transcranial magnetic stimulation and occupational therapy goal setting in patients with chronic stroke: A retrospective analysis of prospectively collected data. Front. Neurol. 2020, 11, 581186. [Google Scholar] [CrossRef] [PubMed]
- Santisteban, L.; Teremetz, M.; Bleton, J.P.; Baron, J.C.; Maier, M.A.; Lindberg, P.G. Upper limb outcome measures used in stroke rehabilitation studies: A systematic literature review. PLoS ONE 2016, 11, e0154792. [Google Scholar] [CrossRef]
- Chen, P.; Liu, T.W.; Tse, M.M.Y.; Lai, C.K.Y.; Tsoh, J.; Ng, S.S.M. The predictive role of hand section of Fugl-Meyer assessment and motor activity log in action research arm test in people with stroke. Front. Neurol. 2022, 13, 926130. [Google Scholar] [CrossRef]
- Fleming, M.K.; Newham, D.J.; Roberts-Lewis, S.F.; Sorinola, I.O. Self-perceived utilization of the paretic arm in chronic stroke requires high upper limb functional ability. Arch. Phys. Med. Rehabil. 2014, 95, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.L.; Potter-Baker, K.A.; Cunningham, D.A.; Li, M.; Sankarasubramanian, V.; Lee, J.; Jones, S.; Sakaie, K.; Wang, X.; Machado, A.G.; et al. Stratifying chronic stroke patients based on the influence of contralesional motor cortices: An inter-hemispheric inhibition study. Clin. Neurophysiol. 2020, 131, 2516–2525. [Google Scholar] [CrossRef] [PubMed]
- Paszkiel, S.; Dobrakowski, P. Brain–computer technology-based training system in the field of motor imagery. IET Sci. Meas. Technol. 2020, 14, 1014–1018. [Google Scholar] [CrossRef]
- Baniqued, P.D.E.; Stanyer, E.C.; Awais, M.; Alazmani, A.; Jackson, A.E.; Mon-Williams, M.A.; Mushtaq, F.; Holt, R.J. Brain-computer interface robotics for hand rehabilitation after stroke: A systematic review. J. Neuroeng. Rehabil. 2021, 18, 15. [Google Scholar] [CrossRef]
- Rossi, S.; Hallett, M.; Rossini, P.M.; Pascual-Leone, A.; Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 2009, 120, 2008–2039. [Google Scholar] [CrossRef] [PubMed]
- Abo, M.; Kakuda, W.; Momosaki, R.; Harashima, H.; Kojima, M.; Watanabe, S.; Sato, T.; Yokoi, A.; Umemori, T.; Sasanuma, J. Randomized, multicenter, comparative study of NEURO versus CIMT in poststroke patients with upper limb hemiparesis: The NEURO-VERIFY Study. Int. J. Stroke 2014, 9, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Thieme, H.; Morkisch, N.; Mehrholz, J.; Pohl, M.; Behrens, J.; Borgetto, B.; Dohle, C. Mirror therapy for improving motor function after stroke. Cochrane Database Syst. Rev. 2018, 7, CD008449. [Google Scholar] [CrossRef]
- Marcolino, M.A.Z.; Hauck, M.; Stein, C.; Schardong, J.; Pagnussat, A.S.; Plentz, R.D.M. Effects of transcutaneous electrical nerve stimulation alone or as additional therapy on chronic post-stroke spasticity: Systematic review and meta-analysis of randomized controlled trials. Disabil. Rehabil. 2020, 42, 623–635. [Google Scholar] [CrossRef]
- Conforto, A.B.; Dos Anjos, S.M.; Bernardo, W.M.; Silva, A.A.D.; Conti, J.; Machado, A.G.; Cohen, L.G. Repetitive Peripheral Sensory Stimulation and Upper Limb Performance in Stroke: A Systematic Review and Meta-analysis. Neurorehabil. Neural Repair 2018, 32, 863–871. [Google Scholar] [CrossRef]
- Costantino, C.; Galuppo, L.; Romiti, D. Short-term effect of local muscle vibration treatment versus sham therapy on upper limb in chronic post-stroke patients: A randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2017, 53, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Carroll, D. A quantitative test of upper extremity function. J. Chronic Dis. 1965, 18, 479–491. [Google Scholar] [CrossRef]
- Lyle, R.C. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. Int. J. Rehabil. Res. 1981, 4, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Hoonhorst, M.H.; Nijland, R.H.; van den Berg, J.S.; Emmelot, C.H.; Kollen, B.J.; Kwakkel, G. How do Fugl-Meyer arm motor scores relate to dexterity according to the action research arm test at 6 months poststroke? Arch. Phys. Med. Rehabil. 2015, 96, 1845–1849. [Google Scholar] [PubMed]
- Ishikawa, A.; Kakuda, W.; Taguchi, K.; Uruma, G.; Abo, M. The reliability and validity of a new subjective assessment scale for poststroke upper limb hemiparesis, the Jikei assessment scale for motor impairment in daily living. Tokyo Jikei Med. J. 2010, 125, 159–167. [Google Scholar]
- Uswatte, G.; Taub, E.; Morris, D.; Vignolo, M.; McCulloch, K. Reliability and validity of the upper-extremity Motor Activity Log-14 for measuring real-world arm use. Stroke 2005, 36, 2493–2496. [Google Scholar] [CrossRef]
- Dionisio, A.; Duarte, I.C.; Patricio, M.; Castelo-Branco, M. The use of repetitive transcranial magnetic stimulation for stroke rehabilitation: A systematic review. J. Stroke Cerebrovasc. Dis. 2018, 27, 1–31. [Google Scholar] [CrossRef]
- Coupar, F.; Pollock, A.; Rowe, P.; Weir, C.; Langhorne, P. Predictors of upper limb recovery after stroke: A systematic review and meta-analysis. Clin. Rehabil. 2012, 26, 291–313. [Google Scholar] [CrossRef]
- Nowak, D.A.; Grefkes, C.; Ameli, M.; Fink, G.R. Interhemispheric competition after stroke: Brain stimulation to enhance recovery of function of the affected hand. Neurorehabil. Neural Repair 2009, 23, 641–656. [Google Scholar]
- Raghavan, P. Upper limb motor impairment after stroke. Phys. Med. Rehabil. Clin. N. Am. 2015, 26, 599–610. [Google Scholar] [CrossRef]
- Taub, E.; Uswatte, G.; Elbert, T. New treatments in neurorehabilitation founded on basic research. Nat. Rev. Neurosci. 2002, 3, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.C.; Lewthwaite, R.; Rocktashel, J.; Winstein, C.J. Self-efficacy and reach performance in individuals with mild motor impairment due to stroke. Neurorehabil. Neural Repair 2019, 33, 319–328. [Google Scholar]
- Rice, D.B.; McIntyre, A.; Mirkowski, M.; Janzen, S.; Viana, R.; Britt, E.; Teasell, R. Patient-centered goal setting in a hospital-based outpatient stroke rehabilitation center. PM R 2017, 9, 856–865. [Google Scholar] [CrossRef]
- Hayward, K.; Barker, R.; Brauer, S. Interventions to promote upper limb recovery in stroke survivors with severe paresis: A systematic review. Disabil. Rehabil. 2010, 32, 1973–1986. [Google Scholar] [CrossRef]
- Hayward, K.S.; Kuys, S.S.; Barker, R.N.; Brauer, S.G. Can stroke survivors with severe upper arm disability achieve a clinically important change in arm function during inpatient rehabilitation? A multicentre, prospective, observational study. NeuroRehabilitation 2014, 35, 17–23. [Google Scholar] [CrossRef]
- Matsuoka, K.; Watanabe, A.; Kawaguchi, T.; Misawa, K.; Murakami, K.; Fukuda, M. Development of a new daily activities scale for the affected hand after stroke. Prog. Rehabil. Med. 2020, 5, 20200031. [Google Scholar] [CrossRef] [PubMed]
- van Andel, C.J.; Wolterbeek, N.; Doorenbosch, C.A.; Veeger, D.H.; Harlaar, J. Complete 3D kinematics of upper extremity functional tasks. Gait Posture 2008, 27, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.C.; Kennedy, N.C.; Clark, A.; Pomeroy, V.M. Getting a kinematic handle on reach-to-grasp: A meta-analysis. Physiotherapy 2018, 104, 153–166. [Google Scholar]
- Cirstea, M.C.; Mitnitski, A.B.; Feldman, A.G.; Levin, M.F. Interjoint coordination dynamics during reaching in stroke. Exp. Brain Res. 2003, 151, 289–300. [Google Scholar]
- Takahashi, K.; Domen, K.; Sakamoto, T.; Toshima, M.; Otaka, Y.; Seto, M.; Irie, K.; Haga, B.; Takebayashi, T.; Hachisuka, K. Efficacy of upper extremity robotic therapy in subacute poststroke hemiplegia: An exploratory randomized trial. Stroke 2016, 47, 1385–1388. [Google Scholar] [CrossRef] [PubMed]
- Skinner, B.F. The Behavior of Organisms: An Experimental Analysis; BF Skinner Foundation: Cambridge, MA, USA, 2019. [Google Scholar]
- Dickinson, A. Actions and habits: The development of behavioural autonomy. Philos. Trans. R. Soc. London. B Biol. Sci. 1985, 308, 67–78. [Google Scholar]
- Jarque-Bou, N.J.; Vergara, M.; Sancho-Bru, J.L.; Gracia-Ibanez, V.; Roda-Sales, A. Hand kinematics characterization while performing activities of daily living through kinematics reduction. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 1556–1565. [Google Scholar] [CrossRef]
- Chen, Y.W.; Liao, W.W.; Chen, C.L.; Wu, C.Y. Kinematic descriptions of upper limb function using simulated tasks in activities of daily living after stroke. Hum. Mov. Sci. 2021, 79, 102834. [Google Scholar] [CrossRef]
- Classen, J.; Liepert, J.; Wise, S.P.; Hallett, M.; Cohen, L.G. Rapid plasticity of human cortical movement representation induced by practice. J. Neurophysiol. 1998, 79, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Nudo, R.J.; Milliken, G.W.; Jenkins, W.M.; Merzenich, M.M. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J. Neurosci. 1996, 16, 785–807. [Google Scholar] [CrossRef] [PubMed]
- Peurala, S.H.; Kantanen, M.P.; Sjogren, T.; Paltamaa, J.; Karhula, M.; Heinonen, A. Effectiveness of constraint-induced movement therapy on activity and participation after stroke: A systematic review and meta-analysis of randomized controlled trials. Clin. Rehabil. 2012, 26, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Han, C.E.; Arbib, M.A.; Schweighofer, N. Stroke rehabilitation reaches a threshold. PLoS Comput. Biol. 2008, 4, e1000133. [Google Scholar] [CrossRef]
- Kim, K.; Song, W.K.; Lee, J.; Lee, H.Y.; Park, D.S.; Ko, B.W.; Kim, J. Kinematic analysis of upper extremity movement during drinking in hemiplegic subjects. Clin. Biomech. 2014, 29, 248–256. [Google Scholar] [CrossRef]
- Veeger, H.E.; Magermans, D.J.; Nagels, J.; Chadwick, E.K.; van der Helm, F.C. A kinematical analysis of the shoulder after arthroplasty during a hair combing task. Clin. Biomech. 2006, 21, S39–S44. [Google Scholar] [CrossRef]
- Brunnstrom, S. Motor testing procedures in hemiplegia: Based on sequential recovery stages. Phys. Ther. 1966, 46, 357–375. [Google Scholar] [CrossRef] [PubMed]
- Hijikata, N.; Kawakami, M.; Ishii, R.; Tsuzuki, K.; Nakamura, T.; Okuyama, K.; Liu, M. Item difficulty of Fugl-Meyer Assessment for upper extremity in persons with chronic stroke with moderate-to-severe upper limb impairment. Front. Neurol. 2020, 11, 577855. [Google Scholar] [CrossRef]
- Tauchi, Y.; Kyougoku, M.; Takahashi, K.; Okita, Y.; Takebayashi, T. Dimensionality and item-difficulty hierarchy of the Fugl-Meyer assessment of the upper extremity among Japanese patients who have experienced stroke. Top Stroke Rehabil. 2021, 29, 1–9. [Google Scholar] [CrossRef]
- Schwarz, A.; Kanzler, C.M.; Lambercy, O.; Luft, A.R.; Veerbeek, J.M. Systematic review on kinematic assessments of upper limb movements after stroke. Stroke 2019, 50, 718–727. [Google Scholar] [CrossRef]
- Desrosiers, J.; Bravo, G.; Hébert, R.; Dutil, E.; Mercier, L. Validation of the Box and Block Test as a measure of dexterity of elderly people: Reliability, validity, and norms studies. Arch. Phys. Med. Rehabil. 1994, 75, 751–755. [Google Scholar] [CrossRef]
- Mathiowetz, V.; Volland, G.; Kashman, N.; Weber, K. Adult norms for the Box and Block Test of manual dexterity. Am. J. Occup. Ther. 1985, 39, 386–391. [Google Scholar] [CrossRef]
- Wolf, S.L.; Catlin, P.A.; Ellis, M.; Archer, A.L.; Morgan, B.; Piacentino, A. Assessing Wolf motor function test as outcome measure for research in patients after stroke. Stroke 2001, 32, 1635–1639. [Google Scholar] [CrossRef]
- Roda-Sales, A.; Vergara, M.; Sancho-Bru, J.L.; Gracia-Ibanez, V.; Jarque-Bou, N.J. Human hand kinematic data during feeding and cooking tasks. Sci. Data 2019, 6, 167. [Google Scholar] [CrossRef]
- Ko, P.H.; Hwang, Y.H.; Liang, H.W. Influence of smartphone use styles on typing performance and biomechanical exposure. Ergonomics 2016, 59, 821–828. [Google Scholar] [CrossRef]
- Zhao, C.G.; Ju, F.; Sun, W.; Jiang, S.; Xi, X.; Wang, H.; Sun, X.L.; Li, M.; Xie, J.; Zhang, K.; et al. Effects of Training with a Brain-Computer Interface-Controlled Robot on Rehabilitation Outcome in Patients with Subacute Stroke: A Randomized Controlled Trial. Neurol. Ther. 2022, 11, 679–695. [Google Scholar]
- Prasad, G.; Herman, P.; Coyle, D.; McDonough, S.; Crosbie, J. Applying a brain-computer interface to support motor imagery practice in people with stroke for upper limb recovery: A feasibility study. J. Neuroeng. Rehabil. 2010, 7, 60. [Google Scholar] [CrossRef]
- Fu, J.; Chen, S.; Jia, J. Sensorimotor Rhythm-Based Brain-Computer Interfaces for Motor Tasks Used in Hand Upper Extremity Rehabilitation after Stroke: A Systematic Review. Brain. Sci. 2022, 13, 56. [Google Scholar] [CrossRef]
- Lin, J.H.; Hsu, M.J.; Sheu, C.F.; Wu, T.S.; Lin, R.T.; Chen, C.H.; Hsieh, C.L. Psychometric comparisons of 4 measures for assessing upper-extremity function in people with stroke. Phys. Ther. 2009, 89, 840–850. [Google Scholar] [CrossRef]
- Carey, L.M.; Matyas, T.A.; Baum, C. Effects of somatosensory impairment on participation after stroke. Am. J. Occup. Ther. 2018, 72, 7203205100p1–7203205100p10. [Google Scholar]
- Schinwelski, M.; Sławek, J. Prevalence of spasticity following stroke and its impact on quality of life with emphasis on disability in activities of daily living. Systematic review. Neurol. Neurochir. Pol. 2010, 44, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Oosterwijk, A.M.; Nieuwenhuis, M.K.; van der Schans, C.P.; Mouton, L.J. Shoulder and elbow range of motion for the performance of activities of daily living: A systematic review. Physiother. Theory Pract. 2018, 34, 505–528. [Google Scholar] [CrossRef]
- Bland, M.D.; Beebe, J.A.; Hardwick, D.D.; Lang, C.E. Restricted active range of motion at the elbow, forearm, wrist, or fingers decreases hand function. J. Hand Ther. 2008, 21, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Turner-Stokes, L.; Ashford, S.; Esquenazi, A.; Wissel, J.; Ward, A.B.; Francisco, G.; Lains, J.; Suputtitada, A.; Serrano, S.; Baguley, I.J.; et al. A comprehensive person-centered approach to adult spastic paresis: A consensus-based framework. Eur. J. Phys. Rehabil. Med. 2018, 54, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, T.; Hada, T.; Sasaki, N.; Kida, H.; Takahashi, J.; Maku, T.; Nakada, R.; Shiraishi, T.; Akiyama, S.; Kitagawa, T.; et al. Effects and safety of high-frequency rTMS in acute intracerebral hemorrhage patients: A pilot study. J. Neurol. Sci. 2022, 443, 120473. [Google Scholar] [CrossRef]
- Simmonds, K.P.; Luo, Z.; Reeves, M. Race/ethnic and stroke subtype differences in poststroke functional recovery after acute rehabilitation. Arch. Phys. Med. Rehabil. 2021, 102, 1473–1481. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).