Do Sleep-Related Metacognitive Strategies Shape My Sleep? The Relationships between Strategies for Controlling Sleep-Related Intrusive Thoughts and Subjective and Objective Sleep Quality in Young Adulthood and Older Age
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Materials
2.2.1. Measures of Subjective and Objective Sleep Quality
2.2.2. Sleep-Related Metacognitive Strategies
2.3. Procedure
2.4. Statistical Analyses
3. Results
3.1. Subjective Sleep Parameters
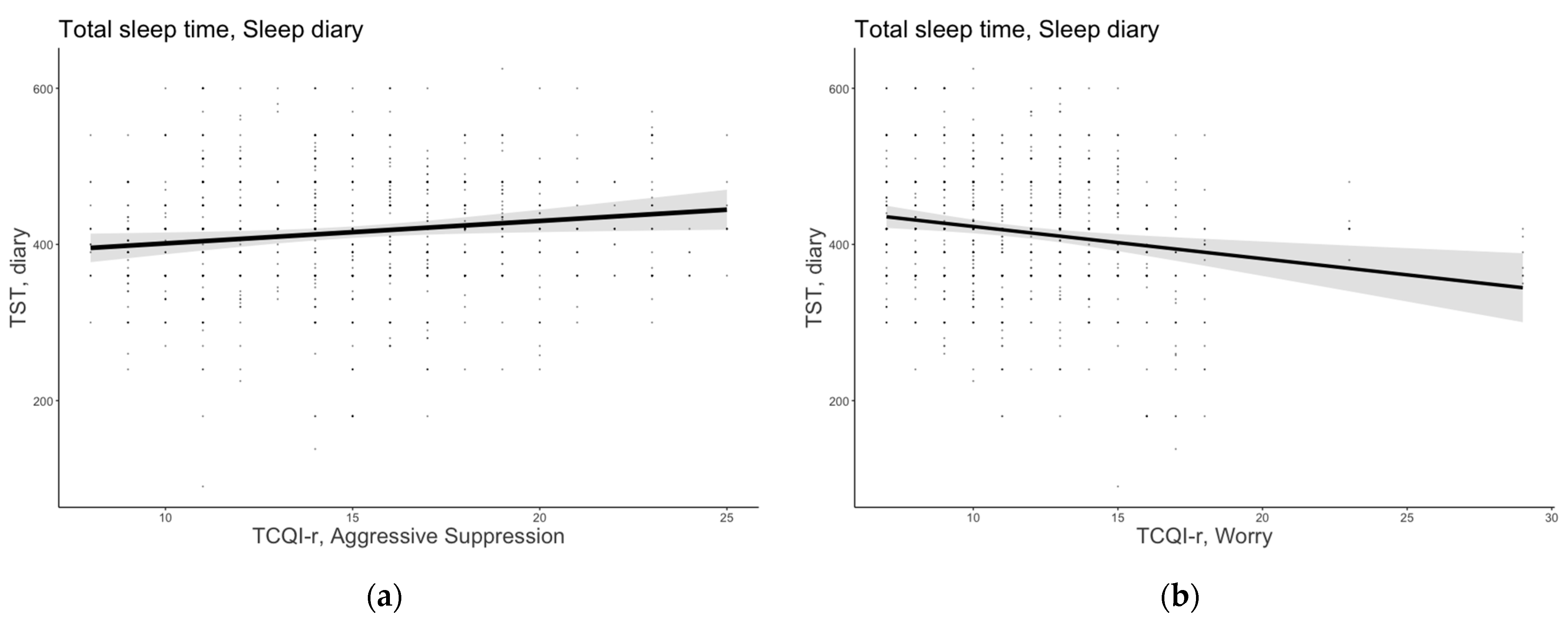
3.1.1. Total Sleep Time
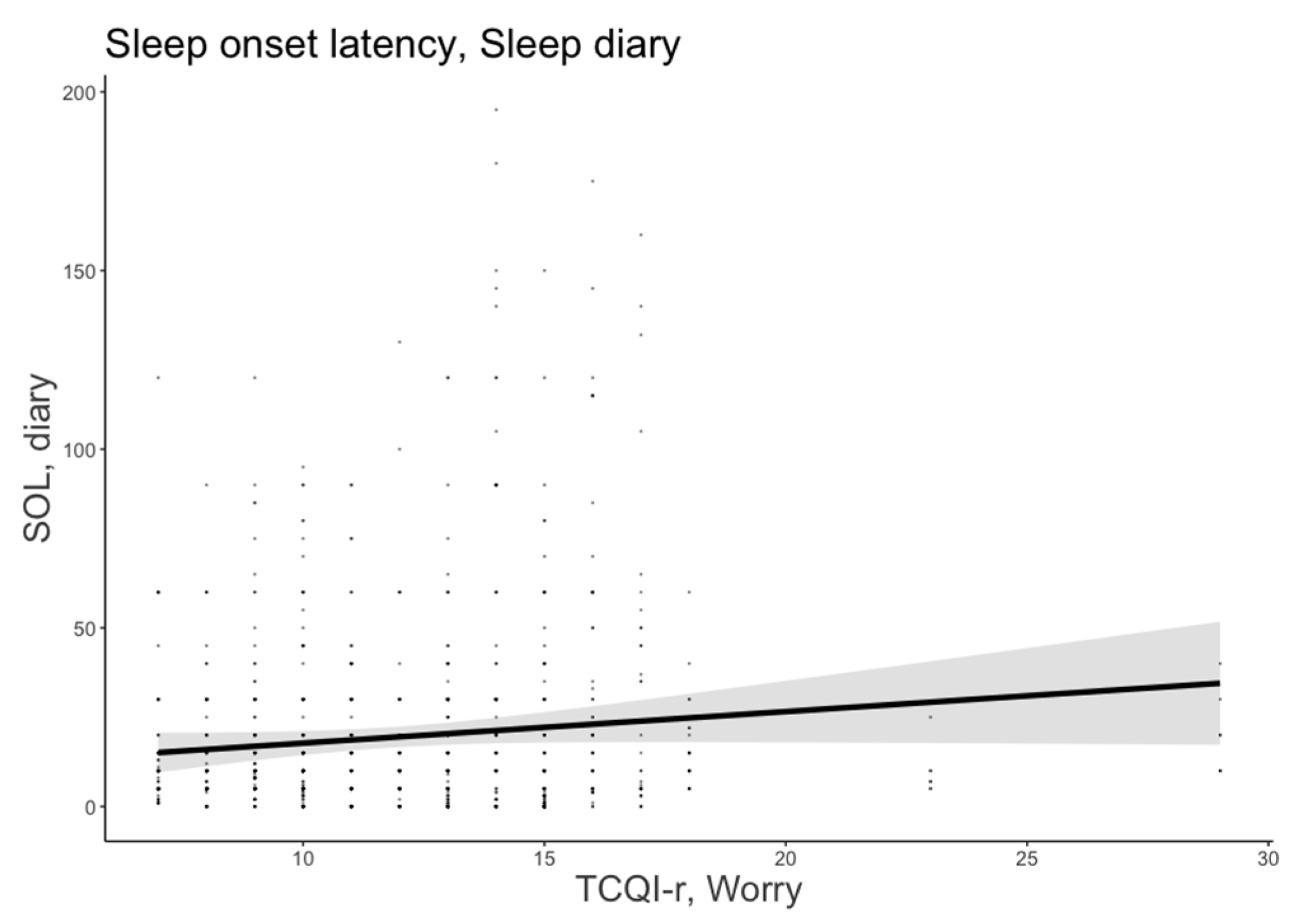
3.1.2. Sleep Onset Latency
3.1.3. Sleep Efficiency
3.2. Objective (Actigraphic) Sleep Parameters
3.2.1. Total Sleep Time
3.2.2. Sleep Onset Latency
3.2.3. Sleep Efficiency
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sella, E.; Miola, L.; Toffalini, E.; Borella, E. The relationship between sleep quality and quality of life in aging: A systematic review and meta-analysis. Health Psychol. Rev. 2021, 1–23. [Google Scholar] [CrossRef]
- Mander, B.A.; Winer, J.R.; Walker, M.P. Sleep and human aging. Neuron 2017, 94, 19–36. [Google Scholar] [CrossRef]
- Ohayon, M.; Wickwire, E.M.; Hirshkowitz, M.; Albert, S.M.; Avidan, A.; Daly, F.J.; Dauvilliers, Y.; Ferri, R.; Fung, C.; Gozal, D.; et al. National Sleep Foundation’s sleep quality recommendations: First report. Sleep Health 2017, 3, 6–19. [Google Scholar] [CrossRef]
- Ancoli-Israel, S. Sleep and its disorders in aging populations. Sleep Med. 2009, 10, S7–S11. [Google Scholar] [CrossRef]
- Martin, J.L.; Ancoli-Israel, S. Sleep disturbances in long-term care. Clin. Geriatr. Med. 2008, 24, 39–50. [Google Scholar] [CrossRef]
- Ree, M.J.; Harvey, A.G.; Blake, R.; Tang, N.K.; Shawe-Taylor, M. Attempts to control unwanted thoughts in the night: Development of the thought control questionnaire-insomnia revised (TCQI-R). Behav. Res. Ther. 2005, 43, 985–998. [Google Scholar] [CrossRef]
- Harvey, A.G. I can’t sleep, my mind is racing! An investigation of strategies of thought control in insomnia. Behav. Cogn. Psychother. 2001, 29, 3–11. [Google Scholar] [CrossRef]
- Sella, E.; Cellini, N.; Miola, L.; Sarlo, M.; Borella, E. The influence of metacognitive beliefs on sleeping difficulties in older adults. Appl. Psychol. Health Well-Being 2019, 11, 20–41. [Google Scholar] [CrossRef] [PubMed]
- Sella, E.; Borella, E. Strategies for controlling sleep-related intrusive thoughts, and subjective and objective sleep quality: How self-reported poor and good sleepers differ. Aging Ment. Health 2021, 25, 1959–1966. [Google Scholar] [CrossRef]
- Ellis, J.; Cropley, M. An examination of thought control strategies employed by acute and chronic insomniacs. Sleep Med. 2002, 3, 393–400. [Google Scholar] [CrossRef]
- Bélanger, L.; Morin, C.M.; Gendron, L.; Blais, F.C. Presleep cognitive activity and thought control strategies in insomnia. J. Cogn. Psychother. 2005, 19, 19–28. [Google Scholar] [CrossRef]
- Harvey, A.G.; Gregory, A.M.; Bird, C. The role of cognitive processes in sleep disturbance: A comparison of Japanese and English university students. Behav. Cogn. Psychother. 2002, 30, 259–270. [Google Scholar] [CrossRef]
- Wicklow, A.; Espie, C.A. Intrusive thoughts and their relationship to actigraphic measurement of sleep: Towards a cognitive model of insomnia. Behav. Res. Ther. 2000, 38, 679–693. [Google Scholar] [CrossRef]
- Harvey, A.G. The attempted suppression of presleep cognitive activity in insomnia. Cognit. Ther. Res. 2003, 27, 593–602. [Google Scholar] [CrossRef]
- Lichstein, K.L.; Rosenthal, T.L. Insomniacs’ perceptions of cognitive versus somatic determinants of sleep disturbance. J. Abnorm. Psychol. 1980, 89, 105. [Google Scholar] [CrossRef]
- Watts, F.N.; East, M.P.; Coyle, K. Insomniacs’ perceived lack of control over sleep. Psychol. Health 1995, 10, 81–95. [Google Scholar] [CrossRef]
- Gellis, L.A.; Park, A. Nighttime thought control strategies and insomnia severity. Cognit. Ther. Res. 2013, 37, 383–389. [Google Scholar] [CrossRef]
- Schmidt, R.E.; Harvey, A.G.; Van der Linden, M. Cognitive and affective control in insomnia. Front. Psychol. 2011, 2, 349. [Google Scholar] [CrossRef]
- Buysse, D.J. Sleep health: Can we define it? Does it matter? Sleep 2014, 37, 9–17. [Google Scholar] [CrossRef]
- Casagrande, M.; Forte, G.; Favieri, F.; Corbo, I. Sleep Quality and Aging: A Systematic Review on Healthy Older People, Mild Cognitive Impairment and Alzheimer’s Disease. IJERPH 2022, 19, 8457. [Google Scholar] [CrossRef]
- Schutte-Rodin, S.; Broch, L.; Buysse, D.; Dorsey, C.; Sateia, M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J. Clin. Sleep Med. 2008, 4, 487–504. [Google Scholar] [CrossRef]
- van den Berg, J.F.; Miedema, H.M.; Tulen, J.H.; Hofman, A.; Neven, A.K.; Tiemeier, H. Sex differences in subjective and actigraphic sleep measures: A population-based study of elderly persons. Sleep 2009, 32, 1367–1375. [Google Scholar] [CrossRef]
- Cellini, N.; Menghini, L.; Mercurio, M.; Vanzetti, V.; Bergamo, D.; Sarlo, M. Sleep quality and quantity in Italian University students: An actigraphic study. Chronobiol. Int. 2020, 37, 1538–1551. [Google Scholar] [CrossRef]
- Carrier, J.; Monk, T.H. Circadian rhythms of performance: New trends. Chronobiol. Int. 2000, 17, 719–732. [Google Scholar] [CrossRef]
- Kitamura, S.; Hida, A.; Watanabe, M.; Enomoto, M.; Aritake-Okada, S.; Moriguchi, Y.; Kamei, Y.; Mishima, K. Evening preference is related to the incidence of depressive states independent of sleep-wake conditions. Chronobiol. Int. 2010, 27, 1797–1812. [Google Scholar] [CrossRef]
- Inomata, Y.; Echizenya, M.; Takeshima, M.; Shimizu, K.; Shimizu, T. Validity and reliability of the Japanese version of the Morningness-Eveningness Questionnaire evaluated from actigraphy. Sleep Biol. Rhythms. 2014, 12, 289–296. [Google Scholar] [CrossRef]
- Vitale, J.A.; Roveda, E.; Montaruli, A.; Galasso, L.; Weydahl, A.; Caumo, A.; Carandente, F. Chronotype influences activity circadian rhythm and sleep: Differences in sleep quality between weekdays and weekend. Chronobiol. Int. 2015, 32, 405–415. [Google Scholar] [CrossRef]
- Yesavage, J.A.; Brink, T.L.; Rose, T.L.; Lum, O.; Huang, V.; Adey, M.; Leirer, V.O. Development and validation of a geriatric depression screening scale: A preliminary report. J. Psychiatr. Res. 1982, 17, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Steer, R.A.; Ball, R.; Ranieri, W.F. Comparison of Beck Depression Inventories-IA and-II in psychiatric outpatients. J. Pers. Assess. 1996, 67, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Spielberger, C.D. State-Trait Anxiety Inventory for Adults; American Psychological Association: Washington, DC, USA, 1983. [Google Scholar]
- Pedrabissi, L.; Santinello, M. Verifica della validità dello STAI forma Y di Spielberger. Boll. Psicol. Appl. 1989, 191–192, 11–12. [Google Scholar]
- Gallina, P.; Saugo, M.; Antoniazzi, M.; Fortuna, P.; Toffanin, R.; Maggi, S. Validazione della Scheda per la Valutazione Multidimensionale dell’Anziano (SVAMA). Tendenze Nuove 2006, 6, 229–264. [Google Scholar]
- De Beni, R.; Borella, E.; Carretti, B.; Marigo, C.; Nava, L.A. Portfolio per la Valutazione del Benessere e delle Abilit a Cognitive nell’età Adulta e Avanzata [The Assesment of Well-Being and Cognitive Abilities in Adulthood and Aging]; Giunti OS: Firenze, Italy, 2008. [Google Scholar]
- Buysse, D.J.; Reynolds III, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Natale, V.; Esposito, M.J.; Martoni, M.; Fabbri, M. Validity of the reduced version of the Morningness–Eveningness Questionnaire. Sleep Biol Rhythms. 2006, 4, 72–74. [Google Scholar] [CrossRef]
- Adan, A.; Almirall, H. Horne & Östberg morningness-eveningness questionnaire: A reduced scale. Pers Individ Differ. 1991, 12, 241–253. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Respironics, P. Actiware Software: Actiwatch Instruction Manual, software version 5.52. 0003; Mini Mitter Company: Bend, OR, USA, 2005. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. lme4: Linear Mixed-Effects Models Using Eigen and S4. R Package Version 1.1-31. 2015. Available online: http://CRAN.R-project.org/package=lme4 (accessed on 28 January 2023).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Pinheiro, J.C.; Bates, D.M. Linear mixed-effects models: Basic concepts and examples. In Mixed-Effects Models in S and S-PLUS; Springer: New York, NY, USA, 2000; pp. 3–56. [Google Scholar] [CrossRef]
- Akaike, H. Maximum likelihood identification of Gaussian autoregressive moving average models. Biometrika 1973, 60, 255–265. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R.; Huyvaert, K.P. AIC model selection and multimodel inference in behavioral ecology: Some background, observations, and comparisons. Behav. Ecol. Sociobiol. 2011, 65, 23–35. [Google Scholar] [CrossRef]
- Wagenmakers, E.J.; Farrell, S. AIC model selection using Akaike weights. Psychon. Bull. Rev. 2004, 11, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Barton, K.; Barton, M.K.; Package ‘MuMin’. R Package Version, 1.47.1. 2015. Available online: https://cran.r-project.org/web/packages/MuMIn/index.html (accessed on 28 January 2023).
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Clancy, F.; Prestwich, A.; Caperon, L.; Tsipa, A.; O’Connor, D.B. The association between worry and rumination with sleep in non-clinical populations: A systematic review and meta-analysis. Health Psychol. Rev. 2020, 14, 427–448. [Google Scholar] [CrossRef] [PubMed]
- Cropley, M.; Dijk, D.J.; Stanley, N. Job strain, work rumination, and sleep in school teachers. Eur. J. Work Organ. Psychol. 2006, 15, 181–196. [Google Scholar] [CrossRef]
- Nota, J.A.; Coles, M.E. Duration and timing of sleep are associated with repetitive negative thinking. Cognit. Ther. Res. 2015, 39, 253–261. [Google Scholar] [CrossRef]
- John, O.P.; Gross, J.J. Healthy and unhealthy emotion regulation: Personality processes, individual differences, and life span development. J. Pers. 2004, 72, 1301–1334. [Google Scholar] [CrossRef] [PubMed]
- Wells, A.; Matthews, G. Self-consciousness and cognitive failures as predictors of coping in stressful episodes. Cogn. Emot. 1994, 8, 279–295. [Google Scholar] [CrossRef]
- Morin, C.M.; Beaulieu-Bonneau, S.; LeBlanc, M.; Savard, J. Self-help treatment for insomnia: A randomized controlled trial. Sleep 2005, 28, 1319–1327. [Google Scholar] [CrossRef]
- Reed, D.L.; Sacco, W.P. Measuring sleep efficiency: What should the denominator be? J. Clin. Sleep Med. 2016, 12, 263–266. [Google Scholar] [CrossRef]
- Grandner, M.A.; Kripke, D.F.; Yoon, I.Y.; Youngstedt, S.D. Criterion validity of the Pittsburgh Sleep Quality Index: Investigation in a non-clinical sample. Sleep Biol. Rhythms. 2006, 4, 129–136. [Google Scholar] [CrossRef]
- Landry, G.J.; Best, J.R.; Liu-Ambrose, T. Measuring sleep quality in older adults: A comparison using subjective and objective methods. Front. Aging Neurosci. 2015, 7, 166. [Google Scholar] [CrossRef]
- Kay, D.B.; Dzierzewski, J.M.; Rowe, M.; McCrae, C.S. Greater night-to-night variability in sleep discrepancy among older adults with a sleep complaint compared to noncomplaining older adults. Behav. Sleep Med. 2013, 11, 76–90. [Google Scholar] [CrossRef]
- Ohayon, M.M.; Carskadon, M.A.; Guilleminault, C.; Vitiello, M.V. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: Developing normative sleep values across the human lifespan. Sleep 2004, 27, 1255–1273. [Google Scholar] [CrossRef] [PubMed]
- Sella, E.; Carbone, E.; Toffalini, E.; Borella, E. Self-reported sleep quality and dysfunctional sleep-related beliefs in young and older adults: Changes in times of COVID-19 lockdown. Sleep Med. 2021, 81, 127–135. [Google Scholar] [CrossRef]
- Sella, E.; Palumbo, R.; Di Domenico, A.; Borella, E. How emotions induced by reading influence sleep quality in young and older adults. Aging Ment. Health 2022, 1–9. [Google Scholar] [CrossRef]
- Bonnet, M.H.; Arand, D.L. Clinical effects of sleep fragmentation versus sleep deprivation. Sleep Med. Rev. 2003, 7, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.; Thayer, J.F.; Germain, A.; Moul, D.; Vasko, R.; Puhl, M.; Miewald, J.; Buysse, D.J. Psychological stress is associated with heightened physiological arousal during NREM sleep in primary insomnia. Behav. Sleep Med. 2007, 5, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Rösler, L.; van Der Lande, G.; Leerssen, J.; Cox, R.; Ramautar, J.R.; van Someren, E.J. Actigraphy in studies on insomnia: Worth the effort? J. Sleep Res. 2022, 32, e13750. [Google Scholar] [CrossRef] [PubMed]
| M | SD | |
|---|---|---|
| Age (years) | 45.35 | 20.53 |
| Female (%) | 60% | - |
| Education (years) | 14.95 | 3.96 |
| Circadian preference | ||
| MEQ-r (score) | 15.71 | 3.54 |
| Sleep quality | ||
| PSQI (score) | 4.99 | 1.77 |
| Sleep diary * | ||
| TST (minutes) | 415.38 | 76.18 |
| SOL (minutes) | 19.46 | 27.75 |
| SE (%) | 92.02 | 11.31 |
| Actigraphy * | ||
| TST (minutes) | 398.84 | 72.99 |
| SOL (minutes) | 10.74 | 17.14 |
| SE (%) | 85.16 | 9.52 |
| Thought control strategies | ||
| TCQI-r, Agg Supp (score) | 14.66 | 3.86 |
| TCQI-r, Cogn Dist (score) | 12.32 | 2.98 |
| TCQI-r, Behav Dist (score) | 10.11 | 3.82 |
| TCQI-r, Reapp (score) | 15.01 | 4.13 |
| TCQI-r, Worry (score) | 11.39 | 2.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sella, E.; Carbone, E.; Borella, E. Do Sleep-Related Metacognitive Strategies Shape My Sleep? The Relationships between Strategies for Controlling Sleep-Related Intrusive Thoughts and Subjective and Objective Sleep Quality in Young Adulthood and Older Age. Brain Sci. 2023, 13, 271. https://doi.org/10.3390/brainsci13020271
Sella E, Carbone E, Borella E. Do Sleep-Related Metacognitive Strategies Shape My Sleep? The Relationships between Strategies for Controlling Sleep-Related Intrusive Thoughts and Subjective and Objective Sleep Quality in Young Adulthood and Older Age. Brain Sciences. 2023; 13(2):271. https://doi.org/10.3390/brainsci13020271
Chicago/Turabian StyleSella, Enrico, Elena Carbone, and Erika Borella. 2023. "Do Sleep-Related Metacognitive Strategies Shape My Sleep? The Relationships between Strategies for Controlling Sleep-Related Intrusive Thoughts and Subjective and Objective Sleep Quality in Young Adulthood and Older Age" Brain Sciences 13, no. 2: 271. https://doi.org/10.3390/brainsci13020271
APA StyleSella, E., Carbone, E., & Borella, E. (2023). Do Sleep-Related Metacognitive Strategies Shape My Sleep? The Relationships between Strategies for Controlling Sleep-Related Intrusive Thoughts and Subjective and Objective Sleep Quality in Young Adulthood and Older Age. Brain Sciences, 13(2), 271. https://doi.org/10.3390/brainsci13020271







