Immunohistochemical Distribution and Neurochemical Characterization of Huntingtin-Associated Protein 1 Immunoreactive Neurons in the Adult Mouse Lingual Ganglia
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Characterization of Primary Antibodies
2.3. Tissue Preparations for Immunohistochemistry
2.4. Single-Label Immunohistochemistry
2.5. Double-Label Immunohistochemistry
2.6. Imaging
2.7. Cell Counting
3. Results
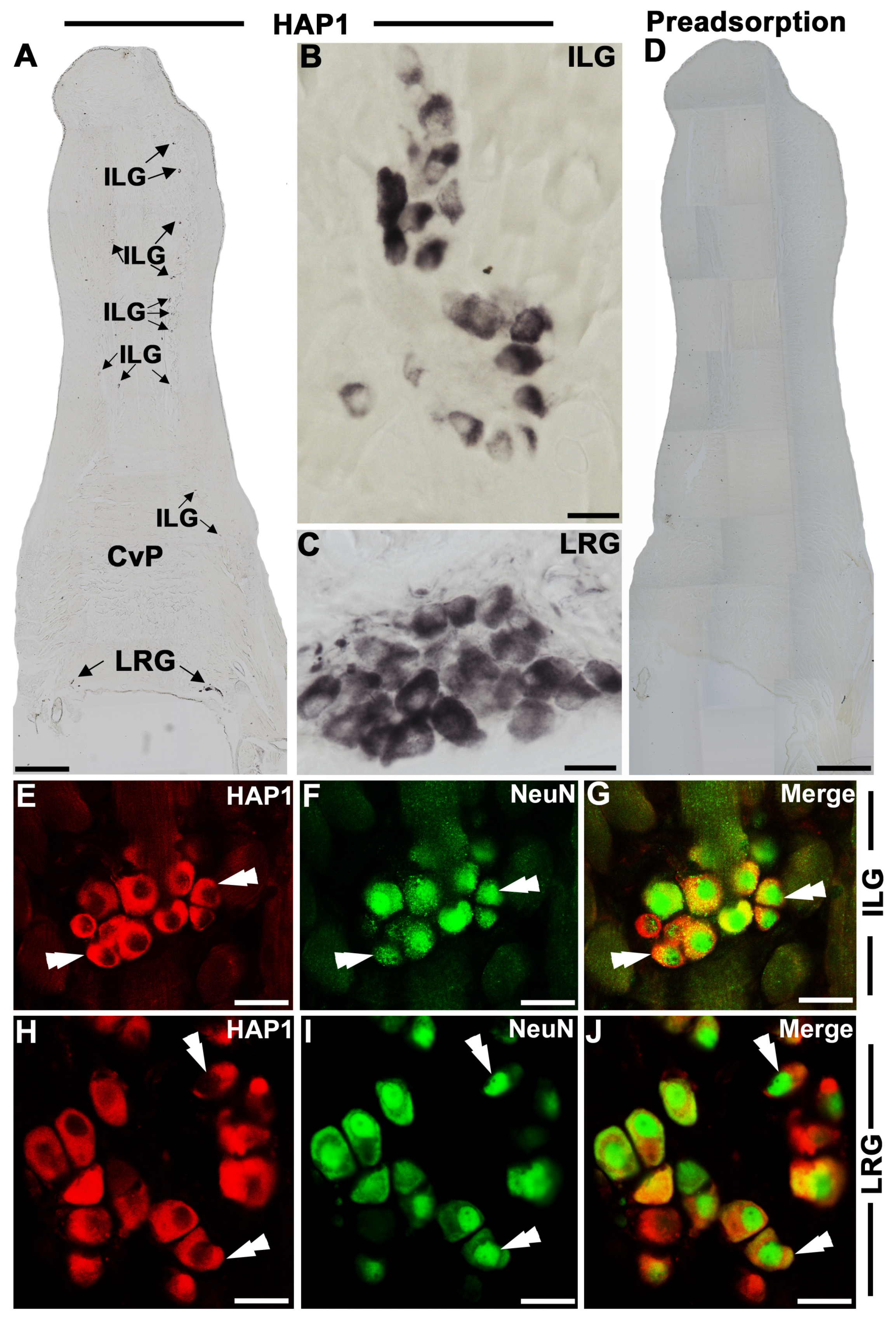
3.1. Immunohistochemical Expression and Distribution of HAP1 in the Lingual Ganglia
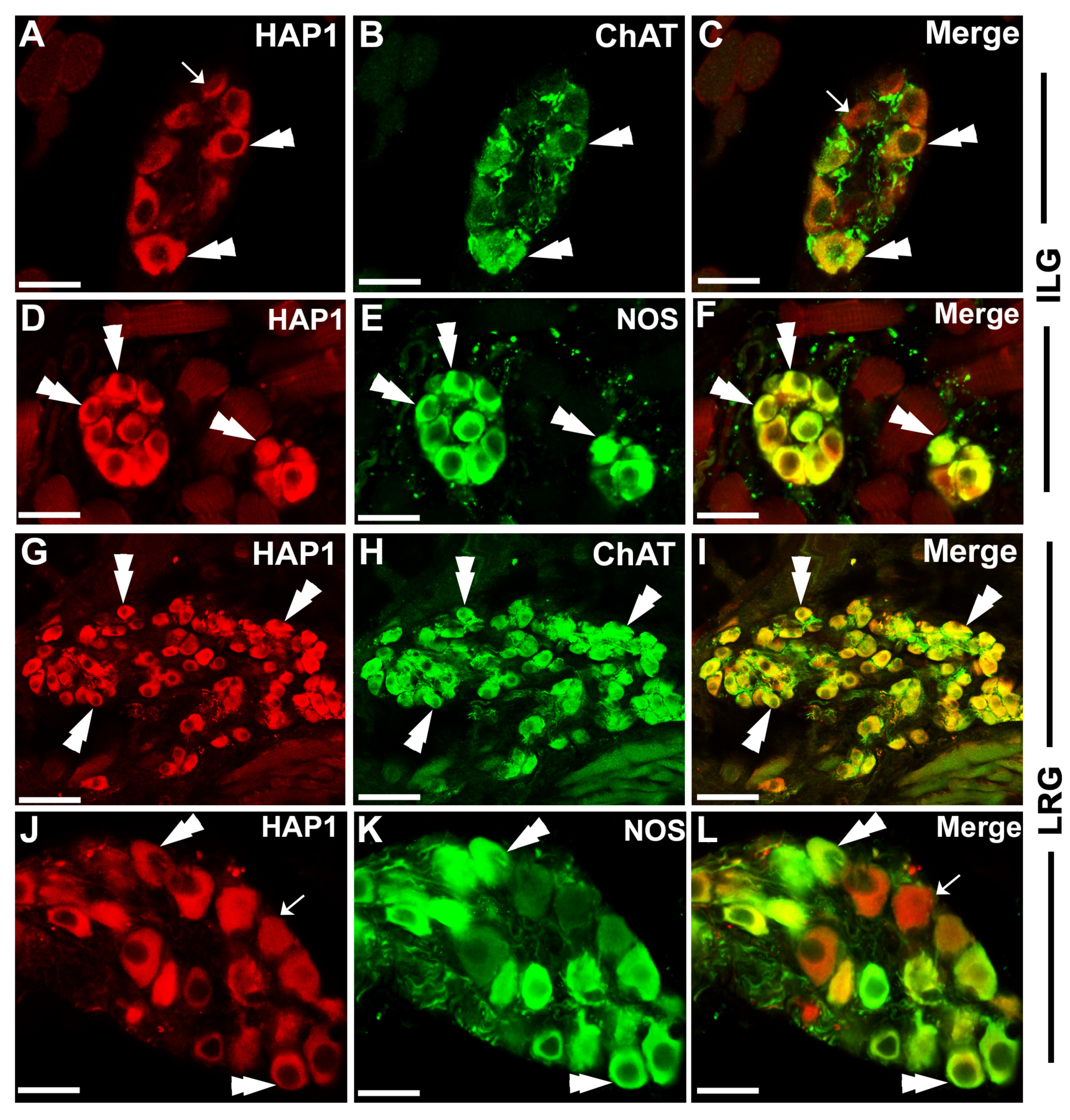
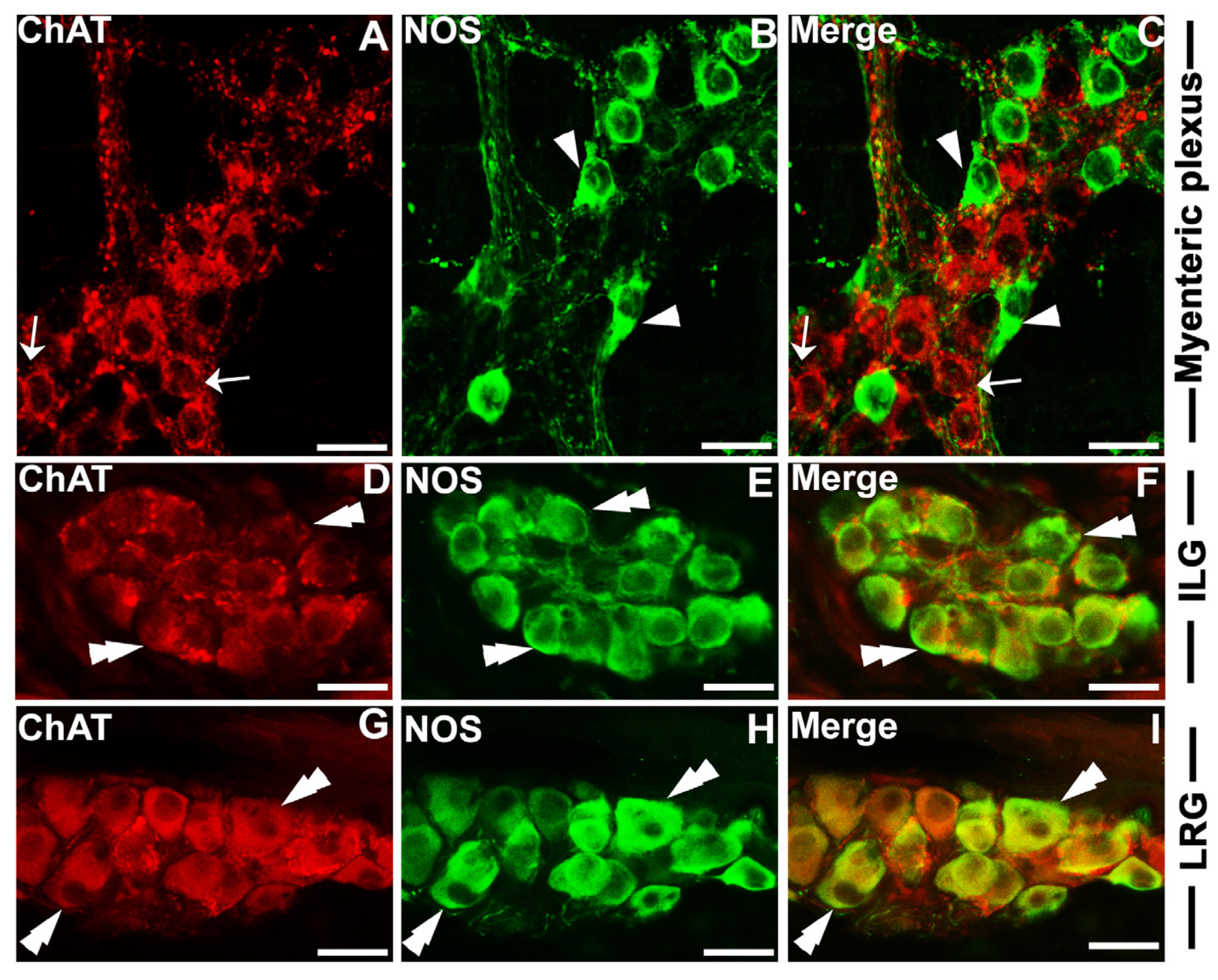
3.2. Immunohistochemical Relationships of HAP1 with the Markers of Parasympathetic Neurons
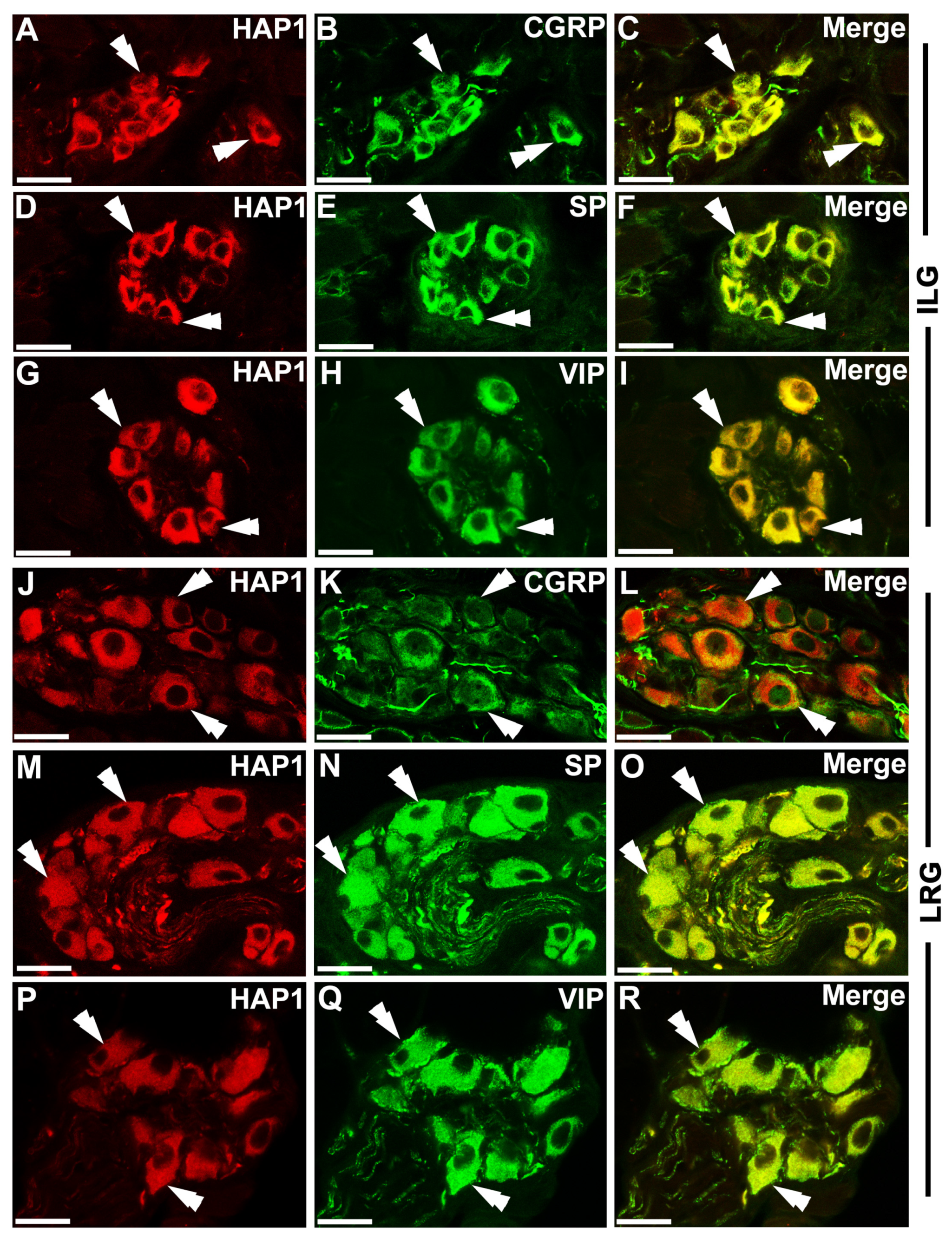
3.3. Immunohistochemical Relationships of HAP1 with CGRP, SP, and VIP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.-J.; Li, S.-H.; Sharp, A.H.; Nucifora, F.C.; Schilling, G.; Lanahan, A.; Worley, P.; Snyder, S.H.; Ross, C.A. A Huntingtin-Associated Protein Enriched in Brain with Implications for Pathology. Nature 1995, 378, 398–402. [Google Scholar] [CrossRef]
- Li, S.-H.; Yu, Z.-X.; Li, C.-L.; Nguyen, H.-P.; Zhou, Y.-X.; Deng, C.; Li, X.-J. Lack of Huntingtin-Associated Protein-1 Causes Neuronal Death Resembling Hypothalamic Degeneration in Huntington’s Disease. J. Neurosci. 2003, 23, 6956–6964. [Google Scholar] [CrossRef]
- Metzger, S.; Rong, J.; Nguyen, H.-P.; Cape, A.; Tomiuk, J.; Soehn, A.S.; Propping, P.; Freudenberg-Hua, Y.; Freudenberg, J.; Tong, L.; et al. Huntingtin-Associated Protein-1 Is a Modifier of the Age-at-Onset of Huntington’s Disease. Hum. Mol. Genet. 2008, 17, 1137–1146. [Google Scholar] [CrossRef]
- Liu, Q.; Cheng, S.; Yang, H.; Zhu, L.; Pan, Y.; Jing, L.; Tang, B.; Li, S.; Li, X.-J. Loss of Hap1 Selectively Promotes Striatal Degeneration in Huntington Disease Mice. Proc. Natl. Acad. Sci. USA 2020, 117, 20265–20273. [Google Scholar] [CrossRef]
- Shinoda, K.; Mori, S.; Ohtsuki, T.; Osawa, Y. An Aromatase-Associated Cytoplasmic Inclusion, the “Stigmoid Body”, In the Rat Brain: I. Distribution in the Forebrain. J. Comp. Neurol. 1992, 322, 360–376. [Google Scholar] [CrossRef]
- Shinoda, K.; Nagano, M.; Osawa, Y. An Aromatase-Associated Cytoplasmic Inclusion, the “Stigmoid Body” In the Rat Brain: II. Ultrastructure (with a Review of Its History and Nomenclature). J. Comp. Neurol. 1993, 329, 1–19. [Google Scholar] [CrossRef]
- Fujinaga, R.; Kawano, J.; Matsuzaki, Y.; Kamei, K.; Yanai, A.; Sheng, Z.; Tanaka, M.; Nakahama, K.-I.; Nagano, M.; Shinoda, K. Neuroanatomical Distribution of Huntingtin-Associated Protein 1-MRNA in the Male Mouse Brain. J. Comp. Neurol. 2004, 478, 88–109. [Google Scholar] [CrossRef]
- Islam, M.N.; Fujinaga, R.; Yanai, A.; Jahan, M.R.; Takeshita, Y.; Kokubu, K.; Shinoda, K. Characterization of the “Sporadically Lurking HAP1-Immunoreactive (SLH) Cells” in the Hippocampus, with Special Reference to the Expression of Steroid Receptors, GABA, and Progenitor Cell Markers. Neuroscience 2012, 210, 67–81. [Google Scholar] [CrossRef]
- Islam, M.N.; Takeshita, Y.; Yanai, A.; Imagawa, A.; Jahan, M.R.; Wroblewski, G.; Nemoto, J.; Fujinaga, R.; Shinoda, K. Immunohistochemical Analysis of Huntingtin-Associated Protein 1 in Adult Rat Spinal Cord and Its Regional Relationship with Androgen Receptor. Neuroscience 2017, 340, 201–217. [Google Scholar] [CrossRef]
- Islam, M.N.; Maeda, N.; Miyasato, E.; Jahan, M.R.; Tarif, A.M.M.; Ishino, T.; Nozaki, K.; Masumoto, K.-h.; Yanai, A.; Shinoda, K. Expression of Huntingtin-Associated Protein 1 in Adult Mouse Dorsal Root Ganglia and Its Neurochemical Characterization in Reference to Sensory Neuron Subpopulations. IBRO Rep. 2020, 9, 258–269. [Google Scholar] [CrossRef]
- Islam, M.N.; Miyasato, E.; Jahan, M.R.; Tarif, A.M.M.; Nozaki, K.; Masumoto, K.; Yanai, A.; Shinoda, K. Mapping of STB/HAP1 Immunoreactivity in the Mouse Brainstem and Its Relationships with Choline Acetyltransferase, with Special Emphasis on Cranial Nerve Motor and Preganglionic Autonomic Nuclei. Neuroscience 2022, 499, 40–63. [Google Scholar] [CrossRef]
- Chen, X.; Xin, N.; Pan, Y.; Zhu, L.; Yin, P.; Liu, Q.; Yang, W.; Xu, X.; Li, S.; Li, X.-J. Huntingtin-Associated Protein 1 in Mouse Hypothalamus Stabilizes Glucocorticoid Receptor in Stress Response. Front. Cell. Neurosci. 2020, 14, 125. [Google Scholar] [CrossRef]
- Takeshita, Y.; Fujinaga, R.; Zhao, C.; Yanai, A.; Shinoda, K. Huntingtin-Associated Protein 1 (HAP1) Interacts with Androgen Receptor (AR) and Suppresses SBMA-Mutant-AR-Induced Apoptosis. Hum. Mol. Genet. 2006, 15, 2298–2312. [Google Scholar] [CrossRef]
- Prigge, J.R.; Schmidt, E.E. HAP1 Can Sequester a Subset of TBP in Cytoplasmic Inclusions via Specific Interaction with the Conserved TBPCORE. BMC Mol. Biol. 2007, 8, 76. [Google Scholar] [CrossRef]
- Takeshita, Y.; Fujinaga, R.; Kokubu, K.; Islam, M.N.; Jahan, M.R.; Yanai, A.; Kakizuka, A.; Shinoda, K. Interaction of Ataxin-3 with Huntingtin-Associated Protein 1 through Josephin Domain. Neuroreport 2011, 22, 232–238. [Google Scholar] [CrossRef]
- Sheng, G.; Xu, X.; Lin, Y.-F.; Wang, C.-E.; Rong, J.; Cheng, D.; Peng, J.; Jiang, X.; Li, S.-H.; Li, X.-J. Huntingtin-Associated Protein 1 Interacts with Ahi1 to Regulate Cerebellar and Brainstem Development in Mice. J. Clin. Investig. 2008, 118, 2785–2795. [Google Scholar] [CrossRef]
- Singh, A.; Dawson, T.M.; Kulkarni, S. Neurodegenerative Disorders and Gut-Brain Interactions. J. Clin. Investig. 2021, 131, e143775. [Google Scholar] [CrossRef]
- Furness, J.B. The Enteric Nervous System and Neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 286–294. [Google Scholar] [CrossRef]
- Tarif, A.M.M.; Islam, M.N.; Jahan, M.R.; Yanai, A.; Nozaki, K.; Masumoto, K.; Shinoda, K. Immunohistochemical Expression and Neurochemical Phenotypes of Huntingtin-Associated Protein 1 in the Myenteric Plexus of Mouse Gastrointestinal Tract. Cell Tissue Res. 2021, 386, 533–558. [Google Scholar] [CrossRef]
- Tarif, A.M.M.; Islam, M.N.; Jahan, M.R.; Afrin, M.; Meher, M.M.; Yanai, A.; Nozaki, K.; Masumoto, K.; Shinoda, K. Neurochemical phenotypes of huntingtin-associated protein 1 in reference to secretomotor and vasodilator neurons in the submucosal plexuses of rodent small intestine. Neurosci. Res. 2022, S0168-0102(22)00324-8. [Google Scholar] [CrossRef]
- Mu, L.; Sanders, I. Human Tongue Neuroanatomy: Nerve Supply and Motor Endplates. Clin. Anat. 2010, 23, 777–791. [Google Scholar] [CrossRef]
- Fagan, S.E.; Roy, W. Anatomy, Head and Neck, Lingual Nerve; Stat Pearls Publishing: Treasure Island, FL, USA, 2022; Volume 52. [Google Scholar]
- Sbarbati, A.; Merigo, F.; Bernardi, P.; Crescimanno, C.; Benati, D.; Osculati, F. Ganglion Cells and Topographically Related Nerves in the Vallate Papilla/von Ebner Gland Complex. J. Histochem. Cytochem. 2002, 50, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Tsumori, T.; Ando, A.; Yasui, Y. A Light and Electron Microscope Study of the Connections between the Preganglionic Fibers and the Intralingual Ganglion Cells in the Rat. Anat. Embryol. 1996, 194, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Hino, N.; Masuko, S.; Katsuki, T. An Immunohistochemical Study of Sensory and Autonomic Innervation of the Dog Tongue with Special Reference to Substance P- and Calcitonin Gene-Related Peptide-Containing Fibers in Blood Vessels and the Intralingual Ganglia. Arch. Histol. Cytol. 1993, 56, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Toda, N. Colocalization of Acetylcholinesterase and Vasoactive Intestinal Peptide (VIP) in Nicotinamide Adenine Dinucleotide Phosphate Diaphorase (NADPH-d) Positive Neurons in the Intralingual Ganglia and Perivascular Nerve Fibers around Lingual Arteries in the Po. Neurosci. Lett. 1997, 222, 147–150. [Google Scholar] [CrossRef]
- Ichikawa, H.; Matsuo, S.; Wakisaka, S.; Akai, M. Ontogeny of Cholinergic Ganglionic Cells Which Take Up L-3,4-Dihydroxyphenylalanine and Contain Vasoactive Intestinal Polypeptide in the Mouse Tongue. Cells Tissues Organs 1991, 140, 135–138. [Google Scholar] [CrossRef]
- Fehér, E.; Batbayar, B.; Zelles, T. Morphological Evidence of Sensory Neurons in the Root of the Rat Tongue. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2005, 286, 848–853. [Google Scholar] [CrossRef]
- Yanai, A.; Islam, M.N.; Hayashi-Okada, M.; Jahan, M.R.; Tarif, A.M.M.; Nozaki, K.; Masumoto, K.; Shinoda, K. Immunohistochemical Relationships of Huntingtin-Associated Protein 1 with Enteroendocrine Cells in the Pyloric Mucosa of the Rat Stomach. Acta Histochem. 2020, 122, 151650. [Google Scholar] [CrossRef]
- Islam, M.N.; Sakimoto, Y.; Jahan, M.R.; Ishida, M.; Tarif, A.M.M.; Nozaki, K.; Masumoto, K.; Yanai, A.; Mitsushima, D.; Shinoda, K. Androgen Affects the Dynamics of Intrinsic Plasticity of Pyramidal Neurons in the CA1 Hippocampal Subfield in Adolescent Male Rats. Neuroscience 2020, 440, 15–29. [Google Scholar] [CrossRef]
- Islam, M.N.; Sakimoto, Y.; Jahan, M.R.; Miyasato, E.; Tarif, A.M.M.; Nozaki, K.; Masumoto, K.; Yanai, A.; Mitsushima, D.; Shinoda, K. Androgen Affects the Inhibitory Avoidance Memory by Primarily Acting on Androgen Receptor in the Brain in Adolescent Male Rats. Brain Sci. 2021, 11, 239. [Google Scholar] [CrossRef]
- Jahan, M.R.; Kokubu, K.; Islam, M.N.; Matsuo, C.; Yanai, A.; Wroblewski, G.; Fujinaga, R.; Shinoda, K. Species Differences in Androgen Receptor Expression in the Medial Preoptic and Anterior Hypothalamic Areas of Adult Male and Female Rodents. Neuroscience 2015, 284, 943–961. [Google Scholar] [CrossRef] [PubMed]
- Nagano, M.; Shinoda, K. Coexistence of the Stigmoid Body and Estrogen Receptor in Some Neuronal Groups Involved in Rat Reproductive Functions. Brain Res. 1994, 634, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Kitada, Y. Parasympathetic Postganglionic Cells in the Glossopharyngeal Nerve Trunk and Their Relationship to Unmyelinated Nerve Fibers in the Fungiform Papillae of the Frog. Anat. Rec. 1991, 230, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.-N.; Huang, Z.-B.; Wang, H.; Rao, X.-R.; Kong, H.; Xu, J.; Li, X.-J.; Yang, C.; Sheng, G.-Q. Brainstem Hap1-Ahi1 Is Involved in Insulin-Mediated Feeding Control. FEBS Lett. 2011, 585, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Fujinaga, R.; Takeshita, Y.; Uozumi, K.; Yanai, A.; Yoshioka, K.; Kokubu, K.; Shinoda, K. Microtubule-Dependent Formation of the Stigmoid Body as a Cytoplasmic Inclusion Distinct from Pathological Aggresomes. Histochem. Cell Biol. 2009, 132, 305–318. [Google Scholar] [CrossRef]
- Fujinaga, R.; Yanai, A.; Nakatsuka, H.; Yoshida, K.; Takeshita, Y.; Uozumi, K.; Zhao, C.; Hirata, K.; Kokubu, K.; Nagano, M.; et al. Anti-Human Placental Antigen Complex X-P2 (HPAX-P2) Anti-Serum Recognizes C-Terminus of Huntingtin-Associated Protein 1A Common to 1B as a Determinant Marker for the Stigmoid Body. Histochem. Cell Biol. 2007, 128, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Wroblewski, G.; Islam, M.N.; Yanai, A.; Jahan, M.R.; Masumoto, K.; Shinoda, K. Distribution of HAP1-Immunoreactive Cells in the Retrosplenial–Retrohippocampal Area of Adult Rat Brain and Its Application to a Refined Neuroanatomical Understanding of the Region. Neuroscience 2018, 394, 109–126. [Google Scholar] [CrossRef]
- Gutekunst, C.-A.; Li, S.-H.; Yi, H.; Ferrante, R.J.; Li, X.-J.; Hersch, S.M. The Cellular and Subcellular Localization of Huntingtin-Associated Protein 1 (HAP1): Comparison with Huntingtin in Rat and Human. J. Neurosci. 1998, 18, 7674–7686. [Google Scholar] [CrossRef]
- Dragatsis, I.; Zeitlin, S.; Dietrich, P. Huntingtin-Associated Protein 1 (Hap1) Mutant Mice Bypassing the Early Postnatal Lethality Are Neuroanatomically Normal and Fertile but Display Growth Retardation. Hum. Mol. Genet. 2004, 13, 3115–3125. [Google Scholar] [CrossRef]
- Sheng, G.; Chang, G.; Lin, J.Y.; Yu, Z.-X.; Fang, Z.-H.; Rong, J.; Lipton, S.A.; Li, S.-H.; Tong, G.; Leibowitz, S.F.; et al. Hypothalamic Huntingtin-Associated Protein 1 as a Mediator of Feeding Behavior. Nat. Med. 2006, 12, 526–533. [Google Scholar] [CrossRef]
- Lin, Y.-F.; Xu, X.; Cape, A.; Li, S.; Li, X.-J. Huntingtin-Associated Protein-1 Deficiency in Orexin-Producing Neurons Impairs Neuronal Process Extension and Leads to Abnormal Behavior in Mice. J. Biol. Chem. 2010, 285, 15941–15949. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-H.; Hosseini, S.H.; Gutekunst, C.-A.; Hersch, S.M.; Ferrante, R.J.; Li, X.-J. A Human HAP1 Homologue. J. Biol. Chem. 1998, 273, 19220–19227. [Google Scholar] [CrossRef] [PubMed]
- Oomori, Y.; Iuchi, H.; Ishikawa, K.; Satoh, Y.; Ono, K. Immunocytochemical Study of Tyrosine Hydroxylase and Dopamine β-Hydroxylase Immunoreactivities in the Rat Pancreas. Histochemistry 1994, 101, 313–323. [Google Scholar] [CrossRef]
- Kittler, J.T.; Thomas, P.; Tretter, V.; Bogdanov, Y.D.; Haucke, V.; Smart, T.G.; Moss, S.J. Huntingtin-Associated Protein 1 Regulates Inhibitory Synaptic Transmission by Modulating γ-Aminobutyric Acid Type A Receptor Membrane Trafficking. Proc. Natl. Acad. Sci. USA 2004, 101, 12736–12741. [Google Scholar] [CrossRef]
- Gauthier, L.R.; Charrin, B.C.; Borrell-Pagès, M.; Dompierre, J.P.; Rangone, H.; Cordelières, F.P.; De Mey, J.; MacDonald, M.E.; Leßmann, V.; Humbert, S.; et al. Huntingtin Controls Neurotrophic Support and Survival of Neurons by Enhancing BDNF Vesicular Transport along Microtubules. Cell 2004, 118, 127–138. [Google Scholar] [CrossRef]
| Name | Immunogen | Code | Source | Dilution | Reference |
|---|---|---|---|---|---|
| Goat Polyclonal anti-HAP1 (R19) | Rat HAP1 C-terminus | Cat# sc-8770, RRID: AB_647322 | Santa Cruz Biotechnology, Santa Cruz, CA, USA | 1:10,000 | [29] |
| Rabbit Polyclonal anti-NeuN | Synthetic peptide of Human NeuN aa 1–100 | Cat# ab177487, RRID: AB_2532109 | Abcam, Cambridge, UK | 1:5000 | [10] |
| Rabbit Polyclonal anti-ChAT | Synthetic peptide within Pig Choline Acetyltransferase aa 150–250 | Cat# ab178850, RRID: AB_2721842 | Abcam, Cambridge, UK | 1:5000 | [11] |
| Goat Polyclonal anti-ChAT | Human placental ChAT | Cat# AB144P, RRID: AB_11214092 | Millipore, Billerica, MA, USA | 1:5000 | [9] |
| Rabbit Polyclonal anti-NOS | C-terminus synthetic peptide of human nNOS coupled to KLH | Cat# 24287, RRID: AB_572256 | Immunostar, Hudson, WI, USA | 1:3000 | [9] |
| Rabbit Polyclonal anti-SP | Synthetic SP coupled to KLH with carbodiimide | Cat# 20064, RRID: AB_572266 | Immunostar, Hudson, WI, USA | 1:2000 | [10] |
| Rabbit Polyclonal anti-CGRP | CGRP-KLH (rat) | Cat# C8198, RRID: AB_259091 | Sigma-Aldrich, St. Louis, MO, USA | 1:2000 | [20] |
| Rabbit Polyclonal anti-VIP | Porcine VIP coupled to bovine thyroglobulin with carbodiimide | Cat# 20077, RRID: AB_572270 | Immunostar, Hudson, WI, USA | 1:5000 | [19] |
| Relationship of HAP1 with Different Markers (ratio %) | ILG | LRG | |
|---|---|---|---|
| ChAT | ChAT/HAP1 | 84.36 ± 2.1 | 99.39 ± 0.2 |
| HAP1/ChAT | 98.42 ± 3.2 | 99.28 ± 0.3 | |
| NOS | NOS/HAP1 | 90.91 ± 1.2 | 86.47 ± 1.9 |
| HAP1/NOS | 99.37 ± 0.3 | 98.54 ± 0.7 | |
| CGRP | CGRP/HAP1 | 81.84 ± 3.2 | 81.69 ± 2.2 |
| HAP1/CGRP | 85.67 ± 2.9 | 89.27 ± 1.7 | |
| SP | SP/HAP1 | 99.68 ± 0.4 | 98.74 ± 0.4 |
| HAP1/SP | 99.57 ± 0.2 | 99.17 ± 0.3 | |
| VIP | VIP/HAP1 | 99.73 ± 0.3 | 89.29 ± 2.8 |
| HAP1/VIP | 98.19 ± 0.2 | 99.69 ± 0.3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.N.; Sakurai, Y.; Hiwaki, Y.; Tarif, A.M.M.; Afrin, M.; Meher, M.M.; Nozaki, K.; Masumoto, K.H.; Yanai, A.; Jahan, M.R.; et al. Immunohistochemical Distribution and Neurochemical Characterization of Huntingtin-Associated Protein 1 Immunoreactive Neurons in the Adult Mouse Lingual Ganglia. Brain Sci. 2023, 13, 258. https://doi.org/10.3390/brainsci13020258
Islam MN, Sakurai Y, Hiwaki Y, Tarif AMM, Afrin M, Meher MM, Nozaki K, Masumoto KH, Yanai A, Jahan MR, et al. Immunohistochemical Distribution and Neurochemical Characterization of Huntingtin-Associated Protein 1 Immunoreactive Neurons in the Adult Mouse Lingual Ganglia. Brain Sciences. 2023; 13(2):258. https://doi.org/10.3390/brainsci13020258
Chicago/Turabian StyleIslam, Md Nabiul, Yoshinori Sakurai, Yurie Hiwaki, Abu Md Mamun Tarif, Marya Afrin, Mirza Mienur Meher, Kanako Nozaki, Koh Hei Masumoto, Akie Yanai, Mir Rubayet Jahan, and et al. 2023. "Immunohistochemical Distribution and Neurochemical Characterization of Huntingtin-Associated Protein 1 Immunoreactive Neurons in the Adult Mouse Lingual Ganglia" Brain Sciences 13, no. 2: 258. https://doi.org/10.3390/brainsci13020258
APA StyleIslam, M. N., Sakurai, Y., Hiwaki, Y., Tarif, A. M. M., Afrin, M., Meher, M. M., Nozaki, K., Masumoto, K. H., Yanai, A., Jahan, M. R., & Shinoda, K. (2023). Immunohistochemical Distribution and Neurochemical Characterization of Huntingtin-Associated Protein 1 Immunoreactive Neurons in the Adult Mouse Lingual Ganglia. Brain Sciences, 13(2), 258. https://doi.org/10.3390/brainsci13020258











