An Interpretable Machine Learning Model to Predict Cortical Atrophy in Multiple Sclerosis
Abstract
1. Introduction
2. Materials and Methods
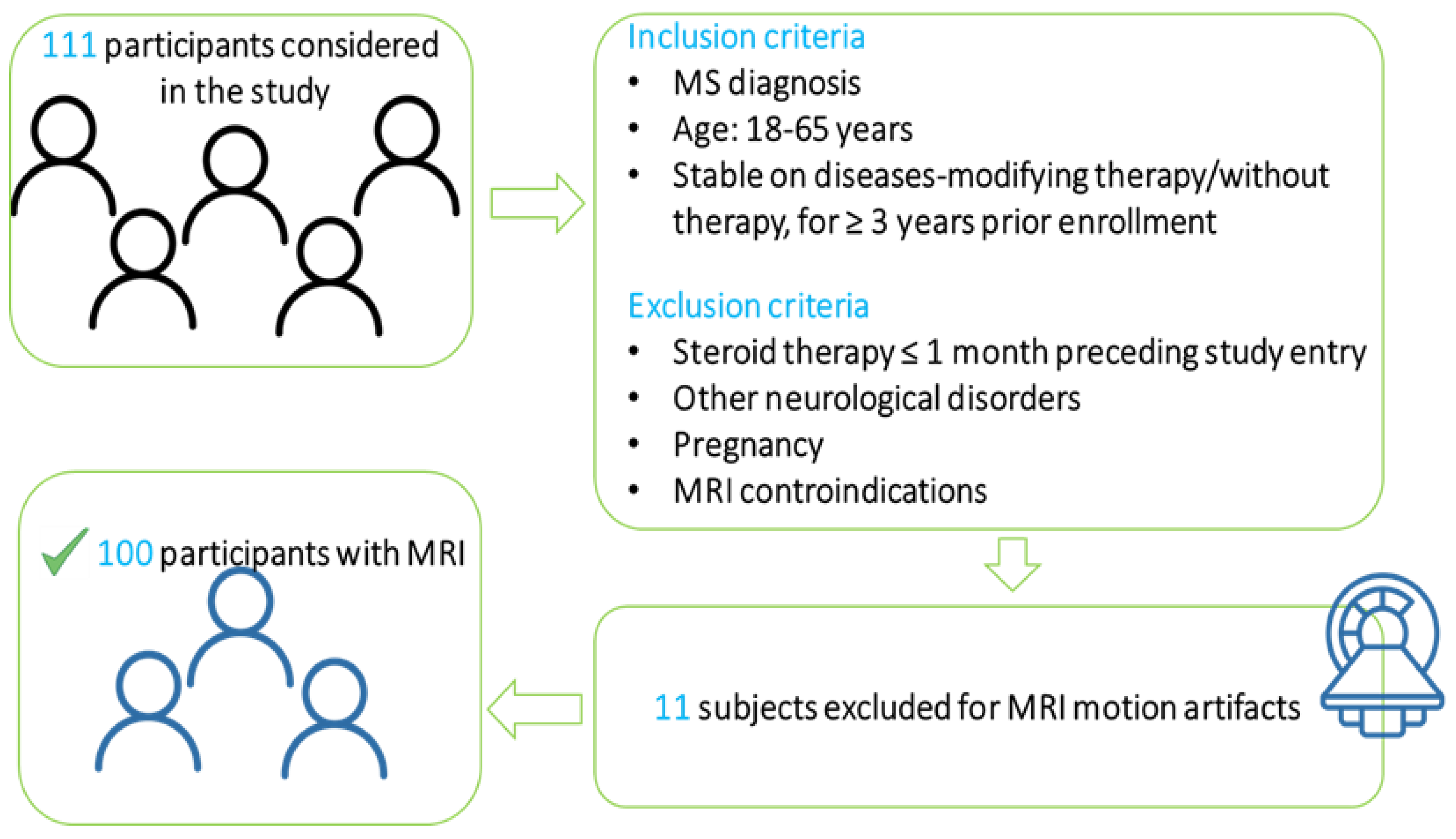
2.1. Patient Population
2.2. MRI Protocol
2.3. Image Processing
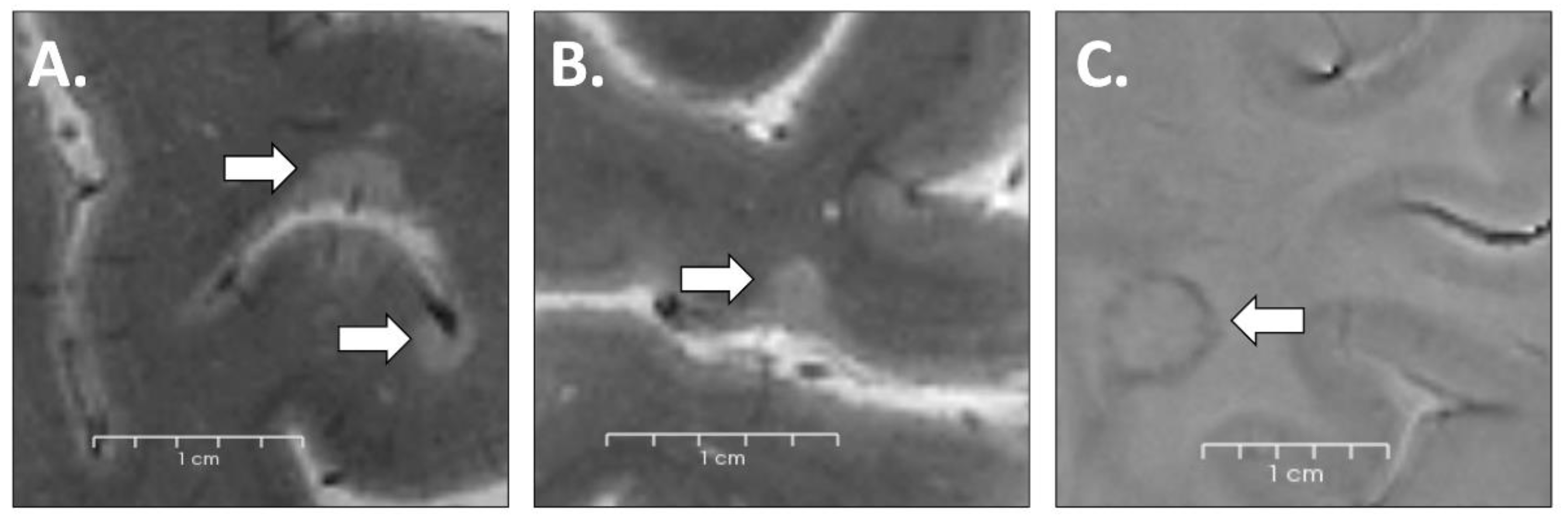
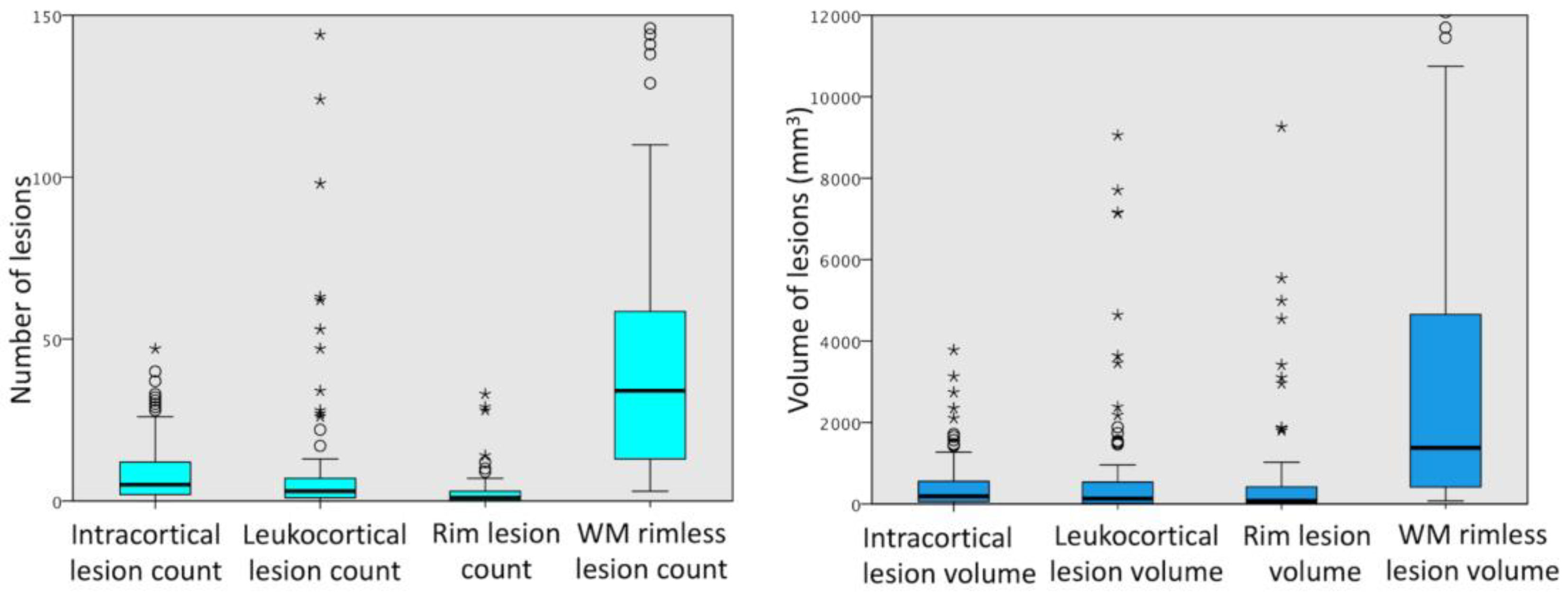
2.4. Lesion Identification
2.5. Predictive Model
- (i)
- We randomly divided the data set in a stratified manner into training and test sets (70% and 30%, respectively).
- (ii)
- We used a grid search (5-fold cross-validation) during training to optimize model hyperparameters (maximum depth of a tree; step size shrinkage used in update to prevent overfitting; the minimum sum of instance weight needed in a child).
- (iii)
- We evaluated model performances by calculating, through the same Python script, the Pearson correlation (r) and p value between the real and predicted values in the test set.
- (iv)
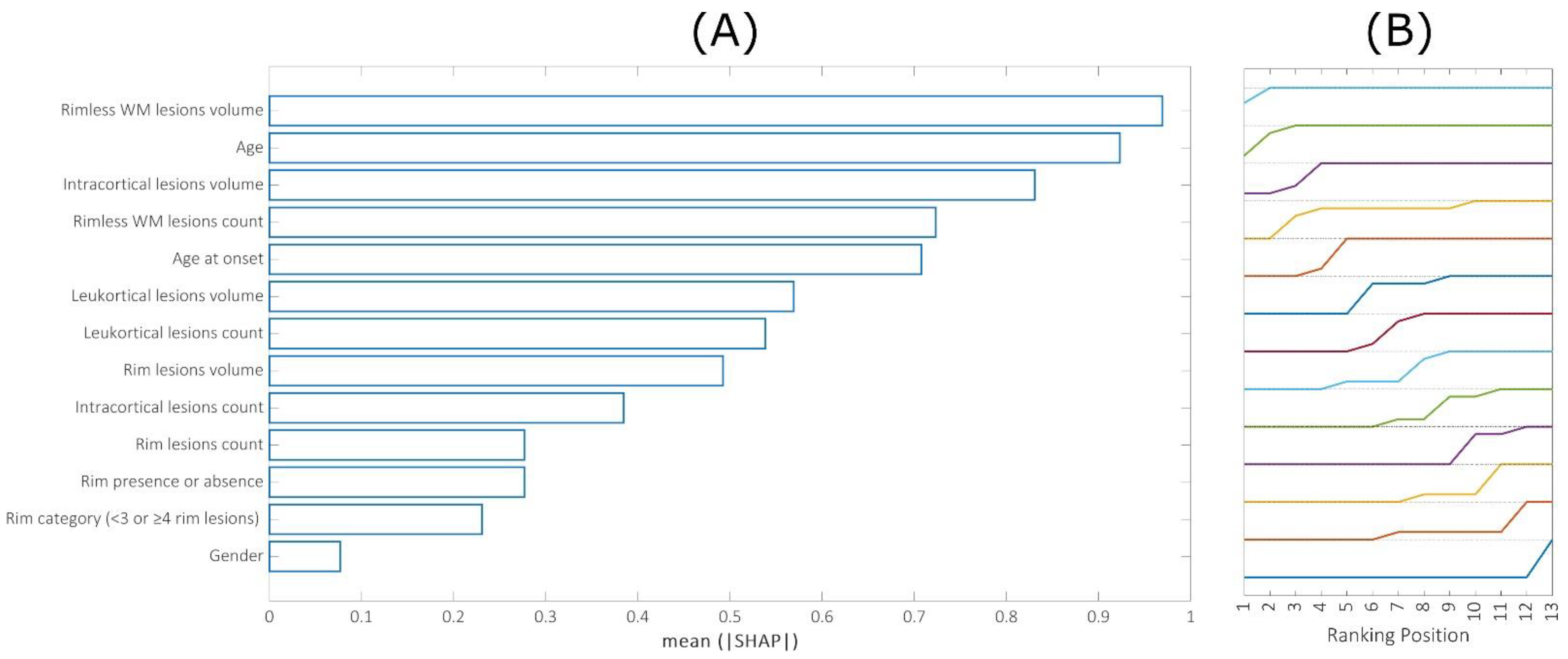
- The individual and cumulative contribution of each feature to the final prediction was assessed by calculating the Shapley additive explanations (SHAP) values [20].
- (v)
- By repeating 50 times all the procedure on different 70/30 randomly split training and test sets, it was possible to obtain a confidence interval for both r- and p-values, as well as average SHAP values across repetitions.
3. Results
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wallin, M.T.; Culpepper, W.J.; Nichols, E.; Bhutta, Z.A.; Gebrehiwot, T.T.; Hay, S.I.; Khalil, I.A.; Krohn, K.J.; Liang, X.; Naghavi, M.; et al. Global, Regional, and National Burden of Multiple Sclerosis 1990-2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 269–285. [Google Scholar] [CrossRef]
- Correale, J.; Marrodan, M.; Ysrraelit, M.C. Mechanisms of Neurodegeneration and Axonal Dysfunction in Progressive Multiple Sclerosis. Biomedicines 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Rocca, M.A. MR Imaging of Gray Matter Involvement in Multiple Sclerosis: Implications for Understanding Disease Pathophysiology and Monitoring Treatment Efficacy. AJNR Am. J. Neuroradiol. 2010, 31, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, M.; Magliozzi, R.; Ciccarelli, O.; Geurts, J.J.; Reynolds, R.; Martin, R. Exploring the Origins of Grey Matter Damage in Multiple Sclerosis. Nat. Rev. Neurosci. 2015, 16, 147–158. [Google Scholar] [CrossRef]
- Fisher, E.; Lee, J.C.; Nakamura, K.; Rudick, R.A. Gray Matter Atrophy in Multiple Sclerosis: A Longitudinal Study. Ann. Neurol. 2008, 64, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Charil, A.; Dagher, A.; Lerch, J.P.; Zijdenbos, A.P.; Worsley, K.J.; Evans, A.C. Focal Cortical Atrophy in Multiple Sclerosis: Relation to Lesion Load and Disability. Neuroimage 2007, 34, 509–517. [Google Scholar] [CrossRef]
- Battaglini, M.; Giorgio, A.; Stromillo, M.L.; Bartolozzi, M.L.; Guidi, L.; Federico, A.; De Stefano, N. Voxel-Wise Assessment of Progression of Regional Brain Atrophy in Relapsing-Remitting Multiple Sclerosis. J. Neurol. Sci. 2009, 282, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Kilsdonk, I.D.; Jonkman, L.E.; Klaver, R.; van Veluw, S.J.; Zwanenburg, J.J.; Kuijer, J.P.; Pouwels, P.J.; Twisk, J.W.; Wattjes, M.P.; Luijten, P.R.; et al. Increased Cortical Grey Matter Lesion Detection in Multiple Sclerosis with 7 T MRI: A Post-Mortem Verification Study. Brain 2016, 139, 1472–1481. [Google Scholar] [CrossRef]
- Pitt, D.; Boster, A.; Pei, W.; Wohleb, E.; Jasne, A.; Zachariah, C.R.; Rammohan, K.; Knopp, M.V.; Schmalbrock, P. Imaging Cortical Lesions in Multiple Sclerosis with Ultra-High-Field Magnetic Resonance Imaging. Arch. Neurol. 2010, 67, 812–818. [Google Scholar] [CrossRef]
- Treaba, C.A.; Herranz, E.; Barletta, V.T.; Mehndiratta, A.; Ouellette, R.; Sloane, J.A.; Klawiter, E.C.; Kinkel, R.P.; Mainero, C. The Relevance of Multiple Sclerosis Cortical Lesions on Cortical Thinning and Their Clinical Impact as Assessed by 7.0-T MRI. J. Neurol. 2021, 268, 2473–2481. [Google Scholar] [CrossRef]
- Absinta, M.; Sati, P.; Masuzzo, F.; Nair, G.; Sethi, V.; Kolb, H.; Ohayon, J.; Wu, T.; Cortese, I.C.M.; Reich, D.S. Association of Chronic Active Multiple Sclerosis Lesions with Disability In Vivo. JAMA Neurol. 2019, 76, 1474–1483. [Google Scholar] [CrossRef]
- Chawla, S.; Kister, I.; Sinnecker, T.; Wuerfel, J.; Brisset, J.C.; Paul, F.; Ge, Y. Longitudinal Study of Multiple Sclerosis Lesions Using Ultra-High Field (7T) Multiparametric MR Imaging. PLoS ONE 2018, 13, e0202918. [Google Scholar] [CrossRef] [PubMed]
- Mainero, C.; Benner, T.; Radding, A.; van der Kouwe, A.; Jensen, R.; Rosen, B.R.; Kinkel, R.P. In Vivo Imaging of Cortical Pathology in Multiple Sclerosis Using Ultra-High Field MRI. Neurology 2009, 73, 941–948. [Google Scholar] [CrossRef]
- Dal-Bianco, A.; Schranzer, R.; Grabner, G.; Lanzinger, M.; Kolbrink, S.; Pusswald, G.; Altmann, P.; Ponleitner, M.; Weber, M.; Kornek, B.; et al. Iron Rims in Patients with Multiple Sclerosis as Neurodegenerative Marker? A 7-Tesla Magnetic Resonance Study. Front. Neurol. 2021, 12, 632749. [Google Scholar] [CrossRef]
- Treaba, C.A.; Conti, A.; Klawiter, E.C.; Barletta, V.T.; Herranz, E.; Mehndiratta, A.; Russo, A.W.; Sloane, J.A.; Kinkel, R.P.; Toschi, N.; et al. Cortical and Phase Rim Lesions on 7 T MRI as Markers Of Multiple Sclerosis Disease Progression. Brain Commun. 2021, 3, fcab134. [Google Scholar] [CrossRef] [PubMed]
- Tozlu, C.; Jamison, K.; Nguyen, T.; Zinger, N.; Kaunzner, U.; Pandya, S.; Wang, Y.; Gauthier, S.; Kuceyeski, A. Structural Disconnectivity from Paramagnetic Rim Lesions Is Related to Disability in Multiple Sclerosis. Brain Behav. 2021, 11, e2353. [Google Scholar] [CrossRef]
- Destrieux, C.; Fischl, B.; Dale, A.; Halgren, E. Automatic Parcellation of Human Cortical Gyri and Sulci Using Standard Anatomical Nomenclature. Neuroimage 2010, 53, 1–15. [Google Scholar] [CrossRef]
- Yao, B.; Bagnato, F.; Matsuura, E.; Merkle, H.; van Gelderen, P.; Cantor, F.K.; Duyn, J.H. Chronic Multiple Sclerosis Lesions: Characterization with High-Field-Strength MR Imaging. Radiology 2012, 262, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Guestrin, C. XGBoost: A Scalable Tree Boosting System. In Proceedings of the KDD ’16, San Francisco, CA, USA, 13–17 August 2016; ACM: New York, NY, USA, 2016; pp. 785–794. [Google Scholar]
- Lundberg, S.M.; Lee, S.-I. A Unified Approach to Interpreting Model Predictions. In Proceedings of the Advances in Neural Information Processing Systems, Long Beach, CA, USA, 4–9 December 2017; Curran Associates, Inc.: New York, NY, USA, 2017; Volume 30. [Google Scholar]
- Brescia, E.; Costantino, D.; Marzo, F.; Massenio, P.R.; Cascella, G.L.; Naso, D. Automated Multistep Parameter Identification of SPMSMs in Large-Scale Applications Using Cloud Computing Resources. Sensors 2021, 21, 4699. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-Learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Lassmann, H. Mechanisms of Demyelination and Tissue Destruction in Multiple Sclerosis. Clin. Neurol. Neurosurg. 2002, 104, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Damasceno, A.; Damasceno, B.P.; Cendes, F. No Evidence of Disease Activity in Multiple Sclerosis: Implications on Cognition and Brain Atrophy. Mult. Scler. 2016, 22, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Bodini, B.; Khaleeli, Z.; Cercignani, M.; Miller, D.H.; Thompson, A.J.; Ciccarelli, O. Exploring the Relationship between White Matter and Gray Matter Damage in Early Primary Progressive Multiple Sclerosis: An In Vivo Study with TBSS and VBM. Hum. Brain Mapp. 2009, 30, 2852–2861. [Google Scholar] [CrossRef] [PubMed]
- Steenwijk, M.D.; Daams, M.; Pouwels, P.J.W.; Balk, L.J.; Tewarie, P.K.; Killestein, J.; Uitdehaag, B.M.J.; Geurts, J.J.G.; Barkhof, F.; Vrenken, H. What Explains Gray Matter Atrophy in Long-Standing Multiple Sclerosis? Radiology 2014, 272, 832–842. [Google Scholar] [CrossRef]
- Geisseler, O.; Pflugshaupt, T.; Bezzola, L.; Reuter, K.; Weller, D.; Schuknecht, B.; Brugger, P.; Linnebank, M. The Relevance of Cortical Lesions in Patients with Multiple Sclerosis. BMC Neurol. 2016, 16, 204. [Google Scholar] [CrossRef]
- Geurts, J.J.G.; Calabrese, M.; Fisher, E.; Rudick, R.A. Measurement and Clinical Effect of Grey Matter Pathology in Multiple Sclerosis. Lancet Neurol. 2012, 11, 1082–1092. [Google Scholar] [CrossRef]
- Weber, C.E.; Krämer, J.; Wittayer, M.; Gregori, J.; Randoll, S.; Weiler, F.; Heldmann, S.; Roßmanith, C.; Platten, M.; Gass, A.; et al. Association of Iron Rim Lesions with Brain and Cervical Cord Volume in Relapsing Multiple Sclerosis. Eur. Radiol. 2022, 32, 2012–2022. [Google Scholar] [CrossRef]
- Rocca, M.A.; Battaglini, M.; Benedict, R.H.; De Stefano, N.; Geurts, J.J.; Henry, R.G.; Horsfield, M.A.; Jenkinson, M.; Pagani, E.; Filippi, M. Brain MRI Atrophy Quantification in MS: From Methods to Clinical Application. Neurology 2017, 88, 403–413. [Google Scholar] [CrossRef]
- Bo, L.; Geurts, J.J.; Mork, S.J.; van der Valk, P. Grey Matter Pathology in Multiple Sclerosis. Acta Neurol. Scand. Suppl. 2006, 183, 48–50. [Google Scholar] [CrossRef]
- Peterson, J.W.; Bo, L.; Mork, S.; Chang, A.; Trapp, B.D. Transected Neurites, Apoptotic Neurons, and Reduced Inflammation in Cortical Multiple Sclerosis Lesions. Ann. Neurol. 2001, 50, 389–400. [Google Scholar] [CrossRef]
- Bo, L.; Vedeler, C.A.; Nyland, H.I.; Trapp, B.D.; Mork, S.J. Subpial Demyelination in the Cerebral Cortex of Multiple Sclerosis Patients. J. Neuropathol. Exp. Neurol. 2003, 62, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Treaba, C.A.; Granberg, T.E.; Sormani, M.P.; Herranz, E.; Ouellette, R.A.; Louapre, C.; Sloane, J.A.; Kinkel, R.P.; Mainero, C. Longitudinal Characterization of Cortical Lesion Development and Evolution in Multiple Sclerosis with 7.0-T MRI. Radiology 2019, 291, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Maranzano, J.; Dadar, M.; Rudko, D.A.; De Nigris, D.; Elliott, C.; Gati, J.S.; Morrow, S.A.; Menon, R.S.; Collins, D.L.; Arnold, D.L.; et al. Comparison of Multiple Sclerosis Cortical Lesion Types Detected by Multicontrast 3T and 7T MRI. AJNR Am. J. Neuroradiol. 2019, 40, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Fjell, A.M.; Westlye, L.T.; Amlien, I.; Espeseth, T.; Reinvang, I.; Raz, N.; Agartz, I.; Salat, D.H.; Greve, D.N.; Fischl, B.; et al. High Consistency of Regional Cortical Thinning in Aging Across Multiple Samples. Cereb. Cortex 2009, 19, 2001–2012. [Google Scholar] [CrossRef]
- Narayana, P.A.; Govindarajan, K.A.; Goel, P.; Datta, S.; Lincoln, J.A.; Cofield, S.S.; Cutter, G.R.; Lublin, F.D.; Wolinsky, J.S.; MRI Analysis Center at Houston; et al. Regional Cortical Thickness in Relapsing Remitting Multiple Sclerosis: A Multi-Center Study. Neuroimage Clin. 2012, 2, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, H.; Goldman, A.L.; Sambataro, F.; Verchinski, B.A.; Meyer-Lindenberg, A.; Weinberger, D.R.; Mattay, V.S. Normal Age-Related Brain Morphometric Changes: Nonuniformity across Cortical Thickness, Surface Area and Gray Matter Volume? Neurobiol. Aging 2012, 33, 617.e1–617.e9. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, M.; Atzori, M.; Bernardi, V.; Morra, A.; Romualdi, C.; Rinaldi, L.; McAuliffe, M.J.; Barachino, L.; Perini, P.; Fischl, B.; et al. Cortical Atrophy Is Relevant in Multiple Sclerosis at Clinical Onset. J. Neurol. 2007, 254, 1212–1220. [Google Scholar] [CrossRef]
- Loncarski, J.; Monopoli, V.G.; Cascella, G.L.; Cupertino, F. SiC-MOSFET and Si-IGBT-Based Dc-Dc Interleaved Converters for EV Chargers: Approach for Efficiency Comparison with Minimum Switching Losses Based on Complete Parasitic Modeling. Energies 2020, 13, 4585. [Google Scholar] [CrossRef]



| Mean Thickness | r-Value | p Value |
|---|---|---|
| Right Hemisphere | 0.47 (0.15) | 0.009 (0.0013) |
| Left Hemisphere | 0.44 (0.18) | 0.016 (0.020) |
| Whole Brain | 0.48 (0.17) | 0.008 (0.011) |
| LEFT HEMISPHERE | RIGHT HEMISPHERE | ||||
|---|---|---|---|---|---|
| REGION | r Value (SD) | p-Pearson (SD) | REGION | r Value (SD) | p-Pearson (SD) |
| Superior frontal gyrus (F1) | 0.436 (0.149) | 0.016 (0.023) | Superior frontal gyrus (F1) | 0.480 (0.125) | 0.007 (0.011) |
| Medial occipitotemporal sulcus (collateral sulcus) and lingual sulcus | 0.537 (0.131) | 0.002 (0.003) | Medial occipitotemporal sulcus (collateral sulcus) and lingual sulcus | 0.539 (0.125) | 0.002 (0.003) |
| Superior temporal sulcus (parallel sulcus) | 0.444 (0.121) | 0.014 (0.019) | Superior temporal sulcus (parallel sulcus | 0.388 (0.212) | 0.034 (0.050) |
| Opercular part of the inferior frontal gyrus | 0.420 (0.157) | 0.021 (0.030) | Middle-posterior part of the cingulate gyrus and sulcus (pMCC) | 0.374 (0.227) | 0.042 (0.060) |
| Long insular gyrus and central sulcus of the insula | 0.371 (0.137) | 0.044 (0.055) | Middle frontal gyrus (F2) | 0.443 (0.178) | 0.014 (0.020) |
| Middle temporal gyrus (T2) | 0.396 (0.134) | 0.030 (0.042) | Anterior transverse collateral sulcus | 0.377 (0.163) | 0.040 (0.056) |
| Medial orbital sulcus (olfactory sulcus) | 0.387 (0.104) | 0.034 (0.043) | Superior occipital sulcus and transverse occipital sulcus | 0.439 (0.116) | 0.015 (0.020) |
| Lateral occipito-temporal sulcus | 0.413 (0.165) | 0.023 (0.033) | |||
| G1 | G2 | |||
|---|---|---|---|---|
| Mean Thickness | r-Value | p Value | r-Value | p Value |
| Right Hemisphere | 0.46 | 0.0006 | 0.19 | 0.21 |
| Left Hemisphere | 0.41 | 0.002 | 0.12 | 0.43 |
| Whole Brain | 0.45 | 0.0007 | 0.19 | 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conti, A.; Treaba, C.A.; Mehndiratta, A.; Barletta, V.T.; Mainero, C.; Toschi, N. An Interpretable Machine Learning Model to Predict Cortical Atrophy in Multiple Sclerosis. Brain Sci. 2023, 13, 198. https://doi.org/10.3390/brainsci13020198
Conti A, Treaba CA, Mehndiratta A, Barletta VT, Mainero C, Toschi N. An Interpretable Machine Learning Model to Predict Cortical Atrophy in Multiple Sclerosis. Brain Sciences. 2023; 13(2):198. https://doi.org/10.3390/brainsci13020198
Chicago/Turabian StyleConti, Allegra, Constantina Andrada Treaba, Ambica Mehndiratta, Valeria Teresa Barletta, Caterina Mainero, and Nicola Toschi. 2023. "An Interpretable Machine Learning Model to Predict Cortical Atrophy in Multiple Sclerosis" Brain Sciences 13, no. 2: 198. https://doi.org/10.3390/brainsci13020198
APA StyleConti, A., Treaba, C. A., Mehndiratta, A., Barletta, V. T., Mainero, C., & Toschi, N. (2023). An Interpretable Machine Learning Model to Predict Cortical Atrophy in Multiple Sclerosis. Brain Sciences, 13(2), 198. https://doi.org/10.3390/brainsci13020198