Abstract
Traumatic brain injury (TBI) is an important health issue for the worldwide population, as it causes long-term pathological consequences for a diverse group of individuals. We are yet to fully elucidate the significance of TBI polypathologies, such as neuroinflammation and tau hyperphosphorylation, and their contribution to the development of chronic traumatic encephalopathy (CTE) and other neurological conditions. To advance our understanding of TBI, it is necessary to replicate TBI in preclinical models. Commonly used animal models include the weight drop model; these methods model human TBI in various ways and in different animal species. However, animal models have not demonstrated their clinical utility for identifying therapeutic interventions. Many interventions that were successful in improving outcomes for animal models did not translate into clinical benefit for patients. It is important to review current animal models and discuss their strengths and limitations within a TBI context. Modelling human TBI in animals encounters numerous challenges, yet despite these barriers, the TBI research community is working to overcome these difficulties. Developments include advances in biomarkers, standardising, and refining existing models. This progress will improve our ability to model TBI in animals and, therefore, enhance our understanding of TBI and, potentially, how to treat it.
1. Introduction
Traumatic brain injury (TBI) is defined as an alteration in brain function, or other evidence of brain pathology, caused by an external force [1]. Awareness surrounding TBI has accelerated, with increasing recognition as a public health challenge that warrants our attention [1]. TBI affects blast-exposed military veterans, professional contact sport athletes, and survivors of domestic violence and road traffic accidents (RTAs) [1,2,3]. Our understanding of the incidence in Scotland estimates a figure of around 445 per 100,000 for men and 195 per 100,000 for women, which is higher than the overall estimate for Europe [4]. Estimating the global burden of TBI requires data from multiple sources and should be interpreted with caution as individuals experiencing TBI in the context of domestic violence or sporting injuries are less likely to seek care for their injury [4].
TBI demonstrates a bimodal incidence pattern with peaks in adolescents aged 15–25 years and older adults aged ≥ 65 years [5]. However, young children aged 0–4 years are at greater risk of mortality from TBI than older children aged 5–14 years [6]. They are also at risk of abusive head trauma (AHT) which is a leading cause of death in this population and is notoriously misdiagnosed by clinicians [7]. In Scotland, the hospital admission rate in children is decreasing, attributed to public health injury prevention methods [4]. However, the incidence of TBI in the elderly is increasing and these individuals are at greater risk for complicated TBI and extended hospitalisation [5]. Our animal models of TBI should highlight age-related differences where possible to allow for more accurate characterisation of the pathology.
TBI exists on a spectrum of severity, with concussion or mild TBI (mTBI) being the most common [8]. Mild TBI is seen as misleading terminology as repeatedly experiencing mTBI can lead to chronic traumatic encephalopathy (CTE) [3]. Whilst CTE is an emerging diagnosis [9], it is recognised as a neurodegenerative disorder associated with repeated mTBI, commonly occurring in contact sports [10]. The terminology has developed over the past century, from Martland’s observations of ‘punch drunk syndrome’ in 1928, ‘dementia pugilistica’ coined in 1937 by Millspaugh, to Critchley’s CTE in 1949 [10]. Regardless of a diagnosis of CTE, TBI is strongly related to neurodegenerative disorders, such as Alzheimer’s disease (AD) [11,12]. Work towards understanding this link is growing and will advance as we deepen our knowledge of TBI itself [12].
Many diseases are complex, but TBI presents an exceptional challenge as the diversity of causes, people affected, and pathology produced make it difficult to model and subsequently treat. Our inability to provide clinical support to this extensive population has frustrated clinicians and scientists alike, as so many promising preclinical successes have failed in the clinical setting [1,13,14]. Neuroprotective treatments, like progesterone and erythropoietin, consistently show promise in animal studies but fail to improve outcomes in clinical trials [13,15]. Our current treatment approach to TBI reflects a logical neuroprotective approach to reduce secondary injury on the brain, through medical and surgical intervention [15]. Nevertheless, researchers continue to explore therapeutic strategies like neurorestoration, with mesenchymal stem cells as an example [15]. Promisingly, data from recent clinical trials suggest that many individuals with moderate or severe TBI regain function over time [16]. These findings can be utilised in a ‘reverse translation’ approach to refine our screening of potential prognostic or therapeutic biomarkers [17].
However, poor bench-to-bedside translation is not specific to TBI itself and we see that other domains of research, like cardiovascular studies, encounter similar challenges [18]. Our efforts to overcome these barriers can be guided by the stroke research community which updated the STAIR guidelines in 2009 to improve consistency in research methods and reporting [19]. Preclinical stroke research has emerged as a leader in the implementation of crucial study design elements such as randomisation and reporting functional outcomes [18,20]. Heterogeneity between preclinical trials remains an issue but the distinct improvement seen in this field indicates a tangible path for TBI to follow.
This review aims to consider our current understanding of the polypathologies of TBI and review the animal models available to illustrate TBI further, whilst acknowledging the various challenges of modelling TBI. Finally, we will explore the discourse around the recent advancements made and whether this has translated into an improved ability to model human TBI in animals.
2. Polypathology of TBI
It is necessary to consider TBI as a ‘polypathology’ as we gain an appreciation of how different disease processes form a conglomerate of neuropathology, with a devastating burden on the individual and society [21]. Whilst mTBI and severe TBI (sTBI) are differentiated using the Glasgow Coma Scale (GCS) [22], there is a growing appreciation of the overlap of the neuropathological consequences of repetitive mTBI and a single moderate or severe TBI [21]. A 2019 review of the current pathology observed in mTBI and sTBI suggested the concept of a ‘TBI dose effect’, as multiple mTBIs or a single sTBI could similarly contribute to a threshold that triggers a neurodegenerative response [23]. Our understanding of the neurodegeneration that follows TBI has expanded rapidly in the past few decades. We have identified many mechanisms following the primary mechanical injury and how they interact, as seen in Figure 1.
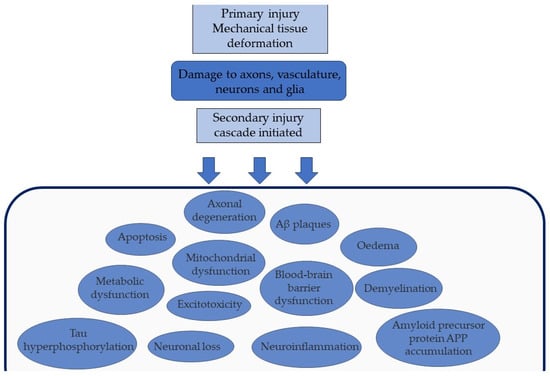
Figure 1.
The sequelae of primary and secondary injuries of TBI. The initial primary mechanical injury damages vital structures such as neurons. This expands to secondary injury, a cascade of pathological events including blood–brain barrier dysfunction and excitotoxicity.
2.1. Diffuse Axonal Injury
One of the most important mechanisms to consider is diffuse axonal injury (DAI). This is a feature of all TBIs, including mTBI, and could represent one of the core substrates that is associated with the development of many secondary injuries [24]. We can identify DAI from histopathology as axonal bulbs, and swellings along axons at the site of injury, which imply failure or disruption of axonal transport [24]. This was evident in autopsy studies of individuals with a moderate–severe TBI, using age-matched controls, demonstrating that this occurs chronically post-TBI, alongside tissue loss in susceptible areas such as the corpus callosum [25]. The evidence for axonal injury developing into chronic axonal neurodegeneration is consistently observed in human sTBI studies and has been observed in gyrencephalic animal models [23]. Whilst axonal injury in mTBI requires further characterisation, mouse models have shown similar axonal pathology after being exposed to repetitive mTBI [8].
2.2. Tau, Amyloid, and TAR DNA-Binding Protein-43
Tau is a protein that provides cytoskeletal support for axonal transport and can be phosphorylated physiologically. Tau becomes pathologically hyperphosphorylated in the setting of disrupted axonal transport [26], eventually aggregating in neurofibrillary tangles seen in CTE and AD [27]. Transgenic mice subjected to TBI demonstrated intensive tau pathology compared to sham mice, which spread to other areas with the greatest neural connectivity [27]. This evidence contributed significantly to the emerging field of TBI research, considering tau to spread in a prion-like manner as a mechanistic explanation for the subsequent neurodegeneration observed diffusely across the brain [28]. Similarly, another protein that accumulates pathologically following axonal disruption is amyloid precursor protein (APP) [11], which is commonly used as an indicator for DAI [3]. We see this translated into the accumulation of amyloid beta (Aβ) plaques, hours after TBI, although the mechanism is yet to be fully elucidated [11]. Amyloid beta plaques are seen as less of a hallmark feature of TBI polypathology, as they are observed less consistently in autopsy studies; 30% of single TBI patients who died in the acute phase post-injury demonstrate Aβ accumulation [12]. TAR DNA-binding protein-43 (TDP-43) is a protein involved in RNA processing, implicated in the pathology of many neurodegenerative diseases, such as frontotemporal dementia (FTD) [29]. TDP-43 immunoreactive neuronal cytoplasmic inclusions are mentioned in the 2016 consensus on diagnosing CTE as a ‘frequently seen’ pathology, alongside Aβ plaques, however, not as part of the diagnostic criteria [9]. This suggests that there is further work to understand the role of TDP-43 and Aβ plaques as neuropathological consequences of TBI.
2.3. Inflammatory Response to TBI
TBI results in a complex neuroinflammatory response, observed up to 18 years after injury [25]. A primary mechanical injury damages the blood–brain barrier (BBB), cell membranes, and vasculature, as shown in Figure 1. This leads to secondary effects like glutamate and calcium release, mitochondrial dysfunction, and cell death pathway activation, as previously reviewed [30]. The cascade continues with cytokines and chemokines, activation of microglia and astrocytes, and recruitment of peripheral immune cells such as T-cells [31]. This response aids repair and clearance mechanisms, however, it can become harmful and contribute to the subsequent neurodegeneration observed. This is exemplified by the dual role microglia play in healing and repairing, as well as propagating secondary injury and neuronal damage, post-TBI [32].
Autopsy studies have shown widespread diffuse BBB disruption after a moderate to severe TBI, compared to age-matched controls [33]. This was observed in the acute phase, but also following long-term survival from TBI, suggesting that it is a chronic pathological process. This is mirrored in further analysis, linking the absence of the BBB in maintaining homeostasis within the brain to the dysregulation of tau phosphorylation seen [34]. Interestingly, the pattern of BBB dysfunction was shown to be preferential towards the crests of gyri, rather than the depths of sulci [33], which contrasts the tau pathology pattern considered to be pathognomonic of CTE [9,12].
3. Current Animal Models of TBI
It is paramount that we attempt to improve the outcomes of those experiencing the polypathologies of TBI. Animal models are essential to understanding the pathological mechanisms behind human TBI. Here we give an overview of the main animal models available, how they induce TBI, and which pathologies can be modelled (Table 1).
3.1. Fluid Percussion Injury (FPI) Model
Fluid percussion injury (FPI) is a commonly used and well-characterised model of TBI [35]. Lateral FPI (applied ≥ 3.5 mm lateral to the sagittal suture) has been used more frequently, to study neuronal degeneration and neuroinflammation [30]. The central FPI (midline applied FPI) is used to produce diffuse and concussive injuries [36] and is increasing in use, alongside the interest in sports and blast injuries [37]. A limitation of both FPI models is that the severity of injury produced is inconsistent, and the brainstem involvement corresponds with greater morbidity [36]. Similarly, it has been observed that the FPI and controlled cortical impact (CCI) model dependably produces progressive tissue atrophy in rodents, whereas this finding is not reliably shown in human TBI [14]. However, when used in aged animals, it produces delayed seizures, which mirrors human TBI and posttraumatic epilepsy [5], which is advantageous to the model. FPI models use head constraints and, while this improves reproducibility, it fails to capture the dynamic nature of TBI in humans [38].
3.2. Weight Drop Model
Weight drop models peaked during the 1990s [39] and are still commonly used as they are easily operated [36]. Whilst Feeney’s original model involves a craniotomy to produce a mainly focal injury, Shohami’s was designed to replicate human closed head injury (CHI) and Marmarou’s intended to recapitulate vehicle accidents or falls using impact acceleration. Finally, the Maryland model was built on Marmarou’s work to emphasise frontal impact, commonly seen in human TBI in sports or RTA [37]. Previous reviews of this model found significant variation in the weight used and the height of the drop [39] and this model is limited by its association with unintentional skull fractures and rebound injury [36]. Additionally, whilst many preclinical trials focus on reporting neuropathologies [5,39], fewer trials are reporting functional outcomes post-TBI. Only 31% of 335 weight drop publications performed the Neurological Scale Score (NSS), which assesses sensorimotor skills [39]. Our models must remain focused on human-orientated functional outcomes as well as pathology, to be of clinical relevance.
3.3. Controlled Cortical Impact (CCI) Model
Controlled cortical impact (CCI) models were developed as part of the early 2000s move away from weight drop towards piston-focused devices [39]. The CCI model is useful for biomechanical studies as it is easy to adjust and measure mechanical parameters, such as velocity, and there is no risk of secondary rebound injury, as seen in gravity-driven devices [36,37]. This method can be used to induce mTBI on an intact skull [36], in the absence of a craniotomy. However, it is important to consider if CCI or FPI models use a craniotomy for their uninjured ‘sham’ mice. Craniotomies have been shown to cause proinflammatory, morphological, and behavioural damage through the surgical procedure alone [40]. It is recommended that these animal models use naïve, anaesthetised animals as a more appropriate control group [41]. Another disadvantage cited of the CCI model is that it does not produce DAI due to the small diameter of the tip delivering the impact and therefore is not a valid model for exploring this significant feature of TBI neuropathology [42]. However, there are models that alter the size of the impactor to improve clinical relevance to DAI [42].
3.4. Closed Head Impact Model of Engineered Rotational Acceleration (CHIMERA) Model
In 2014, Namjoshi developed the closed head impact model of engineered rotational acceleration (CHIMERA) to overcome some of the difficulties facing animal models of TBI [38]. Firstly, this model offers precise delivery and analysis of the biomechanical features of the injury, which are critical for enabling comparison, as human TBI is well-characterised for its biomechanical variation [14]. Additionally, this model does not use a craniotomy which increases its strength as an accurate model of human TBI [43] and allows it to be used for multiple behavioural and neuropathological assessments, following unconstrained head impact to a closed skull [38]. However, for ethical reasons and to comply with relevant animal welfare regulations, animal models are commonly anaesthetised or sedated during injury delivery [42]. A systematic review and meta-analysis of trials using anaesthesia in rodents found it to be neuroprotective, with an estimate of improving neurological outcomes by 30–40% [44]. This may influence the findings of neuropathology and behavioural analysis of TBI animal models, making them less translatable to the human clinical setting [43]. This is a key difficulty in replicating the human condition of TBI in animals.
3.5. Blast TBI Models
To model blast injuries in animals, various methods have been created and have shown extensive, graded effects of blast injuries on rodents [37]. In 2012, Goldstein showed evidence of CTE-like pathology in mice, 2 weeks after a single controlled blast from a compressed gas-driven shock tube, although this is only using male mice [2]. A challenge of blast models is the frequent involvement of other organ systems, such as the respiratory system, leading to studies using thoracic protective vests to prevent associated morbidity [37,45]. The complexity of current animal blast models poses a barrier to further analysis and comparison to other animal models of TBI [39].
3.6. Penetrating TBI Models
Penetrating models of TBI (pTBI) were challenging to develop, due to a high mortality rate associated with the speed of injury delivery [36]. However, using a modified air rifle with a pellet has shown utility in producing histopathological outcomes such as cavitations and gliosis [46]. One of the key advantages of the pTBI is being able to move the injury site, which allows for precise targeting of lesions of the brain [46]. Notably, this model produced a significant increase in reference memory errors during the 7-day testing period using the radial arm maze [46]. However, these findings were produced in a small sample size (n = 10 rats) and this model requires further validation. Additionally, this model needs standardisation, as the variation it allows makes the results produced less reproducible [37].
3.7. Rotational TBI Models
The majority of animal models used in TBI research are rodents [47] which have lissencephalic brains with relatively minimal white matter. This limits our ability to analyse how their brains respond to injury, as their structural differences mean that some neuropathology, such as white matter degeneration, will not be seen in the model [11]. We know that the large size of the gyrencephalic human brain means that it undergoes greater deformation in response to dynamic or rapid accelerations, compared to smaller, lissencephalic brains responding to similar forces [5,47]. This is significant when we analyse the consequence of TBI, as we aim to mirror the characteristics of the human brain undergoing shear stress as closely as possible.
To account for this, Gennarelli used a rotational acceleration model on gyrencephalic primates, to demonstrate the inertial forces associated with motor vehicle crashes [48]. This model illustrated DAI, prolonged loss of consciousness, and induction of coma in non-human primates [14]. However, the specimens were collected from primates culled at different time points, ranging from 2 h to 8 weeks after injury, which may have had a confounding impact on the results [48]. Moreover, it is not feasible to conduct preclinical studies in large animals on the scale required to investigate TBI, due to financial and ethical implications [14,45]. Therefore, it is imperative to refine rodent models further, as they remain central to preclinical models.
3.8. Non-Mammal Models
Alongside the continued use of rodent models, there is a growing appreciation for non-mammal models due to their simplified physiology and cost-efficacy [49].
Drosophila melanogaster flies are used as an animal model that has 70% genetic overlap, a nervous system with glial cells, similar diversity of neurotransmitters, and exhibits most of the behavioural impairment displayed by humans [50]. Using Drosophila flies will augment our ability to explore TBI more efficiently for high-throughput screening of therapeutic compounds, with numerous different models of injury being developed like the omni bead ruptor model for mTBI [49].
Furthermore, zebrafish represent an advantageous model due to their reduced animal husbandry costs and ease of monitoring in batches [51]. A non-surgical closed head injury model in male and female zebrafish produced similar mechanisms of mammalian pathophysiology as well as demonstrating relevant behavioural outcomes to TBI, indicating clear potential for further applications in TBI research [51]. Zebrafish have a shorter lifespan which allows for greater flexibility to use existing weight drop models to investigate the impact of ageing on TBI outcomes [49]. Similarly, Xenopus tadpoles have been shown to demonstrate a wide range of TBI pathologies in response to a focal impact injury model [50]. The variety of models being produced for each non-mammalian species does present a challenge for reducing heterogeneity within the field, as the models have not been characterised as extensively as rodent models of TBI and require further scrutiny as to their utility [49]. Nevertheless, these models will be pivotal to the feasibility of preclinical research using parallel models to improve clinical translation [14].

Table 1.
Overview of current animal models of TBI.
Table 1.
Overview of current animal models of TBI.
| Animal Models References | Experimental Procedure | Animals | Technical Features & Variations Used | Pathology | Strengths | Limitations |
|---|---|---|---|---|---|---|
| FPI model [5,14,36,37,42,45] | Fixed animal’s brain is exposed via a craniotomy A cap is attached to the skull and a reservoir of saline water in a cylindrical tube is attached to the cap At the other end of the reservoir there is a transducer measuring pressure changes A pendulum strikes a piston connected to the transducer which conducts a pressure pulse to the dura of the animal This displaces and deforms the brain tissue, with varying severity | Cat Rabbit Swine Rat Dog Mouse | Position of the craniotomy: central-sagittal, lateral-parietal, para-sagittal Can alter height of pendulum to control severity of injury Variations in tube length, material angle | Can cause mild–severe TBI without skull fracture Central: diffuse contusions, haemorrhages, concussion, neuroinflammation, BBB dysfunction Lateral: focal contusions, diffuse subcortical and contralateral injury, haemorrhage | Motor, behavioural, and cognitive deficits seen, lasting for weeks to months EEG abnormalities corresponding to severity of injury Contusions and axonal damage produced in rodents similar to humans Bradycardia, haemorrhage at grey–white matter interface, increased plasma glucose levels, hypertension | High mortality Difficulty calibrating pendulum (improved with addition of microprocessor-controlled pneumatically driven instruments) Requirement of craniotomy Progressive tissue atrophy consistently seen in rodents—unclear if this mirrors human pathophysiology |
| Weight drop model [14,36,37,45,49,52] | Guided falling of a weight onto the unconstrained skull of an animal: Feeney’s: craniotomy used Marmarou’s: exposed dorsal–ventral skull covered with steel disk resting on foam pad Maryland: impact applied to anterior part of skull Shohami’s: weight applied to one side of unprotected skull resting on hard surface | Rat Mouse Zebrafish | Adjust height of drop Alter the mass, shape, material of weight used With or without craniotomy Change contact surface material or area | Feeney’s: contusion type injury, concussion, traumatic axonal injury, haemorrhage development of a necrotic cavity Shohami’s and Marmarou’s: concussions and traumatic axonal injury primarily, contusions and possible skull fractures Maryland’s: primarily traumatic axonal injury with concussion and haemorrhage | Closely resembles clinical TBI Scalable model as height and mass of weight can be adjusted for severity Simple mechanism and construction Shohami model demonstrates impaired neurological and cognitive outcomes (motor, learning, memory, and anxiety) Use in zebrafish shows genomic changes in CNS injury pathways | Variability seen in injury deliverability Use of metal plate in Marmarou’s/Maryland’s does not reflect human TBI Feeney: craniotomy-associated damage Marmarou’s: higher fatality rate Shohami’s: increased probability of skull fractures |
| CCI [14,36,37,41,53] | Craniotomy performed on restrained animal skull Use of a pneumatic or electromagnetic impact device to deliver an injury to exposed dura Deformation of underlying cortex | Ferret Mouse Rat Monkey Swine Xenopus | Craniotomy can alter the position and depth. Can alter the speed or angle of the impactor, the diameter of the tip, depth of impact | Cortical tissue loss, subdural haematomas, axonal injury, concussion, BBB dysfunction, increased ICP, haemorrhages if severe Pathology can be focal or diffuse, depending on the severity of the injury delivered | Ability to precisely calibrate injury parameters improves accuracy of injury and therefore reproducibility Reduced risk of rebound injury Motor, emotional, and cognitive deficits seen in walking and memory which correlate with severity and persist for up to 1 year Can be used in small and large animal models | Expensive equipment Craniotomy-associated damage Most CCI models cannot produce DAI Dural laceration as a complication |
| Penetrating TBI model [14,37,42,46,49] | Animal placed in a frame and head fixed Frontal sinus removed Exposed to different projectiles: missiles, gunshots, sharp objects Creates severe deformational damage through a visible cavity | Cat Dog Monkey Sheep Rat Mouse Zebrafish | Anatomical path, velocity, and angle of projectile Low-velocity pellet model: non-fatal | Model for moderate to severe TBI Creates a large focal cavity in the brain. White and grey matter damage. Brain swelling seizures, neuroinflammation, and BBB dysfunction Extensive intracerebral haemorrhage Low-velocity pellet model produces a cavity, haemorrhage, oedema, gliosis, and white matter degeneration | Cognitive: specifically memory impairment and sensorimotor impairment Neurofunctional deficits correlate with injury severity Produces clinically relevant outcomes like raised ICP Move injury site to target precise lesions | Extensive haemorrhage produced Heat damage from velocity of projectile Less standardised than other models High mortality rate |
| CHIMERA [14,38,43,54] | Head of animal unconstrained in supine position Pressure-driven piston controlled with a regulator and digital pressure gauge Impact applied to the dorsum of the head, allowing head to flex forward after injury | Rat Mouse Ferret | Can control the parameters of injury including direction, velocity, and impact energy High-speed camera analysis available | Causes axonal injury DAI, neuroinflammation, neurodegeneration, tau, hyperphosphorylation, and white matter inflammation | Non-surgical technique Can be used repeatedly to study long-term effects Semi-automated procedure Variety of dynamic injuries produced Allows for movement of the head after impact Motor, cognitive, and neuropsychiatric outcomes shown with greater consistency than other models | Standardisation of head plates required No large animal model validated for comparison Relatively few publications compared to other models |
| Primary blast injury [2,14,37,42,45,49] | Animal fixed to metal tube Blast generated through a detonation or release of compressed air | Rat Mouse Pig Drosophila | Head can be restrained or unrestrained Addition of Kevlar vests to protect thorax Amount of explosives or pressure of compressed air used Plastic net to protect from debris or shrapnel | DAI, changes in intracranial pressure, BBB dysfunction, brain oedema, tau hyperphosphorylation, and neuroinflammation | Deficits seen in social recognition, spatial memory, and motor coordination Use of thoracic and abdominal protection minimises mortality Low-level blasts increase ICP and cause cognitive defects Head immobilisation during blast was associated with reduced learning and memory deficits | Model does not accurately recapitulate the dynamic nature of a blast injury Protection from systemic injuries or debris removes the important comorbidities accompanying TBI |
| Rotational acceleration model [14,36,37,45] | The animal’s head is secured to a device or helmet Induction of a graded rotational acceleration forces | Pig Non-human primate Rabbit | Head restrained or unrestrained Angle of injury, rotation, grading of forces | Primarily DAI Non-human primates: severe TBI, swine: mild to severe TBI with DAI, BBB dysfunction, and damage to the hippocampus | Head rotation is associated with poor functional and histopathological outcomes Highly clinically relevant as a model for falls or collisions | Model is technically sophisticated and expensive Ethical concerns about use of non-human primates |
Abbreviations: blood–brain barrier (BBB), controlled cortical impact model (CCI), closed head impact model of engineered rotational acceleration (CHIMERA), diffuse axonal injury (DAI), electroencephalogram (EEG), fluid percussion injury (FPI), intracranial pressure (ICP), penetrating ballistic brain injury (pBBI), traumatic brain injury (TBI).
3.9. Outcomes in TBI
One of the key challenges of modelling TBI is our ability to assess the impact of the injury on the animal. We measure the severity of a human TBI traditionally using the Glasgow Coma Scale (GCS) which measures neurological function in three different domains, whereas animal models can be characterised by the severity of the force delivered [55]. Importantly, TBI research increasingly acknowledges the importance of models using clinically relevant functional parameters such as cognitive tests of memory or behavioural measures of anxiety [56]. There are a myriad of tests available, which overlap with stroke research and suffer from the same lack of standardisation in procedure and reporting [20,55].
4. Recent In Vivo Advancements
Considerable in vivo advancements have been made in recent years, changing and increasing our ability to model and replicate TBI in animals. This is exemplified by the 2016 Operation Brain Trauma Therapy (OBTT), which advocates for a novel and rigorous approach to a preclinical trial model for TBI, similar to the efforts of the stroke research guidelines in 2009 [19,57]. Implementation of standardisation across multiple models is notable for its efforts to reduce heterogeneity, as well as an appreciation of the necessity of using large gyrencephalic animals and reporting functional outcomes consistently. The inclusion of biomarker assessments allows us to refine the findings of previous studies, showing their potential utility as a prognostic and therapeutic opportunity in human TBI [22].
4.1. Biomarkers of TBI
Recent TBI research has highlighted biomarkers as a priority, as they would be notable in their clinical utility in countries with less access to advanced imaging technologies [1,58]. To date, cytoplasmic calcium-binding protein S-100β is the most studied biomarker in the setting of TBI, and there is considerable evidence to support its diagnostic and prognostic capabilities [59]. Additionally, glial fibrillary acidic protein (GFAP) has been studied as a biomarker of injured brain tissue in humans and animals [59]. Recent studies suggest that TSPO may be a reliable prognostic biomarker in human TBI, controlling for other prognostic factors like intracranial pressure [60]. This is mirrored in animal studies suggesting further therapeutic potential through the reduction in apoptosis in male rat models [61].
Increasingly, there is a focus on restoring function in neurodegenerative disease, given the limited time frame for optimal treatment in TBI. Zebrafish demonstrate distinct neuroregenerative capabilities, unlike humans. This represents an exciting opportunity to utilise an animal model’s divergence from human physiology to explore the potential of biomarkers like brain-derived neurotrophic factor (BDNF) to harness restoration following TBI or stroke [62,63]. As we explore the utility of these elements, improvements in administration across the BBB through biomaterials like heparan sulphate may enhance our understanding of the utility of biomarkers and their therapeutic potential [62].
Studies have shown microRNAs (miRNAs), which regulate protein synthesis, could be a useful biomarker in human TBI [43]. These developments may improve our ability to model TBI in animals, as we can validate the expression of certain miRNA changes after a CCI model of injury in mice [64], with overlap between specific miRNAs in humans post-TBI [65]. Studying human and animal model biomarkers in parallel reinforces the importance of viewing preclinical models through the lens of translation into clinical care.
4.2. Neuroimaging
Neuroimaging has advanced greatly in recent years and can benefit our ability to model TBI. In 2007, Macdonald showed that diffusion tensor imaging (DTI) could detect DAI in mouse models, unlike conventional magnetic resonance imaging (MRI) [66]. Whilst further research is needed to determine when this imaging should be used in animals and humans for providing prognostic information, it marks a huge step in our ability to assess TBI clinically and preclinically [67]. As we expand our ability to measure human TBI through imaging, this contributes to another new dimension of models—synthetic head models. Researchers have created an idealised gyrencephalic human brain made from polyacrylamide gel and whilst the phantom head has demonstrated early limitations, it has the potential to be refined into a validated model of human TBI; without the use of animals [68].
4.3. Genetic Technologies
TBI modelling has been substantially advanced through genetic technologies, allowing us to create transgenic mice, which can highlight certain pathologies [42]. Focusing on tau, genetically engineering mice to express human tau determined how TBI acts as a risk factor for tauopathies such as AD [27]. This enables us to move beyond the constraints of rodent physiology, to recreate TBI pathology with greater precision and accuracy.
Furthermore, studies have investigated how mTBI polypathology is linked to changes in genetic regulation mechanisms in animal models, whilst comparing this to genome-wide association studies (GWASs) of humans [69]. The overlap between genetic changes seen in rodents exposed to TBI and human genes shown to be causally linked to neurodegenerative diseases such as AD suggests potential mechanisms of how TBI creates a predisposition towards these diseases [69].
Using genetic technologies effectively illustrates the complementary roles of large animal and small animal models, as these techniques are much more viable in smaller animals, including non-mammals [70].
Studies have already shown an upregulation of important TBI-mediator genes in Drosophila, but there is potential to build on this with genetic screens for the genes that make them resistant or susceptible to TBI-related pathology, which could allow for therapeutic opportunities [52].
Advancing our understanding of TBI through ‘omics’ such as genomics and transcriptomics is an important consideration but one that remains in its infancy. A recent review notes that one of the limitations is the statistical challenges associated with analysing vast quantities of data from the diverse cohort of TBI-affected individuals, who present with extensive and variable pathology [71]. However, our ability to interpret ‘omics’ data accurately and reliably is improving, and we can continue to use the data to assess the ability of animal models to recapitulate the human response to TBI.
4.4. CHIMERA Model
The CHIMERA model is an excellent example of a recent improvement in animal modelling. It primarily produces DAI pathology, which is instrumental to our understanding of human polypathology, and it permits standardisation of the injury parameters, necessary for allowing comparison between laboratories [54]. Whilst the technology itself represents development, preclinical research remains slow to use animals of different genders and ages and this continues to be a barrier to our interpretation of preclinical trial models [54]. However, when we consider the simplicity of the original weight drop model, the CHIMERA animal model is a significant leap forward in our ability to model TBI in animals. This exemplifies the exciting in vivo advancements seen in recent years, as we gain a better appreciation of the complexity of human TBI and the elements we could effectively model in animals [1].
5. General Limitations
As we refine rodent models to provide a better, reproducible re-enactment of human TBI, we encounter the same difficulties acknowledged many decades ago. Holburn determined, in 1943, that it is necessary to scale the force of the injury delivery for a smaller brain to fully recapitulate the true effects of human TBI [14]. This involves increasing the size of the inertial forces for a rat brain by 8000%, which is evidently unobtainable. Very few preclinical models discuss how they overcome this distinct barrier to modelling TBI in animals, which calls into question the validity of the models to do so [47].
Further, we understand that TBI is often part of polytrauma, and models should include hypoxia, ischaemia, and potentially substance use, including prescription medications for comorbidities or existing conditions, to provide a more reliable representation of human TBI, as these will exacerbate pathological outcomes [45]. Since this was highlighted in 2005, studies have begun to incorporate these features into CCI models, however, there is still a paucity of studies that utilise this [37]. This demonstrates an area where further advancement is needed.
Furthermore, Bodnar demonstrated that very few studies include aged, female, or young animals [5,39], which suggests that our preclinical models may be of lower utility in evaluating TBI in a large proportion of the population. Evaluation of sex within animal models of TBI presents a largely mixed picture and uncertainty around the specific biological mechanisms underpinning this difference [72]. We can use a lens of ‘mosaicism’ to demonstrate that functional and biological domains are differentially affected in TBI dependent on the sex of the human or animal participant [73]. This furthers the idea of using multiple functional outcomes to evaluate a model, to ensure its relevance to both sexes [73].
Whilst progesterone has not delivered clinical utility in the setting of TBI, its potential neuroprotective qualities were highlighted in trials examining the impact of female sex hormones in TBI outcomes [74]. Our understanding of polypathology and the outcomes associated with TBI will be inherently limited if we exclude female animals from our research.
We know that TBI evolves over years and neuroinflammation is seen to persist for many years [25]. However, a very small proportion of preclinical trials report looking at outcomes beyond 1 month [39], which limits our ability to make valid conclusions about the long-term sequelae of human TBI. Some of these limitations are shared by all models previously discussed and an overview of the barriers to a clinically translatable animal model of human TBI is illustrated below (Figure 2).
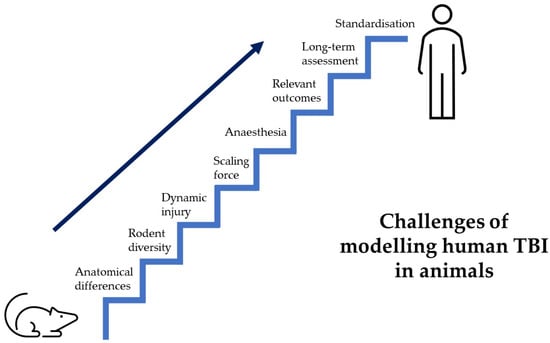
Figure 2.
Hurdles of TBI preclinical research. Displays a staircase, representing the many hurdles faced by preclinical trials to create an animal model that recapitulates human TBI.
6. Conclusions
Overall, to evaluate if in vivo advancements have improved our ability to model human TBI in animal models, we must consider the scale of progress that would signify improvement. Given the difficulties facing preclinical trial models, the advancement we have seen is reflective of distinct improvement. Reflection has been a critical element of this: considering the disappointment seen in the progesterone trials [13], the limitations of the animal models [45], and how the TBI research community needs to alter and coordinate its approach to model the condition. It is imperative to highlight the areas where progress has been slower, the minimal use of female rodents in models as an example. As mandated by the National Institute of Health, biological sex must be considered and characterised as part of future TBI study design [75]. Nonetheless, a key theme to consider is how the advancements in different dimensions of TBI research interact with each other. This is emphasised within the previous review of TBI developments, as we observe how the improvements made to biomarkers have a synergistic effect on our ability to use animal models for evaluating TBI [76]. When viewing the evidence base, notable improvements have been made to our ability to model human TBI in animals. Advancement within preclinical stroke research emerged as improvements within clinical trial design, before seeing clinically useful treatments such as nerinetide appear [77]. We can be hopeful that the trajectory of TBI preclinical research will move towards a similar, standardised approach that will ultimately provide therapeutic options to TBI patients.
Author Contributions
J.L.F. was responsible for conceptualisation, writing (review & editing), supervision. E.F.-J. was responsible for conceptualisation, investigation, writing (original draft and review & editing). W.H.M. was responsible for writing (review & editing) and supervision. L.M.W. was responsible for writing (review & editing) and supervision All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed. All authors have read and agreed to the published version of the manuscript.
Funding
J.L.F. is currently supported by the British Heart Foundation; PG/21/10559.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
E.F.-J. completed this article as part of an intercalated BMed Sci (Hons) degree.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Maas, A.I.R.; Menon, D.K.; David Adelson, P.D.; Andelic, N.; Bell, M.J.; Belli, A.; Bragge, P.; Brazinova, A.; Büki, A.; Chesnut, R.M.; et al. Traumatic Brain Injury: Integrated Approaches to Improve Prevention, Clinical Care, and Research. Lancet Neurol. 2017, 16, 987–1048. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, L.E.; Fisher, A.M.; Tagge, C.A.; Zhang, X.L.; Velisek, L.; Sullivan, J.A.; Upreti, C.; Kracht, J.M.; Ericsson, M.; Wojnarowicz, M.W.; et al. Chronic Traumatic Encephalopathy in Blast-Exposed Military Veterans and a Blast Neurotrauma Mouse Model. Sci. Transl. Med. 2012, 4, 134ra60. [Google Scholar] [CrossRef]
- Hay, J.; Johnson, V.E.; Smith, D.H.; Stewart, W. Chronic Traumatic Encephalopathy: The Neuropathological Legacy of Traumatic Brain Injury. Annu. Rev. Pathol. 2016, 11, 21–45. [Google Scholar] [CrossRef] [PubMed]
- Shivaji, T.; Lee, A.; Dougall, N.; McMillan, T.; Stark, C. The Epidemiology of Hospital Treated Traumatic Brain Injury in Scotland. BMC Neurol. 2014, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- Iboaya, A.; Harris, J.L.; Arickx, A.N.; Nudo, R.J. Models of Traumatic Brain Injury in Aged Animals: A Clinical Perspective. Neurorehabil. Neural Repair 2019, 33, 975–988. [Google Scholar] [CrossRef] [PubMed]
- Araki, T.; Yokota, H.; Morita, A. Pediatric Traumatic Brain Injury: Characteristic Features, Diagnosis, and Management. Neurol. Med. Chir. 2017, 57, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Nikam, R.M.; Kecskemethy, H.H.; Kandula, V.V.R.; Averill, L.W.; Langhans, S.A.; Yue, X. Abusive Head Trauma Animal Models: Focus on Biomarkers. Int. J. Mol. Sci. 2023, 24, 4463. [Google Scholar] [CrossRef]
- Mouzon, B.; Chaytow, H.; Crynen, G.; Bachmeier, C.; Stewart, J.; Mullan, M.; Stewart, W.; Crawford, F. Repetitive Mild Traumatic Brain Injury in a Mouse Model Produces Learning and Memory Deficits Accompanied by Histological Changes. J. Neurotrauma 2012, 29, 2761–2773. [Google Scholar] [CrossRef]
- McKee, A.C.; Cairns, N.J.; Dickson, D.W.; Folkerth, R.D.; Dirk Keene, C.; Litvan, I.; Perl, D.P.; Stein, T.D.; Vonsattel, J.P.; Stewart, W.; et al. The First NINDS/NIBIB Consensus Meeting to Define Neuropathological Criteria for the Diagnosis of Chronic Traumatic Encephalopathy. Acta Neuropathol. 2016, 131, 75–86. [Google Scholar] [CrossRef]
- Perrine, K.; Helcer, J.; Tsiouris, A.J.; Pisapia, D.J.; Stieg, P. Literature Review The Current Status of Research on Chronic Traumatic Encephalopathy. World Neurosurg. 2017, 102, 533–544. [Google Scholar] [CrossRef]
- Johnson, V.E.; Stewart, W.; Smith, D.H. Traumatic Brain Injury and Amyloid-β Pathology: A Link to Alzheimer’s Disease? Nat. Rev. Neurosci. 2010, 11, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.H.; Johnson, V.E.; Stewart, W. Chronic Neuropathologies of Single and Repetitive TBI: Substrates of Dementia? Nat. Rev. Neurol. 2013, 9, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Denier, C.; Oudinet, J.P.; Adams, D.; Guennoun, R. Progesterone Neuroprotection: The Background of Clinical Trial Failure. J. Steroid Biochem. Mol. Biol. 2016, 160, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.H.; Kochanek, P.M.; Rosi, S.; Meyer, R.; Ferland-beckham, C.; Prager, E.M.; Ahlers, S.T.; Crawford, F. Roadmap for Advancing Pre-Clinical Science in Traumatic Brain Injury. J. Neurotrauma 2021, 3221, 3204–3221. [Google Scholar] [CrossRef] [PubMed]
- Galgano, M.; Toshkezi, G.; Qiu, X.; Russell, T.; Chin, L.; Zhao, L.-R. Traumatic Brain Injury. Cell Transplant. 2017, 26, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- McCrea, M.A.; Giacino, J.T.; Barber, J.; Temkin, N.R.; Nelson, L.D.; Levin, H.S.; Dikmen, S.; Stein, M.; Bodien, Y.G.; Boase, K.; et al. Functional Outcomes over the First Year after Moderate to Severe Traumatic Brain Injury in the Prospective, Longitudinal TRACK-TBI Study. JAMA Neurol. 2021, 78, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Gratz, D.; Hund, T.J.; Falvo, M.J.; Wold, L.E. Reverse Translation: Using Computational Modeling to Enhance Translational Research. Circ. Res. 2018, 122, 1496–1498. [Google Scholar] [CrossRef]
- Ramirez, F.D.; Motazedian, P.; Jung, R.G.; Di Santo, P.; Macdonald, Z.D.; Moreland, R.; Simard, T.; Clancy, A.A.; Russo, J.J.; Welch, V.A.; et al. Methodological Rigor in Preclinical Cardiovascular Studies. Circ. Res. 2017, 120, 1916–1926. [Google Scholar] [CrossRef]
- Fisher, M.; Feuerstein, G.; Howells, D.W.; Hurn, P.D.; Kent, T.A.; Savitz, S.I.; Lo, E.H. Update of the Stroke Therapy Academic Industry Roundtable Preclinical Recommendations. Stroke 2009, 40, 2244–2250. [Google Scholar] [CrossRef]
- Hietamies, T.M.; Ostrowski, C.; Pei, Z.; Feng, L.; McCabe, C.; Work, L.M.; Quinn, T.J. Variability of Functional Outcome Measures Used in Animal Models of Stroke and Vascular Cognitive Impairment—A Review of Contemporary Studies. J. Cereb. Blood Flow Metab. 2018, 38, 1872–1884. [Google Scholar] [CrossRef]
- Wilson, L.; Stewart, W.; Dams-O’Connor, K.; Diaz-Arrastia, R.; Horton, L.; Menon, D.K.; Polinder, S. The Chronic and Evolving Neurological Consequences of Traumatic Brain Injury. Lancet Neurol. 2017, 16, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.S.; Stein, S.C.; Swanson, R.; Guan, S.; Garcia, L.; Mehta, D.; Smith, D.H.; Smith, D.H. Blood Biomarkers for Traumatic Brain Injury: A Quantitative Assessment of Diagnostic and Prognostic Accuracy. Front. Neurol. 2019, 10, 446. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.H.; Johnson, V.E.; Trojanowski, J.Q.; Stewart, W. Chronic Traumatic Encephalopathy—Confusion and Controversies. Nat. Rev. Neurol. 2019, 15, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Johnson, V.E.; Stewart, W.; Smith, D.H. Axonal Pathology in Traumatic Brain Injury. Exp. Neurol. 2013, 246, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Johnson, V.E.; Stewart, J.E.; Begbie, F.D.; Trojanowski, J.Q.; Smith, D.H.; Stewart, W. Inflammation and White Matter Degeneration Persist for Years after a Single Traumatic Brain Injury. Brain 2013, 136, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Iliff, J.J.; Chen, M.J.; Plog, B.A.; Zeppenfeld, D.M.; Soltero, X.; Yang, L.; Singh, I.; Deane, R.; Nedergaard, M. Impairment of Glymphatic Pathway Function Promotes Tau Pathology after Traumatic Brain Injury. J. Neurosci. 2014, 34, 16180–16193. [Google Scholar] [CrossRef] [PubMed]
- Edwards, G.; Zhao, J.; Dash, P.K.; Soto, C.; Moreno-Gonzalez, I. Traumatic Brain Injury Induces Tau Aggregation and Spreading. J. Neurotrauma 2020, 37, 80–92. [Google Scholar] [CrossRef]
- Zanier, E.R.; Bertani, I.; Sammali, E.; Pischiutta, F.; Chiaravalloti, M.A.; Vegliante, G.; Masone, A.; Corbelli, A.; Smith, D.H.; Menon, D.K.; et al. Induction of a Transmissible Tau Pathology by Traumatic Brain Injury. Brain 2018, 141, 2685–2699. [Google Scholar] [CrossRef]
- Jo, M.; Lee, S.; Jeon, Y.; Kim, S.; Kwon, Y.; Kim, H. The Role of TDP-43 Propagation in Neurodegenerative Diseases: Integrating Insights from Clinical and Experimental Studies. Exp. Mol. Med. 2020, 52, 1652–1662. [Google Scholar] [CrossRef]
- Simon, D.W.; McGeachy, M.J.; Bayır, H.; Clark, R.S.B.; Loane, D.J.; Kochanek, P.M. The Far-Reaching Scope of Neuroinflammation after Traumatic Brain Injury. Nat. Rev. Neurol. 2017, 13, 171–191. [Google Scholar] [CrossRef]
- Corps, K.N.; Roth, T.L.; McGavern, D.B. Inflammation and Neuroprotection in Traumatic Brain Injury. JAMA Neurol. 2015, 72, 355. [Google Scholar] [CrossRef] [PubMed]
- Loane, D.J.; Byrnes, K.R. Role of Microglia in Neurotrauma. Neurotherapeutics 2010, 7, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Hay, J.R.; Johnson, V.E.; Young, A.M.H.; Smith, D.H.; Stewart, W. Blood-Brain Barrier Disruption Is an Early Event That May Persist for Many Years After Traumatic Brain Injury in Humans. J. Neuropathol. Exp. Neurol. 2015, 74, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Doherty, C.P.; O’Keefe, E.; Wallace, E.; Loftus, T.; Keaney, J.; Kealy, J.; Humphries, M.M.; Molloy, M.G.; Meaney, J.F.; Farrell, M.; et al. Blood-Brain Barrier Dysfunction as a Hallmark Pathology in Chronic Traumatic Encephalopathy. J. Neuropathol. Exp. Neurol. 2016, 75, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.I.; McIntosh, T.K.; Maxwell, W.L.; Nicoll, J.A. Recent Advances in Neurotrauma. J. Neuropathol. Exp. Neurol. 2000, 59, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Rostami, E. Traumatic Brain Injury Models in Animals. In Injury Models of the Central Nervous System: Methods and Protocols; Kobeissy, F.H., Dixon, C.E., Hayes, R.L., Mondello, S., Eds.; Springer: New York, NY, USA, 2016; pp. 47–59. ISBN 978-1-4939-3816-2. [Google Scholar]
- Xiong, Y.; Mahmood, A.; Chopp, M. Animal Models of Traumatic Brain Injury. Nat. Rev. Neurosci. 2013, 14, 128–142. [Google Scholar] [CrossRef]
- Cheng, W.H.; Martens, K.M.; Carr, M.; Wilkinson, A.; Fan, J.; Robert, J.; Hayat, A.; Namjoshi, D.R.; Wellington, C.L.; McInnes, K.A.; et al. Merging Pathology with Biomechanics Using CHIMERA (Closed-Head Impact Model of Engineered Rotational Acceleration): A Novel, Surgery-Free Model of Traumatic Brain Injury. Mol. Neurodegener. 2014, 9, 218–252. [Google Scholar]
- Bodnar, C.N.; Roberts, K.N.; Higgins, E.K.; Bachstetter, A.D. A Systematic Review of Closed Head Injury Models of Mild Traumatic Brain Injury in Mice and Rats. J. Neurotrauma 2019, 36, 1683–1706. [Google Scholar] [CrossRef]
- Cole, J.T.; Yarnell, A.; Kean, W.S.; Gold, E.; Lewis, B.; Ren, M.; McMullen, D.C.; Jacobowitz, D.M.; Pollard, H.B.; O’Neill, J.T.; et al. Craniotomy: True Sham for Traumatic Brain Injury, or a Sham of a Sham? J. Neurotrauma 2011, 28, 359–369. [Google Scholar] [CrossRef]
- Fournier, M.-L.; Clément, T.; Aussudre, J.; Plesnila, N.; Obenaus, A.; Badaut, J. Contusion Rodent Model of Traumatic Brain Injury: Controlled Cortical Impact. In Wound Regeneration: Methods and Protocols; Das, H., Ed.; Springer: New York, NY, USA, 2021; pp. 49–65. ISBN 978-1-0716-0845-6. [Google Scholar]
- Zhang, Y.P.; Cai, J.; Shields, L.B.E.; Liu, N.; Xu, X.M.; Shields, C.B. Traumatic Brain Injury Using Mouse Models. Transl. Stroke Res. 2014, 5, 454–471. [Google Scholar] [CrossRef]
- Najem, D.; Rennie, K.; Ribecco-Lutkiewicz, M.; Ly, D.; Haukenfrers, J.; Liu, Q.; Nzau, M.; Fraser, D.D.; Bani-Yaghoub, M. Traumatic Brain Injury: Classification, Models, and Markers. Biochem. Cell Biol. 2018, 96, 391–406. [Google Scholar] [CrossRef] [PubMed]
- Archer, D.P.; McCann, S.K.; Walker, A.M.; Premji, Z.A.; Rogan, K.J.; Hutton, M.J.H.; Gray, L.J. Neuroprotection by Anaesthetics in Rodent Models of Traumatic Brain Injury: A Systematic Review and Network Meta-Analysis. Br. J. Anaesth. 2018, 121, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Cernak, I. Animal Models of Head Trauma. NeuroRx 2005, 2, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Plantman, S.; Ng, K.C.; Lu, J.; Davidsson, J.; Risling, M. Characterization of a Novel Rat Model of Penetrating Traumatic Brain Injury. J. Neurotrauma 2012, 29, 1219–1232. [Google Scholar] [CrossRef] [PubMed]
- Johnson, V.E.; Meaney, D.F.; Cullen, D.K.; Smith, D.H. Animal Models of Traumatic Brain Injury. Handb. Clin. Neurol. 2015, 127, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Gennarelli, T.A.; Thibault, L.E.; Adams, J.H.; Graham, D.I.; Thompson, C.J.; Marcincin, R.P. Diffuse Axonal Injury and Traumatic Coma in the Primate. Ann. Neurol. 1982, 12, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Zulazmi, N.A.; Arulsamy, A.; Ali, I.; Zainal Abidin, S.A.; Othman, I.; Shaikh, M.F. The Utilization of Small Non-Mammals in Traumatic Brain Injury Research: A Systematic Review. CNS Neurosci. Ther. 2021, 27, 381–402. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Behnke, J.A.; Hardin, K.R.; Zheng, J.Q. Drosophila Melanogaster as a Model to Study Age and Sex Differences in Brain Injury and Neurodegeneration after Mild Head Trauma. Front. Neurosci. 2023, 17, 1150694. [Google Scholar] [CrossRef]
- McCutcheon, V.; Park, E.; Liu, E.; Sobhebidari, P.; Tavakkoli, J.; Wen, X.-Y.; Baker, A.J. A Novel Model of Traumatic Brain Injury in Adult Zebrafish Demonstrates Response to Injury and Treatment Comparable with Mammalian Models. J. Neurotrauma 2016, 34, 1382–1393. [Google Scholar] [CrossRef]
- Shah, E.J.; Gurdziel, K.; Ruden, D.M. Mammalian Models of Traumatic Brain Injury and a Place for Drosophila in TBI Research. Front. Neurosci. 2019, 13, 409. [Google Scholar] [CrossRef]
- Spruiell Eldridge, S.L.; Teetsel, J.F.K.; Torres, R.A.; Ulrich, C.H.; Shah, V.V.; Singh, D.; Zamora, M.J.; Zamora, S.; Sater, A.K. A Focal Impact Model of Traumatic Brain Injury in Xenopus Tadpoles Reveals Behavioral Alterations, Neuroinflammation, and an Astroglial Response. Int. J. Mol. Sci. 2022, 23, 7578. [Google Scholar] [CrossRef]
- McNamara, E.H.; Grillakis, A.A.; Tucker, L.B.; McCabe, J.T. The Closed-Head Impact Model of Engineered Rotational Acceleration (CHIMERA) as an Application for Traumatic Brain Injury Pre-Clinical Research: A Status Report. Exp. Neurol. 2020, 333, 113409. [Google Scholar] [CrossRef] [PubMed]
- DeWitt, D.S.; Hawkins, B.E.; Dixon, C.E.; Kochanek, P.M.; Armstead, W.; Bass, C.R.; Bramlett, H.M.; Buki, A.; Dietrich, W.D.; Ferguson, A.R.; et al. Pre-Clinical Testing of Therapies for Traumatic Brain Injury. J. Neurotrauma 2018, 35, 2737–2754. [Google Scholar] [CrossRef] [PubMed]
- Shultz, S.R.; McDonald, S.J.; Corrigan, F.; Semple, B.D.; Salberg, S.; Zamani, A.; Jones, N.C.; Mychasiuk, R. Clinical Relevance of Behavior Testing in Animal Models of Traumatic Brain Injury. J. Neurotrauma 2020, 37, 2381–2400. [Google Scholar] [CrossRef] [PubMed]
- Lafrenaye, A.; Mondello, S.; Povlishock, J.; Gorse, K.; Walker, S.; Hayes, R.; Wang, K.; Kochanek, P.M. Operation Brain Trauma Therapy: An Exploratory Study of Levetiracetam Treatment Following Mild Traumatic Brain Injury in the Micro Pig. Front. Neurol. 2021, 11, 586958. [Google Scholar] [CrossRef]
- Deshetty, U.M.; Periyasamy, P. Potential Biomarkers in Experimental Animal Models for Traumatic Brain Injury. J. Clin. Med. 2023, 12, 3923. [Google Scholar] [CrossRef]
- Wang, K.K.; Yang, Z.; Zhu, T.; Shi, Y.; Rubenstein, R.; Tyndall, J.A.; Manley, G.T. An Update on Diagnostic and Prognostic Biomarkers for Traumatic Brain Injury. Expert Rev. Mol. Diagn. 2018, 18, 165–180. [Google Scholar] [CrossRef]
- Bao, Q.; Yuan, X.; Bian, X.; Wei, W.; Jin, P.; Jiang, W. Prognostic Significance of Translocator Protein in Brain Tissue Following Traumatic Brain Injury. Turk. Neurosurg. 2022, 33, 736–744. [Google Scholar] [CrossRef]
- Soustiel, J.F.; Vlodavsky, E.; Milman, F.; Gavish, M.; Zaaroor, M. Improvement of Cerebral Metabolism Mediated by Ro5-4864 Is Associated with Relief of Intracranial Pressure and Mitochondrial Protective Effect in Experimental Brain Injury. Pharm. Res. 2011, 28, 2945–2953. [Google Scholar] [CrossRef]
- Houlton, J.; Abumaria, N.; Hinkley, S.F.R.; Clarkson, A.N. Therapeutic Potential of Neurotrophins for Repair after Brain Injury: A Helping Hand from Biomaterials. Front. Genet. 2019, 10, 790. [Google Scholar] [CrossRef]
- Cacialli, P. Neurotrophins Time Point Intervention after Traumatic Brain Injury: From Zebrafish to Human. Int. J. Mol. Sci. 2021, 22, 1585. [Google Scholar] [CrossRef] [PubMed]
- Meissner, L.; Gallozzi, M.; Balbi, M.; Schwarzmaier, S.; Tiedt, S.; Terpolilli, N.A.; Plesnila, N. Temporal Profile of MicroRNA Expression in Contused Cortex after Traumatic Brain Injury in Mice. J. Neurotrauma 2016, 33, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Bhomia, M.; Balakathiresan, N.S.; Wang, K.K.; Papa, L.; Maheshwari, R.K. A Panel of Serum MiRNA Biomarkers for the Diagnosis of Severe to Mild Traumatic Brain Injury in Humans. Sci. Rep. 2016, 6, 28148. [Google Scholar] [CrossRef] [PubMed]
- Mac Donald, C.L.; Dikranian, K.; Song, S.K.; Bayly, P.V.; Holtzman, D.M.; Brody, D.L. Detection of Traumatic Axonal Injury with Diffusion Tensor Imaging in a Mouse Model of Traumatic Brain Injury. Exp. Neurol. 2007, 205, 116–131. [Google Scholar] [CrossRef] [PubMed]
- Alosco, M.L.; Culhane, J.; Mez, J. Neuroimaging Biomarkers of Chronic Traumatic Encephalopathy: Targets for the Academic Memory Disorders Clinic. Neurotherapeutics 2021, 18, 772–791. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, A.K.; Vidhate, S.; McIlvain, G.; Luster, J.; Galindo, E.J.; Johnson, C.L.; Pham, D.L.; Butman, J.A.; Mejia-Alvarez, R.; Tartis, M.; et al. Characterization of Material Properties and Deformation in the ANGUS Phantom during Mild Head Impacts Using MRI. J. Mech. Behav. Biomed. Mater. 2023, 138, 105586. [Google Scholar] [CrossRef]
- Meng, Q.; Zhuang, Y.; Ying, Z.; Agrawal, R.; Yang, X.; Gomez-Pinilla, F. Traumatic Brain Injury Induces Genome-Wide Transcriptomic, Methylomic, and Network Perturbations in Brain and Blood Predicting Neurological Disorders. EBioMedicine 2017, 16, 184–194. [Google Scholar] [CrossRef]
- Grovola, M.R.; von Reyn, C.; Loane, D.J.; Cullen, D.K. Understanding Microglial Responses in Large Animal Models of Traumatic Brain Injury: An Underutilized Resource for Preclinical and Translational Research. J. Neuroinflamm. 2023, 20, 67. [Google Scholar] [CrossRef]
- Abu Hamdeh, S.; Tenovuo, O.; Peul, W.; Marklund, N. “Omics” in Traumatic Brain Injury: Novel Approaches to a Complex Disease. Acta Neurochir. 2021, 163, 2581–2594. [Google Scholar] [CrossRef]
- Rubin, T.G.; Lipton, M.L. Sex Differences in Animal Models of Traumatic Brain Injury. J. Exp. Neurosci. 2019, 13, 117906951984402. [Google Scholar] [CrossRef]
- Gupte, R.; Brooks, W.; Vukas, R.; Pierce, J.; Harris, J. Sex Differences in Traumatic Brain Injury: What We Know and What We Should Know. J. Neurotrauma 2019, 36, 3063–3091. [Google Scholar] [CrossRef] [PubMed]
- Biegon, A. Considering Biological Sex in Traumatic Brain Injury. Front. Neurol. 2021, 12, 576366. [Google Scholar] [CrossRef] [PubMed]
- Clayton, J.A.; Collins, F.S. Policy: NIH to Balance Sex in Cell and Animal Studies. Nature 2014, 509, 282–283. [Google Scholar] [CrossRef] [PubMed]
- Chinnery, P.F. Traumatic Brain Injury Advances since 2017: What Has Changed? Lancet Neurol. 2022, 21, 953–954. [Google Scholar] [CrossRef]
- Hill, M.D.; Goyal, M.; Menon, B.K.; Nogueira, R.G.; McTaggart, R.A.; Demchuk, A.M.; Poppe, A.Y.; Buck, B.H.; Field, T.S.; Dowlatshahi, D.; et al. Efficacy and Safety of Nerinetide for the Treatment of Acute Ischaemic Stroke (ESCAPE-NA1): A Multicentre, Double-Blind, Randomised Controlled Trial. Lancet 2020, 395, 878–887. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).