Memory, Emotion, and Quality of Life in Patients with Long COVID-19
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Data Collection
2.2.1. Neuropsychological Assessment
- -
- Montreal Cognitive Assessment (MoCA) [32]: Providing a global assessment of cognitive function.
- -
- Wechsler Memory Scale III (WMS III) Word List [33]: Focusing on verbal memory capabilities.
- -
- -
- Wechsler Memory Scale III (WMS III) Digit Span Forward and Backward Test [33]: Assessing attention and working memory.
- -
- Trail Making Test A and B [35]: Examining visual–motor processing speed and cognitive flexibility.
- -
- Letter Cancellation Task, WAIS-III Digit Symbol Coding [33]: Measuring attention, speed, and executive functions.
- -
- Verbal Fluency Task, Boston Naming Test [36]: Assessing language-related cognitive abilities.
- -
- Rivermead Prospective Memory Tasks (from the Rivermead Behavioural Memory Test) [37]: Exploring prospective memory abilities.
2.2.2. Psychological Assessment
- -
- Modified Fatigue Impact Scale (MFIS) [38]: Examining the impact of fatigue on daily functioning.
- -
- Beck Depression Inventory (BDI-2) [39]: Assessing the severity of depressive symptoms.
- -
- -
- Short Form-12 Health Survey (SF-12) [41]: Measuring overall health-related quality of life.
- -
- Memory Failures of Everyday (MFE) [42]: Exploring everyday memory lapses.
- -
- Oviedo Sleep Questionnaire [43]: Assessing sleep patterns and quality.
2.2.3. Procedure
2.3. Analysis
- (1)
- Assumption checks: Prior to conducting the main analyses, the normality of the data distribution was assessed through the Shapiro–Wilk test for the control group (n = 29) and through the Kolmogorov–Smirnov test for the experimental group (n = 57) to ensure that the data met the assumptions necessary for parametric statistical tests (Table S1).
- (2)
- Descriptive analysis: Statistical comparisons, using Student’s t-test for independent samples and the Mann–Whitney U test for the two independent samples, were conducted to quantify the variables related to cognitive abilities and psychological factors (anxiety, depression), quality of sleep, and fatigue of patients diagnosed with PASC versus asymptomatic patients and/or non-COVID-19-infected patients.
3. Results
3.1. Results Pertaining to Demographic Variables
3.2. Results Pertaining to Cognitive Variables
- Incidental Learning (Key Numbers Task): Significant differences were found in incidental learning within the Key Numbers Task (U = 462.5, p = 0.001). This suggests variations in the ability to learn new information incidentally between the two groups.
- Direct Digit Span: Statistically significant differences were detected in the direct digit span (U = 562, p = 0.022), indicating variations in working memory capacity for forward digit span between groups.
- Inverse Digit Span: However, there were no statistically significant differences in the inverse digit span (U = 632.5, p = 0.105), suggesting that the backward working memory capacity was similar between the groups.
- Rivermead Behavioral Memory Test (RBMT) Prospective Memory Tasks: Significant differences were found in the RBMT prospective memory tasks for remembering appointments (U = 610, p = 0.020) and object recall (U = 681.5, p = 0.032). However, no significant differences were observed for location recall (U = 693.5, p = 0.082).
- Verbal Fluency Task: In both phonemic (p- and s-) (t = −2.190, p = 0.031) and semantic (animals) (t = −2.277, p = 0.025) verbal fluency tasks, statistically significant differences were noted, indicating variations in participants’ verbal fluency between the groups (see Figure 1).
3.3. Results Pertaining to Psychological Variables
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Visco, V.; Vitale, C.; Rispoli, A.; Izzo, C.; Virtuoso, N.; Ferruzzi, G.J.; Santopietro, M.; Melfi, A.; Rusciano, M.R.; Maglio, A.; et al. Post-COVID-19 Syndrome: Involvement and Interactions between Respiratory, Cardiovascular and Nervous Systems. J. Clin. Med. 2022, 11, 524. [Google Scholar] [CrossRef]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef] [PubMed]
- Espinar Herranz, K.; Delgado-Losada, M.L. Alteraciones cognitivas pacientes COVID persistente. In Investigaciones DACIU 2022–2023: Una Publicación que Refleja los Trabajos Realizados por los Participantes en el Programa DACIU, 1st ed.; Flaño Lombardo, A., Flaño Romero, A., Eds.; Avanza: Madrid, Spain, 2023; pp. 113–118. [Google Scholar]
- Ceban, F.; Ling, S.; Lui, L.M.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav. Immun. 2021, 101, 93–135. [Google Scholar] [CrossRef] [PubMed]
- Chiappelli, F. Towards neuro-COVID-19. Bioinformation 2020, 16, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Pezzini, A.; Padovani, A. Lifting the mask on neurological manifestations of COVID-19. Nat. Rev. Neurol. 2020, 16, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Nasserie, T.; Hittle, M.; Goodman, S.N. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: A systematic review. JAMA Netw. Open 2021, 4, e2111417. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’Em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef]
- Del Rio, C.; Collins, L.F.; Malani, P. Long-term health consequences of COVID-19. JAMA 2020, 324, 1723–1724. [Google Scholar] [CrossRef]
- Taquet, M.; Dercon, Q.; Luciano, S.; Geddes, R.J.; Husain, M.; Harrison, P.J. Incidence, co-occurrence, and evolution of long-COVID features: A 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021, 18, e1003773. [Google Scholar] [CrossRef]
- Ziauddeen, N.; Gurdasani, D.; O’hara, M.E.; Hastie, C.; Roderick, P.; Yao, G.; Alwan, N.A. Characteristics and impact of Long COVID: Findings from an online survey. PLoS ONE 2022, 17, e0264331. [Google Scholar] [CrossRef]
- Singal, C.M.S.; Jaiswal, P.; Seth, P. SARS-CoV-2, more than a respiratory virus: Its potential role in neuropathogenesis. ACS Chem. Neurosci. 2020, 11, 1887–1899. [Google Scholar] [CrossRef]
- Aparisi, Á.; Ybarra-Falcón, C.; Iglesias-Echeverría, C.; García-Gómez, M.; Marcos-Mangas, M.; Valle-Peñacoba, G.; Carrasco-Moraleja, M.; Fernández-de-Las-Peñas, C.; Guerrero, Á.L.; García-Azorín, D. Cardio-Pulmonary Dysfunction Evaluation in Patients with Persistent Post-COVID-19 Headache. Int. J. Environ. Res. Public Health 2022, 19, 3961. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, F.; Pezzuto, F.; Fortarezza, F. Pulmonary pathology and COVID-19: Lessons from autopsy. The experience of European Pulmonary Pathologists. Virchows Arch. 2020, 477, 359–372. [Google Scholar] [CrossRef]
- Sommer, N.; Schmeck, B. Pulmonale Manifestationen bei Long-COVID [Pulmonary manifestations in long COVID]. Inn. Med. 2022, 63, 819–829. [Google Scholar]
- Tian, S.; Xiong, Y.; Liu, H. Pathological study of the 2019 novel coronavirus disease (COVID-19) through post-mortem core biopsies. Mod. Pathol. 2020, 33, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Iqbal, K.; Ali, S.A.; Azim, D.; Farid, E.; Baig, M.D.; Arif, B.T.; Raza, M. The COVID-19 sequelae: A cross-sectional evaluation of post-recovery symptoms and the need for rehabilitation of COVID-19 survivors. Cureus 2021, 13, e13080. [Google Scholar] [CrossRef]
- Nouraeinejad, A. A proposal to apply brain injury recovery treat ments for cognitive impairment in COVID-19 survivors. Int. J. Neurosci. 2022, 5, 1–2. [Google Scholar]
- Xu, Z.; Shi, L.; Wang, Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Tran, V.T.; Porcher, R.; Pane, I.; Ravaud, P. Course of post COVID-19 disease symptoms overtime in the ComPaRe long COVID prospective e-cohort. Nat. Commun. 2022, 13, 1812. [Google Scholar] [CrossRef]
- Zheng, J.; Wong, L.-Y.R.; Li, K.; Verma, A.K.; Ortiz, M.E.; Wohlford-Lenane, C.; Leidinger, M.R.; Knudson, C.M.; Meyerholz, D.K.; McCray, P.B., Jr.; et al. COVID-19 treatments and pathogenesis including anosmia in K18-hACE2 mice. Nature 2021, 589, 603–607. [Google Scholar] [CrossRef]
- Montalvan, V.; Lee, J.; Bueso, T.; De Toledo, J.; Rivas, K. Neurological manifestations of COVID-19 and other coronavirus infections: A systematic review. Clin. Neurol. Neurosurg. 2020, 194, 105921. [Google Scholar] [CrossRef] [PubMed]
- Grof, D.; Sun, A.; Ssentongo, A.E.; Ba, D.M.; Parsons, N.; Poudel, G.R.; Lekoubou, A.; Oh, J.S.; Ericson, J.E.; Ssentongo, P.; et al. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: A systematic review. JAMA Netw. Open 2021, 4, e2128568. [Google Scholar] [CrossRef] [PubMed]
- Yelin, D.; Margalit, I.; Nehme, M.; Bordas-Martínez, J.; Pistelli, F.; Yahav, D.; Guessous, I.; Durà-Miralles, X.; Carrozzi, L.; Shapira-Lichter, I.; et al. Patterns of Long COVID Symptoms: A Multi-Center Cross Sectional Study. J. Clin. Med. 2022, 11, 898. [Google Scholar] [CrossRef] [PubMed]
- Taquet, M.; Geddes, J.R.; Husain, M.; Luciano, S.; Harrison, P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: A retrospective cohort study using electronic health records. Lancet Psychiatry 2021, 8, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, K.; Lin, Y.; Prewitt, K.-R.M.; Potter, D.A. Multidisciplinary Approach to Brain Fog and Related Persisting Symptoms Post COVID-19. J. Health Serv. Psychol. 2022, 48, 31–38. [Google Scholar] [CrossRef]
- Crivelli, L.; Calandri, I.; Corvalán, N.; Carello, M.A.; Keller, G.; Martínez, C.; Arruabarrena, M.; Allegri, R. Cognitive consequences of COVID-19: Results of a cohort study from South America. Arq. Neuro-Psiquiatria 2021, 80, 240–247. [Google Scholar] [CrossRef]
- Ani Nalbandian, K.S.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; Ahluwalia, N. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Alemanno, F.; Houdayer, E.; Parma, A.; Spina, A.; Del Forno, A.; Scatolini, A.; Angelone, S.; Brugliera, L.; Tettamanti, A.; Beretta, L.; et al. COVID-19 cognitive deficits after respiratory assistance in the subacute phase: A COVID-rehabilitation unit experience. PLoS ONE 2021, 16, e0246590. [Google Scholar] [CrossRef]
- Beaud, V.; Crottaz-Herbette, S.; Dunet, V.; Vaucher, J.; Bernard-Valnet, R.; Du Pasquier, R.; Bart, P.-A.; Clarke, S. Pattern of cognitive deficits in severe COVID-19. J. Neurol. Neurosurg. Psychiatry 2020, 92, 567–568. [Google Scholar] [CrossRef]
- Ortelli, P.; Ferrazzoli, D.; Sebastianelli, L.; Engl, M.; Romanello, R.; Nardone, R.; Bonini, I.; Koch, G.; Saltuari, L.; Quartarone, A.; et al. Neuropsychological and neurophysiological correlates of fatigue in post-acute patients with neurological manifestations of COVID-19: Insights into a challenging symptom. J. Neurol. Sci. 2021, 420, 117271. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool For Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Weschler, D. WAIS-III: Escala de Inteligencia de Weschler para Adultos-III: Manual Técnico, 2nd ed.; TEA Ediciones: Madrid, Spain, 2001. [Google Scholar]
- Rey, A. Test de Copia de una Figura Compleja. In Manual de la Adaptación Española, 2nd ed.; TEA Ediciones: Madrid, Spain, 1942. [Google Scholar]
- Reitan, R.M. Validity of the Trail Making Test as an Indicator of Organic Brain Damage. Percept. Mot. Ski. 1958, 8, 271–276. [Google Scholar] [CrossRef]
- Kaplan, E.; y Goodglass, H. Test de Vocabulario de Boston; Editorial Panamericana: Madrid, Spain, 1986. [Google Scholar]
- Wilson, B.A. RBMT: Test de Memoria Conductual de Rivermead: Manual de Aplicación y Corrección; Pearson: Madrid, Spain, 1998. [Google Scholar]
- Larson, R.D. Psychometric properties of the modified fatigue impact scale. Int. J. MS Care 2013, 15, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Steer, R.A.; y Brown, G. Inventario de Depresión de Beck-II (BDI-II); Pearson Educación: Madrid, Spain, 2011. [Google Scholar]
- Spielberger, C.D.; Gorsuch, R.L.; y Lushene, R.E. STAI Cuestionario de Ansiedad Estado Rasgo; TEA Ediciones: Madrid, Spain, 2015. [Google Scholar]
- Alonso, J. Valores poblacionales de referencia de la versión española del Cuestionario de Salud SF-36. Med. Clin. 1998, 111, 410–416. [Google Scholar]
- García-Martínez, J.; Sánchez-Cánovas, J. Adaptación del cuestionario de fallos de memoria en la vida cotidiana (MFE). Boletín Psicol. 1993, 43, 89–105. [Google Scholar]
- Bobes García, J.; González, G.; Portilla, M.P.; Martínez, S.; Alejandra, P.; Fernández, B.; Teresa, M.; Celso, I.A.; Domínguez, F.; María, J. Propiedades psicométricas del cuestionario Oviedo de sueño. Psicothema 2000, 12, 107–112. [Google Scholar]
- Becker, J.H.; Lin, J.J.; Doernberg, M.; Stone, K.; Navis, A.; Festa, J.R.; Wisnivesky, J.P. Assessment of Cognitive Function in Patients After COVID-19 Infection. JAMA Netw. Open 2021, 4, e2130645. [Google Scholar] [CrossRef]
- Miskowiak, K.; Johnsen, S.; Sattler, S.; Nielsen, S.; Kunalan, K.; Rungby, J.; Lapperre, T.; Porsberg, C. Cognitive impairments four months after COVID-19 hospital discharge: Pattern, severity and association with illness variables. Eur. Neuropsychopharmacol. 2021, 46, 39–48. [Google Scholar] [CrossRef]
- Hampshire, A.; Trender, W.; Chamberlain, S.R.; Jolly, A.E.; Grant, J.E.; Patrick, F.; Mazibuko, N.; Williams, S.C.; Barnby, J.M.; Hellyer, P.; et al. Cognitive deficits in people who have recovered from COVID-19. eClinicalMedicine 2021, 39, 101044. [Google Scholar] [CrossRef]
- Frontera, J.A.; Yang, D.; Lewis, A.; Patel, P.; Medicherla, C.; Arena, V.; Fang, T.; Andino, A.; Snyder, T.; Madhavan, M.; et al. A prospective study of long-term outcomes among hospitalized COVID-19 patients with and without neurological complications. J. Neurol. Sci. 2021, 426, 117486. [Google Scholar] [CrossRef]
- Sasannejad, C.; Ely, E.W.; Lahiri, S. Long-term cognitive impairment after acute respiratory distress syndrome: A review of clinical impact and pathophysiological mechanisms. Crit. Care 2019, 23, 352. [Google Scholar] [CrossRef] [PubMed]
- Crivelli, L.; Palmer, K.; Calandri, I.; Guekht, A.; Beghi, E.; Carroll, W.; Frontera, J.; García-Azorín, D.; Westenberg, E.; Winkler, A.S.; et al. Changes in cognitive functioning after COVID-19: A systematic review and meta-analysis. Alzheimer’s Dement. 2022, 18, 1047–1066. [Google Scholar] [CrossRef] [PubMed]
- Carfì, A.; Bernabei, R.; Landi, F. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.S.; Malsy, J.; Pöttgen, J.; Zai, S.S.; Ufer, F.; Hadjilaou, A.; Schmiedel, S.; Addo, M.M.; Gerloff, C.; Heesen, C.; et al. Frequent neurocognitive defcits after recovery from mild COVID-19. Brain Commun. 2020, 2, fcaa205. [Google Scholar] [CrossRef] [PubMed]
- Daroische, R.; Hemminghyth, M.S.; Eilertsen, T.H.; Breitve, M.H.; Chwiszczuk, L.J. Cognitive impairment after COVID-19—A review on objective test data. Front. Neurol. 2021, 12, 699582. [Google Scholar] [CrossRef]
- Premraj, L.; Kannapadi, N.V.; Briggs, J.; Seal, S.M.; Battaglini, D.; Fanning, J.; Suen, J.; Robba, C.; Fraser, J.; Cho, S.-M. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: A meta-analysis. J. Neurol. Sci. 2022, 434, 120162. [Google Scholar] [CrossRef]
- Ferrucci, R.; Dini, M.; Rosci, C.; Capozza, A.; Groppo, E.; Reitano, M.R.; Allocco, E.; Poletti, B.; Brugnera, A.; Bai, F.; et al. One-year cognitive follow-up of COVID-19 hospitalized patients. Eur. J. Neurol. 2022, 29, 2006–2014. [Google Scholar] [CrossRef]
- Zhou, H.; Lu, S.; Chen, J.; Wei, N.; Wang, D.; Lyu, H.; Shi, C.; Hu, S. The landscape of cognitive function in recovered COVID-19 patients. J. Psychiatr. Res. 2020, 129, 98–102. [Google Scholar] [CrossRef]
- Søraas, A.; Bø, R.; Kalleberg, K.T.; Støer, N.C.; Ellingjord-Dale, M.; Landrø, N.I. Self-reported memory problems 8 months after COVID-19 infection. JAMA Netw. Open 2021, 4, e2118718. [Google Scholar] [CrossRef]
- Graham, E.L.; Clark, J.R.; Orban, Z.S.; Lim, P.H.; Szymanski, A.L.; Taylor, C.; DiBiase, R.M.; Jia, D.T.; Balabanov, R.; Ho, S.U.; et al. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized COVID-19 “long haulers”. Ann. Clin. Transl. Neurol. 2021, 8, 1073–1085. [Google Scholar] [CrossRef]
- Delgado-Alonso, C.; Valles-Salgado, M.; Delgado-Álvarez, A.; Yus, M.; Gómez-Ruiz, N.; Jorquera, M.; Polidura, C.; Gil, M.J.; Marcos, A.; Matías-Guiu, J.; et al. Cognitive dysfunction associated with COVID-19: A comprehensive neuropsychological study. J. Psychiatr. Res. 2022, 150, 40–46. [Google Scholar] [CrossRef]
- Xie, Y.; Al-Aly, Z. Risks and burdens of incident diabetes in long Covid: A cohort study. Lancet Diabetes Endocrinol. 2022, 10, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Calabria, M.; García-Sánchez, C.; Grunden, N.; Pons, C.; Arroyo, J.A.; Gómez-Anson, B.; García, M.d.C.E.; Belvís, R.; Morollón, N.; Igual, J.V.; et al. Post-COVID-19 fatigue: The contribution of cognitive and neuropsychiatric symptoms. J. Neurol. 2022, 269, 3990–3999. [Google Scholar] [CrossRef] [PubMed]
- Bungenberg, J.; Humkamp, K.; Hohenfeld, C.; Rust, M.I.; Ermis, Y.; Dreher, M.; Hartmann, N.U.K.; Marx, G.; Binkofski, F.; Finke, C.; et al. Long COVID-19: Objectifying most self-reported neurological symptoms. Ann. Clin. Transl. Neurol. 2022, 9, 141–154. [Google Scholar] [CrossRef]
- Whiteside, D.M.; Oleynick, V.; Holker, E.; Waldron, E.J.; Porter, J.; Kasprzak, M. Neurocognitive deficits in severe COVID-19 infection: Case series and proposed model. Clin. Neuropsychol. 2021, 35, 799–818. [Google Scholar] [CrossRef] [PubMed]
- Vagheggini, G.; Marzetti, F.; Miniati, M.; Bernardeschi, L.; Miccoli, M.; Brivio, G.B.; Meini, S.; Panait, E.; Cini, E.; Gemignani, A. Pulmonary Function and Psychological Burden Three Months after COVID-19: Proposal of a Comprehensive Multidimensional Assessment Protocol. Healthcare 2022, 10, 612. [Google Scholar] [CrossRef]

| Sex | |||
|---|---|---|---|
| Total n (%) | Experimental n (%) | Control n (%) | |
| Man | 38 (44.2) | 17 (29.8) | 21 (72.4) |
| Woman | 48 (55.8) | 40 (70.2) | 8 (27.6) |
| t-statistic: t = 4.605; p = 0.000 * | |||
| Age | Mean (SD) | Mean (SD) | Mean (SD) |
| 46.76 (10.212) | 48.06 (8.22) | 44.22 (13.188) | |
| U-statistic: U = 664; p = 0.210 | |||
| Marital status | |||
| Total n (%) | Experimental n (%) | Control n (%) | |
| Single | 22 (25.6) | 14 (24.6) | 8 (27.6) |
| Married | 43 (50) | 28 (49.1) | 15 (51.7) |
| Divorced | 3 (3.5) | 3 (5.3) | 0 (0) |
| Cohabiting couple | 11 (12.8) | 7 (12.3) | 4 (13.8) |
| No data | 7 (8.1) | 5 (8.8) | 2 (6.9) |
| U-statistic: U = 670; p = 0.714 | |||
| Educational level | |||
| Total n (%) | Experimental n (%) | Control n (%) | |
| Primary school studies | 4 (4.7) | 1 (1.8) | n = 3 (10.3) |
| High school studies | 20 (23.3) | 15 (26.3) | n = 5 (17.2) |
| University studies | 33 (38.4) | 25 (43.9) | n = 8 (27.6) |
| Master’s/PhD | 25 (29.1) | 12 (21.1) | n = 13 (44.8) |
| No data | 4 (4.7) | 4 (7) | n = 0 (0) |
| Employment | |||
| Total n (%) | Experimental n (%) | Control n (%) | |
| Non-qualified (homemakers’ collective) | 1 (1.3) | 0 (0) | 1 (3.4) |
| Qualified for a manual trade (bricklayer, seamstress …) | 7 (8.8) | 3 (5.3) | 4 (13.8) |
| Qualified for a non-manual trade (administrative, technician …) | 24 (29.1) | 21 (36.8) | 4 (13.8) |
| Professionals (university workers) | 38 (44.2) | 23 (40.4) | 15 (51.7) |
| Manager | 5 (5.8) | 4 (7) | 1 (3.4) |
| Student | 3 (3.5) | 0 (0) | 3 (10.3) |
| Retired | 1 (1.2) | 0 (0) | 1 (3.4) |
| No data | 6 (7) | 6 (10.5) | 0 (0) |
| U-statistic: U = 633.5; p = 0.253 | |||
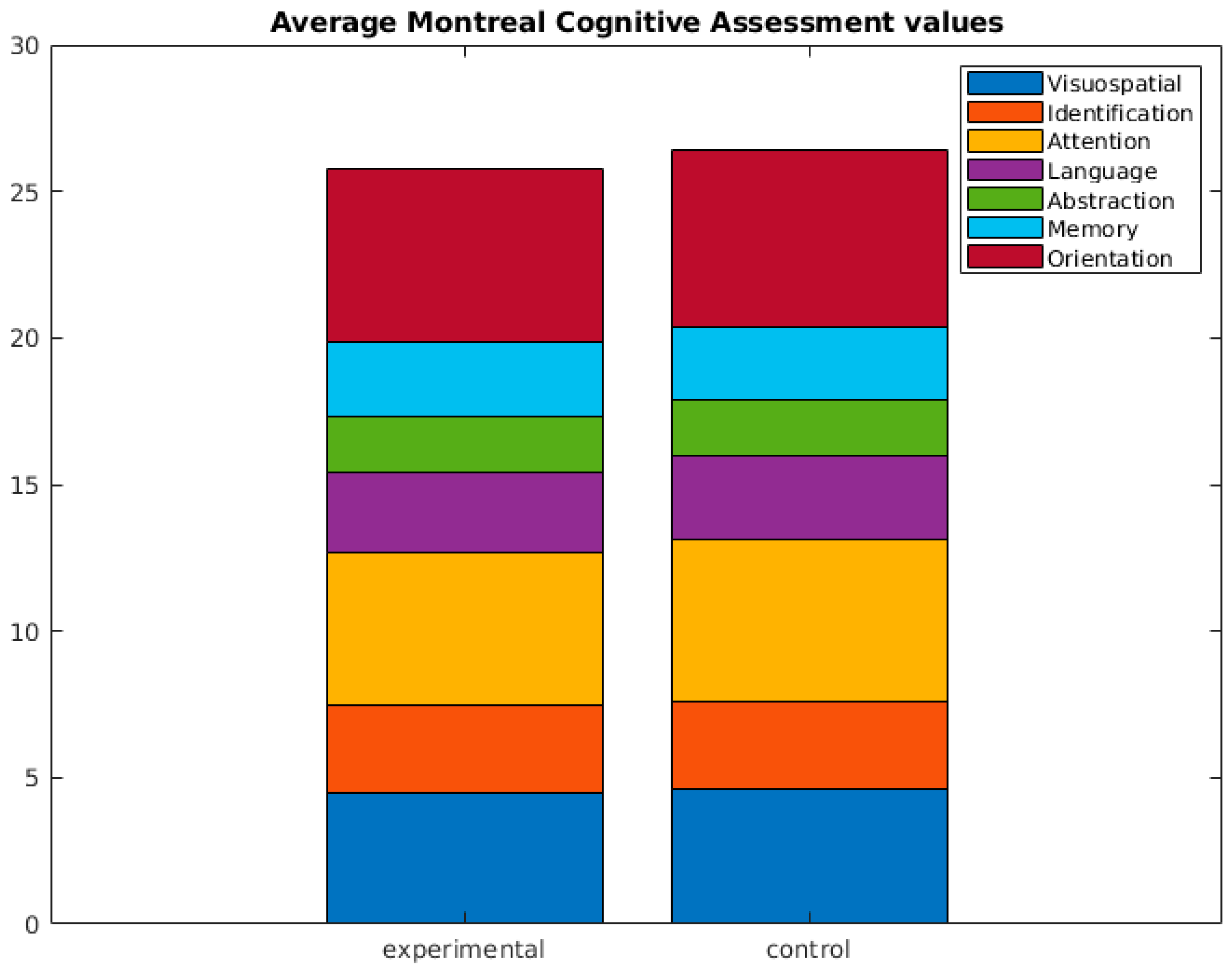
| Montreal Cognitive Assessment (MOCA) | ||||
|---|---|---|---|---|
| Experimental n = 57 Mean (SD) | Control n = 29 Mean (SD) | Statistic (U, p) | ||
| Visuospatial | 4.49 (0.658) | 4.59 (0.733) | 732.5 (0.318) | |
| Identification | 2.98 (0.132) | 3.00 (0.00) | 812 (0.476) | |
| Attention | 5.19 (1.076) | 5.52 (0.829) | 672 (0.115) | |
| Language | 2.74 (0.518) | 2.90 (0.409) | 699.5 (0.078) | |
| Abstraction | 1.91 (0.285) | 1.90 (0.310) | 813.5 (0.813) | |
| Memory | 2.53 (1.324) | 2.48 (1.595) | 811 (0.885) | |
| Orientation | 5.91 (0.285) | 6.00 (0.000) | 754 (0.102) | |
| Total | 25.81 (2.057) | 26.38 (2.321) | 680 (0.174) | |
| List of words | ||||
| Experimental (n = 57) Mean (SD) | Control (n = 29) Mean (SD) | Statistic (t, p) | ||
| Immediate memory | 31.58 (6.636) | 31.07 (4.480) | 0.372 (0.711) | |
| Delayed memory | 7.65 (3.044) | 7.48 (2.721) | 0.248 (0.805) | |
| Recognition | 22.56 (2.163) | 22.90 (1.698) | 750 (0.457) | |
| Rey–Osterrieth Complex Figure Test | ||||
| Experimental (n = 57) Mean (SD) | Control (n = 29) Mean (SD) | Statistic (U, p) | ||
| Copy | Time | 132.29 (39.707) | n = 27; 129 (51.909) | 0.311 (0.757) |
| Score | 33.68 (2.329) | 33.52 (2.879) | 806.5 (0.853) | |
| Immediate memory | Time | 108.46 (53.733) | n = 27; 95.44 (36.297) | 711.5 (0.578) |
| Score | 21.09 (6.602) | 21.26 (7.199) | 807 (0.859) | |
| Delayed memory | Time | 82.44 (35.263) | n = 27; 71.07 (31.006) | 634 (0.194) |
| Score | 19.96 (6.744) | 21.38 (7.009) | 719.5 (0.328) | |
| Digital Span Task | ||||
| Experimental (n = 57) Mean (SD) | Control (n = 29) Mean (SD) | Statistic (U, p) | ||
| Digit Span Forward Test | 3.98 (1.077) | 4.75 (1.481) | 562 (0.022 *) | |
| Digit Span Backward Test | 2.84 (0.941) | 3.18 (1.020) | 632.5 (0.105) | |
| Total Forward Digits | 6.72 (2.374) | 7.25 (2.675) | 707.5 (0.393) | |
| Total Backward Digits | 4.75 (1.864) | 5.07 (1.698) | 690 (0.304) | |
| Trail Making Test (TMT) | ||||
| Experimental (n = 57) Mean (SD) | Control (n = 29) Mean (SD) | Statistic (U, p) | ||
| TMTa | Trials | 23.95 (0.225) | 24 (0) | 783 (0.211) |
| Errors | 0.09 (0.285) | 0 (0) | 754 (0.102) | |
| Time | 38.89 (14.495) | 35.97 (14.618) | 687 (0.202) | |
| TMTb | Trials | 22.74 (1.110) | 22.17 (2.726) | 769 (0.297) |
| Errors | 0.39 (1.176) | 0.83 (2.578) | 773 (0.447) | |
| Time | 77.26 (30.181) | 77.86 (49.713) | 689 (0.209) | |
| Cancellation Task | ||||
| Experimental (n = 57) Mean (SD) | Control (n = 29) Mean (SD) | Statistic (U, p) | ||
| TOT Effectiveness | 285.95 (96.666) | 290.41 (98.925) | 755.5 (0.517) | |
| CON Concentration index | 18.25 (5.190) | 20.48 (4.556) | t = −1.966 (0.053) | |
| TR Total of responses | 296.89 (92.640) | 317.48 (74.601) | 686.5 (0.201) | |
| TA Total of trials | 18.54 (5.352) | 20.48 (4.672) | 667 (0.144) | |
| Commissions | 0.11 (0.409) | 0.10 (0.310) | 802 (0.637) | |
| Omissions | 3.54 (3.576) | 3.24 (2.116) | 776 (0.641) | |
| Rivermead Behavioural Test (RMBT) | ||||
| Experimental (n = 57) Mean (SD) | Control (n = 29) Mean (SD) | Statistic (U. p) | ||
| Recalling the date | 1.51 (0.691) | 1.90 (0.724) | 610 (0.020 *) | |
| Recalling of object | 1.82 (0.475) | 2.00; (0) | 693.5 (0.032 *) | |
| Recalling of place | 1.80 (0.487) | 1.97; (0.186) | 693.5 (0.082) | |
| Digit Symbol Coding | ||||
| Experimental (n = 57) Mean (SD) | Control (n = 29) Mean (SD) | Statistic (U. p) | ||
| Score | 69.157 (17.034) | 75.241 (14.032) | 631 (0.074) | |
| Incidental memory | 4.67 (2.423) | 6.59 (2.529) | 462.5 (0.001 *) | |
| Boston Vocabulary Test | ||||
| Experimental (n = 57) Mean (SD) | Control (n = 29) Mean (SD) | Statistic (U. p) | ||
| Spontaneous answers | 55.42 (3.635) | 55.55 (2.910) | 803.5 (0.832) | |
| Semantic key | 0.32 (0.540) | 0.28 (0.528) | 795 (0.709) | |
| Phonological key | 3.12; 2.673 | 2.38; 1.821 | 729; 0.367 | |
| Verbal Fluency Task | ||||
| Experimental (n = 57) Mean (SD) | Control (n = 29) Mean (SD) | Statistic (t. p) | ||
| Words with p- (Spanish language) | 17.11 (4.742) | 19.07 (4.765) | −2.190 (0.031 *) | |
| Words with s- (Spanish language) | 15.84 (4.279) | 18.00 (4.226) | ||
| Animals | 21.91 (5.149) | 24.52 (4.741) | −2.277 (0.025 *) | |
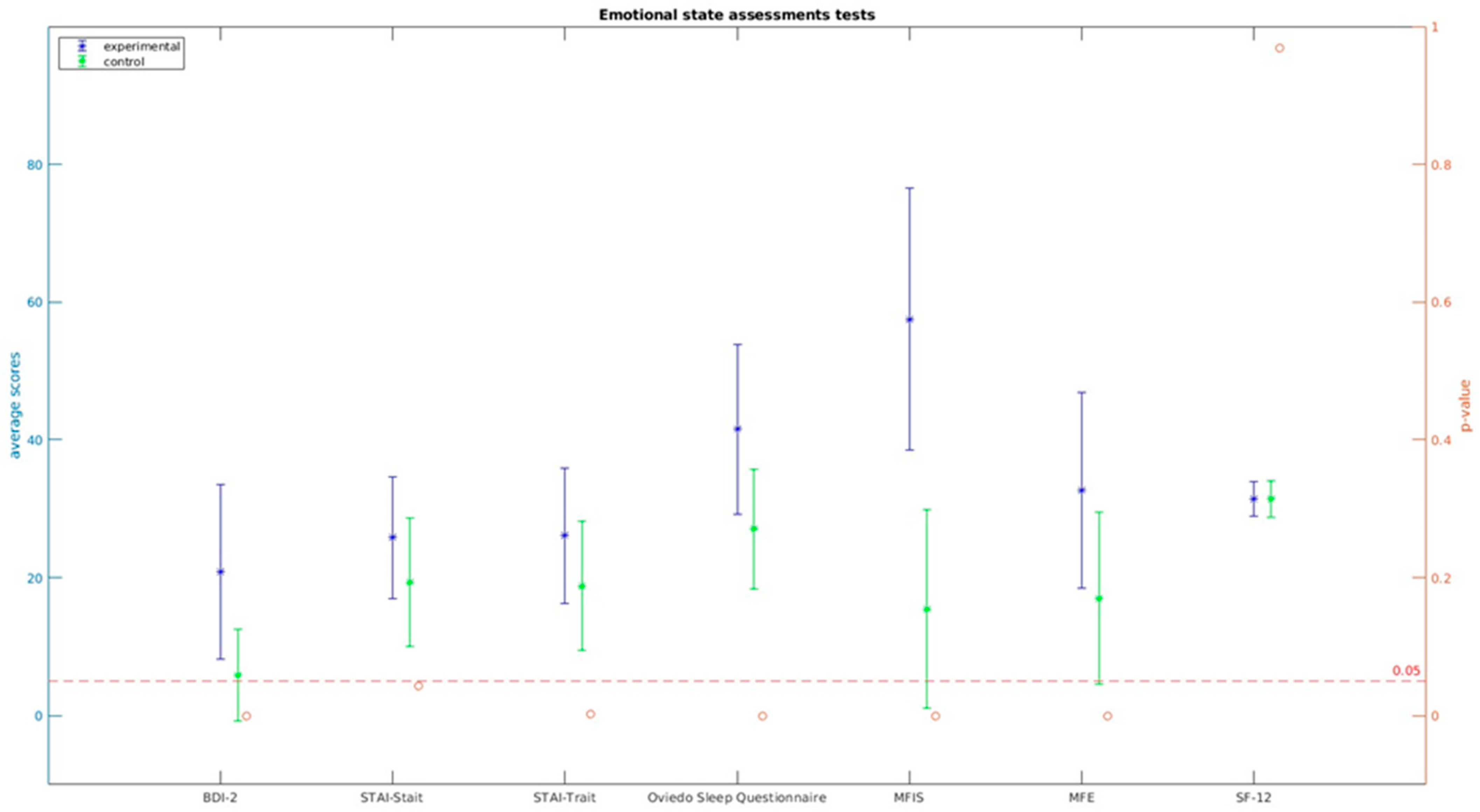
| Experimental (n = 55) Mean (SD) | Control (n = 25) Mean (SD) | Statistic (U. p) | |
|---|---|---|---|
| BDI-2 | 20.88 (12.684) | 5.83 (6.627) | 167.5 (0.0 **) |
| STAI-Stait | 25.79 (8.800) | n = 24; 19.33 (9.375) | 479.5 (0.043 *) |
| STAI-Trait | 26.09 (9.823) | n = 24; 18.79 (9.413) | t = 3.082 (0.003) |
| Oviedo Sleep Questionnaire | 41.55 (12.357) | 27.08 (8.717) | 226 (0.0 **) |
| MFIS | 57.48 (19.012) | 15.48 (14.353) | 72 (0.0 **) |
| MFE | 32.64 (14.189) | 17.00 (12.437) | 306.5 (0.0 **) |
| SF-12 | 31.46 (2.515) | 31.44 (2.599) | 0.040 (0.968) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinar-Herranz, K.; Delgado-Lima, A.H.; Villatoro, B.S.; Garaboa, E.M.; Gómez, V.S.; Vides, L.G.; Bouhaben, J.; Delgado-Losada, M.L. Memory, Emotion, and Quality of Life in Patients with Long COVID-19. Brain Sci. 2023, 13, 1670. https://doi.org/10.3390/brainsci13121670
Espinar-Herranz K, Delgado-Lima AH, Villatoro BS, Garaboa EM, Gómez VS, Vides LG, Bouhaben J, Delgado-Losada ML. Memory, Emotion, and Quality of Life in Patients with Long COVID-19. Brain Sciences. 2023; 13(12):1670. https://doi.org/10.3390/brainsci13121670
Chicago/Turabian StyleEspinar-Herranz, Katrina, Alice Helena Delgado-Lima, Beatriz Sequeira Villatoro, Esther Marín Garaboa, Valeria Silva Gómez, Leonela González Vides, Jaime Bouhaben, and María Luisa Delgado-Losada. 2023. "Memory, Emotion, and Quality of Life in Patients with Long COVID-19" Brain Sciences 13, no. 12: 1670. https://doi.org/10.3390/brainsci13121670
APA StyleEspinar-Herranz, K., Delgado-Lima, A. H., Villatoro, B. S., Garaboa, E. M., Gómez, V. S., Vides, L. G., Bouhaben, J., & Delgado-Losada, M. L. (2023). Memory, Emotion, and Quality of Life in Patients with Long COVID-19. Brain Sciences, 13(12), 1670. https://doi.org/10.3390/brainsci13121670






