The Novel Antipsychotic Lumateperone (Iti-007) in the Treatment of Schizophrenia: A Systematic Review
Abstract
:1. Introduction
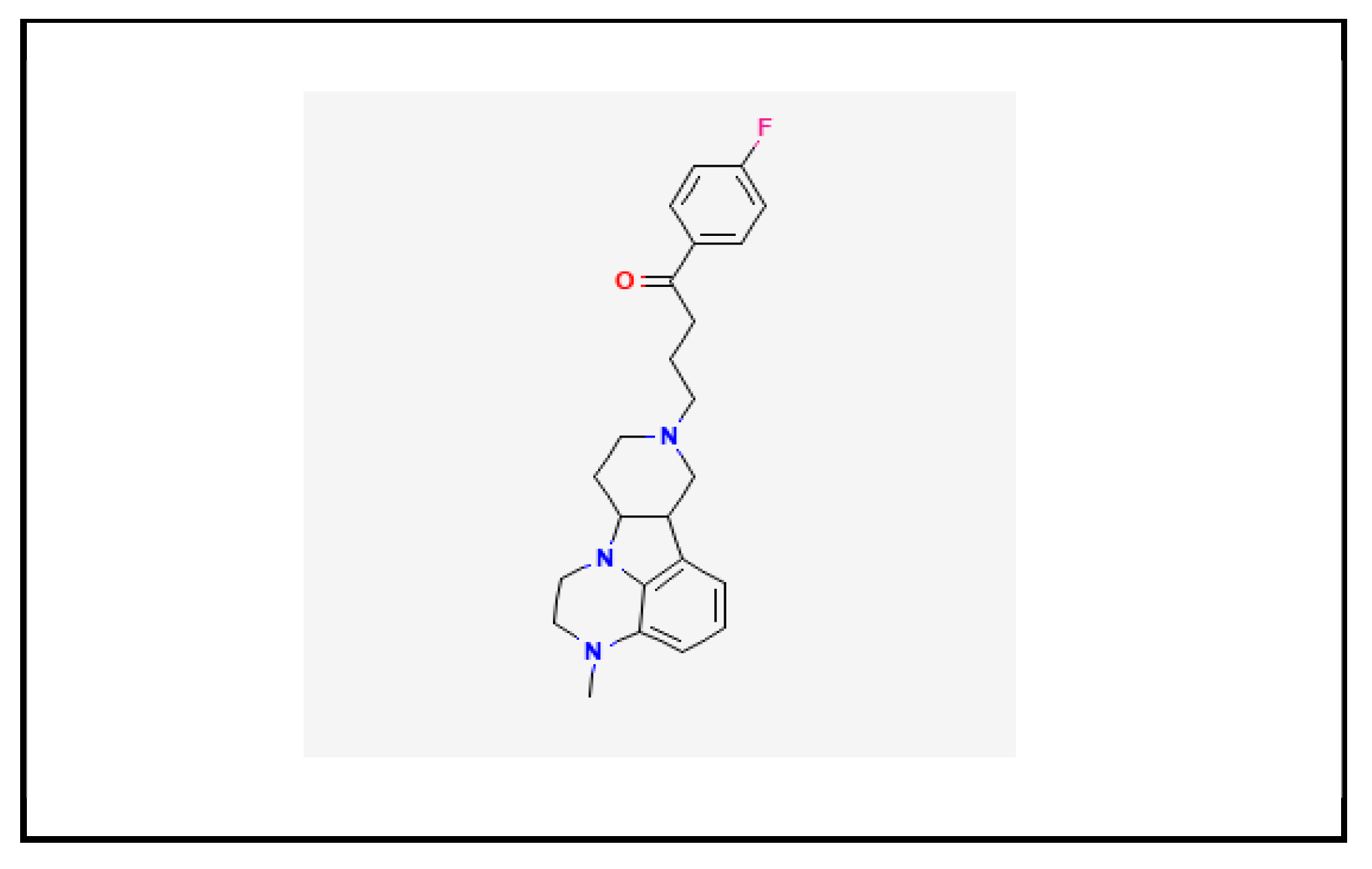
1.1. Chemistry
1.2. Pharmacokinetic Profile
1.3. Pharmacodynamic Profile
1.4. Preclinical Studies
1.5. Posology and Pharmacological Formulation
1.6. Pregnancy and Breastfeeding
1.7. Aims of the Study
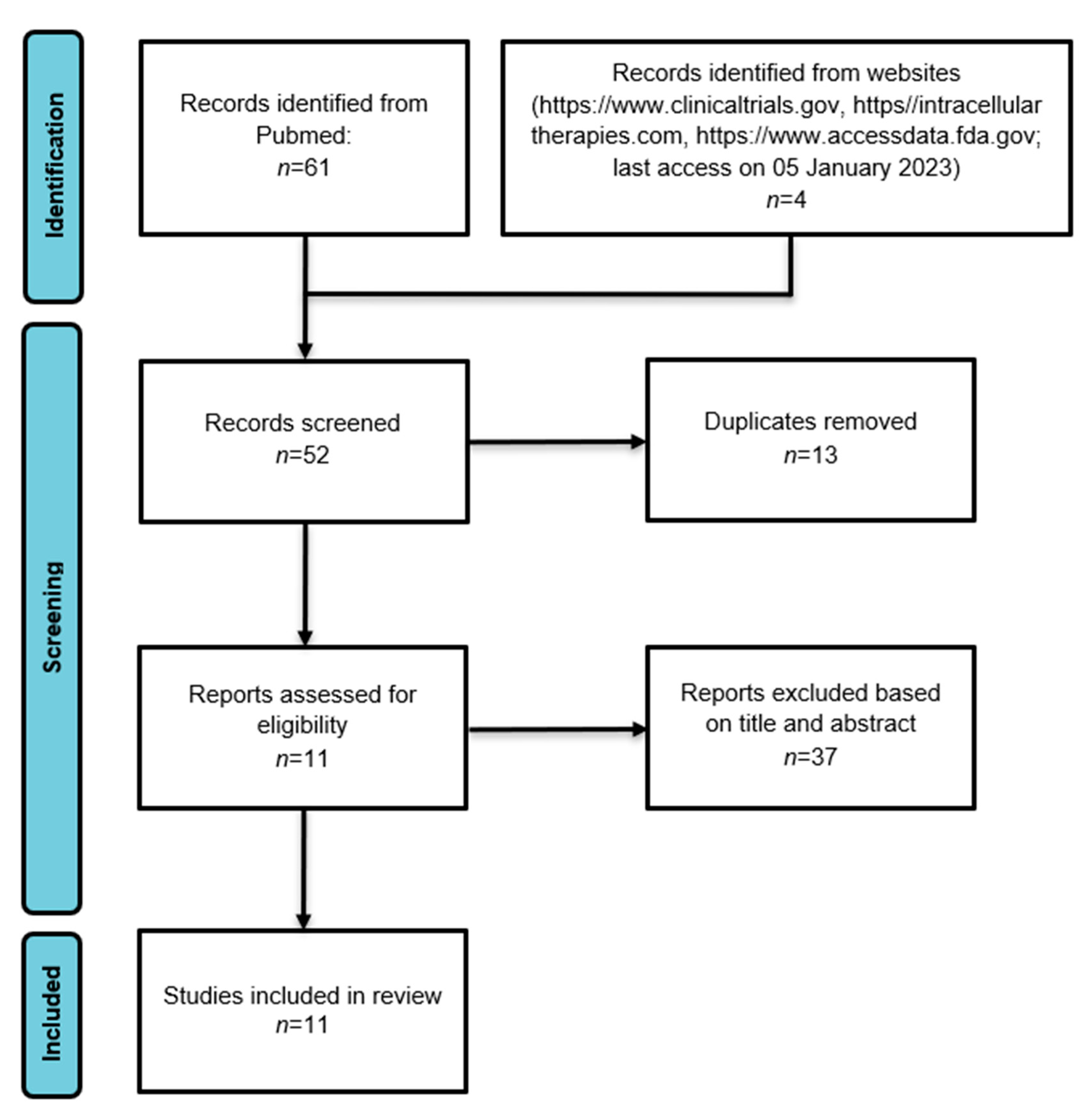
2. Materials and Methods
3. Results
3.1. Phase I Clinical Trials
3.2. Phase II Clinical Trials
3.3. Phase III Clinical Trials
3.4. Further Studies
3.5. Ongoing Studies
4. Discussion
Limitation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Davis, R.; Correll, C.U. ITI-007 in the treatment of schizophrenia: From novel pharmacology to clinical outcomes. Expert. Rev. Neurother. 2016, 16, 601–614. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, J.; Acharya, R.B.; Marcellus, V.; Rey, J.A. Lumateperone: A Novel Antipsychotic for Schizophrenia. Ann. Pharmacother. 2021, 55, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Mazza, M.; Marano, G.; Traversi, G.; Sani, G.; Janiri, L. Evidence on the New Drug Lumateperone (ITI-007) for Psychiatric and Neurological Disorders. CNS Neurol. Disord. Drug Targets 2020, 19, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Reddy, H.M.; Poole, J.S.; Maguire, G.A.; Stahl, S.M. New Medications for Neuropsychiatric Disorders. Psychiatr. Clin. N. Am. 2020, 43, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.A. Lumateperone: First Approval. Drugs 2020, 80, 417–423. [Google Scholar] [CrossRef]
- Intra-Cellular Therapies Inc. U.S. FDA Approval of CAPLYTA (Lumateperone) for the Treatment of Bipolar Depression in Adults; News Release; Intra-Cellular Therapies: New York, NY, USA, 2021; Available online: https://ir.intracellulartherapies.com/news-releases/news-release-details/intra-cellular-therapies-announces-us-fda-approval-caplytar (accessed on 4 February 2022).
- Syed, A.B.; Brašić, J.R. The role of lumateperone in the treatment of schizophrenia. Ther. Adv. Psychopharmacol. 2021, 11, 20451253211034019. [Google Scholar] [CrossRef] [PubMed]
- Intra-Cellular Therapies Inc. An Open-Label Study of Lumateperone as Adjunctive Therapy in the Treatment of Patients with Major Depressive Disorder. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04985942 (accessed on 4 February 2022).
- Ahmed, M.; Malik, M.; Teselink, J.; Lanctôt, K.L.; Herrmann, N. Current Agents in Development for Treating Behavioral and Psychological Symptoms Associated with Dementia. Drugs Aging 2019, 36, 589–605. [Google Scholar] [CrossRef]
- Davis, R.; Saillard, J. Safety and Tolerability of ITI-007 in patients with dementia: A novel treatment designed to treat behavioral disturbances associated with dementia and related disorders. J. Prev. Alzheimers Dis. 2014, 1, 287–288. [Google Scholar]
- Correll, C.U.; Davis, R.E.; Weingart, M.; Saillard, J.; O’Gorman, C.; Kane, J.M.; Lieberman, J.A.; Tamminga, C.A.; Mates, S.; Vanover, K.E. Efficacy and Safety of Lumateperone for Treatment of Schizophrenia: A Randomized Clinical Trial. JAMA Psychiatry 2020, 77, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, Q.; Robichaud, A.J.; Lee, T.; Tomesch, J.; Yao, W.; Beard, J.D.; Snyder, G.L.; Zhu, H.; Peng, Y.; et al. Discovery of a tetracyclic quinoxaline derivative as a potent and orally active multifunctional drug candidate for the treatment of neuropsychiatric and neurological disorders. J. Med. Chem. 2014, 57, 2670–2682. [Google Scholar] [CrossRef]
- Vyas, P.; Hwang, B.J.; Brasic, J.R. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert. Opin. Pharmacother. 2020, 21, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Maini, K.; Hollier, J.W.; Gould, H.; Bollich, V.; John LaForge, J.; Cornett, E.M.; Edinoff, A.N.; Kaye, A.M.; Kaye, A.D. Lumateperone tosylate, A Selective and Concurrent Modulator of Serotonin, Dopamine, and Glutamate, in the Treatment of Schizophrenia. Health Psychol. Res. 2021, 9, 24932. [Google Scholar] [CrossRef]
- Vanover, K.E.; Davis, R.E.; Zhou, Y.; Ye, W.; Brašić, J.R.; Gapasin, L.; Saillard, J.; Weingart, M.; Litman, R.E.; Mates, S.; et al. Dopamine D2 receptor occupancy of lumateperone (ITI-007): A Positron Emission Tomography Study in patients with schizophrenia. Neuropsychopharmacology 2019, 44, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Snyder, G.L.; Vanover, K.E.; Zhu, H.; Miller, D.B.; O’Callaghan, J.P.; Tomesch, J.; Li, P.; Zhang, Q.; Krishnan, V.; Hendrick, J.P.; et al. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology 2015, 232, 605–621. [Google Scholar] [CrossRef] [PubMed]
- Vanover, K.; Glass, S.; Kozauer, S.; Saillard, J.; Sanchez, J.; Weingart, M.; Mates, S.; Satlin, A.; Davis, R. Lumateperone (ITI-007) for the Treatment of Schizophrenia: Overview of Placebo-Controlled Clinical Trials and an Open-label Safety Switching Study. CNS Spectr. 2019, 24, 190–191. [Google Scholar] [CrossRef]
- Li, P.; Snyder, G.L.; Vanover, K.E. Dopamine Targeting Drugs for the Treatment of Schizophrenia: Past, Present and Future. Curr. Top. Med. Chem. 2016, 16, 3385–3403. [Google Scholar] [CrossRef]
- Kantrowitz, J.T. The Potential Role of Lumateperone-Something Borrowed? Something New? JAMA Psychiatry 2020, 77, 343–344. [Google Scholar] [CrossRef]
- Davis, R.; Vanover, K.E.; Zhou, Y.; Brašić, J.R.; Guevara, M.; Bisuna, B.; Ye, W.; Raymont, V.; Willis, W.; Kumar, A.; et al. ITI-007 demonstrates brain occupancy at serotonin 5-HT2A and dopamine D2 receptors and serotonin transporters using positron emission tomography in healthy volunteers. Psychopharmacology 2015, 232, 2863–2872. [Google Scholar] [CrossRef]
- Sutherland, C.; Cohen, P. The alpha-isoform of glycogen synthase kinase-3 from rabbit skeletal muscle is inactivated by p70 S6 kinase or MAP kinase-activated protein kinase-1 in vitro. FEBS Lett. 1994, 338, 37–42. [Google Scholar] [CrossRef]
- Nakazawa, T.; Komai, S.; Tezuka, T.; Hisatsune, C.; Umemori, H.; Semba, K.; Mishina, M.; Manabe, T.; Yamamoto, T. Characterization of Fyn-mediated tyrosine phosphorylation sites on GluRε2 (NR2B) subunit of the N-methyl-D-aspartate receptor. J. Biol. Chem. 2001, 276, 693–699. [Google Scholar] [CrossRef]
- Beaulieu, J.M.; Gainetdinov, R.R.; Caron, M.G. Akt/GSK3 signaling in the action of psychotropic drugs. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 327–347. [Google Scholar] [CrossRef] [PubMed]
- Titulaer, J.; Radhe, O.; Danielsson, K.; Dutheil, S.; Marcus, M.M.; Jardemark, K.; Svensson, T.H.; Snyder, G.L.; Ericson, M.; Davis, R.E.; et al. Lumateperone-mediated effects on prefrontal glutamatergic receptor-mediated neurotransmission: A dopamine D1 receptor dependent mechanism. Eur. Neuropsychopharmacol. 2022, 62, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Svensson, T.; Dutheil, S.; Hendrick, J.; Zhang, L.; Wennogle, L.; O’Gorman, C.; Snyder, G.; Marcus, M.; Sharon, M.; Vanover, K.; et al. Lumateperone uniquely enhcances glutamatergic neurotransimission through activation of both NMDA and AMPA channels via a dopamine D1 receptor dependent mechanism: Implication for treatment of mood disorders. Nuropsychopharmacology 2017, 44, 160. [Google Scholar]
- Lieberman, J.A.; Davis, R.E.; Correll, C.U.; Goff, D.C.; Kane, J.M.; Tamminga, C.A.; Mates, S.; Vanover, K.E. ITI-007 for the Treatment of Schizophrenia: A 4-Week Randomized, Double-Blind, Controlled Trial. Biol. Psychiatry 2016, 79, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Caplyta ® (lumateperone; 2018). NDA 209500. Multi-Disciplinary Review and Evaluation. Version Date: 12 October 2018. Reference ID: 4537404. Multi-Discipline Review. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/209500Orig1s000MultidisciplineR.pdf (accessed on 6 February 2022).
- Mecklenburg, L.; Kusewitt, D.; Kolly, C.; Treumann, S.; Adams, E.T.; Diegel, K.; Yamate, J.; Kaufmann, W.; Müller, S.; Danilenko, D.; et al. Proliferative and non-proliferative lesions of the rat and mouse integument. J. Toxicol. Pathol. 2013, 26 (Suppl. S3), 27S–57S. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, H.; Na, X.L.; Dong, S.Y.; Dong, H.W.; Yu, J.; Jia, L.; Wu, Y.H. Aniline Induces Oxidative Stress and Apoptosis of Primary Cultured Hepatocytes. Int. J. Environ. Res. Public Health 2016, 13, 1188. [Google Scholar] [CrossRef]
- Ma, H.; Wang, J.; Abdel-Rahman, S.Z.; Boor, P.J.; Khan, M.F. Oxidative DNA damage and its repair in rat spleen following subchronic exposure to aniline. Toxicol. Appl. Pharmacol. 2008, 233, 247–253. [Google Scholar] [CrossRef]
- Lenz, B.; Braendli-Baiocco, A.; Engelhardt, J.; Fant, P.; Fischer, H.; Francke, S.; Fukuda, R.; Gröters, S.; Harada, T.; Harleman, H.; et al. Characterizing Adversity of Lysosomal Accumulation in Nonclinical Toxicity Studies: Results from the 5th ESTP International Expert Workshop. Toxicol. Pathol. 2018, 46, 224–246. [Google Scholar] [CrossRef]
- Daniel, W.A. Mechanisms of cellular distribution of psychotropic drugs. Significance for drug action and interactions. Prog. Neuropsychopharmacol. Biol. Psychiatry 2003, 27, 65–73. [Google Scholar] [CrossRef]
- Eftekhari, A.; Azarmi, Y.; Parvizpur, A.; Eghbal, M.A. Involvement of oxidative stress and mitochondrial/lysosomal cross-talk in olanzapine cytotoxicity in freshly isolated rat hepatocytes. Xenobiotica 2016, 46, 369–378. [Google Scholar] [CrossRef]
- Lumateperone. In Drugs and Lactation Database (LactMed); National Institute of Child Health and Human Development: Bethesda, MD, USA, 2006. Available online: https://www.ncbi.nlm.nih.gov/books/NBK582100/ (accessed on 30 November 2022).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Intra-Cellular Therapies Inc. Pharmacokinetics, Safety, and Tolerability of Lumateperone Long-Acting Injectable in Patients with Schizophrenia. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04709224 (accessed on 7 January 2023).
- Intra-Cellular Therapies Inc. Safety, Tolerability and Pharmacokinetics of Lumateperone in Pediatric Patients with Schizophrenia or Schizoaffective Disorder. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04779177 (accessed on 7 January 2023).
- Davis, R.; Dmitrienko, A.; Glass, S.; Kozauer, S.; Saillard, J.; Weingart, M.; Satlin, A.; Mates, S.; Correll, C.; Vanover, K. F46. Lumateperone (ITI-007): Favorable safety profile in an open label safety switching study from standard-of-care antipsychotic therapy in patients with schizophrenia. Schizophr. Bull. 2018, 44 (Suppl. S1), S236–S237. [Google Scholar] [CrossRef]
- Correll, C.U.; Vanover, K.E.; Durgam, S.; Davis, R.; Rowe, W.; Mates, S.; Satlin, A. Results from a 12-month Open-label safety study of Lumateperone (ITI-007) in patients with stable symptoms of Schizophrenia. CNS Spectr. 2020, 25, 317–318. [Google Scholar] [CrossRef]
- Kane, J.M.; Vanover, K.E.; Durgam, S.; Davis, R.; Satlin, A.; Rowe, W.; Mates, S.; Tamminga, C. 185 The Safety and Tolerability of Lumateperone 42 mg for the Treatment of Schizophrenia: A Pooled Analysis of 3 Randomized Placebo-Controlled Trials. CNS Spectr. 2020, 25, 316–317. [Google Scholar] [CrossRef]
- Durgam, S.; Satlin, A.; Davis, R.E.; Vanover, K.E.; Mates, S.; Kane, J.M. T205. Lumateperone in the treatment of schizophrenia: Evaluation of extrapyramidal and motor symptoms in 4 late-phase clinical trialS. Schizophr. Bull. 2020, 46, 310. [Google Scholar] [CrossRef]
- Intra-Cellular Therapies Inc. Lumateperone for the Prevention of Relapse in Patients with Schizophrenia. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04959032 (accessed on 5 February 2022).
- Corponi, F.; Fabbri, C.; Bitter, I.; Montgomery, S.; Vieta, E.; Kasper, S.; Pallanti, S.; Serretti, A. Novel antipsychotics specificity profile: A clinically oriented review of lurasidone, brexpiprazole, cariprazine and lumateperone. Eur. Neuropsychopharmacol. 2019, 29, 971–985. [Google Scholar] [CrossRef] [PubMed]
- Edinoff, A.; Wu, N.; deBoisblanc, C.; Feltner, C.O.; Norder, M.; Tzoneva, V.; Kaye, A.M.; Cornett, E.M.; Kaye, A.D.; Viswanath, O.; et al. Lumateperone for the Treatment of Schizophrenia. Psychopharmacol. Bull. 2020, 50, 32–59. [Google Scholar]
- Limandri, B.J. Lumateperone: New Drug or Same Old Drug With a New Dress? J. Psychosoc. Nurs. Ment. Health Serv. 2020, 58, 9–12. [Google Scholar] [CrossRef]
- Cooper, D.; Gupta, V. Lumateperone. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Kumar, B.; Kuhad, A.; Kuhad, A. Lumateperone: A new treatment approach for neuropsychiatric disorders. Drugs Today 2018, 54, 713–719. [Google Scholar] [CrossRef]
- Kahn, R.S.; Sommer, I.E.; Murray, R.M.; Meyer-Lindenberg, A.; Weinberger, D.R.; Cannon, T.D.; O’Donovan, M.; Correll, C.U.; Kane, J.M.; van Os, J.; et al. Schizophrenia. Nat. Rev. Dis. Primers 2015, 1, 150–167. [Google Scholar] [CrossRef]
- Riboldi, I.; Cavaleri, D.; Capogrosso, C.A.; Crocamo, C.; Bartoli, F.; Carrà, G. Practical Guidance for the Use of Long-Acting Injectable Antipsychotics in the Treatment of Schizophrenia. Psychol. Res. Behav. Manag. 2022, 15, 3915–3929. [Google Scholar] [CrossRef] [PubMed]
- Lumateperone. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012. Available online: https://www.ncbi.nlm.nih.gov/books/NBK574493/ (accessed on 30 November 2022).
- Bergman, H.; Rathbone, J.; Agarwal, V.; Soares-Weiser, K. Antipsychotic reduction and/or cessation and antipsychotics as specific treatments for tardive dyskinesia. Cochrane Database Syst. Rev. 2018, 2, 459. [Google Scholar] [CrossRef] [PubMed]
- FDA. Lumateperone Drug Prescribing. Highlights of Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/209500s005s006lbl.pdf (accessed on 5 February 2022).
| Clinical Trials.gov ID Number (Current Status) Period of Study | Peer-Reviewed Publication and/or Abstract Conference | Study Design | Endpoint Classification | Aim/Hypothesis | Sample Characteristics | Study Description | Outcomes | Adverse Effects Observed during the Studies | |
|---|---|---|---|---|---|---|---|---|---|
| Primary | Secondary | ||||||||
| NCT04709224 ITI-007-025 (completed) November 2021–December 2021. | Unpublished [36] | Open-label study-Phase I. | Pharmacokinetics/Safety/Tolerability. | Study examining the safety, pharmacokinetics, and tolerability of progressively increasing doses of the long-acting injectable formulation of lumateperone in adults diagnosed with schizophrenia, who are clinically stable and have been free from acute exacerbation for a minimum of 3 months. | n = 37. Age: 18–50 years. Diagnosis: schizophrenia diagnosis, clinically stable. | Length: 4 wks. double-blind treatment. Experimental: LAI lumateperone 50 mg subcutaneous on the abdomen, 100 mg subcutaneous on the abdomen, 200 mg subcutaneous on the abdomen, 100–200 mg subcutaneous in the outer area of the upper arm | Cmax Tmax AUC T ½ | Safety/ Tolerability as Measured by Number of Participants with AEs; Change from baseline in: - systolic and diastolic blood pressure; - platelet count; - white blood cell count; - alanine aminotransferase - hemoglobin; - aspartate aminotransferase; - glucose; - creatine kinase; - ECG QTc interval. | Data not available |
| ITI-007-020; NCT04779177 (completed) March 2021–December 2022. | Unpublished [37] | Open-label study-Phase I. | Pharmacokinetics/Safety/Tolerability. | Study assessing the pharmacokinetics, safety, and tolerability of a single oral dose of lumateperone in adolescent patients diagnosed with schizophrenia or schizoaffective disorder, who are clinically stable and have been free from acute exacerbation for at least 3 months. | n = 26. Age: 13–17 yy. Diagnosis: schizophrenia and schizoaffective disorder diagnosis, clinically stable. | Length: 30 days. Experimental: 28 mg/d, 42 mg/d | Cmax Tmax AUC T ½ CL/F | Safety/ Tolerability as Measured by Number of subjects with treatment-emergent adverse events. Change from baseline in: - systolic and diastolic blood pressure; - white blood cell count; - alanine aminotransferase; - aspartate aminotransferase; - hemoglobin; - ECG QTc interval; - Abnormal Involuntary Movement Scale (AIMS). | Data not available |
| NCT01499563 ITI-007-005 (completed) December 2011–November 2013. | [26] | Double-blind, placebo-controlled, randomized, study-Phase II. | Safety/Efficacy study. | Study investigating the safety and effectiveness of lumateperone in adult patients undergoing an acute exacerbation of schizophrenia. | n = 335. Age: 18–55 y. Diagnosis: schizophrenia diagnosis, current psychotic episode. | Length: 4 wks. double-blind treatment. Experimental: 60 mg/d (n = 76), 120 mg/d (n = 80) of oral lumateperone. Comparator: placebo (n = 80) orally. Active comparator: risperidone 4 mg/d (n = 75) | PANSS | PANSS subscales CDSS. | Dry mouth, worsening of schizophrenia, somnolence/sedation, nausea, dizziness. |
| NCT03817528 ITI-007-303a (completed) March 2019–March 2021. | [17,38] | Open-label study-Phase II. | Safety/Efficacy study. | Study exploring efficacy and safety of lumateperone in adults patients affected by stable schizophrenia, who were switched from SOC. | n = 302. Age: 18–60. Diagnosis: schizophrenia diagnosis, stable. | Length: 6 wks. Fw-up: 40 wks. Experimental: lumateperone 60 mg once daily. | TEAEs | PANSS. | Somnolence, dry mouth, headache, migraine, panic attack, hepatic enzyme augmentation, non-cardiac chest pain. |
| NCT03817528 ITI-007-303b Continuation of study ITI-007-303a. | [39] | Open-label study-Phase II. | Safety/Efficacy study. | Study exploring efficacy and safety of lumateperone in adults patients affected by stable schizophrenia, who were switched from SOC. | n= 603. Age: 18–60. Diagnosis: schizophrenia diagnosis, stable. | Length: 120 wk. Experimental: lumateperone 60 mg once daily. | TEAEs | PANSS CDSS. | Anxiety, suicidal ideation, EPS, dizziness, diarrhea, nausea, vomiting, insomnia, fatigue. |
| NCT02288845 ITI-007-008 (completed) October 2014–September 2015. | [15] | Open-label study-Phase II. | Receptor Occupancy/Safety/ Tolerability and Pharmacokinetics | Study evaluating D2 receptor occupancy, tolerability and safety of lumateperone in adult patients affected by schizophrenia in clinical remission. | n = 14. Age:18–60 yy. Diagnosis: schizophrenia diagnosis, in clinical remission and free from acute exacerbation of psychosis. | Length: 2 wks. Experimental: 60 mg/d of oral lumateperone. | Brain Receptor Occupancy as Measured by PET. | Safety/ Tolerability as Measured by Number of Participants with AEs. | Mild to moderate headache, mild sedation. |
| NCT0228276 ITI-007-301 (completed) November 2014– September 2015. | [11] | Double-blind, placebo-controlled, randomized, study-Phase III. | Safety/Efficacy study. | Study assessing the safety and effectiveness of lumateperone in adult patients going through an acute exacerbation of schizophrenia. | n = 450. Age: 18–60 yy. Diagnosis: schizophrenia diagnosis, current psychotic episode. | Length: 4 wks. double-blind treatment. Experimental: 40 mg/d (n = 150), 60 mg/d (n = 150) of oral lumateperone. Comparator: placebo (n = 150) orally. | PANSS | PANSS subscales; CGI-S, CDSS, PSP. | Somnolence, sedation, fatigue, constipation, headache, nausea, dry mouth, dizziness. |
| NCT02469155 ITI-007-302 (completed) June 2015– August 2016. | [17] | Double-blind, placebo-controlled, randomized with active comparator, study-Phase III | Efficacy study | Study evaluating the efficacy of lumateperone in adult patients experiencing an acute exacerbation of schizophrenia. | n = 696. Age: 18–60 yy. Diagnosis: schizophrenia diagnosis, current psychotic episode. | Length: 6 wks. double-blind treatment. Experimental: 20 mg/d (n = 174), 60 mg/d (n = 174) of oral lumateperone. Comparator: placebo (n = 174) orally. Active comparator: risperidone 4 mg/d (n = 174) | PANSS | PANSS subscales. | Somnolence, dry mouth and headache. |
| References | Study Design | Study Description |
|---|---|---|
| [40] | Pooled analysis: NCT00282761 NCT02469155 NCT01499563 | A pooled analysis of three phase II/III clinical trials carried out on individuals with schizophrenia to assess the safety and efficacy of lumateperone. |
| [41] | Pooled analysis: NCT00282761 NCT02469155 NCT01499563 NCT03817528 (b) | A pooled analysis of four phase II/III clinical trials carried out on individuals with schizophrenia in order to evaluate motor symptoms and EPS. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longo, G.; Cicolini, A.; Orsolini, L.; Volpe, U. The Novel Antipsychotic Lumateperone (Iti-007) in the Treatment of Schizophrenia: A Systematic Review. Brain Sci. 2023, 13, 1641. https://doi.org/10.3390/brainsci13121641
Longo G, Cicolini A, Orsolini L, Volpe U. The Novel Antipsychotic Lumateperone (Iti-007) in the Treatment of Schizophrenia: A Systematic Review. Brain Sciences. 2023; 13(12):1641. https://doi.org/10.3390/brainsci13121641
Chicago/Turabian StyleLongo, Giulio, Angelica Cicolini, Laura Orsolini, and Umberto Volpe. 2023. "The Novel Antipsychotic Lumateperone (Iti-007) in the Treatment of Schizophrenia: A Systematic Review" Brain Sciences 13, no. 12: 1641. https://doi.org/10.3390/brainsci13121641
APA StyleLongo, G., Cicolini, A., Orsolini, L., & Volpe, U. (2023). The Novel Antipsychotic Lumateperone (Iti-007) in the Treatment of Schizophrenia: A Systematic Review. Brain Sciences, 13(12), 1641. https://doi.org/10.3390/brainsci13121641








